Abstract
Activated protein C (APC) is a natural anticoagulant that plays a pivotal role in coagulation homeostasis. Severe inherited or acquired deficiency results in a clinical syndrome called purpura fulminans. In addition, APC also appears to have potent cytokine-modifying properties and is protective in animal models of sepsis. The dual functional properties of APC are particularly relevant to severe meningococcemia, where acquired PC deficiency is accompanied by multiorgan failure and purpura fulminans. The authors conducted an open-label prospective study assessing the efficacy of PC replacement therapy in patients with severe meningococcal septicemia, purpura fulminans, and multiorgan failure. The morbidity and mortality were compared with predicted morbidity using the Glasgow Meningococcal Septicemia Prognostic Score. Thirty-six patients with a mean age of 12 years (range 3 months to 72 years) were enrolled in the study. The mean ± SD for plasma PC was 18 ± 7 IU/mL. PC was significantly lower than antithrombin or protein S and was also significantly lower than PC levels in a cohort of patients who developed meningococcemia without multiorgan failure and purpura fulminans. A total of 3 of 36 (8%) patients died, which compares favorably with predicted mortality of 18 of 36 (50%). Amputations were required in 4 of 33 (12%) survivors and in 2 of 31 (6.5%) patients who received PC within 24 hours of admission into the hospital, in comparison with the predicted amputation rate of 11 of 33 (30%). In conclusion, PC replacement therapy in severe meningococcal septicemia was associated with a reduction in predicted morbidity and mortality. The beneficial effect of PC replacement may reflect both the anticoagulant and anti-inflammatory properties of the PC pathway.
Introduction
Meningococcemia in association with purpura fulminans and hemodynamic deterioration continues to have a mortality rate in excess of 50%.1,2 In patients who survive the acute phase of the severe illness, mutilating complications and end-organ failure are common.1,2 Like other inflammatory response syndromes, meningococcemia is associated with clinical and laboratory evidence of disseminate intravascular coagulation (DIC). However, the reduction in protein C (PC) activity is far more severe in this particular sepsis syndrome than in other related conditions.3-5 The precise reason circulating PC should drop to a greater extent than either antithrombin (AT) or protein S (PS) is not fully understood. What is known, however, is that in meningococcemia there is a strong correlation between the severity of the acquired PC deficiency, the extent of the thrombotic skin lesions, and a negative clinical outcome.6 7
The PC anticoagulant mechanism functions in vivo to suppress thrombotic phenomena. This pathway is activated in the microcirculation, where activated PC (APC) is generated “on demand” from PC following the binding of thrombin to the endothelial receptor thrombomodulin.8 APC in collaboration with its cofactor, PS, which circulates free or complexed with the complement regulatory protein C4bBP (60%), inactivates 2 of the cofactors critical for thrombin generation, factors Va and VIIIa, and at the same time promotes fibrinolysis.8 A receptor for PC is located on the vascular endothelium and is called the endothelial protein C receptor (EPCR).9 EPCR augments PC activation on the endothelium by bringing PC into close proximity to the thrombin:thrombomodulin complex and is quickly up-regulated in response to endotoxin, a response that appears to be mediated by thrombin because it is blocked by hirudin thrombin inhibitor.10 11These observations suggest that EPCR plays an important role in the activation of PC and in the regulation of coagulation homeostasis, especially in response to endotoxin.
The activation of PC to APC represents an important host defense mechanism against excessive fibrin formation, and when this pathway fails, purpura fulminans ensues. This syndrome is characterized by DIC and microvascular thrombosis in the dermis, which may ultimately result in renal failure, skin necrosis, gangrene, and amputation. The 3 most common clinical situations where purpura fulminans is seen are severe meningococcal disease, homozygous PC or PS deficiency, and autoimmune PS or PC deficiency.
In addition to its anticoagulant properties, the PC pathway appears to negatively regulate a variety of proinflammatory mediators. APC is protective in animal models of sepsis and down-regulates lipopolysacharide-induced tumor necrosis factor (TNF)-α and interleukin-1β production in monocytes.12-14 This finding is particularly relevant to severe meningococcemia, where TNF-α and interleukin-1β appear to play a pivotal role in the development of multiorgan failure.15-17 Therefore, the acquired PC deficiency that accompanies severe meningococcemia may not only result in purpura fulminans but may also remove a potentially important negative regulator of the host inflammatory response and thus contribute to the mortality.
Recent case reports and one small series has suggested that PC replacement therapy is associated with a reduction in morbidity and mortality in patients with severe meningococcemia and purpura fulminans.18-20 We hypothesized that PC replacement in meningococcemia would reverse purpura fulminans and at the same time improve multiorgan failure associated with proinflammatory cytokine production. We report the results of an open-label prospective study assessing the efficacy of PC replacement therapy in patients with severe meningococcal septicemia, purpura fulminans, and multiorgan failure.
Patients, materials, and methods
Study subjects
Between January 1996 and June 1999, 36 consecutive patients (17 males; 19 females) with severe meningococcemia were treated with PC replacement therapy (Table 1, group I). The mean age ± SD was 12 ± 16.4 years (range 3 months to 76 years). Patients were eligible to receive PC replacement therapy if they had a presumptive diagnosis of meningococcemia with septic shock and purpura fulminans. Septic shock was defined as hypotension (systolic blood pressure below 75 mm Hg for patients younger than 4 years of age and below 80 mm Hg for patients 4 years of age and older) that did not correct with fluid resuscitation and required ionotropic support. Purpura fulminans was defined as the presence of extensive purpuric coalescing skin lesions. The patients were classified by the primary physician at the time of initial consultation with the hematology service. The Glasgow Meningococcal Septicemia Prognostic Score (GMSPS) (Table2 and Table3) was recorded on each patient prior to treatment with PC replacement therapy and used to predict morbidity and mortality.21 22 All patients were treated with conventional antibiotics and fluid resuscitation (40 mL/kg of body weight of colloid solution) and required ionotropic support (with adrenaline, noradrenaline, or dobutamine) and assisted ventilation. Unfractionated heparin at a dose of 10 to 15 IU/kg per hour was used to maintain the patency of dialysis circuits and to inhibit microvascular thrombus formation. We maintained the platelet count and fibrinogen levels above 50 × 109/L and 2 g/L, respectively. A higher than normal threshold was used for fibrinogen concentration because of the use of both heparin and PC concentrate.
Summary of clinical data from patients with severe meningococcemia and purpura fulminans who were treated with PC concentrate
| Parameter . | Results . |
|---|---|
| Mean age (range) | 12 (3 months-72 years) |
| Sex | 17 males; 18 females |
| Mechanical ventilation | 35/36 |
| Ionotropic support | 36/36 |
| PC replacement therapy | 36/36 |
| Mean time to PC therapy (range) | 12 (2-72 hours) |
| Antithrombin III replacement therapy | 2/36 |
| Unfractionated heparin | 26/36 |
| Continuous venovenous hemodiafiltration | 19/36 |
| Peritoneal dialysis | 2/36 |
| Mean GMSPS ± SD (range) | 12 ± 2 (8-15) |
| Predicted mortality | 18/36 (50%) |
| Actual mortality | 3/36 (8%) |
| Predicted amputation rate | 11/33 (30%) |
| Actual amputation rate | 4/33 (12%) |
| Amputation rate in patients who received PC within 24 hours of admission | 2/31 (6.5%) |
| Skin grafting only | 2/33 (13%) |
| Chronic renal failure requiring dialysis | 1/33 |
| Ischemic stroke | 1/33 |
| Severe meningoencephalopathy | 1/33 |
| Full recovery with no complications | 26/36 (72%) |
| Parameter . | Results . |
|---|---|
| Mean age (range) | 12 (3 months-72 years) |
| Sex | 17 males; 18 females |
| Mechanical ventilation | 35/36 |
| Ionotropic support | 36/36 |
| PC replacement therapy | 36/36 |
| Mean time to PC therapy (range) | 12 (2-72 hours) |
| Antithrombin III replacement therapy | 2/36 |
| Unfractionated heparin | 26/36 |
| Continuous venovenous hemodiafiltration | 19/36 |
| Peritoneal dialysis | 2/36 |
| Mean GMSPS ± SD (range) | 12 ± 2 (8-15) |
| Predicted mortality | 18/36 (50%) |
| Actual mortality | 3/36 (8%) |
| Predicted amputation rate | 11/33 (30%) |
| Actual amputation rate | 4/33 (12%) |
| Amputation rate in patients who received PC within 24 hours of admission | 2/31 (6.5%) |
| Skin grafting only | 2/33 (13%) |
| Chronic renal failure requiring dialysis | 1/33 |
| Ischemic stroke | 1/33 |
| Severe meningoencephalopathy | 1/33 |
| Full recovery with no complications | 26/36 (72%) |
Glasgow Meningococcal Septicemia Prognostic Score21
| Parameter . | Points . |
|---|---|
| Blood pressure < 75 mm Hg systolic, age < 4 y | 3 |
| Blood pressure < 80 mm Hg systolic, age ≥ 4 y | 3 |
| Skin/rectal temperature difference > 3°C | 3 |
| Glasgow coma score, modified coma scale score < 8, or deterioration of ≥ 3 points in 1 hour | 3 |
| Deterioration in hour before scoring | 2 |
| Absence of meningism | 2 |
| Extending purpura or widespread ecchymoses | 1 |
| Base deficit > 8 | 1 |
| Maximum score | 15 |
| Parameter . | Points . |
|---|---|
| Blood pressure < 75 mm Hg systolic, age < 4 y | 3 |
| Blood pressure < 80 mm Hg systolic, age ≥ 4 y | 3 |
| Skin/rectal temperature difference > 3°C | 3 |
| Glasgow coma score, modified coma scale score < 8, or deterioration of ≥ 3 points in 1 hour | 3 |
| Deterioration in hour before scoring | 2 |
| Absence of meningism | 2 |
| Extending purpura or widespread ecchymoses | 1 |
| Base deficit > 8 | 1 |
| Maximum score | 15 |
Glasgow coma score as used in the GMSPS
| Modified coma scale parameter . | Points . |
|---|---|
| Eyes open | |
| Spontaneously | 4 |
| To speech | 3 |
| To pain | 2 |
| None | 1 |
| Best verbal response | |
| Oriented | 6 |
| Words | 4 |
| Vocal sounds | 3 |
| Cries | 2 |
| None | 1 |
| Best motor response | |
| Obeys commands | 6 |
| Localizes pain | 4 |
| Moves to pain | 1 |
| None | 0 |
| Modified coma scale parameter . | Points . |
|---|---|
| Eyes open | |
| Spontaneously | 4 |
| To speech | 3 |
| To pain | 2 |
| None | 1 |
| Best verbal response | |
| Oriented | 6 |
| Words | 4 |
| Vocal sounds | 3 |
| Cries | 2 |
| None | 1 |
| Best motor response | |
| Obeys commands | 6 |
| Localizes pain | 4 |
| Moves to pain | 1 |
| None | 0 |
Coagulation parameters were also measured in a control group of 23 consecutive patients (12 males; 11 females) who developed meningococcemia without multiorgan failure or purpura fulminans (group II). The mean age ± SD of these patients was 8 ± 14 years (range 3 months to 72 years).
Ethical approval was obtained from the institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Protein C concentrate
The PC concentrate used in this study was manufactured by monoclonal antibody purification of viral-inactivated prothrombin complex concentrate by Baxter Hyland Immuno (Vienna, Austria). This concentrate undergoes viral inactivation by solvent detergent and vapor-heating methods. After reconstitution, the concentrate contains 125 IU/mL of PC. One unit is defined as the amount of PC in 1 mL of pooled normal plasma. The concentrate was initially administered intravenously as a test dose (10 IU/kg over 10 minutes) followed by a loading dose of 100 IU/kg and a continuous infusion of 10 IU/kg per hour. Thereafter, the dose was adjusted on a daily basis with the aim of maintaining a plasma PC level of 80 IU/mL to 120 IU/mL.
Antithrombin concentrate
AT concentrate (Atenativ, antithrombin III, Kiba Pharmacia, Stockholm, Sweden) was used in 2 patients who had AT levels below 0.30 IU/mL. The AT concentrate was heat treated and purified by affinity chromatography on heparin-Sepharose gel.
Laboratory investigations
Venous whole blood was collected into 0.109-mol/L sodium citrate (Sarstedt Monovette 9NC/3mL) tubes and the plasma separated by centrifugation at 3800g for 10 minutes. PC and PS were measured by clotting assays (Instrumentation Laboratory, Lexicon, MA). AT was measured by chromogenic assay (Instrumentation Laboratory, Milan, Italy). The reference ranges in our laboratory for PC, PS, and AT were 80 to 130 IU/mL, 80 to 140 IU/mL, and 70 to 120 IU/mL, respectively. D-dimers were measured by latex agglutination (Fibronsticon, Organon Teknika, Boxtel, The Netherlands), and a normal value was defined as less than 500 μg/mL. Fibrinogen was determined by Klauss method (Thromboscreen Pacific Haemostasis) with a reference range of 1.5 to 4 g/L. Plasminogen-activator inhibitor-1 (PAI-1) antigen was measured by bioimmunoassay (Chromolize PAI-1, Biopool International, Sweden). The reference range for this assay in our laboratory was 4 to 43 ng/mL.
Results
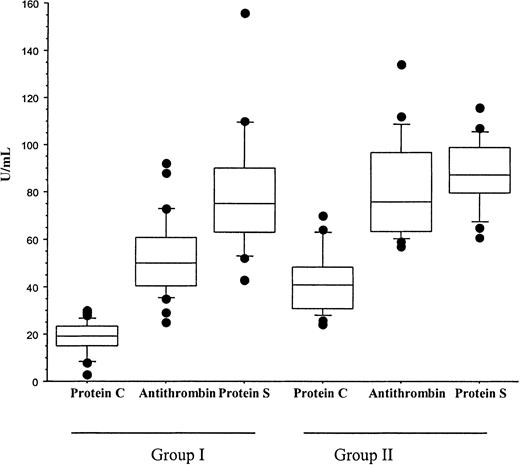
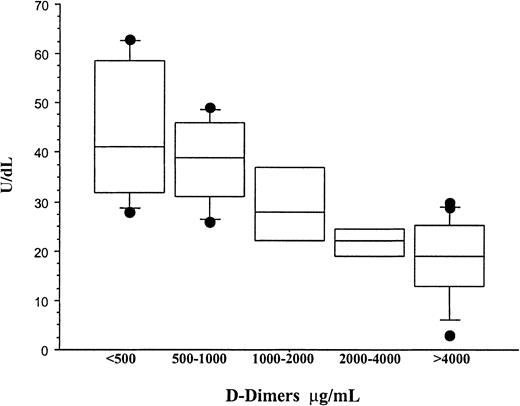
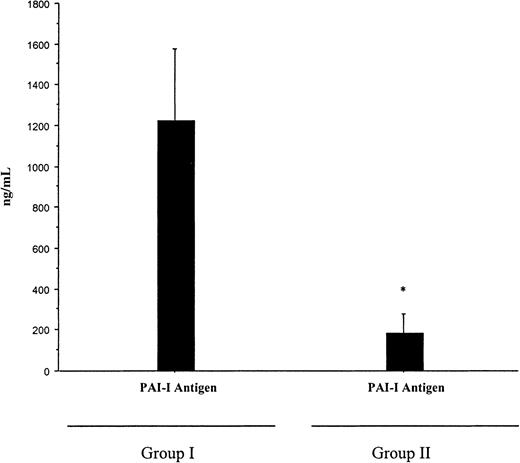
A diagnosis of Neisseria meningitidis was made in all patients by either peripheral blood polymerase chain reaction, blood cultures, or skin scrappings.23 Plasma PC and AT were significantly lower in patients who developed multiorgan failure (group I) than in the cohort patients who had meningococcemia without multiorgan failure or purpura fulminans (group II). The mean ± SD for PC was 18 ± 7 IU/mL versus 41.6 ± 13.3 IU/mL,P < .001; for AT, 53 ± 16 IU/mL versus 81 ± 20 IU/mL, P < .001; and for PS, 74.9 ± 18.8 IU/mL versus 87.4 ± 14.1 IU/mL, P = ns. In addition, plasma PC was significantly reduced in comparison with AT and PS within both groups,P < .01 (Figure 1). While the reduction in PC in all patients was inversely proportional to the concentration of D-dimers, it was still significantly reduced in patients with a normal D-dimer assay (Figure2). This suggests that the reduction in PC does not solely reflect increased consumption. PAI-1 levels were significantly higher in patients with severe meningococcemia (group I) than those with milder disease (group II), 1222.9 ± 1319 ng/mL versus 185 ± 296 ng/mL, P = .02 (Figure3).
PC, PS, and AT in meningococcemia.
This diagram represents PC, AT, and PS values obtained at the time of diagnosis in patients with meningococcemia who developed multiorgan failure and purpura fulminans (group I) and patients who did not develop multiorgan failure and purpura fulminans (group II). PC was significantly lower than AT and PS in patients within group I and group II (P < .001 and P < .001, respectively). In addition, PC and AT levels were significantly lower in group I than group II (P < .001 and P < .001, respectively). Statistical analysis was performed using a Mann-Whitney test.
PC, PS, and AT in meningococcemia.
This diagram represents PC, AT, and PS values obtained at the time of diagnosis in patients with meningococcemia who developed multiorgan failure and purpura fulminans (group I) and patients who did not develop multiorgan failure and purpura fulminans (group II). PC was significantly lower than AT and PS in patients within group I and group II (P < .001 and P < .001, respectively). In addition, PC and AT levels were significantly lower in group I than group II (P < .001 and P < .001, respectively). Statistical analysis was performed using a Mann-Whitney test.
The relationship between the concentration of PC and D-dimers in meningococcemia.
Plasma PC and D-dimers were measured at diagnosis in all patients who developed meningococcemia with or without multiorgan failure or purpura fulminans (groups I and II). D-dimers were measured by a semiquantitative latex agglutination assay, and the patients were separated into distinct groups on the basis of D-dimer values. The mean ± SD PC concentrations for patients with D-dimer values of less than 500 μg/mL, 500 to 1000 μg/mL, 1000 to 2000 μg/mL, 2000 to 4000 μg/mL, and more than 4000 μg/mL were 43.8 ± 14.35 IU/dL, 38.3 ± 9.5 IU/dL, 29.5 ± 9.7 IU/dL, 21.7 ± 3.3 IU/dL, and 17.76 ± 8.5 IU/dL, respectively. Although the concentration of PC was inversely proportional to the D-dimer values, patients with a normal D-dimer assay (< 500 μg/mL) still had significant reduction in PC. This suggests that consumptive coagulopathy is not solely responsible for acquired PC deficiency and that other mechanisms must also be involved.
The relationship between the concentration of PC and D-dimers in meningococcemia.
Plasma PC and D-dimers were measured at diagnosis in all patients who developed meningococcemia with or without multiorgan failure or purpura fulminans (groups I and II). D-dimers were measured by a semiquantitative latex agglutination assay, and the patients were separated into distinct groups on the basis of D-dimer values. The mean ± SD PC concentrations for patients with D-dimer values of less than 500 μg/mL, 500 to 1000 μg/mL, 1000 to 2000 μg/mL, 2000 to 4000 μg/mL, and more than 4000 μg/mL were 43.8 ± 14.35 IU/dL, 38.3 ± 9.5 IU/dL, 29.5 ± 9.7 IU/dL, 21.7 ± 3.3 IU/dL, and 17.76 ± 8.5 IU/dL, respectively. Although the concentration of PC was inversely proportional to the D-dimer values, patients with a normal D-dimer assay (< 500 μg/mL) still had significant reduction in PC. This suggests that consumptive coagulopathy is not solely responsible for acquired PC deficiency and that other mechanisms must also be involved.
The concentration of PAI-1 levels in patients with meningococcemia.
PAI-1 levels were measured by immunologic assay at the time of diagnosis in patients with meningococcemia who developed multiorgan failure and purpura fulminans (group I) and patients who did not develop multiorgan failure and purpura fulminans (group II). PAI-1 was higher in group I (mean ± SD of 1222.9 ± 1319 ng/mL) than in group II (185 ± 296 ng/mL), *P = .02. Statistical analysis was performed using a Mann-Whitney test.
The concentration of PAI-1 levels in patients with meningococcemia.
PAI-1 levels were measured by immunologic assay at the time of diagnosis in patients with meningococcemia who developed multiorgan failure and purpura fulminans (group I) and patients who did not develop multiorgan failure and purpura fulminans (group II). PAI-1 was higher in group I (mean ± SD of 1222.9 ± 1319 ng/mL) than in group II (185 ± 296 ng/mL), *P = .02. Statistical analysis was performed using a Mann-Whitney test.
PC concentrate was commenced within 18 hours in 35 of 37 patients. The mean interval to commencement of PC was 12 hours (range 2 to 72). Nineteen patients underwent continuous venovenous hemodiafiltration, and 2 patients underwent peritoneal dialysis. Heparin was administered to 26 patients. The reason for not using heparin in the remaining patients was either physician preference or failure to correct the coagulation parameters prior to death. No patient who received heparin developed hemorrhagic complications. AT concentrate was administered to 2 patients in whom the AT level was below 30 IU/mL. Both patients made a full and uneventful recovery.
The mean ± SD GMSPS was 12 ± 2, which predicted a mortality of 18 of 36 (50%).22 The actual mortality was 3 of 36 (8%). One patient died from cerebral edema secondary to meningoencephalopathy. One patient died within 1 hour of admission to the hospital from refractory hypotension. The remaining patient died from intracerebral hemorrhage. The PC and fibrinogen levels were undetectable at diagnosis in this patient, and hemorrhage occurred despite replacement therapy with PC concentrate, cryoprecipitate (2 units per 10 kg of body weight of cryoprecipitate), fresh frozen plasma 40 (mL/kg), and platelets (postplatelet count 90 × 109/L). The fibrinogen and PC levels were not measured after replacement therapy and before the intracerebral hemorrhage. This patient did not receive heparin because fibrinogen and platelet counts were below the threshold level at the time of death. One patient recovered but suffered irreversible brain damage. This most likely resulted from meningoencephalopathy or anoxic injury sustained during a prolonged cardiac arrest, which occurred at time of admission prior to PC replacement. No hemorrhage was identified on computed tomography scan of the brain.
Four of the 33 patients who survived the acute phase of the illness required amputation (12%). This compares favorably with the predicted risk of amputation of 33% (11 of 33).7,22,24 25 Two of the patients who required amputation had nonviable limbs prior to the initiation of PC 48 and 72 hours after admission to the hospital. One of these patients also suffered an ischemic stroke. The amputation rate for patients treated within 24 hours of admission to the hospital was 2 of 31 (6.5%). In both cases, PC was commenced within 5 hours after hospital admission. One of the remaining 28 survivors underwent skin grafting, and another patient required chronic hemodialysis. A total of 26 of 36 (72%) of the patients fully recovered with no complications.
Discussion
First described by Vieusseaux in 1805, invasive meningococcal disease is a worldwide public health problem.26 Localized outbreaks in Ireland, which has one of the highest incidences in Europe, continues to cause serious alarm as a result of the fulminant pattern of disease and higher incidence among children and adolescents. Several scoring systems have been used to predict mortality and morbidity for meningococcemia. We selected the GMSPS (Table 2, Table3).21 This score contains several clinical values plus the base deficit, is quick and easy to perform in most clinical settings, and has been retrospectively validated.22 The GMSPS is an accepted scoring system for meningococcal disease and has been used in the recent evaluation of novel treatment strategies in this condition.27,28 In the initial series, a score above 8 predicted 100% mortality. More recent reports describe survival at higher scores, with a mortality of 30% and 50% for scores of 8 and 12, respectively.24 In addition, a score of 10 or greater appears to be associated with a 30% risk of amputation.24The mean ± SD GMSPS in the patients who received PC concentrate was 12 ± 2, which predicted a mortality of 18 of 36 (50%). The actual mortality in this cohort was only 3 of 36 (8%). These patients were treated in 8 different hospitals and therefore do not reflect the experience of a single highly resourced center. Furthermore, the mortality is far lower than previous published data on cohorts of patients that included those with mild, moderate, and severe disease.1,2,6,7 29
It is unlikely that PC replacement had either a positive or negative impact on the outcome of the patient who died from cerebral edema secondary to meningoencephalopathy or the one who survived with the severe neurologic deficit presumed secondary to meningoencephalopathy or anoxic injury. In addition, PC replacement was ineffective in the patient who was premorbid on admission to the hospital, which suggests that this therapeutic option may fail to salvage patients who are in the terminal phase of the sepsis syndrome. Intracerebral hemorrhage has been previously reported in meningococcal infection.30-33Although the mechanism for this complication is unclear, it is likely that the severe deficiency of fibrinogen was an important contributing factor in the patient in this study. The correction of PC to within the normal range would not normally be expected to increase the risk of bleeding. However, it is possible that PC replacement disturbed a finely balanced equilibrium between severe deficiencies of natural anticoagulants and procoagulant and thereby increased the risk of hemorrhage.
We have demonstrated a lower than expected amputation rate of 4 of 33 (12%) in patients with severe meningococcemia and purpura fulminans. This compares favorably with previously published data where 30% to 50% of survivors with similar disease severity required amputations.7,22,25 Furthermore, 2 patients who required amputations already had nonviable limbs at the time of initiation of PC, 48 and 72 hours after admission to the hospital, resulting in an amputation rate for patients treated with PC within 24 hours of admission to the hospital of 2 of 31 (6.5%). These 2 cases demonstrate that early PC replacement may fail to prevent the morbidity associated with purpura fulminans. It is possible that this failure reflects the biological properties of PC, which primarily prevents clot formation rather than lyse established thrombi. However, it is likely that the absolute level of PC is not the sole arbiter in determining either the development of purpura fulminans or response to therapy. This is supported by the absence of purpura fulminans in patients with similar reduction in PC associated with inherited deficiency states.34 35 Furthermore, although patients with severe meningococcemia and purpura fulminans (group I, Figure 2.1) have significantly lower PC levels than those patients with milder disease (group II, Figure 2.1), there is considerable overlap between the 2 groups. Therefore, additional defects within the PC pathway may contribute both to the pathophysiology of purpura fulminans and the effectiveness of PC replacement.
The function of the PC pathway in meningococcemia may be further compromised by increased C4B binding protein36 resulting in decreased free protein S or by a decreased endogenous activation of PC due to down-regulation of endothelial thrombomodulin or EPCR. A reduction in free PS has not been reported in severe meningococcal disease, and we only detected mild reduction in PS using a clotting-based assay that should be sensitive to free PS. However, in vitro studies have demonstrated that endotoxin down-regulates endothelial thrombomodulin, and EPCR expression has been shown to be reduced in biopsies taken from the purpuric skin lesions of patients with meningococcemia.37,38 The possibility that patients who develop purpura fulminans harbor an inherited thrombophilic predisposition was recently investigated.39 The prevalence of the genetic risk factors for thrombosis was found to be no higher than expected on the basis of their prevalence in the general population (PS deficiency, PC deficiency, AT deficiency, APC resistance, factor V Leiden mutation). Other studies have looked at the possible role of the fibrinolytic pathway in meningococcemia. PAI-1 levels appear to correlate with TNF-α concentrations and were found to be approximately twice as high in nonsurvivors at a similar TNF-α concentration.40 This is supported by data from the current study in which PAI-1 levels were significantly higher in patients with severe disease in comparison with those with milder disease (Figure 3). Increased PAI-1 levels have been associated with the 4G to 5G polymorphism within the promoter region of PAI-1 gene, and recent data suggest that this polymorphism is associated with an adverse outcome in severe meningococcal infection.41,42Therefore, the development of purpura fulminans in meningococcemia is likely to result from failure of the PC pathway because of a combination of interactive factors, including activation of the coagulation cascade due to increased monocyte tissue factor expression,43 a rapid reduction in circulating PC, impaired PC activation due to a decreased concentration of endothelial thrombomodulin or EPCR, hypofibrinolysis secondary to increased PAI-1, and poor tissue perfusion due to septic shock, which may be exacerbated by protracted use of potent vasoconstrictive agents. The development of purpura fulminans in other conditions is associated with failure of the PC pathway because of severe inherited or acquired PC or PS deficiency.44 The demonstration that PC is reduced in meningococcemia and that the level is predictive of outcome, together with the reduction in predicted mortality associated with replacement therapy, strongly suggests that acquired PC deficiency plays a important role in the development of purpura fulminans in these patients. However, in some patients APC may be more effective at preventing the complications associated with purpura fulminans because it does not require endogenous activation. Although this treatment strategy is likely to be associated with increased bleeding complications, it does merit investigation especially in those patients with extending purpura fulminans despite PC replacement.
PC was reduced to a far greater extent than the other natural anticoagulants, which is consistent with previously published data (Figure 1) and suggests that it does not solely result from increased consumption. This is supported by the fact that PC was reduced in some patients despite the presence of a normal D-dimer assay (Figure 2). Recent data suggest that TNF-α inhibits the transcription of PC in liver cells45 and that PC inhibitors are increased in sepsis.46 The disproportionate reduction in PC may also result from increased binding to the endothelial PC receptor or monocytes.9 46-49 Therefore, the cause of PC deficiency in meningococcemia may involve consumptive coagulopathy, loss from the intravascular space due to capillary leak, decreased hepatic synthesis, increased binding to PC inhibitors, and increased ligand binding following up-regulation of PC binding sites on mononuclear phagocytes and large-vessel endothelium.
There are always hazards in comparing a nonrandomized clinical trial with contemporary and historical controls. Nevertheless, these data suggest that PC replacement therapy reduces morbidity and mortality in severe meningococcal septicemia. Although PC is not the sole determinant of purpura fulminans, PC replacement therapy may provide a safe and effective means of correcting a critical component in the pathophysiology of this condition. In addition, the anti-inflammatory properties of the PC pathway may negatively regulate the host inflammatory response and improve multiorgan failure and survival. Further work is required to confirm the benefits of PC infusion in severe meningococcemia and to identify more effective treatment strategies for patients who fail to respond to replacement therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Owen P. Smith, Consultant Paediatric Haematologist, National Centre for Inherited Coagulation Disorders, Department of Haematology, St James's Hospital, James's St, Dublin 8, Ireland; e-mail: osmith@stjames.ie.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal