Abstract
High serum concentrations of vascular endothelial growth factor (S-VEGF) and basic fibroblast growth factor (S-bFGF) are associated with unfavorable clinical characteristics in cancer. The combined effect of S-VEGF and S-bFGF on the survival of 200 patients with non-Hodgkin lymphoma (NHL) was studied. High S-VEGF and S-bFGF at diagnosis were associated with poor survival with the medians, the highest tertiles, or the highest quartiles as the cutoff values. The highest prognostic power was obtained when S-VEGF and S-bFGF were examined as a combination. Patients who had both S-VEGF and S-bFGF within the highest quartiles had only a 21% 5-year survival rate in contrast to a 64% 5-year survival rate among patients with both factors within the 3 lowest quartiles (P < .0001). Simultaneous elevation of S-VEGF and S-bFGF was associated with poor survival in different grades of lymphomas and in the largest histologic subgroup, the large-cell diffuse and immunoblastic lymphomas. S-VEGF (relative risk [RR], 1.83; P = .019) and S-bFGF (RR, 2.02; P = .0049) had independent influences on survival in multivariate models when tested together with the components of the International Prognostic Index (IPI). Patients with both S-VEGF and S-bFGF within the highest quartiles had nearly 3 times higher risk for death (RR, 2.90; 95% confidence interval [CI], 1.56-5.40;P = .0008) than the rest of the patients. This RR was higher than the relative risks associated with any of the components of the IPI in the same model. The authors conclude that the combination of S-VEGF and S-bFGF is a powerful prognostic variable in NHL.
Introduction
During tumorigenesis, the vasculature becomes activated to grow new capillaries. This process, which is called angiogenesis, is essential for the growth and dissemination of cancer (reviewed by Folkman1). Angiogenesis is regulated by a balance of various positive and negative angiogenic molecules (reviewed by Hanahan and Folkman2 and Iruela-Arispe and Dvorak3). Vascular endothelial growth factor (VEGF), also called vascular permeability factor (reviewed by Dvorak et al4 and Ferrara and Davis-Smyth5) and basic fibroblast growth factor (bFGF), also called fibroblast growth factor-2 (FGF-2) (reviewed by Friesel and Maciag6 and Bikfalvi et al7) are secreted multifunctional cytokines that are mitogens for endothelial cells and potent inducers of angiogenesis in vivo. Expression of VEGF in tumor xenografts in nude mice has been shown to enhance tumor growth and tumor angiogenesis.8,9In a transgenic mouse model, the angiogenic switch in bovine papilloma virus–induced fibrosarcoma correlates with the export of bFGF from cells.10 The important role of these angiogenic factors has been shown in vivo by immunoneutralizing antibodies against VEGF11-13 and bFGF,14 which inhibit tumor growth in nude mice.
High serum concentrations of VEGF (S-VEGF) in patients with cancer are associated with several unfavorable clinical parameters. These include short tumor volume doubling time,15 progressive disease,16,17 extensive disease,17-21 and poor patient survival.22-24 Similarly, high concentrations of bFGF have been detected in the urine or serum of cancer patients.16,21,25-28 A high serum concentration of bFGF (S-bFGF) has been found to be associated with a large tumor size in head and neck cancer29 and with a short tumor volume doubling time in colorectal cancer.15 In chronic lymphocytic leukemia, the elevated intracellular level of bFGF correlates with stage and is also associated with resistance to chemotherapy.30 Recently, we found that a high pretreatment S-bFGF level is an independent predictor of poor prognosis in non-Hodgkin lymphoma (NHL).31 We now wanted to compare the predictive values of S-VEGF and S-bFGF in NHL and measured them from the serum samples of 200 lymphoma patients taken at the time of the diagnosis and stored at −20°C. We found that a simultaneous elevation in the serum concentrations of both growth factors characterized a subgroup of NHL patients with particularly poor outcome.
Patients, materials, and methods
Patients
Serum VEGF and bFGF concentrations were measured in 200 randomly selected adult patients with non-Hodgkin lymphoma, diagnosed and treated in the Department of Oncology, Helsinki University Central Hospital (Helsinki, Finland), from 1981 to 1987, for whom frozen serum taken at the time of diagnosis but before lymphoma treatment was available. Forty-seven (23.5%) lymphomas had been classified as low grade, 96 (48%) as intermediate grade, and 49 (24.5%) as high grade according to the Working Formulation Scheme32 by a pathologist with a special interest in lymphoma. Eight (4%) patients were considered as unclassifiable. The histologic types of lymphomas in the series, according to the Working Formulation Scheme, were small lymphocytic, consistent with chronic lymphocytic leukemia (CLL; n = 12; 6%); small lymphocytic, plasmacytoid (n = 2; 1%); follicular, predominantly small cleaved cell (n = 24; 12%); follicular, mixed small and large cell (n = 4; 2%); follicular, predominantly large cell (n = 3; 2%); diffuse, small cleaved cell (n = 18; 9%); diffuse, mixed small and large cell (n = 8; 4%); diffuse, large cell (n = 56; 28%); large cell immunoblastic (n = 22; 11%); lymphoblastic (n = 6; 3%); small noncleaved cell, non-Burkitt (n = 6; 3%); small noncleaved cell, Burkitt type (n = 3; 2%); other (n = 36; 18%). Clinical staging was performed according to the Ann Arbor classification system. Evaluation of clinical status, chest radiography, computerized tomography (CT) of the mediastinum and the abdomen, and bone marrow biopsy were performed as staging examinations. Seventy-two (36%) of the patients had stage I, 50 (25%) had stage II, 35 (17.5%) had stage III, and 43 (21.5%) had stage IV disease at diagnosis. Thirty-six (18%) had B symptoms (weight loss, unexplained fever, or night sweats). The IPI33 could be determined in all. In total, 145 patients were treated with standard combination chemotherapy. Patients with intermediate or high-grade lymphoma and disseminated disease were usually treated with bleo-CHOP (bleomycin, cyclophosphamide, doxorubicin, vincristine, prednisone), M-BACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone), or another anthracycline-containing combination chemotherapy regimen. Patients with low-grade lymphomas were usually treated with a single-agent chlorambucil if they were symptomatic. Sixty-five patients received megavoltage radiotherapy. Patients were regularly followed-up at intervals of a few months in an outpatient department. All patients were observed for 5 years or until death. During the follow-up time, 105 patients died. The median follow-up time for the surviving patients was 86 months (range, 62 to 180 months).
Methods
Venous blood samples.
Peripheral venous blood samples were collected in sterile test tubes a few hours or a few days before lymphoma therapy was begun. Samples were centrifuged at 2000g for 10 minutes and stored at −20 °C.
Serum VEGF and bFGF immunoassay.
Serum VEGF concentrations were determined as serum VEGF immunoreactivity using a quantitative sandwich enzyme immunoassay technique (Quantikine Human VEGF Immunoassay; R&D Systems, Minneapolis, MN) as described earlier.22 Serum bFGF concentrations were determined as serum bFGF immunoreactivity using a quantitative sandwich enzyme immunoassay technique (Quantikine High-Sensitivity Human FGF Basic Immunoassay; R&D Systems) as described earlier.31Both systems use a solid-phase monoclonal and an enzyme-linked polyclonal antibody raised against recombinant human VEGF or bFGF. For each analysis, 100 μL serum was used. All analyses and calibrations were carried out in duplicate. The calibrations on each microtiter plate included recombinant human VEGF or bFGF standards. Optical densities were determined using a microtiter palate reader (Multiscan RC Type 351; Labsystems, Helsinki, Finland) at 450 nm for VEGF and at 490 nm for bFGF. The blank was subtracted from the duplicate readings for each standard and sample. A standard curve was created by plotting the logarithm of the mean absorbance of each standard versus the logarithm of the cytokine concentration. Concentrations are reported as picograms per milliliter.
Statistical analysis.
Statistical analyses were performed using the software package Stat-View 5.01 (SAS Institute, Cary, NC). The Mann-Whitney test, the Spearman rank correlation coefficient, the Fisher exact test, and the Kruskal-Wallis test were used to compare serum VEGF and bFGF concentrations in different groups. Cumulative survival was computed according to the product-limit method of Kaplan-Meier from the date of the diagnosis. The log rank (Mantel-Cox) test was used to compare survival rates of the different subgroups of patients. The relative influence of different variables on survival was studied in multivariate survival analyses using the parametric model of Weibull and the nonparametric proportional hazards model of Cox. Prognostic factors introduced in the models are commonly accepted and have been reported33 by the International Non-Hodgkin's Lymphoma Prognostic Factors Project on a large series of patients. All Pvalues are 2-tailed.
Results
Serum VEGF and bFGF concentrations in NHL patients at diagnosis
Serum VEGF concentrations at diagnosis among the 200 patients with NHL ranged from undetectable to 1290 pg/mL (median, 262 pg/mL; mean, 338 pg/mL). One third of the patients had S-VEGF greater than or equal to 390 pg/mL (the highest tertile), and one fourth had S-VEGF greater than or equal to 462 pg/mL (the highest quartile). The bFGF concentrations in the same serum samples ranged from undetectable to 34.7 pg/mL (median, 3.3 pg/mL; mean, 4.4 pg/mL). One third of the patients had S-bFGF greater than or equal to 4.9 pg/mL (the highest tertile), and one fourth had S-bFGF greater than or equal to 5.5 pg/mL (the highest quartile).
Serum VEGF and bFGF concentrations and clinical features
There was no significant difference in the serum VEGF or bFGF concentrations between patients with low-grade lymphoma and those with intermediate or high-grade lymphoma (P > .1 for both comparisons). A high pretreatment S-VEGF was associated with a low World Health Organization (WHO) performance status (0-1 vs 2-4;P = .0015). Women were found to have higher S-bFGF levels than men had (median, 4.2 pg/mL; range, 0-34.7 pg/mL vs median, 2.7 pg/mL; range, 0-15.9 pg/mL, respectively; P = .037). No significant associations were found between pretreatment S-VEGF or S-bFGF and age at diagnosis, Ann Arbor stage, histologic grade, number of extranodal tumor sites, or presence of B symptoms (P > .1 for all comparisons). When patients with different histologic types of lymphoma (according to the Working Formulation Scheme) were compared, no significant differences were found in the S-VEGF or S-bFGF levels between the different histologic types (P > .1 for all comparisons). Pretreatment serum levels of VEGF or bFGF did not associate with the patients' assessed response to lymphoma therapy (P > .1 for all comparisons). Because bFGF may clear through the kidney,34we compared the patients' serum bFGF and VEGF levels to kidney function. Analyses showed that serum bFGF or VEGF levels in the patients with high (greater than 100 μmol/L) serum creatinine were comparable to those found in patients with normal serum creatinine (P > .1 for both comparisons).
We also studied how the combination of VEGF and bFGF relates to the clinical features. Patients with both S-VEGF and S-bFGF simultaneously within the highest quartiles (S-VEGF 462 pg/mL or more and S-bFGF 5.5 pg/mL or more) were compared to the rest of the patients. However, no significant associations were found between the combination of elevated S-VEGF and S-bFGF and the age at diagnosis, gender, the Ann Arbor stage, the number of extranodal tumor sites, or the presence of B symptoms (P > .1 for all comparisons). Simultaneous elevation of S-VEGF and S-bFGF was associated with a low WHO performance status (P = .015), but the association was weaker than the association between WHO performance and high S-VEGF alone (S-VEGF greater than or equal to vs less than 462 pg/mL;P = .0027).
Serum VEGF and bFGF concentrations and hematologic variables
High S-VEGF was associated with a high serum lactate dehydrogenase (LDH) level (P = .0036, Spearman rank correlation; Table1). S-bFGF and serum LDH were also positively associated, but their association was considerably weaker (P = .046; Table 1). High S-VEGF was strongly associated with a high leukocyte count and a high thrombocyte count (P < .0001 for both comparisons; Table 1). A high thrombocyte count was also strongly associated with a high S-bFGF (P < .0001; Table 1), but there was no association between S-bFGF and the leukocyte count (P > .05; Table1). In addition, a high S-VEGF was associated with a high S-bFGF (P = .0003; Table 1).
Associations between serum VEGF and bFGF concentrations and hematologic variables
| . | S-VEGF . | S-bFGF . | S-LDH . |
|---|---|---|---|
| S-bFGF | 0.0003 | * | 0.046 |
| S-LDH | 0.0036 | 0.046 | * |
| Leukocyte count | <0.0001 | NS | NS |
| Thrombocyte count | <0.0001 | <0.0001 | NS |
| . | S-VEGF . | S-bFGF . | S-LDH . |
|---|---|---|---|
| S-bFGF | 0.0003 | * | 0.046 |
| S-LDH | 0.0036 | 0.046 | * |
| Leukocyte count | <0.0001 | NS | NS |
| Thrombocyte count | <0.0001 | <0.0001 | NS |
Spearman rank correlation was determined. P > .05 was considered nonsignificant (NS).
Serum VEGF and bFGF concentrations in univariate survival analyses
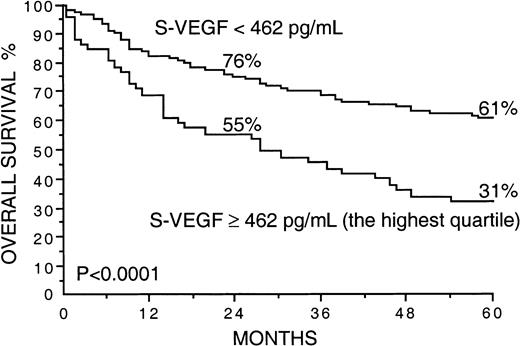
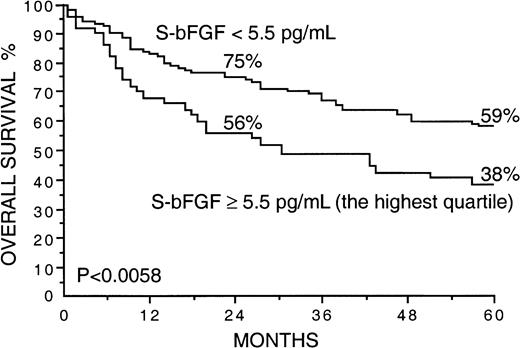
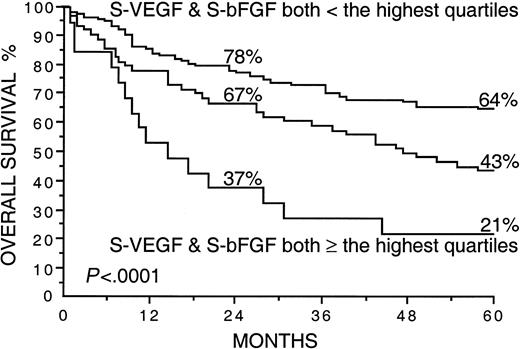
Several factors correlated strongly with overall survival in univariate survival analyses in the current series (Table2). Patients with high S-VEGF or S-bFGF concentrations at diagnosis had inferior overall survival rates than those with lower serum concentrations of VEGF or bFGF (Table 2). The 5-year survival rate of patients with S-VEGF within the highest quartile (S-VEGF, 462 pg/mL or more) was only 31% in comparison to the 61% 5-year survival rate found among patients with S-VEGF lower than 462 pg/mL (P < .0001; Figure1, Table 2). Similarly, the 5-year survival rate of the patients with S-bFGF within the highest quartile (S-bFGF, 5.5 pg/mL or more) was 38%, in contrast to the 59% 5-year survival rate of those patients with S-bFGF lower than 5.5 pg/mL (P = .0058; Figure 2, Table2). High S-VEGF or S-bFGF levels were also associated with poor prognosis when the medians (P = .027 andP = .029, respectively) or the highest tertiles (P = .0039 and P = .013, respectively) were used as cutoff values in univariate survival analyses. However, the use of the highest quartiles as cutoff values provided the highest statistical significance for both S-VEGF and S-bFGF. Consequently, the quartiles were used as cutoff values in further analyses. The strongest prognostic power in univariate analyses was obtained when S-VEGF and S-bFGF were used as a combined parameter. Patients who had both S-VEGF and S-bFGF within the highest quartiles of the respective measurement had as low as 21% 5-year survival rate, in contrast to the 64% 5-year survival rate of those patients with both S-VEGF and S-bFGF within the 3 lowest quartiles (P < .0001; Figure3; Table 2). As can be seen in Figure 3, the patients with one factor within the highest quartile and the other factor within the 3 lowest quartiles had a survival rate that was between the survival rates of the patients with both factors simultaneously elevated or simultaneously at a lower level.
Univariate survival analyses of 200 NHL patients
| Factor . | 5-year survival (%) . | P . |
|---|---|---|
| IPI | ||
| ≤1 | 74 | <.0001 |
| >1 | 30 | |
| Age at diagnosis (y) | ||
| ≤60 | 60 | <.0001 |
| >60 | 39 | |
| WHO performance status | ||
| 0-1 | 60 | <.0001 |
| 2-4 | 21 | |
| Ann Arbor stage | ||
| I-II | 64 | <.0001 |
| III-IV | 37 | |
| Serum LDH at diagnosis | ||
| Normal | 64 | <.0001 |
| Abnormal | 28 | |
| Presence of B symptoms | ||
| No | 60 | <.0001 |
| Yes | 25 | |
| Histologic grade | ||
| Low | 68 | .0028 |
| Intermediate or high | 49 | |
| No. extranodal sites | ||
| 0-1 | 56 | .0064 |
| >1 | 28 | |
| Serum VEGF at diagnosis | ||
| <462 pg/mL | 61 | <.0001 |
| ≥462 pg/mL (highest quartile) | 31 | |
| Serum bFGF at diagnosis | ||
| <5.5 pg/mL | 59 | .0058 |
| ≥5.5 pg/mL (highest quartile) | 38 | |
| Serum VEGF and bFGF at diagnosis | ||
| both < highest quartile | 64 | <.0001 |
| both within highest quartile | 21 |
| Factor . | 5-year survival (%) . | P . |
|---|---|---|
| IPI | ||
| ≤1 | 74 | <.0001 |
| >1 | 30 | |
| Age at diagnosis (y) | ||
| ≤60 | 60 | <.0001 |
| >60 | 39 | |
| WHO performance status | ||
| 0-1 | 60 | <.0001 |
| 2-4 | 21 | |
| Ann Arbor stage | ||
| I-II | 64 | <.0001 |
| III-IV | 37 | |
| Serum LDH at diagnosis | ||
| Normal | 64 | <.0001 |
| Abnormal | 28 | |
| Presence of B symptoms | ||
| No | 60 | <.0001 |
| Yes | 25 | |
| Histologic grade | ||
| Low | 68 | .0028 |
| Intermediate or high | 49 | |
| No. extranodal sites | ||
| 0-1 | 56 | .0064 |
| >1 | 28 | |
| Serum VEGF at diagnosis | ||
| <462 pg/mL | 61 | <.0001 |
| ≥462 pg/mL (highest quartile) | 31 | |
| Serum bFGF at diagnosis | ||
| <5.5 pg/mL | 59 | .0058 |
| ≥5.5 pg/mL (highest quartile) | 38 | |
| Serum VEGF and bFGF at diagnosis | ||
| both < highest quartile | 64 | <.0001 |
| both within highest quartile | 21 |
Cumulative survival from the diagnosis was computed using the product-limit method. The log rank test was used to compare the different groups.
Overall survival rates of 200 patients with non-Hodgkin lymphoma by the pretreatment S-VEGF.
The highest quartile (S-VEGF, 462 pg/mL or more) was used as the cut-off value. Survival rates at 24 and 60 months are given.
Overall survival rates of 200 patients with non-Hodgkin lymphoma by the pretreatment S-VEGF.
The highest quartile (S-VEGF, 462 pg/mL or more) was used as the cut-off value. Survival rates at 24 and 60 months are given.
Overall survival rates of 200 patients with non-Hodgkin lymphoma by the pretreatment S-bFGF.
The highest quartile (S-bFGF, 5.5 pg/mL or more) was used as the cutoff value. Survival rates at 24 and 60 months are given.
Overall survival rates of 200 patients with non-Hodgkin lymphoma by the pretreatment S-bFGF.
The highest quartile (S-bFGF, 5.5 pg/mL or more) was used as the cutoff value. Survival rates at 24 and 60 months are given.
Overall survival rates of 200 patients with non-Hodgkin lymphoma by the pretreatment S-VEGF and S-bFGF.
The highest quartiles (S-VEGF, 462 pg/mL or more; S-bFGF, 5.5 pg/mL or more) were used as the cutoff values. Survival rates at 24 and 60 months are given. The plotted cumulative survival lines are shown for the patients with both S-VEGF and S-bFGF within the highest quartiles, for the patients with both S-VEGF and S-bFGF within the 3 lowest quartiles, and for the patients who had one of the factors within the highest quartile and the other factor within the 3 lowest quartiles (the line in the middle).
Overall survival rates of 200 patients with non-Hodgkin lymphoma by the pretreatment S-VEGF and S-bFGF.
The highest quartiles (S-VEGF, 462 pg/mL or more; S-bFGF, 5.5 pg/mL or more) were used as the cutoff values. Survival rates at 24 and 60 months are given. The plotted cumulative survival lines are shown for the patients with both S-VEGF and S-bFGF within the highest quartiles, for the patients with both S-VEGF and S-bFGF within the 3 lowest quartiles, and for the patients who had one of the factors within the highest quartile and the other factor within the 3 lowest quartiles (the line in the middle).
In patients with intermediate or high-grade lymphoma (n = 145) the 5-year survival rate of the patients within the highest quartile of S-VEGF (S-VEGF, 487 pg/mL or more) was only 32% in contrast to the 55% 5-year survival rate of those patients with a lower S-VEGF (P = .0035; Table 3). Similarly, the 5-year survival rate of the patients having S-bFGF within the highest quartile (S-bFGF, 6.1 pg/mL or more) was 34%, whereas the 5-year survival rate among patients with lower S-bFGF was 54% (P = .014; Table 3). The high predictive power of the combined assessment of S-VEGF and S-bFGF was evident; patients with intermediate or high-grade lymphoma had serum concentrations for both factors within the highest quartiles that gave them only an 8% 5-year survival rate, in contrast to the 57% 5-year survival rate of patients with both S-VEGF and S-bFGF within the 3 lower quartiles (P < .0001; Table 3). Results were similar to those in low-grade lymphoma (n = 47), where patients with both a high S-VEGF and a high S-bFGF had especially poor outcome (the 5-year survival rate 14%; P = .0010; Table 3).
Summary of univariate survival analyses by pretreatment S-VEGF and S-bFGF in different subgroups of 200 patients with NHL
| . | n . | 5-year survival (%) . | P . |
|---|---|---|---|
| Intermediate and high-grade lymphomas (n = 145) | |||
| Serum VEGF at diagnosis | |||
| < highest quartile | 109 | 55 | <.0035 |
| highest quartile | 36 | 32 | |
| Serum bFGF at diagnosis | |||
| < highest quartile | 109 | 54 | .014 |
| highest quartile | 36 | 34 | |
| Serum VEGF and bFGF at diagnosis | |||
| both < highest quartile | 85 | 57 | <.0001 |
| both highest quartile | 12 | 8 | |
| Low grade lymphomas (n = 47) | |||
| Serum VEGF at diagnosis | |||
| < highest quartile | 35 | 79 | .016 |
| highest quartile | 12 | 39 | |
| Serum bFGF at diagnosis | |||
| < highest quartile | 35 | 74 | .26 |
| highest quartile | 12 | 54 | |
| Serum VEGF and bFGF at diagnosis | |||
| both < highest quartile | 28 | 75 | .0010 |
| both highest quartile | 7 | 14 | |
| Large cell diffuse and immunoblastic lymphomas (n = 78) | |||
| Serum VEGF at diagnosis | |||
| < highest quartile | 58 | 53 | .0095 |
| highest quartile | 20 | 30 | |
| Serum bFGF at diagnosis | |||
| < highest quartile | 58 | 55 | .020 |
| highest quartile | 20 | 25 | |
| Serum VEGF and bFGF at diagnosis | |||
| both < highest quartile | 46 | 57 | <.0001 |
| both highest quartile | 8 | 0 |
| . | n . | 5-year survival (%) . | P . |
|---|---|---|---|
| Intermediate and high-grade lymphomas (n = 145) | |||
| Serum VEGF at diagnosis | |||
| < highest quartile | 109 | 55 | <.0035 |
| highest quartile | 36 | 32 | |
| Serum bFGF at diagnosis | |||
| < highest quartile | 109 | 54 | .014 |
| highest quartile | 36 | 34 | |
| Serum VEGF and bFGF at diagnosis | |||
| both < highest quartile | 85 | 57 | <.0001 |
| both highest quartile | 12 | 8 | |
| Low grade lymphomas (n = 47) | |||
| Serum VEGF at diagnosis | |||
| < highest quartile | 35 | 79 | .016 |
| highest quartile | 12 | 39 | |
| Serum bFGF at diagnosis | |||
| < highest quartile | 35 | 74 | .26 |
| highest quartile | 12 | 54 | |
| Serum VEGF and bFGF at diagnosis | |||
| both < highest quartile | 28 | 75 | .0010 |
| both highest quartile | 7 | 14 | |
| Large cell diffuse and immunoblastic lymphomas (n = 78) | |||
| Serum VEGF at diagnosis | |||
| < highest quartile | 58 | 53 | .0095 |
| highest quartile | 20 | 30 | |
| Serum bFGF at diagnosis | |||
| < highest quartile | 58 | 55 | .020 |
| highest quartile | 20 | 25 | |
| Serum VEGF and bFGF at diagnosis | |||
| both < highest quartile | 46 | 57 | <.0001 |
| both highest quartile | 8 | 0 |
Highest quartiles of S-VEGF and S-bFGF within the respective subgroups were used as cutoff values.
The association between S-VEGF, S-bFGF, and survival was also studied separately in the subgroup of large cell diffuse and immunoblastic lymphomas. This was the largest histologic subgroup (n = 78) in the current series. When the highest quartile of S-VEGF within this subgroup (S-VEGF, 499 pg/mL or more) was used as the cutoff value, the patients within the highest quartile had a 5-year survival rate of 30% in comparison to the 53% 5-year survival rate of the patients with lower S-VEGF (P = .0095; Table 3). Patients within the highest quartile of S-bFGF (S-bFGF, 6.2 pg/mL or more) had a 5-year survival rate of 25% in contrast to the 55% 5-year survival rate of the patients with lower S-bFGF (P = .020; Table 3). Patients with large cell diffuse or immunoblastic lymphoma, who had both S-VEGF and S-bFGF within their highest quartiles, had 2- and 5-year survival rates as low as 13% and 0%, whereas those patients with both S-VEGF and S-bFGF had 2- and 5-year survival rates of 76% and 57% (P < .0001; Table 3).
Because VEGF in the circulation is found in blood cells, including platelets and leukocytes,35 36 we studied whether platelet count or leukocyte count had any prognostic value in the current series. However, no associations were found between platelet count or leukocyte count and survival (tested using the medians as the cutoff values; P > .1 for both comparisons).
Serum VEGF and bFGF concentrations and the components of the International Prognostic Index in univariate survival analyses
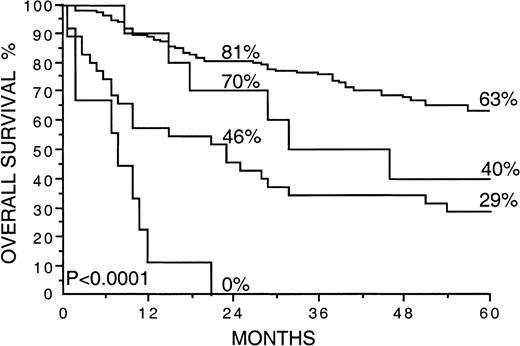
A high S-VEGF (greater than or equal to that in the highest quartile) correlated strongly with a high IPI score (P < .0001), whereas no significant association was found between a high S-bFGF (greater than or equal to that in the highest quartile) and the IPI (P = .074). The relation between the serum levels of VEGF and bFGF and the IPI is shown in Table4. In univariate survival analysis, patients with 2 or more adverse features in the IPI had only a 30% 5-year survival rate, in contrast to a 74% 5-year survival rate for patients with 0 to 1 adverse features in the IPI (P < .0001; Table 2). We next striated the patients with 0 to 1 or 3 to 5 adverse features by the IPI by S-VEGF and S-bFGF (both factors greater than or equal to or less than the highest quartiles; Figure 4). Interestingly, S-VEGF and S-bFGF appeared to provide additional information to the IPI because the 5-year survival rate of the patients with a high IPI score and high S-VEGF and S-bFGF was as low as 0%. In contrast, the 5-year survival rate of those patients with a low IPI score (0-1) and low S-VEGF and S-bFGF (both factors less than the highest quartiles) was 63% (P < .0001).
S-VEGF and S-bFGF in relation to the International Prognostic Index
| . | Both factors low, n (%) . | One factor elevated, n (%) . | Both factors elevated, n (%) . |
|---|---|---|---|
| All patients (n = 200) | |||
| IPI 0-1 | 74 (71) | 25 (24) | 5 (5) |
| IPI 2-3 | 36 (45) | 34 (43) | 10 (13) |
| IPI 4-5 | 7 (50) | 3 (21) | 4 (29) |
| Subgroup of large cell diffuse and immunoblastic lymphomas (n = 78) | |||
| IPI 0-1 | 31 (72) | 11 (26) | 1 (2) |
| IPI 2-3 | 14 (48) | 11 (38) | 4 (14) |
| IPI 4-5 | 1 (17) | 2 (33) | 3 (50) |
| . | Both factors low, n (%) . | One factor elevated, n (%) . | Both factors elevated, n (%) . |
|---|---|---|---|
| All patients (n = 200) | |||
| IPI 0-1 | 74 (71) | 25 (24) | 5 (5) |
| IPI 2-3 | 36 (45) | 34 (43) | 10 (13) |
| IPI 4-5 | 7 (50) | 3 (21) | 4 (29) |
| Subgroup of large cell diffuse and immunoblastic lymphomas (n = 78) | |||
| IPI 0-1 | 31 (72) | 11 (26) | 1 (2) |
| IPI 2-3 | 14 (48) | 11 (38) | 4 (14) |
| IPI 4-5 | 1 (17) | 2 (33) | 3 (50) |
Highest quartiles were used as cutoff values for the elevation of a factor.
Overall survival rates of 200 patients with non-Hodgkin lymphoma by the International Prognostic Index and the pretreatment S-VEGF and S-bFGF.
The cut-off values used are greater than or equal to 2 adverse features by the IPI and the highest quartiles for S-VEGF and S-bFGF (462 pg/mL or more and 5.5 pg/mL or more, respectively). Survival rates at 24 and 60 months are given. The plotted cumulative survival lines from the top are: (1) less than 2 adverse features by the IPI, and S-VEGF and S-bFGF less than the highest quartiles; (2) less than 2 adverse features by the IPI, and S-VEGF and S-bFGF greater than or equal to the highest quartiles; (3) greater than or equal to 2 adverse features by the IPI and S-VEGF, and S-bFGF less than the highest quartiles; (4) greater than or equal to 2 adverse features by the IPI and S-VEGF, and S-bFGF greater than or equal to the highest quartiles.
Overall survival rates of 200 patients with non-Hodgkin lymphoma by the International Prognostic Index and the pretreatment S-VEGF and S-bFGF.
The cut-off values used are greater than or equal to 2 adverse features by the IPI and the highest quartiles for S-VEGF and S-bFGF (462 pg/mL or more and 5.5 pg/mL or more, respectively). Survival rates at 24 and 60 months are given. The plotted cumulative survival lines from the top are: (1) less than 2 adverse features by the IPI, and S-VEGF and S-bFGF less than the highest quartiles; (2) less than 2 adverse features by the IPI, and S-VEGF and S-bFGF greater than or equal to the highest quartiles; (3) greater than or equal to 2 adverse features by the IPI and S-VEGF, and S-bFGF less than the highest quartiles; (4) greater than or equal to 2 adverse features by the IPI and S-VEGF, and S-bFGF greater than or equal to the highest quartiles.
Serum VEGF and bFGF concentrations and the components of the International Prognostic Index in multivariate survival analyses
In the current series, the relative risk (eβ) of death of those patients having an IPI score of 2 or greater was 6.34 (95% CI, 3.56-11.32; P < .0001; Table5). To find out whether high pretreatment serum concentrations of VEGF and bFGF have an independent influence on survival, they were entered in multivariate analyses together with the components of the IPI. The combination of S-VEGF and S-bFGF was entered in the model, together with the age, the WHO performance status, the Ann Arbor stage, the number of extranodal tumor sites, and the serum LDH at diagnosis. In multivariate analysis, the combination of the pretreatment serum concentrations of VEGF and bFGF had a strong independent influence on survival. The relative risk for death of patients with both S-VEGF and S-bFGF within the highest quartiles was, using the Weibull model, estimated to be as high as 2.90 (95% CI, 1.56-5.40; P = .0008; Table 5) when compared to the rest of the patients. This RR was higher than the relative risks associated with any of the components of the IPI in the same model (Table 5). The RR comparing patients with both S-VEGF and S-bFGF within the highest quartile to those patients with both factors lower than the highest quartile was even higher (RR 3.56; 95% CI, 1.82-6.96;P = .0002). Pretreatment levels of S-VEGF (RR 1.83; 95% CI, 1.10-3.02; P = .019; Table 5) and S-bFGF (RR 2.02; 95% CI, 1.24-3.28; P = .0049; Table 5) had an independent influence on survival when they were entered in the model separately, together with the components of the IPI, but their prognostic influence as single factors was clearly weaker than in combination.
Serum VEGF and bFGF concentrations and components of the International Prognostic Index in multivariate analyses
| Factor . | Relative risk (RR, eβ) . | 95% CI for RR . | P . |
|---|---|---|---|
| IPI | |||
| ≥2 | 6.34 | 3.56-11.32 | <.0001 |
| Serum VEGF and bFGF at diagnosis both ≥ highest quartiles | 2.90 | 1.56-5.40 | .0008 |
| WHO performance status ≥2 | 2.74 | 1.60-4.68 | .0002 |
| Age at diagnosis | |||
| >60 y | 2.49 | 1.56-3.97 | .0001 |
| Serum LDH at diagnosis | |||
| Abnormal | 2.46 | 1.53-3.96 | .0002 |
| Ann Arbor stage | |||
| ≥III | 2.29 | 1.42-3.70 | .0007 |
| No. extranodal tumor sites | |||
| >1 | 1.81 | 0.90-3.63 | .094 |
| Serum bFGF at diagnosis | |||
| ≥5.5 pg/mL (highest quartile) | 2.02 | 1.24-3.28 | .0049 |
| Serum VEGF at diagnosis | |||
| ≥462 pg/mL (highest quartile) | 1.83 | 1.10-3.02 | .019 |
| Factor . | Relative risk (RR, eβ) . | 95% CI for RR . | P . |
|---|---|---|---|
| IPI | |||
| ≥2 | 6.34 | 3.56-11.32 | <.0001 |
| Serum VEGF and bFGF at diagnosis both ≥ highest quartiles | 2.90 | 1.56-5.40 | .0008 |
| WHO performance status ≥2 | 2.74 | 1.60-4.68 | .0002 |
| Age at diagnosis | |||
| >60 y | 2.49 | 1.56-3.97 | .0001 |
| Serum LDH at diagnosis | |||
| Abnormal | 2.46 | 1.53-3.96 | .0002 |
| Ann Arbor stage | |||
| ≥III | 2.29 | 1.42-3.70 | .0007 |
| No. extranodal tumor sites | |||
| >1 | 1.81 | 0.90-3.63 | .094 |
| Serum bFGF at diagnosis | |||
| ≥5.5 pg/mL (highest quartile) | 2.02 | 1.24-3.28 | .0049 |
| Serum VEGF at diagnosis | |||
| ≥462 pg/mL (highest quartile) | 1.83 | 1.10-3.02 | .019 |
When the same data were entered to the proportional hazards model of survival, the results were comparable to those obtained using the Weibull model. In the proportional hazards model, the estimated RR for a high S-VEGF (greater than or equal to the highest quartile) was 1.61 (95% CI, 1.06-2.46; P = .027), and for a high S-bFGF (greater than or equal to the highest quartile), the RR was 1.82 (95% CI, 1.18-2.80; P = .0066). In the proportional hazards model, the patients with both S-VEGF and S-bFGF within the highest quartiles had more than twice the risk for death (RR 2.40; 95% CI, 1.38-4.16; P = .0019) than the rest of the patients.
Discussion
The results of the current study show that the serum concentrations of the angiogenic growth factors VEGF and bFGF have an independent prognostic influence on survival in non-Hodgkin lymphoma. Moreover, combining the results of S-VEGF and S-bFGF measurements further improved their prognostic value and enabled us to identify a subgroup of NHL patients with particularly poor outcome. In a univariate analysis, the 5-year survival of the patients with a high S-VEGF and a high S-bFGF was only 21% in contrast to the 64% 5-year survival of those patients with both factors at a lower level. The prognostic power of the simultaneous measurement of S-VEGF and S-bFGF was also seen in the subgroup with low-grade lymphomas and in the groups with intermediate or high-grade lymphomas. Similarly, the combination of S-VEGF and S-bFGF showed its prognostic power when the largest histologic subgroup within the current series, the large cell diffuse and immunoblastic lymphomas, was studied separately. In a multivariate model of survival, the patients with both S-VEGF and S-bFGF simultaneously within the highest quartiles were estimated to have nearly 3 times higher risk for death than the rest of the patients. The relative risk for death was even higher when patients with both factors simultaneously elevated were compared to those patients with both factors simultaneously at a lower level. Interestingly, we also observed a strong positive association between S-VEGF and S-bFGF. All these findings support the hypothesis that a high serum VEGF or bFGF content may reflect active angiogenesis and lymphoma growth. The synergy between VEGF and bFGF in the induction of angiogenesis has been demonstrated in an in vitro model.37Several studies suggest that like solid tumors, hematologic malignancies progress together with an induction of angiogenesis. Bone marrow biopsy samples taken from children with leukemia show significantly higher microvessel density than those from controls, suggesting that leukemia cells induce angiogenesis in the bone marrow and that leukemia might be angiogenesis dependent.38 In non-Hodgkin lymphomas significantly higher microvessel counts have been found in high-grade lymphomas than in low-grade lymphomas, implying that angiogenesis in NHL increases with tumor malignancy grade.39,40 In addition, NHL has been found to express angiogenic molecules, including VEGF and VEGF-C.41 42
Recent data show that most, if not all, VEGF in the circulation is found in blood cells, including platelets and leukocytes, indicating that the VEGF detected in serum samples is released from the blood cells during the coagulation process.35,36 However, even when the leukocyte and platelet counts are taken into account, the levels of circulating VEGF are generally higher in cancer patients than in healthy persons, and leukocytes and platelets isolated from cancer patients contain highly elevated amounts of VEGF per blood cell.36 The findings of the current study are in line with this because the platelet count and the leukocyte count did not have any prognostic value, whereas the serum VEGF concentration was strongly associated with survival. Thus, it appears that it is the amount of VEGF per blood cell that is elevated in lymphoma patients with poor outcome, and S-VEGF is an independent prognostic factor irrespective of the blood cell counts. A possible up-regulator of VEGF biosynthesis in blood cells could be hypoxia, which has been shown to induce VEGF gene expression in human monocytes.43 In addition, placental growth factor induces the expression of VEGF in mononuclear cells.44
In the current series, a high thrombocyte count was strongly associated with a high S-bFGF. However, unlike S-VEGF, S-bFGF did not have an association with the leukocyte count. These results suggest that the origins of VEGF and bFGF in the serum samples of patients with lymphoma are not identical. Peripheral blood platelets and marrow megakaryocytes have been shown to contain bFGF.45 Peripheral blood mononuclear cells,46 T cells,47,48 and granulocytes45 have also been reported to contain bFGF mRNA or protein. Known up-regulators of bFGF expression in blood cells include hypoxia49 and transforming growth factor beta 1.50 Various lymphoblastoid and leukemic cell lines have been shown to secrete bFGF.51,52 Interestingly, peripheral blood mononuclear cells derived from patients with hairy cell leukemia secreted relatively high levels of bFGF in culture media, whereas bFGF was not detectable in peripheral blood mononuclear cell cultures from healthy donors.53 In a transgenic mouse fibrosarcoma model, there is a change in the localization of bFGF from its normal cell-associated state to extracellular release in the later stages of the multistep development of fibrosarcoma. This change was concomitant with the neovascularization seen in vivo. Thus, in this multistep tumorigenesis model, there appears to be a switch to the angiogenic phenotype that is associated with the export and release of bFGF.10 In a tumor-bearing mouse model, the origin of elevated bFGF levels in the urine has been found to be almost solely from tumor cells.54
Non-Hodgkin lymphomas are a heterogeneous group of lymphoproliferative malignancies with differing patterns of behavior and responses to treatment.55 Although many patients with NHL are cured by therapy, the remainder are not cured and ultimately die of their disease. Widely accepted clinical models such as the IPI aid in the identification of specific patient risk groups and in the ongoing comparison of different therapeutic approaches. In this work, the simultaneous measurement of S-VEGF and S-bFGF enabled us to distinguish lymphoma patients with different outcomes after treatment. These results suggest that the simultaneous use of S-VEGF and S-bFGF measurements may provide prognostic information additional to that gained from the IPI. Furthermore, various endogenous inhibitors of angiogenesis may also be found in the bloodstream. A circulating form of human endostatin has been identified.56 Intriguingly, the concentrations of soluble endostatin found in the serum samples of healthy human donors57 are similar to the concentrations that efficiently inhibit endothelial cell proliferation in vitro.58 These findings suggest that circulating forms of endostatin may be involved in the homeostatic control of angiogenesis. Hence, it might be possible to obtain an angiogenic profile of a cancer patient's blood sample by measuring the concentrations of several circulating angiogenic and antiangiogenic molecules. This angiogenic profile might be used to monitor cancer therapy or it might be a predictor of outcome after cancer has been diagnosed and even aid in the selection of the proper anti-angiogenic treatment.
Acknowledgment
We thank Kati Konola for exellent technical assistance.
Supported by grants from the Sigrid Juselius Foundation, the Finnish Medical Foundation, the Maud Kuistila Memorial Foundation, and the Helsinki University Central Hospital Research Funds.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Petri Salven, Department of Cell Biology, New York University School of Medicine, 550 First Ave, New York, NY 10016; e-mail: salvep01@popmail.med.nyu.edu; petri.salven@helsinki.fi.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal