Abstract
Screening for hereditary hemochromatosis (HHC) by means of transferrin saturation (TS) levels has been advocated and will identify many patients who are asymptomatic. The purposes of this study were (1) to determine HFE genotypes among asymptomatic HHC patients and correlate this profile with the degree of iron overload and (2) to evaluate the relationship between mobilized iron (mob Fe), age, serum ferritin (SF), and quantitative hepatic iron (QHI) in this population. One hundred twenty-three asymptomatic HHC patients were evaluated; all had quantitative phlebotomy to determine mob Fe and genotyping for C282Y and H63D mutations. Liver biopsies with QHI determinations were performed on 72 of the 123 patients. Of the entire group, 60% were homozygous for C282Y, and 13% were compound heterozygotes (C282Y/H63D). Among asymptomatic patients, the prevalence of homozygous C282Y is lower compared with previous studies that include clinically affected patients. Of those patients with more than 4 g mob Fe, 77% were homozygous C282Y. Asymptomatic patients with lower iron burdens frequently had genotypes other than homozygous C282Y. There was no correlation between age and mob Fe in these patients; however, there was a correlation between mob Fe and both SF (r = 0.68) and QHI (r = 0.75). In conclusion, asymptomatic patients with moderate iron overload had a different genotypic profile than was seen in advanced iron overload. The significance of identifying patients with modest degrees of iron loading, who may not be homozygous for C282Y, must be addressed if routine TS screening is to be implemented.
Introduction
Hereditary hemochromatosis (HHC) is a common disorder with a prevalence of 3 to 8 per 1000.1,2 Patients with HHC have enhanced gastrointestinal absorption of iron and may accumulate excessive iron stores, causing organ dysfunction.3,4 A clinical diagnosis of HHC can often be established on the basis of serum iron studies5-8; however, a liver biopsy and determination of quantitative hepatic iron (QHI)9-11 are often required to confirm the presence of tissue iron overload. Noninvasive alternatives to liver biopsy include quantitative phlebotomy with determination of mobilized iron (mob Fe)12 and, more recently, testing for HFE mutations.13 14
Two point mutations in the HFE gene, C282Y and H63D (single-letter amino acid codes), have been described in HHC patients; 64% to 100% of HHC cases are homozygous C282Y, with other identified patients being compound heterozygotes, H63D homozygotes, heterozygotes for either mutation, or normal.15 These 2 HFE mutations have different prevalence rates and degrees of penetrance in causing iron overload. The C282Y mutation has a gene frequency of 0.029 to 0.07 and thus a heterozygote prevalence of 5.8% to 14%.16-19The penetrance of this mutation has been speculated to be high, but these conclusions are based on only a few tested individuals. The H63D mutation is fairly prevalent in control (presumed normal) subjects, with a heterozygote prevalence of 25% and a homozygote prevalence of 3.62%. The penetrance is presumed to be low and estimated by one group at 0.017. Recently, another mutation in the HFE gene has been identified and designated S65C.20 The prevalence of this mutation and its role in clinically significant iron overload remain to be determined.
Many features of HHC make it a rational candidate for population screening,21-24 and both phenotypic and genotypic screening strategies have been advocated. Phenotypic screening using serum transferrin saturation (TS) levels has the advantages of being inexpensive and clinically relevant. Some practitioners are implementing this approach. The role of HFE genotyping as a confirmatory test in patients with suspected HHC has not been fully studied. Some experts have suggested that HFE genotyping in the setting of clinically suspected HHC can help confirm the diagnosis, precluding the need for liver biopsy in some cases.14 25
The variability in the reported prevalence of homozygous C282Y in HHC patients may reflect ethnic differences as well as the manner in which patients are identified, hence reflecting total body iron burden. Our center has offered phlebotomy treatment to all patients with a clinical diagnosis of HHC. This report describes the distribution of HFE genotypes and body iron burdens in asymptomatic patients with presumed HHC identified by TS screening in one of the following settings: our screening study, family screening, or routine screening by their primary care physicians. In addition, we evaluated the relationship between mob Fe and age, serum ferritin (SF), and QHI in patients who were C282Y homozygotes. As routine screening for HHC using TS testing becomes more commonplace, it is necessary to have a comprehensive understanding of the laboratory phenotypes and genotypes that can be seen in identified patients.
Patients and methods
The 123 patients in this report were identified by TS testing and referred to our treatment center. The mechanisms used include our primary care screening study,2 family screening of first-degree relatives of known patients, and routine screening by primary care physicians. Of the 33 patients identified by family screening, 11 have at least 1 first-degree relative who is also part of our cohort. Thus, this group of 123 patients represents 113 distinct families. The 62 patients identified by “routine screening” either were found to have an elevated serum iron level on a routine chemistry profile or were screened with serum iron studies at the discretion of their primary care physician. The rationale for screening is unknown to us and was probably not the same for each patient; however, when these patients were evaluated in our treatment center, they were all asymptomatic with no history or findings that would have warranted testing for HHC. This group includes patients with biopsy-proven HHC and patients presumed to have HHC on the basis of clinical and laboratory parameters. The clinically diagnosed cases include patients with elevated TS and SF values who have no known underlying disorder associated with iron loading such as hepatitis, alcoholic liver disease, thalassemia, or sideroblastic anemia. (Although patients with increased TS and with such disorders were referred to us, they are not included in this analysis.) Liver biopsies were performed in 72 of the 123 patients. All patients underwent therapeutic phlebotomy to achieve a state of iron depletion (SF lower than 25), enabling us to determine their mob Fe stores. Mob Fe was determined on the basis of the total blood volume removed prior to achieving a state of iron depletion with the use of the following formula: grams of iron = Hct/3 × weight of blood × 0.0035.
HFE genotyping was performed by means of a method known as TaqMan. This technology uses the 5′ to 3′ exonuclease activity of DNA Taq polymerase and 2 fluorescence-tagged DNA oligonucleotide probes to differentiate the wild-type sequence and the mutant sequence during the DNA amplification process. The genotyping and amplification processes were carried out simultaneously in the Applied Biosystem's Prism 7700 Sequence Detection System. The fluorescent output of probes was calculated by the manufacturer's software to define the 3 genotypes (homozygous normal, heterozygous, and homozygous mutation) of each locus. The validity of this genotyping process has been extensively tested, and results are comparable to those with other methodologies.
Comparison of the distribution of genotypes between groups was made by means of Fisher's exact test. Comparisons of iron load were made with the Mann-Whitney test. Spearman rank correlation was used to assess the association between measures of iron load.
Results
Table 1 shows the distribution of HFE genotypes in 123 asymptomatic patients cared for in our HHC center who were identified by screening. There was a trend toward a higher prevalence of homozygous C282Y in patients identified by family screening compared with other screening modalities, but differences in genotype distributions were not statistically significant (P = .10). Overall, there was a lower prevalence of homozygous C282Y among our cohort of asymptomatic patients compared with clinically affected patients reported in other series.16,18,19,26 27 Since asymptomatic patients are likely to have lower body iron stores, we examined the degree of iron loading relative to genotype (Table 2).
Distribution of HFE genotype in relation to method of patient identification
| Genotype . | Family screening . | Routine screening . | Screening study . | Total . |
|---|---|---|---|---|
| C282Y homozygous | 23 (70%) | 38 (62%) | 13 (46%) | 74 (60%) |
| Compound heterozygous | 2 (6%) | 9 (15%) | 5 (18%) | 16 (13%) |
| H63D homozygous | 2 (6%) | 4 (7%) | 2 (7%) | 8 (7%) |
| C282Y heterozygous | 4 (12%) | 3 (5%) | 2 (7%) | 9 (7%) |
| H63D heterozygous | 2 (6%) | 5 (7%) | 3 (11%) | 10 (8%) |
| Normal | 0 | 3 (5%) | 3 (11%) | 6 (5%) |
| Total | 33 | 62 | 28 | 123 |
| Genotype . | Family screening . | Routine screening . | Screening study . | Total . |
|---|---|---|---|---|
| C282Y homozygous | 23 (70%) | 38 (62%) | 13 (46%) | 74 (60%) |
| Compound heterozygous | 2 (6%) | 9 (15%) | 5 (18%) | 16 (13%) |
| H63D homozygous | 2 (6%) | 4 (7%) | 2 (7%) | 8 (7%) |
| C282Y heterozygous | 4 (12%) | 3 (5%) | 2 (7%) | 9 (7%) |
| H63D heterozygous | 2 (6%) | 5 (7%) | 3 (11%) | 10 (8%) |
| Normal | 0 | 3 (5%) | 3 (11%) | 6 (5%) |
| Total | 33 | 62 | 28 | 123 |
C, Y, H, and D indicate single-letter amino acid codes.
Patients were identified either through the screening study, routine screening by their primary physician, or family screening. All patients were asymptomatic. Patients identified by family screening contained the highest percentage of C282Y homozygotes (70%) followed by those identified by routine screening by their primary physicians (62%). Only 46% of the patients identified through the screening study were C282Y homozygotes.
Distribution of HFE genotype in relation to degree of iron overload
| Genotype . | Mobilized iron (g) . | Total . | ||
|---|---|---|---|---|
| (< 1.5) . | (1.5 to 4) . | (> 4) . | ||
| C282Y homozygous | 5 (63%) | 32 (48%) | 37 (77%) | 74 (60%) |
| Compound heterozygous | 0 | 14 (21%) | 2 (4%) | 16 (13%) |
| H63D homozygous | 0 | 5 (7%) | 3 (6%) | 8 (7%) |
| C282Y heterozygous | 1 (12%) | 5 (7%) | 3 (6%) | 9 (7%) |
| H63D heterozygous | 1 (12%) | 6 (9%) | 3 (6%) | 10 (8%) |
| Normal | 1 (12%) | 5 (7%) | 0 | 6 (5%) |
| Total | 8 | 67 | 48 | 123 |
| Genotype . | Mobilized iron (g) . | Total . | ||
|---|---|---|---|---|
| (< 1.5) . | (1.5 to 4) . | (> 4) . | ||
| C282Y homozygous | 5 (63%) | 32 (48%) | 37 (77%) | 74 (60%) |
| Compound heterozygous | 0 | 14 (21%) | 2 (4%) | 16 (13%) |
| H63D homozygous | 0 | 5 (7%) | 3 (6%) | 8 (7%) |
| C282Y heterozygous | 1 (12%) | 5 (7%) | 3 (6%) | 9 (7%) |
| H63D heterozygous | 1 (12%) | 6 (9%) | 3 (6%) | 10 (8%) |
| Normal | 1 (12%) | 5 (7%) | 0 | 6 (5%) |
| Total | 8 | 67 | 48 | 123 |
Patients were stratified on the basis of the amount of mobilized iron (mob Fe). Of the patients, 39% had > 4 g mob Fe (48/123 patients). Of these patients, 37 of 48 (77%) were homozygous C282Y. Patients with lower degrees of iron loading were more likely to have genotypes other than C282Y/C282Y; notably, 21% of patients with mob Fe between 1.5 and 4 g were compound heterozygotes.
Eight patients had less than 1.5 g mob Fe; 5 of these 8 patients were homozygous C282Y yet had no evidence of significant iron overload. Of the patients with iron burdens lower than 4 g identified by screening, 50% had genotypes other than C282Y/C282Y. In the 48 patients with greater than 4 g mob Fe, 77% were C282Y homozygotes. There were 30 patients with greater than 5 g mob Fe; 27 of 30 (90%) of this group were homozygous C282Y. Thus, as the degree of iron overload increased, so did the likelihood of having the homozygous C282Y genotype.
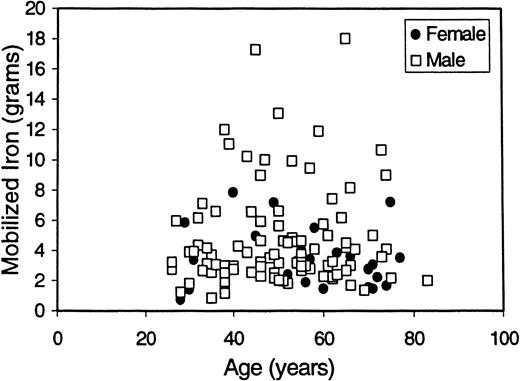
It is known that iron stores increase throughout an individual's lifetime.28 It is also known that because of the physiologic blood loss associated with menses, pregnancy, and childbirth, women with HHC accumulate iron more gradually than men. Therefore, we evaluated our mob Fe data in the context of patient age and sex among patients who are C282Y homozygotes (Figure1). C282Y homozygous men had greater iron loading than women (male median mob Fe = 4.6 g; female median mob Fe = 3.4 g; P = .012). In neither sex was there a significant correlation between age and degree of iron loading (r = 0.22, P = .12 for men; r = −0.01,P = .98 for women).
Distribution of mob Fe in relation to age and sex.
Mob Fe is plotted here as a function of age and sex for C282Y homozygotes. Overall, women had lower degrees of iron loading than men (median = 3.4 g for women; median = 4.6 g for men). In this asymptomatic population of men and women identified by screening, there was no correlation between age and degree of iron loading (r = 0.22 for men; r = −0.01 for women). The mean age for patients with greater than 4 g mob Fe was 48.9 years, and the mean age for patients with between 1.5 and 4 g mob Fe was 51.2 years.
Distribution of mob Fe in relation to age and sex.
Mob Fe is plotted here as a function of age and sex for C282Y homozygotes. Overall, women had lower degrees of iron loading than men (median = 3.4 g for women; median = 4.6 g for men). In this asymptomatic population of men and women identified by screening, there was no correlation between age and degree of iron loading (r = 0.22 for men; r = −0.01 for women). The mean age for patients with greater than 4 g mob Fe was 48.9 years, and the mean age for patients with between 1.5 and 4 g mob Fe was 51.2 years.
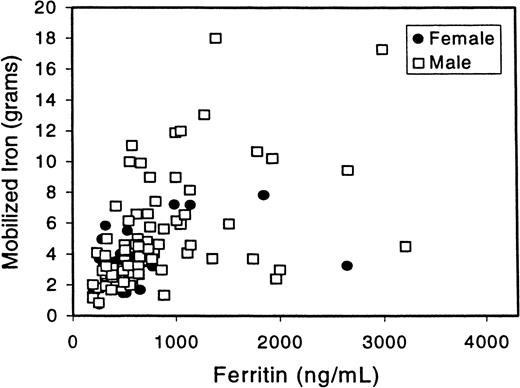
SF is a rough indicator of total body iron, and SF levels are used to monitor phlebotomy therapy. There is evidence that end organ damage such as cirrhosis is rare in patients with SF values lower than 750 to 1000 μg per liter.29 We therefore looked at the relationship between SF and mob Fe in patients who are homozygous C282Y (Figure 2). We focused on this subgroup of patients because it is a more defined, genotypically uniform population. There was a significant correlation between mob Fe and SF of 0.68 (P < .001). There was also a correlation between mob Fe and SF in the entire group of patients (r = 0.59,P < .001; data not shown). In patients with greater than 4 g mob Fe, the range of SF was 289 to 2995 μg per liter. It is notable that some of these patients have significant iron overload despite an SF that is only slightly above the upper limit of normal.
Correlation between SF and mob Fe.
SF values from the homozygous C282Y patients are plotted here against mob Fe. The Spearman rank correlation between mob Fe and ferritin is r = 0.68 (P < .001). The correlation between mob Fe and ferritin in all 123 patients is r = 0.59 (P < .001; data not shown).
Correlation between SF and mob Fe.
SF values from the homozygous C282Y patients are plotted here against mob Fe. The Spearman rank correlation between mob Fe and ferritin is r = 0.68 (P < .001). The correlation between mob Fe and ferritin in all 123 patients is r = 0.59 (P < .001; data not shown).
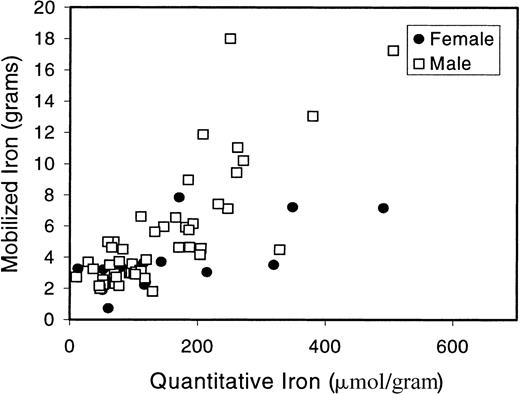
Finally, we evaluated the correlation between QHI and mob Fe in C282Y homozygous patients who underwent liver biopsy (Figure3). The correlation between QHI and mob Fe in C282Y homozygotes was 0.75 (P < .001). When the entire patient population (all genotypes) was evaluated, the correlation was 0.70 (P < .001; data not shown).Of the 72 patients who underwent liver biopsy, 47 were C282Y homozygotes; 13 of these patients had a hepatic iron index (HII) less than 1.9. Of the C282Y/C282Y patients, 2 had cirrhosis; one of these had typical histologic features of HHC and an HII of 6.4, and the other had steatohepatitis and a modest degree of iron overload (QHI 52.1 μmol/gram). Cirrhosis was not seen in any patients with the other genotypes.
Correlation between quantitative hepatic iron and mob Fe.
Liver biopsy and QHI determinations were done for 47 C282Y/C282Y patients. QHI is plotted here against mob Fe. The Spearman rank correlation between QHI and mob Fe is r = 0.75 (P < .001).
Correlation between quantitative hepatic iron and mob Fe.
Liver biopsy and QHI determinations were done for 47 C282Y/C282Y patients. QHI is plotted here against mob Fe. The Spearman rank correlation between QHI and mob Fe is r = 0.75 (P < .001).
Discussion
Many questions remain regarding the optimal screening strategy for HHC, the need for liver biopsy, the role of HFE genotyping, and the relationship between HFE genotype and clinical measures of iron stores.23 Population screening using TS levels has been advocated by some, and efforts to implement TS screening are under way. However, there are many unanswered questions regarding the diagnosis, natural history, and management of HHC in asymptomatic patients identified by screening.23 The purpose of this study was to analyze the distribution of HFE genotypes in asymptomatic patients diagnosed with HHC following TS testing and to further delineate the phenotypes of identified patients. A better understanding of the penetrance and natural history of the disease in patients identified in this manner is crucial before population screening by means of TS levels is widely implemented.
The definition of HHC for the purpose of this study is a clinical one. All patients with repeat TS values greater than 45%, SF greater than 200, and no clinically identifiable secondary cause of iron loading were considered to have presumed HHC and underwent quantitative phlebotomy. Liver biopsy was performed when indicated. We recognize that not all these individuals may meet strict genotypic or phenotypic disease definitions. Advocates of genotypic disease definition would argue that only C282Y homozygotes have true HHC. Of our patients, 60% were C282Y homozygotes, and an additional 13% were compound heterozygotes for the C282Y and H63D mutations. However, significant iron loading is seen in the absence of these genotypes,30and 9 out of 33 (27%) of our patients with other genotypes had more than 4 g of mob Fe.
A strict phenotypic definition requires documented tissue iron loading established by liver biopsy with quantitative iron determination (HII of 1.9 or more or QHI of 71 μmol/gram). However, liver biopsy is invasive and probably not necessary in all cases. A more practical approach to TS screening would be to perform therapeutic phlebotomy and estimate mob Fe in identified patients that have suspected HHC. Thresholds of 4 and 5 g have been used to define clinically significant iron overload. Many C282Y homozygotes do not have enough iron overload to meet such strict phenotypic definitions. Of C282Y homozygotes in our population, 37 of 74 (50%) had mob Fe less than 4 g, and 5 of 74 (7%) had Mob Fe less than 1.5 g. This is consistent with recently reported population screening studies in which 50% of C282Y homozygotes had no clinical manifestations, 19% had normal SF levels, and 19% had an HII of 1.9 or less.31 However, 94% had an elevated TS and would have been detected by TS screening.
Mob Fe is a measure of total body iron stores and, in this study, correlated reasonably well with QHI in patients who had a liver biopsy (R = 0.75). Our data are consistent with other reports that show a rough correlation between QHI and mob Fe.12 Our patient population differs from populations in previous reports in that all patients analyzed were asymptomatic C282Y homozygotes. Although the correlation between mob Fe and SF was 0.68, we identified some patients with clinically significant mob Fe who had SF values only at the upper limit of normal. Thus, a relatively low ferritin level (300 to 500 μg per liter) may not exclude clinically relevant iron overload.
Our study showed a poor correlation between mob Fe and age in both men and women in this cohort of patients. The value of this analysis is limited since our study, by definition, excludes those homozygotes with normal SF levels who did not undergo therapeutic phlebotomy and also excludes symptomatic patients with higher degrees of iron loading. Our data are consistent with other series of modestly iron-loaded patients in which iron load correlates poorly with age.12 It is well known that iron stores in a given individual increase during that person's lifetime.28 One would predict that inclusion of all homozygotes in the population would show an increase of iron stores with age. However, our data demonstrate that among this asymptomatic cohort, there is low enough correlation between age and iron stores that the value of the HII in this setting is questionable. In fact, 13 of 47 C282Y homozygotes who underwent liver biopsy had an HII of 1.9 or lower. Women were more likely than men to have a low HII; 6 of 15 females and 7 of 32 males had an HII of 1.9 or lower.
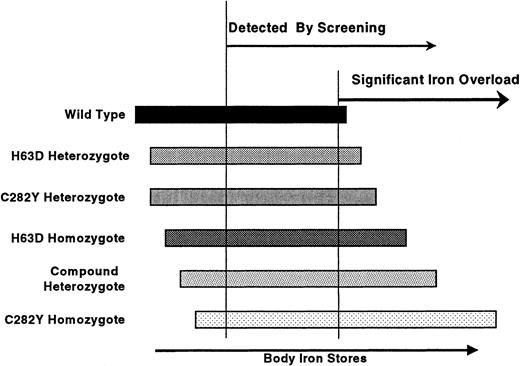
Our study demonstrates that TS screening will identify many individuals with only modest degrees of iron loading who may not meet traditional phenotypic diagnostic criteria but who have genotypes associated with iron loading. Those with lower degrees of iron overload are less likely to be C282Y homozygotes. This concept is shown schematically in Figure4.
Iron overload: genotype versus phenotype.
The range of possible iron stores for each genotype is schematically represented. Homozygosity for C282Y leads to the greatest amount of iron loading, but many homozygotes may have normal iron stores. Screening identifies individuals with lower body iron stores and thus a higher proportion of individuals with genotypes other than homozygous C282Y.
Iron overload: genotype versus phenotype.
The range of possible iron stores for each genotype is schematically represented. Homozygosity for C282Y leads to the greatest amount of iron loading, but many homozygotes may have normal iron stores. Screening identifies individuals with lower body iron stores and thus a higher proportion of individuals with genotypes other than homozygous C282Y.
Patients with modest degrees of iron overload (mob Fe 1.5 to 4 g) are less likely to have classic clinical features of HHC. The natural history of these patients is not clear. Although some may, over time, develop clinically significant iron overload, it is possible that others may never be affected by their elevated iron stores. Modest iron loading may potentially have other adverse effects, as suggested by epidemiological studies showing an association with cardiovascular disease.32 Until the natural history of patients with modest iron loading is better defined, we would recommend empiric phlebotomy treatments, particularly in those with genotypes considered at risk, in an effort to maintain normal body iron stores.
TS screening will also identify many cases of HHC with significant iron overload. Although SF is a useful measurement, it is only a rough predictor of the degree of iron loading. When liver biopsy is either unnecessary to the clinician or unacceptable to the patient, quantitative phlebotomy with determination of mob Fe is a useful diagnostic and therapeutic tool. Most individuals with significant iron overload are homozygous for C282Y. The significance and natural history of other genotypes with iron overload identified by TS screening need to be determined by large-scale prospective studies. In the meantime, implementation of TS screening seems reasonable provided practitioners understand the clinical implications associated with detection in this manner.
Acknowledgment
The authors thank the Centers for Disease Control and Prevention for processing the HFE genotyping.
Supported in part by Agency for Health Care Policy and Research grant RO1 HS07616 and by the National Heart, Lung and Blood Institute grant RO1 HL61428-01A1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ronald L. Sham, Rochester General Hospital, 1425 Portland Ave, Rochester, NY 14621; e-mail: ronald.sham@viahealth.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal