Abstract
Anaplastic large cell lymphoma (ALCL) represents a generally recognized group of large cell lymphomas. Defining features consist of a proliferation of predominantly large lymphoid cells with strong expression of the cytokine receptor CD30 and a characteristic growth pattern. With the use of molecular and clinical criteria, 3 entities of ALCL have been identified: primary systemic anaplastic lymphoma kinase (ALK)+ ALCL, primary systemic ALK− ALCL, and primary cutaneous ALCL. ALK expression is caused by chromosomal translocations, most commonly t(2;5). ALK+ ALCL predominantly affects young male patients and, if treated with chemotherapy, has a favorable prognosis. It shows a broad morphologic spectrum, with the “common type,” the small cell variant, and the lymphohistiocytic variant being most commonly observed. The knowledge of the existence of these variants is essential in establishing a correct diagnosis. ALK− ALCL occurs in older patients, affecting both genders equally and having an unfavorable prognosis. The morphology and the immunophenotype of primary cutaneous ALCL show an overlap with that of lymphomatoid papulosis. Both diseases have an excellent prognosis, and secondary systemic dissemination is only rarely observed. The described ALCL entities usually derive from cytotoxic T cells. In contrast, large B-cell lymphomas with anaplastic morphology are believed to represent not a separate entity but a morphologic variant of diffuse large B-cell lymphoma. Malignant lymphomas with morphologic features of both Hodgkin disease and ALCL have formerly been classified as Hodgkin-like ALCL . Recent immunohistologic studies, however, suggest that ALCLs Hodgkin-like represent either cases of tumor cell–rich classic Hodgkin disease or (less commonly) ALK+ ALCL or ALK− ALCL.
Introduction
In 1985, a number of the authors1described—under the term anaplastic large cell lymphoma (ALCL)—a novel lymphoma category defined by a frequently cohesive proliferation of large pleomorphic blasts and a constant expression of the CD30 molecule on all neoplastic cells. Despite these common features, heterogeneity in the cytology and in the antigen profile of the tumor cells, as well as in the clinical features of patients affected by this condition, was noticed in the original description.1 This led to the distinction of several morphologic, immunophenotypic, and clinical subforms of ALCL.1-17 A morphologic and immunophenotypic overlap with classic Hodgkin disease was also recognized.1,10,13,18,19 In the late 1980s, a translocation between chromosomes 2 and 5 was assigned to a proportion of ALCL cases.20-23 All of these findings raised the questions of whether the morphologic, immunophenotypic, clinical, or genetic forms represent variants of the same disease or different diseases and what their relation is to Hodgkin disease. In the last 5 years, there has been great progress in the molecular characterization of ALCL and Hodgkin disease, the result being that most of the questions raised can now be answered.
How ALCL was recognized
In 1982, Stein's group24,25 discovered a new molecule that was initially termed Ki-1 and subsequently designated CD30.26-28 CD30 is strongly expressed on Hodgkin and Reed-Sternberg cells of classic Hodgkin disease, but is absent from the cells of all normal tissues except for scattered activated large lymphoid blasts preferentially located around B-cell follicles. Biochemical studies29 and molecular cloning30have revealed that CD30 is a 120-kd transmembrane cytokine receptor of the tumor necrosis factor receptor family, for which the ligand (CD30L) was identified.31 A soluble 85-kd form of CD3032 was found to be released from the membrane-bound molecule by proteolytic cleavage33 and can be detected in the sera of patients with CD30+ reactive and neoplastic lesions. Immunohistochemical analysis of a large range of human tumors has shown that CD30 is constantly expressed not only by Hodgkin and Reed-Sternberg cells, but also by a subset of diffuse large cell neoplasms, most of which had originally been diagnosed as malignant histiocytosis, regressing atypical histiocytosis, anaplastic (metastatic) carcinoma, malignant melanoma, seminoma, or even as malignant fibrous histiocytoma.1,3,10,28,34 The frequent presence of lymphoid markers and the consistent absence of molecules associated with histiocytic or other cell lineages indicated a lymphocytic origin for this new tumor category.1 The lymphoid nature was further confirmed by the demonstration of clonal rearrangements in antigen receptor genes.1,35,36 Because of the constant Ki-1/CD30 expression and the frequent anaplastic features, this tumor form was initially called anaplastic large cell lymphoma (ALCL)1 and then, variably, lymphoma large cell anaplastic CD30+ (Kiel classification37), Ki-1 lymphoma,2 or Ki-1+ large cell lymphoma.3 However, the 2 latter terms are inadequate because CD30 is also expressed in some unrelated neoplasms, such as Hodgkin disease1,25,28 or embryonal carcinoma.38 The term anaplastic large cell is also not adequate because the tumor cells in the small cell variant are not large and the monomorphic or immunoblastic variants are not anaplastic. Despite this and because of the absence of a better designation, the term ALCL has now been adopted by the Revised European American Lymphoma (REAL)13 and the new World Health Organization (WHO) classifications.39
Morphologic features and subforms
The histologic appearance of ALCL was originally described as a preferential paracortical involvement of lymph nodes with intrasinusoidal dissemination (Figure1C); although this growth pattern is evident in partially involved lymph nodes, it remains otherwise diffuse.1 Because of the wide histologic spectrum of the tumor cell population and the admixture of reactive cells, several groups proposed the subclassification of ALCL into the subforms listed in Table 1.
Morphology and immunophenotype of anaplastic large cell lymphoma, common type.
(A) Cytologic features of ALCL, common type, in a paraffin section (hematoxylin and eosin). (B) Cytologic features of ALCL, common type, in a touch imprint (May-Grünwald-Giemsa). (C) Dissemination of ALCL cells in a marginal sinus. MS indicates marginal sinus; GC, reactive germinal center (hematoxylin and eosin). (D) CD30 immunoreactivity of ALCL, common type. All tumor cells are CD30+. Note the sinusoidal dissemination (arrow) and the perifollicular homing of the CD30+ tumor cells (antibody clone Ber-H2; alkaline phosphatase–antialkaline phosphatase [APAAP]). (E) Immunoreactivity of an ALCL of common type for the cytotoxic molecule perforin. The sinusoidal dissemination of the tumor cells (arrow) and a small reactive cytotoxic cell (arrowhead) are indicated (antibody clone P1-8; APAAP). (F) Immunoreactivity of an ALCL of common type for the anaplastic lymphoma kinase (ALK). There is strong labeling in the cytoplasm as well as in the nucleus (antibody clone ALKc; APAAP). (G) An ALCL of common type with labeling for ALK restricted to the cytoplasm (antibody clone ALKc; APAAP).
Morphology and immunophenotype of anaplastic large cell lymphoma, common type.
(A) Cytologic features of ALCL, common type, in a paraffin section (hematoxylin and eosin). (B) Cytologic features of ALCL, common type, in a touch imprint (May-Grünwald-Giemsa). (C) Dissemination of ALCL cells in a marginal sinus. MS indicates marginal sinus; GC, reactive germinal center (hematoxylin and eosin). (D) CD30 immunoreactivity of ALCL, common type. All tumor cells are CD30+. Note the sinusoidal dissemination (arrow) and the perifollicular homing of the CD30+ tumor cells (antibody clone Ber-H2; alkaline phosphatase–antialkaline phosphatase [APAAP]). (E) Immunoreactivity of an ALCL of common type for the cytotoxic molecule perforin. The sinusoidal dissemination of the tumor cells (arrow) and a small reactive cytotoxic cell (arrowhead) are indicated (antibody clone P1-8; APAAP). (F) Immunoreactivity of an ALCL of common type for the anaplastic lymphoma kinase (ALK). There is strong labeling in the cytoplasm as well as in the nucleus (antibody clone ALKc; APAAP). (G) An ALCL of common type with labeling for ALK restricted to the cytoplasm (antibody clone ALKc; APAAP).
Morphologic subforms of ALCL and their correlation with immunophenotype
| Histologic subform . | CD30 . | Immunophenotype . | EMA+cases . | ALK+ cases (%) . |
|---|---|---|---|---|
| Common (classic) type* 3,10 13 | Positive | Most T/null | Majority | 60-90 |
| Giant cell-rich3,10 13 | Positive | Most T/null | Minority | 30-40 |
| Small cell11 13 | Positive (mainly large cells) | T | Most† | 100 |
| Lymphohistiocytic3,8,10 13 | Positive | T | Most† | 80-100 |
| Hodgkin-like3,10,13 65 | Positive | Null (rarely T) | Minority | ∼ 15 |
| Rare subforms | ||||
| Sarcomatoid42 | Positive | NDA | NDA | NDA |
| Neutrophil rich13,44 45 | ||||
| Eosinophil rich43 | ||||
| Signet ring15 46 |
| Histologic subform . | CD30 . | Immunophenotype . | EMA+cases . | ALK+ cases (%) . |
|---|---|---|---|---|
| Common (classic) type* 3,10 13 | Positive | Most T/null | Majority | 60-90 |
| Giant cell-rich3,10 13 | Positive | Most T/null | Minority | 30-40 |
| Small cell11 13 | Positive (mainly large cells) | T | Most† | 100 |
| Lymphohistiocytic3,8,10 13 | Positive | T | Most† | 80-100 |
| Hodgkin-like3,10,13 65 | Positive | Null (rarely T) | Minority | ∼ 15 |
| Rare subforms | ||||
| Sarcomatoid42 | Positive | NDA | NDA | NDA |
| Neutrophil rich13,44 45 | ||||
| Eosinophil rich43 | ||||
| Signet ring15 46 |
The most relevant subforms described are indicated in bold. NDA indicates no data available.
The monomorphic subform represents a variant of the common type.
Usually detectable on the larger cells.
The common type1,10,13,17 is characterized by sheets of large lymphoid cells with chromatin-poor horseshoe-shaped nuclei containing multiple nucleoli (Figure 1A). Cells with these cytologic features have been called hallmark cells40 because they are encountered in all ALCL variants, including the small cell and lymphohistiocytic variants. Multinucleated cells with Reed-Sternberg–like appearance may also occur. The tumor cells have an abundant cytoplasm which, in imprint preparations, frequently shows numerous vacuoles (Figure 1B). The monomorphic subform7probably represents a variant of the common type. Because of the cytologic resemblance of the latter to immunoblastic lymphoma, it can easily be confused with nonanaplastic large cell lymphomas when immunohistochemistry is not applied. In the giant cell–rich type,3,10,13,17 a large number of the tumor cells contain more than one nucleus. The small cell variant11 is characterized by a mixture of small, medium-sized, and large lymphoid cells (Figure 2). The nuclei of the small and medium-sized cell population are often irregular. Large cells surrounding small vessels are a frequent and characteristic finding. This is particularly evident following immunostaining for CD30, which highlights the large anaplastic cells. In contrast, heterogeneity is seen in CD30 expression in the smaller cell population, with many small cells being CD30− (more commonly) or weakly positive (Figure 2B). In the past, the small cell variant was usually diagnosed as peripheral T-cell lymphoma, and some authors still prefer this interpretation (reported by D. Weisenburger at a workshop on the findings of the International Non-Hodgkin Lymphoma Classification Porject, organized by J. Armitage and D. Weisenburger, September 8-10, 1997, Omaha, NE). The small cell variant may, however, contain areas with the morphology of ALCL common type (ie, sheets of CD30+ blasts) and can transform into the common type and vice versa.11,40,41 These observations strongly suggest that the small cell variant is part of the histologic spectrum of ALCL. Cytogenetic studies11 showing the t(2;5) and more recently the nucleophosmin (NPM)-ALK fusion protein in the small and large cells of this subform have strongly supported this concept (discussed later in detail). The dominant feature of the lymphohistiocytic subform3,8,10,13 is the large number of histiocytes, which may mask the anaplastic tumor cell population (Figure 2). The latter can be highlighted by CD30 immunostaining. Occasionally, the histiocytes show signs of erythrophagocytosis and often display a monomorphic appearance with eccentric nuclei, a feature that in the past often led to the misdiagnosis of malignant histiocytosis.8 However, the histiocytes hardly proliferate, as revealed by their negativity for the nuclear proliferation marker Ki-67, and are therefore reactive. In contrast, the tumor cells, making up only a minor component of the entire infiltrate, are negative for histiocytic markers but strongly positive for CD30 and Ki-67.8 They are usually smaller than in the common ALCL type, and therefore this subtype may be related to the small cell variant. Because of the small-sized tumor cell component, the lymphohistiocytic subform was not regarded as a variant of ALCL in the Kiel classification, but was erroneously categorized as a peripheral T-cell lymphoma.37
Morphology and immunophenotype of the small cell variant and the lymphohistiocytic variant of ALCL.
(A) Small cell variant of ALCL. Most of the cells have a small irregular nucleus, the cytoplasm is light, and larger cells are located in close vicinity to small vessels (Giemsa). (B) Immunoreactivity of a small cell variant of ALCL for CD30. The larger tumor cells are strongly positive, whereas the small tumor cells are only weakly positive (alkaline phosphatase–antialkaline phosphatase [APAAP]). (C) Immunoreactivity of a small cell variant of ALCL for ALK. The larger cells (often located in the vicinity of small vessels) are positive in the cytoplasm and the nucleus, whereas in the smaller cells, the labeling is restricted mainly to the nucleus (APAAP). (D) Lymphohistiocytic variant of ALCL. Note the broad cytoplasm and the frequently slightly eccentrically located nuclei, which are revealed immunohistologically as belonging to reactive histiocytes (see panel E) (hematoxylin and eosin). (E) Immunoreactivity of the same case as in (D) with the CD68 antibody clone PG-M1. Nearly all of the larger cells are positive. These PG-M1–positive cells, representing nonneoplastic bystander macrophages, obscure the few CD30+ tumor cells (see Figure 2F) (APAAP). (F) Immunoreactivity of the same case as in (D) for CD30. Only a minority of the large cells are positive and represent the tumor cell population (APAAP). (G) Immunoreactivity of the same case as in (D) and (E) for ALK. The labeled cells vary greatly in size, with a predominance of smaller cells. The smaller the cells, the more the labeling is restricted to the nucleus. The negative cells are the bystander macrophages (antibody clone ALK1; APAAP). (H) Immunoreactivity of the same case as in (D), (E), and (G) for perforin. The tumor cells are strongly positive, whereas the bystander macrophages are negative (antibody clone P1-8;APAAP).
Morphology and immunophenotype of the small cell variant and the lymphohistiocytic variant of ALCL.
(A) Small cell variant of ALCL. Most of the cells have a small irregular nucleus, the cytoplasm is light, and larger cells are located in close vicinity to small vessels (Giemsa). (B) Immunoreactivity of a small cell variant of ALCL for CD30. The larger tumor cells are strongly positive, whereas the small tumor cells are only weakly positive (alkaline phosphatase–antialkaline phosphatase [APAAP]). (C) Immunoreactivity of a small cell variant of ALCL for ALK. The larger cells (often located in the vicinity of small vessels) are positive in the cytoplasm and the nucleus, whereas in the smaller cells, the labeling is restricted mainly to the nucleus (APAAP). (D) Lymphohistiocytic variant of ALCL. Note the broad cytoplasm and the frequently slightly eccentrically located nuclei, which are revealed immunohistologically as belonging to reactive histiocytes (see panel E) (hematoxylin and eosin). (E) Immunoreactivity of the same case as in (D) with the CD68 antibody clone PG-M1. Nearly all of the larger cells are positive. These PG-M1–positive cells, representing nonneoplastic bystander macrophages, obscure the few CD30+ tumor cells (see Figure 2F) (APAAP). (F) Immunoreactivity of the same case as in (D) for CD30. Only a minority of the large cells are positive and represent the tumor cell population (APAAP). (G) Immunoreactivity of the same case as in (D) and (E) for ALK. The labeled cells vary greatly in size, with a predominance of smaller cells. The smaller the cells, the more the labeling is restricted to the nucleus. The negative cells are the bystander macrophages (antibody clone ALK1; APAAP). (H) Immunoreactivity of the same case as in (D), (E), and (G) for perforin. The tumor cells are strongly positive, whereas the bystander macrophages are negative (antibody clone P1-8;APAAP).
The sarcomatoid form of ALCL42 mimics soft-tissue tumors, especially of malignant fibrous histiocyte type. The neoplastic cells of this rare variant are large, bizarre, and often spindle-shaped, and express CD30. Multinucleated forms are present in varying numbers. The distinction from malignant fibrous histiocytoma is easily accomplished by immunohistology because these and other soft-tissue tumors consistently lack CD30 and other lymphoid markers. Other rare subforms of ALCL are characterized by an abundant admixture of eosinophils or neutrophils.13,43-45 Such cases may easily be mistaken as Hodgkin disease, true histiocytic malignancies, or even as an acute inflammatory process.44,45 This is especially valid for the neutrophil-rich subform because it may mimic an acute inflammation and, in the skin, a pustular lesion.44 An ALCL subform with signet-ring appearance has also been described.15 46
ALCL may overlap with Hodgkin disease
Although there is accumulating evidence that ALCL and Hodgkin disease are biologically distinct, the morphologic and immunophenotypic border between these disease categories is not sharp in all instances.10,18,19,47 This applies especially to Hodgkin disease cases rich in tumor cells, with lymphocyte depletion, nodular sclerosis grade 2, or syncytial growth pattern. To keep the entities of Hodgkin disease and ALCL distinct, investigators in the late 1980s created a category (basket) under the term ALCL–HD-related. The borderline cases, or gray-zone cases, could then be collected in this basket for further studies.3,10,48 Under the designation Hodgkin-like ALCL, this type was adopted by the REAL classification as a preliminary category.13 The tumors falling into this category show features of both ALCL and Hodgkin disease. These ambiguous cases contain relatively dense nodules or sheets of tumor cells with features of classic Hodgkin and Reed-Sternberg cells (Figure3). Tumor cells are usually present within sinuses and, because of capsule thickening and nodular or diffuse fibrosis, the sinusoidal dissemination is on occasion recognizable only by immunolabeling for CD30. The proportion of admixed reactive inflammatory cells is lower than that found in typical cases of Hodgkin disease. The change from the term ALCL–HD-related to ALCL–HD-like reflects the tendency in the early 1990s to believe that most of these gray-zone lymphomas represent ALCL mimicking Hodgkin disease. However, the frequent expression of the B-cell–specific activation protein BSAP (PAX5) in the absence of the protein ALK (discussed later) favors the opinion that most cases of ALCL–HD-like represent a tumor cell–rich variant of classic Hodgkin disease and not a true ALCL because the mentioned expression pattern is characteristic for Hodgkin disease.49 Accordingly, the new WHO classification has abandoned this subform and subsumes these cases under classic Hodgkin disease.39,50 51
Hodgkin-like ALCL.
(A-D) Hodgkin-like ALCL, ALK+. (A) Morphology at low magnification (hematoxylin and eosin). Note the nodular sclerosis. (B) Same case as in (A) at higher magnification. The large tumor cells resemble Hodgkin- and Reed-Sternberg cells. (C) Same case as in (A) immunostained for EMA (antibody clone E29). All tumor cells are labeled. (D) Same case as in (A) immunostained for ALK (antibody clone ALK1). The tumor cells show a strong positivity of their cytoplasm. (E,F) Hodgkin-like ALCL, ALK−. (E) Morphology by Giemsa stain. (F) Immunoreactivity for CD30 (antibody clone Ber-H2; alkaline phosphatase–antialkaline phosphatase). All tumor cells are strongly CD30-positive, but ALK-negative (not shown).
Hodgkin-like ALCL.
(A-D) Hodgkin-like ALCL, ALK+. (A) Morphology at low magnification (hematoxylin and eosin). Note the nodular sclerosis. (B) Same case as in (A) at higher magnification. The large tumor cells resemble Hodgkin- and Reed-Sternberg cells. (C) Same case as in (A) immunostained for EMA (antibody clone E29). All tumor cells are labeled. (D) Same case as in (A) immunostained for ALK (antibody clone ALK1). The tumor cells show a strong positivity of their cytoplasm. (E,F) Hodgkin-like ALCL, ALK−. (E) Morphology by Giemsa stain. (F) Immunoreactivity for CD30 (antibody clone Ber-H2; alkaline phosphatase–antialkaline phosphatase). All tumor cells are strongly CD30-positive, but ALK-negative (not shown).
Immunophenotype
Immunohistochemical screening of a large number of undifferentiated large cell malignancies has revealed that the tumor cells of all ALCL cases show a strong expression of CD30 on the cell membrane and in the Golgi region (Figure 1D; diffuse cytoplasmic CD30 positivity is of dubious significance), so that the membrane-associated expression of CD30 was included in the definition of ALCL.1,3,10,13,28 The analysis of conventional T- and B-cell markers revealed 3 immunophenotypes (Table2), with the T-cell type being the most frequent.1,6,52 The ε-chain of the T-cell receptor CD3 complex (TCR) is one of the most constantly expressed T-cell antigens; a minority of cases express CD4 or CD8, with a predominance of CD4.1,10,53 The frequency of the null cell type depends on the number of T-cell antigens investigated. Most, if not all, of the null cell cases belong to the T-cell type. This becomes evident when a large number of T-cell antigens, or the configuration of the TCR genes (see below), is investigated and the studies are extended to cytotoxic molecules. The vast majority of the T/null ALCLs proved to harbor clonally rearranged TCR γ and β genes and expressed the cytotoxic molecules perforin, granzyme B, and T-cell–restricted intracellular antigen-1 (TIA-1) (Figures 1E,2H, 12D), regardless of the expression of CD4 or CD8.36,53,54 Because cytotoxic molecules are expressed not only by cytotoxic T cells but also by natural killer (NK) cells,55,56 and activated NK cells may express CD30,57 there is the possibility that a minority of the T/null ALCLs are derived from NK cells rather than cytotoxic T cells. In support of this possibility is the expression of the NK cell–associated marker CD56 in some cases of ALCL54 and the finding that approximately 10% of ALCLs lack detectable TCR gene rearrangements.36 Further studies are required to clarify unequivocally the relation of some ALCL cases to NK cells.
CD30+ large cell lymphomas with anaplastic morphology: immunophenotype, antigen-receptor genotype, and EBV infection
| Immunophenotype . | Incidence . | Clonal rearrangements . | EBV . | Cytotoxic molecules . | Chimeric ALK* . | |
|---|---|---|---|---|---|---|
| TCRγ/β . | IgH . | |||||
| T null | 55% to 76%1,6,52,53 58 | + (90%) | − | − | +/− | + (53% to 89%)40,66 69 |
| 6% to 25%36 40 | + (90%) | − | − | +/− | ||
| B | 5% to 21%1,36,52 59 | − | +/− | +/− | − | −† |
| Immunophenotype . | Incidence . | Clonal rearrangements . | EBV . | Cytotoxic molecules . | Chimeric ALK* . | |
|---|---|---|---|---|---|---|
| TCRγ/β . | IgH . | |||||
| T null | 55% to 76%1,6,52,53 58 | + (90%) | − | − | +/− | + (53% to 89%)40,66 69 |
| 6% to 25%36 40 | + (90%) | − | − | +/− | ||
| B | 5% to 21%1,36,52 59 | − | +/− | +/− | − | −† |
Refers to systemic ALCL. + indicates all cases positive; +/−, majority of cases positive; −/+, minority of cases positive; and −, all cases negative.
Large cell lymphomas with anaplastic morphology that express B-cell antigens are relatively rare1,6,58 (Table 2). They have been incorporated into the Kiel classification as a separate entity. However, according to the REAL and the new WHO classifications, they are not accepted as a distinct entity but are regarded as a morphologic and immunophenotypic variant of diffuse large B-cell lymphoma.13 Recent studies further support this view.59 Therefore, in the current article, these tumors are referred to as anaplastic large B-cell lymphomas. ALCLs that express both B-cell and T-cell antigens have been detected so far only by immunolabeling of frozen sections,1 possibly because of the higher sensitivity of this approach. The meaning of the double expression of T- and B-cell markers is obscure.
Antigenic markers can be useful in the distinction of the different clinical subforms of ALCL (further discussed later). Unlike the systemic form, the primary cutaneous ALCL is usually negative for epithelial membrane antigen (EMA)4,12 and for the ALK protein60-63 (Table 3). Moreover, nearly half of the cases arising in the skin are positive for the cutaneous lymphocyte antigen recognized by monoclonal antibody HECA 452.12
Correlation between clinical features, morphology, and immunophenotype in ALCL
| Clinical form of ALCL . | Morphology . | Phenotype . | EMA . | Chimeric ALK . |
|---|---|---|---|---|
| Systemic | All variants | Mostly T/null | Frequently positive | Positive (∼ 60%) |
| Primary cutaneous | Common type to lymphomatoid papulosis | T/null | Some | Negative3-150 |
| HIV-related | Common or giant cell–rich | Mostly B | NDA | NDA |
| Rarely T/null | NDA | NDA | ||
| Secondary | Variable | T/null3-151 | Negative | Negative |
| Clinical form of ALCL . | Morphology . | Phenotype . | EMA . | Chimeric ALK . |
|---|---|---|---|---|
| Systemic | All variants | Mostly T/null | Frequently positive | Positive (∼ 60%) |
| Primary cutaneous | Common type to lymphomatoid papulosis | T/null | Some | Negative3-150 |
| HIV-related | Common or giant cell–rich | Mostly B | NDA | NDA |
| Rarely T/null | NDA | NDA | ||
| Secondary | Variable | T/null3-151 | Negative | Negative |
NDA indicates no data available.
Rare cases reported to be ALK+ probably represent systemic ALCL with skin involvement, in which the extracutaneous disease escaped recognition.
Immunophenotype depends on the preceding lymphoma disease.
Immunophenotypic differential diagnosis of ALCL versus Hodgkin disease
As mentioned earlier, ALCL and Hodgkin disease share several morphologic and immunophenotypic features and, in some cases, assignment to one of these entities is not possible.3,10,13,64,65 This situation is particularly true for cases that lack expression of T-cell and B-cell antigens. The overlap between these conditions has vanished for the cases expressing ALK fusion proteins (chimeric ALK) because this protein is consistently absent from the tumor cells of all cases of Hodgkin disease.66-69 However, there is still an overlap (Figure4) between ALK− ALCL and Hodgkin disease. In 1994, at the European Association of Hematopathology Workshop in Toledo, Spain, it became evident that markers such as CD15, BNH.9, and EMA—despite initial optimism—do not help in this distinction.19 This proved to be valid also for cytotoxic molecules because these may also be expressed by Reed-Sternberg cells of Hodgkin disease36,53,70 as well as by the tumor cells in ALCL. Recently, BSAP71 has been found to be expressed by Reed-Sternberg cells but not by cells of T-cell or null-cell–type ALCL.49,72 A preliminary study has demonstrated the usefulness of this antigen in the differential diagnosis of ALCL and Hodgkin disease.49
Current morphologic and immunophenotypic overlaps of true ALCLs and anaplastic large B-cell lymphomas with classic Hodgkin disease.
The REAL classification refers to the overlapping neoplasms as ALCL–HD-like because they cannot, because of missing morphologic and immunophenotypic criteria, be allocated to one of the established lymphoma entities. The overlap between ALK+ ALCL and Hodgkin disease has disappeared with the availability of monoclonal antibodies to ALK protein, which strongly stain the tumor cells of all cases of ALCL with a rearranged ALK gene but consistently fail to stain Hodgkin and Reed-Sternberg cells of Hodgkin disease.
Current morphologic and immunophenotypic overlaps of true ALCLs and anaplastic large B-cell lymphomas with classic Hodgkin disease.
The REAL classification refers to the overlapping neoplasms as ALCL–HD-like because they cannot, because of missing morphologic and immunophenotypic criteria, be allocated to one of the established lymphoma entities. The overlap between ALK+ ALCL and Hodgkin disease has disappeared with the availability of monoclonal antibodies to ALK protein, which strongly stain the tumor cells of all cases of ALCL with a rearranged ALK gene but consistently fail to stain Hodgkin and Reed-Sternberg cells of Hodgkin disease.
Immunoglobulin (Ig) and TCR gene rearrangements
Initial studies73,74 on the configuration of the antigen receptor genes in ALCL, which were performed using the Southern blot technique, demonstrated a surprising divergence between immunophenotype and Ig and TCR gene rearrangements. More recent investigations using the PCR in conjunction with family-specific primers have, however, demonstrated an almost complete concordance between the T-cell and B-cell antigen profile and the presence of clonally rearranged TCR genes (Table 2).36 The demonstration of clonally rearranged TCR β and γ genes in 90% of cases of T- and null-type ALCL36 indicates that most, if not all, of the null ALCLs are immunophenotypic variants of T-type ALCL and that ALCLs originating from NK cells seem to be rare, despite the recently reported (although somewhat controversial) expression of CD56 in approximately one third of ALCLs.54
Putative normal counterparts of ALCL
Normal lymphoid tissue contains a small population of large lymphoid blasts that express CD30.1,24,25 These nonneoplastic CD30+ blasts resemble the neoplastic cells of systemic ALCL1 25 in their cytologic features and tissue distribution (preferentially perifollicular and occasionally intrasinusoidal localization). It is therefore tempting to assume that they represent the normal precursor cells of systemic ALCL. The question to be answered now is whether these normal CD30+blasts have the same cytotoxic T-cell phenotype and genotype as systemic ALCLs. Such studies are in progress in the authors' laboratory.
So far, no likely candidate for the normal precursor cell for the primary cutaneous ALCL can be identified. The anaplastic large B-cell lymphoma might be derived from CD30+ germinal center B cells (as are most diffuse large B-cell lymphomas) because they carry—like reactive germinal center B cells and the nonanaplastic CD30− diffuse large B-cell lymphoma—somatic mutations in their rearranged variable Ig genes.75 This supports the close relation between diffuse large B-cell lymphomas with and without anaplastic morphology and is consistent with the concept of the REAL classification that anaplastic large B-cell lymphoma is a variant of diffuse large B-cell lymphoma.
Structure and pathogenic role of the NPM-ALK gene
In the late 1980s, it was found that a proportion of ALCLs were associated with a 2;5 chromosomal translocation.20-23 As demonstrated by Morris et al76 in 1994, the 2;5 translocation causes the NPM gene located at 5q35 to fuse with a gene at 2p23 encoding the receptor tyrosine kinase anaplastic lymphoma kinase (ALK). The properties of wild-type NPM and ALK as well as their chimerized genes and proteins are summarized in Table4 and in Figure5. Wild-type NPM (also known as B23) was first identified in the late 1970s and early 1980s78,79as a ubiquitous acidic 37-kd phosphoprotein associated with nucleoli. NPM shuttles continuously between the cytoplasm and the nucleolus and thus functions as a carrier of newly synthesized proteins into the nucleolus.80 The NPM molecule exercises this function through an oligomerization motif81 at the N-terminal region and 2 nuclear localizing signals at the C-terminal domain82 (Figure 5). The wild-type ALK protein is a 200-kd transmembrane receptor that is most closely related to leukocyte tyrosine kinase (LTK)76,83-85 and whose postnatal expression is restricted physiologically to a few scattered cells in the nervous system (some glial cells, a few endothelial cells, and some pericytes).66 The intracellular tail of the ALK molecule carries the tyrosine kinase catalytic domain (Figure 5), which becomes physiologically activated as a result of homodimerization following ligand binding.86
Properties of NPM, ALK, and NPM-ALK
| Property . | NPM . | ALK . | NPM-ALK . |
|---|---|---|---|
| Synonyms | B23, numatrin, NPM, NO38, nucleophosmin76 78 | ALK (anaplastic lymphoma kinase)76 | — |
| Chromosomal localization | 5q3576 | 2p2376 | 5q35 after t(2;5)76 |
| Molecular weight; length of cDNA (bp); number of amino acid residues (aa) | 32 500; about 1200 bp; 294 aa81 | 200 000; 6226 bp; 1620 aa83 84 | 80 000; 2040 bp; 680 aa (residues 1-116 from NPM; residue 117 generated by breakpoint; residues 118-680 from ALK corresponding to residue 1058-1620 of the ALK sequence)76 85 |
| Length of the gene; number of exons | 25 kB; 11 exons77 | No data available | Promoter and the first 4 exons of the NPM gene and the whole cytoplasmic domain of the ALK sequence77 |
| Cellular localization of the protein | Nucleolar81 | Transmembranous76,83 84 | Cytoplasmic and/or nuclear66,69 107 |
| Related proteins | Nucleoplasmin (nucleosome assembly factor)81 | Member of the insulin receptor family; LTK (leukocyte tyrosine kinase)76,83-85 103 | — |
| Function of the protein | Involved in preribosomal assembly, shuttles between nucleus and cytoplasm, represents one of the main silver-stained proteins (AgNORs)81 87 | Receptor tyrosine kinase with an unknown ligand; putative role during the development of the brain and peripheral neurons83 84 | Contains the oligomerization motif of NPM and the tyrosine kinase domain of ALK; malignant transformation in vitro86,91 107 and in vivo90 |
| Molecular occurrence | Homohexamer81 | Dimerization after stimulation/ligand binding83 84 | Homodimers and heterodimers86 107 |
| Expression pattern | Ubiquitous81 | Brain, particularly prenatal66,83 84 | All cells with t(2;5); expression regulated by the NPM promoter66,76 104 |
| Property . | NPM . | ALK . | NPM-ALK . |
|---|---|---|---|
| Synonyms | B23, numatrin, NPM, NO38, nucleophosmin76 78 | ALK (anaplastic lymphoma kinase)76 | — |
| Chromosomal localization | 5q3576 | 2p2376 | 5q35 after t(2;5)76 |
| Molecular weight; length of cDNA (bp); number of amino acid residues (aa) | 32 500; about 1200 bp; 294 aa81 | 200 000; 6226 bp; 1620 aa83 84 | 80 000; 2040 bp; 680 aa (residues 1-116 from NPM; residue 117 generated by breakpoint; residues 118-680 from ALK corresponding to residue 1058-1620 of the ALK sequence)76 85 |
| Length of the gene; number of exons | 25 kB; 11 exons77 | No data available | Promoter and the first 4 exons of the NPM gene and the whole cytoplasmic domain of the ALK sequence77 |
| Cellular localization of the protein | Nucleolar81 | Transmembranous76,83 84 | Cytoplasmic and/or nuclear66,69 107 |
| Related proteins | Nucleoplasmin (nucleosome assembly factor)81 | Member of the insulin receptor family; LTK (leukocyte tyrosine kinase)76,83-85 103 | — |
| Function of the protein | Involved in preribosomal assembly, shuttles between nucleus and cytoplasm, represents one of the main silver-stained proteins (AgNORs)81 87 | Receptor tyrosine kinase with an unknown ligand; putative role during the development of the brain and peripheral neurons83 84 | Contains the oligomerization motif of NPM and the tyrosine kinase domain of ALK; malignant transformation in vitro86,91 107 and in vivo90 |
| Molecular occurrence | Homohexamer81 | Dimerization after stimulation/ligand binding83 84 | Homodimers and heterodimers86 107 |
| Expression pattern | Ubiquitous81 | Brain, particularly prenatal66,83 84 | All cells with t(2;5); expression regulated by the NPM promoter66,76 104 |
Molecular structure of nucleophosmin (NPM), anaplastic lymphoma kinase (ALK), and ALK fusion proteins.
The NPM molecule consists of an oligomerization domain (residues 1-83), a metal-binding domain (MB; residues 104-115), 2 acidic amino acid clusters (AC; residues 120-132 and 161-188) that function as acceptor regions for nucleolar targeting signals,82 and 2 nuclear localization signals (NLS). The oligomerization domain was defined by means of deletion mutants.81 The ALK protein is a transmembrane tyrosine kinase receptor containing a transmembrane domain (TM) and a tyrosine kinase domain (TKD) in the N-terminal part of the intracytoplasmic tail. In the NPM-ALK fusion protein, the extracellular and transmembrane domains of ALK are replaced by the oligomerization domain of NPM (in approximately 75%) or of other proteins (X), schematically represented in Figure 7. The fused part of NPM contains, in addition to the oligomerization domain, the metal-binding region. The fusion point is at codon 117. N indicates amino terminal; C, carboxy terminal.
Molecular structure of nucleophosmin (NPM), anaplastic lymphoma kinase (ALK), and ALK fusion proteins.
The NPM molecule consists of an oligomerization domain (residues 1-83), a metal-binding domain (MB; residues 104-115), 2 acidic amino acid clusters (AC; residues 120-132 and 161-188) that function as acceptor regions for nucleolar targeting signals,82 and 2 nuclear localization signals (NLS). The oligomerization domain was defined by means of deletion mutants.81 The ALK protein is a transmembrane tyrosine kinase receptor containing a transmembrane domain (TM) and a tyrosine kinase domain (TKD) in the N-terminal part of the intracytoplasmic tail. In the NPM-ALK fusion protein, the extracellular and transmembrane domains of ALK are replaced by the oligomerization domain of NPM (in approximately 75%) or of other proteins (X), schematically represented in Figure 7. The fused part of NPM contains, in addition to the oligomerization domain, the metal-binding region. The fusion point is at codon 117. N indicates amino terminal; C, carboxy terminal.
The 2;5 translocation juxtaposes the portion of the NPM gene encoding the N-terminal domain of NPM (amino acids 1-117) (Figure 5) to the part of the ALK gene that codes for the entire cytoplasmic region of the ALK protein.76,87 As a consequence, the ALK gene comes under the control of the NPM promoter, which induces a permanent and ubiqitous transcription of the NPM-ALK hybrid gene, resulting in the production of an 80-kd chimeric protein termed NPM-ALK76 or p80.88 This NPM-ALK protein contains the NPM oligomerization domain and the intracytoplasmic region of ALK. The C-terminal NPM domain carrying the nuclear localization signals and the extracellular and transmembrane region of the ALK are absent.76,87 The NPM-ALK protein can form homodimers (by cross-linking with other NPM-ALK molecules) or heterodimers (by cross-linking with wild-type NPM) (Figure6). The formation of homodimers results in the constitutive activation of the catalytic ALK domain contained in the NPM-ALK fusion protein.86,87 The activated ALK domain has been shown to bind GRB288,89 and the SH2 domains of phospholipase C-γ,89 interactions that have been demonstrated to induce mitogenic activity and are likely to be involved in neoplastic transformation.86,89 Transfection of murine hematopoietic cells with the NPM-ALK fusion gene induces transplantable lymphoid tumors.90 NPM-ALK was also found to transform rat fibroblasts in vitro.91 Both of these latter findings further support the oncogenic property of this fusion protein.
Patterns of oligomerization and cellular distribution of NPM-ALK fusion and variant ALK-fusion proteins in the various subforms of ALK+ ALCL.
Patterns of oligomerization and cellular distribution of NPM-ALK fusion and variant ALK-fusion proteins in the various subforms of ALK+ ALCL.
Methods for the demonstration of NPM-ALK and the subcellular distribution of this fusion protein
The presence of the NPM-ALK translocation was initially demonstrated in tissue samples by Southern blot analysis,92 reverse transcriptase–polymerase chain reaction (RT-PCR),93-97 in situ hybridization,67 and, more recently, by 2-color fluorescence in situ hybridization (FISH).98 The application of these techniques has confirmed the association of ALCL with the 2;5 translocation. The Southern blot and RT-PCR techniques have, however, produced discrepant results over the frequency of the NPM-ALK fusion gene in ALCL and the occurrence of this anomaly in large B-cell lymphoma, Hodgkin disease, and even in normal cells.99-101 Because RT-PCR is prone to artifact102 and FISH is time-consuming and difficult to apply to paraffin sections, the production and use of polyclonal85,103,104 and monoclonal antibodies specific for fixative-resistant epitopes on the cytoplasmic tail of the ALK protein66,69 and also on the N-terminal domain of NPM105 represented a significant advance in the detection of the NPM-ALK anomaly. Because ALK protein is absent in all normal tissues, with the exception of scattered cells in the brain,66 a positive immunohistochemical staining in tissues (other than brain) indicates anomalous ALK expression, usually in the form of the t(2;5)-associated NPM-ALK fusion protein.66,69,103-105 Thanks to the generation of a monoclonal antibody against the N-terminus of NPM, the molecular association of the detected ALK with NPM can also be demonstrated immunohistologically because, in the presence of NPM-ALK, this antibody stains both the cytoplasm and the nucleus,105 whereas in tissues devoid of NPM-ALK, the labeling is restricted to the nucleus.105,106 The putative mechanism that might account for the different subcellular distribution of NPM-ALK is represented in Figure 6. The lack of nuclear localization signals in the chimeric NPM-ALK protein suggests that its transportation into the nuclei of tumor cells most likely occurs through the formation of heterodimers of NPM-ALK with wild-type NPM,107 which contains 2 nuclear localization signals.82 The availability of anti-ALK and anti-NPM antibodies applicable to archival paraffin-embedded tissues allowed the screening of large numbers of neoplasms, leading to a clear perception of the presence and frequency of the NPM-ALK fusion protein and the possibility of variant ALK proteins in lymphomas.66,69 106
ALK proteins other than NPM-ALK
In 3 large series of ALCL, 15% to 28% of chimeric ALK+ lymphomas were found to be negative for the t(2;5) translocation (as detected by immunohistochemistry), and it was suggested that they may represent cases in which the ALK gene fuses to a partner other than NPM to produce variant X-ALK protein(s).40,66,69,106 Such X-ALK+ lymphomas are characterized by a cytoplasm-restricted expression of the ALK protein (Figures 6 and 7) and a nucleus-restricted expression of wild-type NPM.106Additional evidence to support the presence of chimeric ALK proteins other than NPM-ALK has been obtained from reports of genetic abnormalities affecting the ALK gene in ALK+ ALCL. These include the inversion (2)(p23;q35) and the translocations (1;2)(q21;p23) and (2;3)(p23;q21),108-110 suggesting the existence of genes other than NPM that can deregulate the ALK gene. The existence of variant ALK proteins has been confirmed by immunobiochemical studies using the monoclonal antibodies to ALK and NPM (N-terminal domain).111 Western blotting studies have demonstrated the presence of variant ALK proteins of 85, 97, 104, and 113 kd.111 These new ALK fusion partners have now been identified by 5′ rapid amplification of cDNA ends (RACE) studies (Figure 7). Lamant et al112 described the 104-kd ALK protein as being TPM3 (nonmuscle tropomyosin)-ALK in a tumor exhibiting the (1;2)(q21;p23) translocation. The 85- and the 97-kd ALK proteins were found to be generated by a fusion of the ALK gene with the TFG (tropomyosin receptor kinase–fused gene).113 The larger TFG-ALK fusion protein (TFG-ALKlong) contains an additional 165-bp TFG sequence113 and is associated with the (2;3)(p23;q21) translocation.110 Both of the TPM3 and TFG genes have been found to be involved in the deregulation of the kinase domain of other oncogenic tyrosine kinases present in carcinomas.114,115 In common with NPM, both TFG and TPM3 proteins contain dimerization regions. The possibility therefore exists that the formation of homodimers of TPM3-ALK or TFG-ALK (to mimic ligand binding) results in the constitutive activation of the ALK kinase domain, conferring oncogenic activity on these variant ALK proteins. In support of this is the finding that both TFG-ALK and TPM3-ALK proteins are capable of auto-phosphorylation in vitro.112 The 2 other ALK fusion partners recently identified are ATIC (5-aminoimidazole-4-carboxamide-1-beta-d-ribonucleotide transformylase/inosine monophosphate cyclohydrolase),116-118 caused by the inversion (2)(p23;q35), and CLTCL (clathrin heavy polypeptide–like gene),119 which occurs as a result of the (2;22)(p23;q11) translocation (Figure 7). Recent studies have also been able to document the frequency with which the newly found ALK fusion variants occur (Figure 7).112,113 116-119 It is of pathogenic significance that all chimeric ALK variants contain the same functional kinase domain of ALK as that present in the NPM-ALK protein (Figure 7). The lack of nuclear localization signals in the variant fusion proteins (other than NPM-ALK) accounts for the absence of these fusion proteins from the nucleus and their distribution only in the cytoplasm (Figure 6).
Features of the various ALK fusion proteins occurring in ALK+ anaplastic large cell lymphomas.
§ indicates tyrosine kinase domain of the ALK protein; *, also occurs as an 85-kd (TFG-ALK short) fusion protein; **, 5 aminoimidazole-4-carboxamide-1-beta-d-ribonucleotide transformylase/IMP cyclohydrolase.
Features of the various ALK fusion proteins occurring in ALK+ anaplastic large cell lymphomas.
§ indicates tyrosine kinase domain of the ALK protein; *, also occurs as an 85-kd (TFG-ALK short) fusion protein; **, 5 aminoimidazole-4-carboxamide-1-beta-d-ribonucleotide transformylase/IMP cyclohydrolase.
Evidence has also been obtained for the presence of an exceedingly rare category of ALK+/IgA+ large B-cell lymphoma with immunoblastic (rather than anaplastic) morphology.120Immunocytochemical labeling and Western blotting studies using polyclonal and monoclonal antibodies recognizing the N-terminal and the C-terminal of ALK, respectively, have demonstrated the presence of a 200-kd full-length ALK protein in the tumor cells.120 It appears likely that the ALK+ B-cell tumors recently described by Gascoyne et al101 may also represent cases of this rare category of B-cell lymphoma (R. Gascoyne, personal communication, August 1999). Unfortunately, this possibility could not be clarified because of the unavailability of tissue sections from these cases for re-evaluation by other laboratories.
Occurrence of ALK expression outside lymphoid tumors
ALK proteins may also be expressed in tumors other than lymphoid neoplasms. Falini et al69 reported the presence of full-length ALK protein in 1 of 35 cases of rhabdomyosarcoma. This is in keeping with the earlier observation by Morris et al76 that the rhabdomyosarcoma cell line RH30 expresses the 200-kd wild-type ALK protein. More recently, expression of ALK proteins has been observed in inflammatory myofibroblastic tumors121and in neuroblastomas.122 In the former tumor lesion, the ALK expression appears to be due to a rearrangement of the 2p23 region where the ALK gene is located.
Specificity of ALK gene expression and its correlation with immunophenotype and morphology in lymphoma
Using the highly specific monoclonal anti-ALK antibodies ALK1 and ALKc, investigators found the expression of the chimeric ALK protein to be confined to ALCL of T/null type, with a frequency ranging from 53% to 89%.40,66,69 The only other lymphoid neoplasms to express ALK proteins are the rare large B-cell lymphomas reported by Delsol et al120 and by Gascoyne et al.101 The ALK molecule could not be detected in any other lymphoma types, including most cases of CD30+ primary cutaneous lymphoproliferative disorders (primary cutaneous ALCL and lymphomatoid papulosis), Hodgkin disease, and the majority of cases (more than 85%) of ALCL with Hodgkin-like appearance.40,66,69ALK+ ALCLs usually encompass a wide morphologic spectrum ranging from the small cell to the giant cell–rich variant of ALCL,40,69,123 with most cases falling into the category of ALCL common type. There is a correlation between the size of the ALK+ tumor cells and the subcellular distribution of the ALK protein: The large anaplastic tumor cells are usually positive both in the cytoplasm and the nucleus (less commonly only in the cytoplasm), whereas the small tumor cells exhibit a nuclear-restricted ALK protein expression69 (Figures 1F-G; 2C,H; 3D; 6). The large ALK+ tumor cells make up the dominant population in the common and giant cell types. However, the latter types usually contain, in addition, a small proportion of small-sized elements (sometimes regarded as nonneoplastic on morphologic grounds alone) that express the ALK protein.69 In contrast, the ALK+ small cell elements represent the dominant population in the small cell and lymphohistiocytic variants,69 in which a small proportion of large ALK+ tumor cells is usually detectable, often located around vessels (Figure 2).
The fact that the ALK protein is detectable in both the small and large tumor cells indicates that the genetic lesion leading to the anomalous ALK expression is present in both cell populations, and thus the large and the small cells belong to the same neoplastic clone. For this reason, we can dismiss the hypothesis that the large cells observed in the small cell variant of ALCL represent a subclone that has arisen in a (2;5) translocation–negative low-grade (small cell) lymphoma by acquiring the (2;5) translocation. According to a recent study,41 the transformation of the ALK+ small cell ALCL variant into the ALCL common type may be linked, at least in some cases, to the acquisition of additional chromosomal abnormalities (eg, those involving the sex chromosome and chromosomes 6, 7, 9, and 15).
From the available data, it can be concluded that the wide morphologic spectrum observed in chimeric ALK+ lymphomas is due to the following: (1) the different ratio between the large and the small tumor elements (variable from case to case and also within a given case at presentation and relapse), which appears to be dependent on whether the NPM-ALK protein is dimerized with wild-type NPM (Figure 6); (2) the different tissue distribution of neoplastic cells (eg, perivascular pattern in the small cell and lymphohistiocytic variants); (3) the occasional occurrence of nodular sclerosis (producing a Hodgkin-like appearance); and (4) the presence of different reactive cells (eg, histiocytes in the lymphohistiocytic variant).69 123
Epstein-Barr virus
Epstein-Barr virus (EBV) infection of the tumor cells in primary systemic and cutaneous ALCL of T/null-cell type is rare or absent.124-126 In contrast, EBV is frequently detectable in the anaplastic large B-cell lymphomas (Table 2),127,128with the incidence of infection exceeding that of sporadic Burkitt lymphoma.127 In patients infected with human immunodeficiency virus (HIV), the infection rate of large B-cell lymphomas with anaplastic morphology is increased to more than 50% of the cases.129 In contrast to Burkitt lymphoma, EBV infection in anaplastic large B-cell lymphomas is usually associated with expression of the latent membrane protein-1.129
Clinical features and subforms
ALCL has been clinically subdivided into a primary form (de novo) and a secondary form (anaplastic transformation from another lymphoma). Among the primary ALCLs, systemic and cutaneous categories have been recognized both in immunocompetent patients and in HIV-positive patients (rarely) (Table 3 and Figure8).
Classification of primary large cell lymphoma with anaplastic morphology (ALCL).
*indicates that about 20% of ALCL-type cases may lack anaplastic features129; †, includes all the morphologic variants discussed in Table 5; ‡, in the Kiel classification, included as a separate entity, and in the REAL classification, included as a variant of diffuse large B-cell lymphoma.
Classification of primary large cell lymphoma with anaplastic morphology (ALCL).
*indicates that about 20% of ALCL-type cases may lack anaplastic features129; †, includes all the morphologic variants discussed in Table 5; ‡, in the Kiel classification, included as a separate entity, and in the REAL classification, included as a variant of diffuse large B-cell lymphoma.
Primary systemic ALCL
Primary systemic ALCL is the most frequent subform, accounting for 2% to 8% of non-Hodgkin lymphomas in adults10,13,130 and approximately 20%-30% of large cell lymphomas in children.2,131 Wright et al132 reported that the clinical features and the outcomes of systemic ALCL varied in different studies and argued that this is probably due to differences in the diagnostic criteria used, the varying age distribution of patients, and the inclusion, in clinical trials, of provisional categories such as ALCL–HD-like and ALCL of B phenotype, as well as of primary cutaneous forms of ALCL. An additional bias appears to be that in previous studies, ALK expression was not investigated, and therefore the 2 emerging entities of systemic ALCL (ALK+ and ALK−) were not distinguishable. Because there is now evidence that the clinical features and outcomes of systemic ALCL differ significantly in cases harboring and cases lacking a dysregulated ALK gene, we will discuss ALK+ and ALK− ALCL cases separately.
ALK+ALCL.
ALK+ ALCL mostly occurs in the first 3 decades of life (Figure 9),40,69,101,133,135with male predominance being particularly striking in the second and third decades of life (male/female ratio 6.5).133 This lymphoma frequently presents as an aggressive stage III to IV disease, usually associated with systemic symptoms (75%), especially high fever.133 Extranodal involvement is frequent (60%), with approximately 40% of patients showing 2 or more extranodal sites of the disease. In a large study,133 the frequency of extranodal sites of lymphoma involvement was as follows: skin 21%, bone (solitary or multiple lesions) 17% (Figure 10), soft tissues 17% (Figure 10), lung 11%, and liver 8%, with involvement of the gut and central nervous system (CNS) being a rare event. The incidence of bone marrow involvement is approximately 11% when analyzed with hematoxylin and eosin stains and approximately 30% when checked with immunohistochemistry because scattered ALCL cells are detectable in bone marrow trephines only when they are specifically labeled.134
Extranodal involvement in ALK+ ALCL.
(A) Large osteolytic lesions of the skull in a 14-year-old boy. (B) Involvement of the right obturator muscle (arrows) in a 25-year-old man.
Extranodal involvement in ALK+ ALCL.
(A) Large osteolytic lesions of the skull in a 14-year-old boy. (B) Involvement of the right obturator muscle (arrows) in a 25-year-old man.
ALK+ ALCL appears to benefit from chemotherapy more than ALK− forms of systemic ALCL. This prognostic difference between ALK+ and ALK− ALCL (as defined by immunostaining with a polyclonal anti-p80 antibody) was first described by Shiota et al,135 who reported that p80 (ALK+) ALCL cases showed a far better 5-year survival rate (79.8%) than p80 (ALK−) ones (32.9%). The authors regarded it as unlikely that the younger age of the ALK+patient group could have accounted for this difference in survival because similar findings were observed in patients of both groups (p80+ and p80−) who were less than 30 years of age.135 More recently, the different prognosis of ALK+ ALCL and ALK− ALCL was confirmed in 2 large series of ALCL patients (Figure11)101,133 in which ALK expression in tissue biopsy specimens was determined with anti-ALK monoclonal antibodies. In these studies, the 5-year overall survival of ALK+ versus ALK− ALCL was 71% ± 6% versus 15% ± 11% in one study, respectively (Figure11)133 and 79% versus 46% in the other.101Other studies have also reported an excellent outcome for systemic ALCL occurring in pediatric136-138 and young adult patients.139,140 Although ALK expression was not investigated in these studies, the young age suggests that these series of patients contained a relatively large proportion of chimeric ALK+ ALCL cases. In 2 patients, transformation of the ALK+ small variant to common-type ALCL was found to be predictive of a rapid clinical course,41 but this finding must be confirmed by the study of a larger number of patients. ALCLs expressing an ALK fusion protein other than NPM-ALK were found to resemble typical NPM-ALK+ cases. They were of T or null phenotype, usually occurred in young male patients, presented with advanced disease associated with systemic symptoms and extranodal involvement, and showed an excellent prognosis (14 of 15 patients alive at a median follow-up of 2.26 years).106 These findings suggest that lymphomas carrying variants of the NPM-ALK fusion protein can be grouped with classic t(2;5)-positive tumors as a single entity (ALK+ ALCL or “ALKoma”) with a better prognosis than ALK− ALCL.106
The difference in overall survival between ALK+ ALCL and ALK− ALCL.
With the exception of 2 cases, all patients were treated with anthracyclin-containing regimens.133
The difference in overall survival between ALK+ ALCL and ALK− ALCL.
With the exception of 2 cases, all patients were treated with anthracyclin-containing regimens.133
It is noteworthy that, within the good prognostic group of chimeric ALK+ cases, Falini et al133 demonstrated that the 5-year survival was 94% ± 5% for the low/intermediate risk group (age-adjusted International Prognostic Index [IPI] 0-1) and 41% ± 12% for the high/intermediate risk group (age-adjusted IPI 2). Similar findings were observed by Gascoyne et al.101 These findings are in contrast to a recent report by the Non-Hodgkin's Lymphoma Classification Project, in which no significant difference was observed between ALCL patients with a low or a high IPI.130 However, this latter result was obtained in a less well-defined group of ALCL patients who were not characterized with anti-ALK antibodies.
The opportunity to use immunohistochemical labeling techniques to identify cases of ALCL with good prognosis (ALK+ lymphomas) and to further classify this homogeneous disease into low- and high-risk cases according to the IPI might be of great relevance for the design of optimal therapeutic strategies. The excellent outcome of low-risk ALK+ lymphomas (age-adjusted IPI 0-1) warrants the randomized comparison of less versus more intensive conventional chemotherapy in this category of patients. This concept particularly applies to ALCL occurring in children, whose treatment, because of the large growth fraction and the clinical aggressiveness of the disease, has been mainly based on the highly aggressive regimens used for lymphoblastic leukemia and lymphoma. The low frequency (less than 5%) of CNS involvement by ALK+ lymphomas133 also questions the general policy of intrathecal therapy as prophylaxis for CNS involvement in children. Finally, these findings do not support the use of high-dose therapy followed by autologous bone marrow transplantation as a first-line treatment in ALK+ lymphomas with age-adjusted IPI of 0 to 1. This form of treatment has been proposed for “primary systemic CD30 (Ki-1)+lymphomas”141 (for which data on ALK expression were not available at the time of the study), and that probably included heterogeneous entities (ALK+ and ALK− ALCL as well as cases on the borderline between ALCL and Hodgkin disease).
In contrast, patients with high-risk ALCL, ie, ALK+lymphomas with an age-adjusted IPI of ≥2 or lacking ALK, should probably be enrolled in clinical studies aimed at comparing the efficacy of conventional polychemotherapy versus high-dose chemotherapy followed by stem cell support.141,142 It will also be important to investigate the efficiency of innovative forms of therapy to improve the prognosis of ALK+ lymphomas with high IPI and that of ALK− ALCL. For example, the survival of mice bearing xenografts of human CD30+ ALCL has been prolonged when treated with anti-CD30 immunotoxin.143 In this context, it is interesting that NPM-ALK also binds to the intracellular domain of CD30.144 Thus, anti-CD30 monoclonal antibodies linked to toxins or radioisotopes may provide new tools for the treatment of poor prognostic categories of ALCL. Other novel approaches to the therapy of ALCL might be the use of specific inhibitors for NPM-ALK145 or the induction of a T-cell immune response to ALK protein.
ALK− systemic ALCL.
ALK− systemic ALCL occurs in older individuals (Figure 9) and is associated with a lower male/female ratio (0.9), as well as a lower incidence of stage III to IV disease and extranodal involvement in one study,133 but not in another.101 In both studies, ALK− ALCL showed a poorer prognosis than ALK+ ALCL (Figure 11). A search for innovative therapeutic modalities is needed for this category of lymphoma.
Hodgkin-like ALCL.
This subform occurs in young patients (like ALK+ ALCL) but shows clinical features differing from those observed in ALK+ ALCL. For example, there is a high frequency of mediastinal involvement (bulky disease in approximately 60% of cases), frequent stage II presentation, and lack of skin and bone involvement.64 These clinical findings, together with the absence of ALK protein and expression of BSAP (PAX-5 gene product; see earlier) in most of the cases, further support the view that cases classified as Hodgkin-like ALCL are, in most instances, more related to Hodgkin disease than to ALCL.
Primary cutaneous ALCL
Primary cutaneous ALCL differs from the systemic form in its site of origin, its clinical features, and its almost invariable absence of the ALK protein.60-62,146 Primary cutaneous ALCL arises de novo in the skin and affects older patients with a median age of approximately 60 years. It accounts for approximately 9% of cutaneous lymphomas.147 The lesion usually presents as a solitary, asymptomatic, cutaneous or subcutaneous reddish-violet tumor, which can be superficially ulcerated148 (Figure12). Less commonly, the disease is characterized by multiple tumor nodules aggregated in a circumscribed area or as multicentric tumors at multiple sites. Primary cutaneous ALCL has a more favorable prognosis than systemic ALCL (which may secondarily involve the skin). Approximately 25% of patients with primary cutaneous ALCL show partial or complete spontaneous regression, accounting for the previous designation of regressing atypical histiocytosis. Treatment of localized lesions usually includes excision with or without radiation and is associated with long-term survival.12,147,149 However, patients with disseminated skin disease seem to be at greater risk of developing extracutaneous involvement and may benefit from systemic polychemotherapy.148
Primary cutaneous anaplastic large cell lymphomas.
(A) Macroscopic appearance: ulcerated reddish-violet tumor of the skin. (B) Histologic appearance of the same lesion as in (A) at low magnification (hematoxylin and eosin). (C) Cytologic composition of the same lesion as in (A) and (B). Note the presence of many large anaplastic cells; they are strongly CD30+ (not shown) (hematoxylin and eosin). (D) Immunoreactivity of the same lesion as in (A), (B), and (C) for the cytotoxic molecule granzyme B. All of the tumor cells are strongly positive (antibody clone GrB-7, avidin-biotin-peroxidase).
Primary cutaneous anaplastic large cell lymphomas.
(A) Macroscopic appearance: ulcerated reddish-violet tumor of the skin. (B) Histologic appearance of the same lesion as in (A) at low magnification (hematoxylin and eosin). (C) Cytologic composition of the same lesion as in (A) and (B). Note the presence of many large anaplastic cells; they are strongly CD30+ (not shown) (hematoxylin and eosin). (D) Immunoreactivity of the same lesion as in (A), (B), and (C) for the cytotoxic molecule granzyme B. All of the tumor cells are strongly positive (antibody clone GrB-7, avidin-biotin-peroxidase).
Despite some differences, primary cutaneous ALCL and lymphomatoid papulosis overlap in histologic, immunophenotypic, and clinical features (Table 5). The major clinical difference is that lymphomatoid papulosis, despite relapses, runs a benign clinical course, with spontaneous disappearance of individual skin lesions in most instances.148 Given that a histologic and immunohistochemical distinction between primary cutaneous ALCL and lymphomatoid papulosis is often not possible because of the overlapping features,147,148 clinical criteria should be applied to determine whether the patient has a locally progressive disease that requires treatment (ALCL) or a relapsing condition that needs no treatment (lymphomatoid papulosis).151
Comparison of primary cutaneous ALCL with lymphomatoid papulosis
| Features . | Cutaneous ALCL . | Lymphomatoid papulosis . |
|---|---|---|
| Gross morphology | Nodules | Papules |
| Histology | Clusters/sheets of CD30+ blasts, Hodgkin-like cellular background | Few CD30+ blasts, variable number of cerebriform T cells, many inflammatory cells |
| Distribution | Localized | Regional or generalized |
| Extracutaneous spread | Occasional | Very rare |
| Self-healing | Variable | Always |
| Features . | Cutaneous ALCL . | Lymphomatoid papulosis . |
|---|---|---|
| Gross morphology | Nodules | Papules |
| Histology | Clusters/sheets of CD30+ blasts, Hodgkin-like cellular background | Few CD30+ blasts, variable number of cerebriform T cells, many inflammatory cells |
| Distribution | Localized | Regional or generalized |
| Extracutaneous spread | Occasional | Very rare |
| Self-healing | Variable | Always |
Adapted from Kadin.150
HIV-related ALCL
True ALCL, especially the form bearing chimeric ALK proteins, is rare in HIV-infected patients (Falini et al, manuscript in preparation). Most ALCLs reported to occur in HIV-infected patients are different from true ALCL and appear to be related to the anaplastic variant of diffuse large cell B-cell lymphoma because they are of B-cell origin and are infected by EBV in most instances.152 153 Their prognosis usually relates to the immune status of the patient.
Secondary ALCL
These neoplasms may arise in the progression of other lymphomas, most commonly during the course of mycosis fungoides, peripheral T-cell lymphomas, Hodgkin disease, or lymphomatoid papulosis.10,154 Such tumors mainly occur in older adults,10 are usually ALK−,63,66 and have a poor prognosis,10 154 indicating that the appearance of CD30 expression in a previously CD30− lymphoma (most frequently being primary cutaneous T-cell lymphomas) is an unfavorable prognostic sign.
Soluble CD30 in ALCL and host immune response to the NPM-ALK protein
Increased levels of the soluble form of the CD30 molecule (higher than those in Hodgkin disease) have been detected in virtually all patients with ALCL at diagnosis.155 Interestingly, the soluble CD30 level returned to the normal range on achievement of a complete response, but increased again after relapse.156Moreover, a correlation has been reported between a risk of lower survival and increased pretreatment levels of soluble CD30.156 Recently, a preliminary study has shown that NPM-ALK tyrosine kinase and possibly other ALK-variant fusion proteins consistently elicit an antibody response to the ALK protein in patients with ALK+ ALCL.157 Further studies are, however, necessary to ascertain whether the determination of soluble CD30 or levels of anti-ALK antibodies can serve as independent prognostic and disease monitoring indicators.
Reproducibility of the diagnosis of ALCL
The availability of monoclonal antibodies directed against the CD30 and ALK protein was an enormous achievement in the recognition and diagnosis of ALCL. The detection of CD30 (in conjunction with other lymphoid and nonlymphoid markers) is also important, not only in the differential diagnosis between ALCL and nonlymphoid anaplastic large cell tumors, but also in the distinction between ALCL and other types of lymphomas. It has recently been shown that the reproducibility of the diagnosis of ALCL on morphologic grounds is 46%, but it can be increased to 85% by immunostaining for CD30.130Application of EMA might further improve the reproducibility because its expression is mainly restricted to ALK+ ALCL. The detection of ALK protein adds to our potential to distinguish ALCL of small cell and lymphohistiocytic types (whose small tumor cell components often lack CD30) from peripheral T-cell lymphomas and even reactive conditions,40,66,69 and will thus greatly increase the diagnostic reproducibility of the ALK+ ALCL to practically 100%. In addition, given the absence of ALK protein in all normal tissues but the brain, ALK antibodies can be used to detect single tumor cells at the time of initial staging procedures or after therapy (minimal residual disease, eg, in the bone marrow).66 133
Summary and conclusions
ALCL represents a distinct category of large cell lymphomas defined by a strong expression of the cytokine receptor CD30 on all or most neoplastic cells and a so-called anaplastic cytology, usually associated with a characteristic growth pattern of the tumor cells, sinusoidal dissemination, and perifollicular or perivascular homing. On the basis of the morphologic, immunophenotypic, and clinical heterogeneity, several subtypes of ALCLs have been recognized, the most important ones being primary systemic ALCL (common type, lymphohistiocytic type, small cell variant, giant cell–rich type, and Hodgkin-like type); primary cutaneous ALCL, which belongs to the spectrum of so-called CD30+ cutaneous lymphoproliferative disorders; and secondary ALCL.
Within the group of primary systemic ALCLs, a new distinct clinicopathologic entity has emerged (Figure 8), accounting for 50% to 60% of the cases. It is defined by the expression of an ALK fusion protein, which is NPM-ALK in approximately 72% to 85% of cases and consists of a fusion protein containing ALK and other gene product(s) in the remaining cases. The chimeric ALK+ ALCLs show a wide morphologic spectrum, comprising varying proportions of the common type (60% to 90%), giant cell–rich type (30% to 40%), and Hodgkin-like type (less than 20%), and nearly all cases of the small cell and the lymphohistiocytic type. Distinction of ALK+ ALCLs and ALK− ALCLs (which is only achievable by immunohistochemistry) is clinically important because the former usually affects younger patients and shows a more favorable clinical course.
In addition to the primary systemic group of ALCLs, a primary cutaneous form has been distinguished. It arises de novo in the skin and may rarely affect other organs secondarily. It shows a better prognosis than systemic ALCL and requires different therapy, at least in the early stages. It overlaps with lymphomatoid papulosis, with the result that on the basis of morphologic, immunophenotypic, and genetic findings, a distinction between primary cutaneous ALCL and lymphomatoid papulosis is frequently not possible (Table 5), and a definite diagnosis depends on knowledge of the clinical course.
Anaplastic large B-cell lymphomas show overlapping features with diffuse large B-cell lymphomas, classic Hodgkin disease (which has been found to be B-cell derived in most instances), and Hodgkin-like ALCL (for which the cell of origin is not yet clarified but which might be B-cell derived in the majority of cases; Figure 4). It is clearly different from ALCL of T/null-cell type, as supported by the consistent absence of the ALK fusion protein in all but one study. CD30+ large cell lymphomas expressing B-cell antigens must not be confused with rare cases of CD30− large B-cell lymphomas, which show an immunoblastic appearance, contain cytoplasmic IgA, and consistently express the wild-type (full-length) ALK protein. Whether CD30+ large B-cell lymphomas with anaplastic morphology represent a distinct disease entity or a variant large B-cell lymphoma is a matter of some debate. In the Kiel classification, it is included as a separate disease entity, whereas in the REAL classification and the new WHO classification, it is regarded as a variant of diffuse large B-cell lymphoma.
Secondary ALCL may arise by transformation from another, usually CD30−, lymphoma (most frequently cutaneous T-cell lymphomas). This transformation is usually associated with a more aggressive behavior and shorter survival.
Although the findings reviewed in this article indicate enormous progress in the characterization of ALCL and its subforms since the first description of this lymphoma category 15 years ago, a number of relevant questions remain to be answered. Is the ALK−primary systemic ALCL of T/null-cell type one disease or a mixture of different diseases? Are all anaplastic large B-cell lymphomas variants of diffuse large B-cell lymphomas, or do all or a proportion of them represent a distinct disease entity? What is the mechanism by which the ALK fusion proteins cause malignant growth? Will it be possible to exploit the CD30 expression or an interference with chimeric ALK+ activity for new, more specific therapeutic strategies? Can all interface or gray-zone cases between Hodgkin disease and ALCL be assigned to one of these categories when more and more specific antigenic and genetic markers have been identified? What are the transforming events in ALK− systemic and primary cutaneous ALCL? Providing answers to these questions will certainly increase our understanding of the tumorigenesis and the diagnostic reproducibility of the ALK− ALCL subforms as well as improve the therapeutic management of patients suffering from ALCL.
Acknowledgments
We thank M. Schindler and H. Krosch for their help with the schematic representations and L. Udvarhelyi for his editorial assistance.
Supported by grants from the Deutsche Krebshilfe (70-2202-Mü3), the Deutsche Forschungsgemeinschaft DFG (Ste 318/5-2, SFB 366 B4), the Berliner Krebsgesellschaft, grant 9464 from the Leukemia Research Fund, A.I.R.C. (Associazione Italiana per la Ricerca sul Cancro), Projet Hospitalier de Recherche Clinique (PHRC 98), and Lique Nationale Contra le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
After submission of manuscript, Elias Campo and colleagues identified a further variant ALK fusion protein, which consists of Moesin and ALK (MSN-ALK). The variant was reported at the meeting of the International Lymphoma Study Group in New York City, October 2-4, 2000. Unlike other ALK fusion proteins, the expression of MSN-ALK protein is restricted to the cell membrane.
Author notes
Harald Stein, Institute of Pathology, Benjamin Franklin University Hospital, Free University Berlin, Hindenburgdamm 30, 12200 Berlin, Germany.

![Fig. 1. Morphology and immunophenotype of anaplastic large cell lymphoma, common type. / (A) Cytologic features of ALCL, common type, in a paraffin section (hematoxylin and eosin). (B) Cytologic features of ALCL, common type, in a touch imprint (May-Grünwald-Giemsa). (C) Dissemination of ALCL cells in a marginal sinus. MS indicates marginal sinus; GC, reactive germinal center (hematoxylin and eosin). (D) CD30 immunoreactivity of ALCL, common type. All tumor cells are CD30+. Note the sinusoidal dissemination (arrow) and the perifollicular homing of the CD30+ tumor cells (antibody clone Ber-H2; alkaline phosphatase–antialkaline phosphatase [APAAP]). (E) Immunoreactivity of an ALCL of common type for the cytotoxic molecule perforin. The sinusoidal dissemination of the tumor cells (arrow) and a small reactive cytotoxic cell (arrowhead) are indicated (antibody clone P1-8; APAAP). (F) Immunoreactivity of an ALCL of common type for the anaplastic lymphoma kinase (ALK). There is strong labeling in the cytoplasm as well as in the nucleus (antibody clone ALKc; APAAP). (G) An ALCL of common type with labeling for ALK restricted to the cytoplasm (antibody clone ALKc; APAAP).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3681/5/m_h82300432001.jpeg?Expires=1769081162&Signature=exdGm0fOwAJhp4qmQkCgODv3msNqmH8yBrHJgBW6fPvcmSzMAoAxxWuZ5W~OCLE2z~c3FdP-uZlqeQCdv~9t-x0jJN1d0xwlfxTlkgWzjPzyZI68UROTNFd5WN3wS7Lieo1nsnn848A8W05n7xkpE3~EP3efEtaq3Bj7t9RmN4qU3AEp-S6qb3KYxJsfXnIY9v9hbRI8VrLd0LCyXQznHhwTmUfUOkaxUmkIqwlG5MATwj4y36onoan~KRgiZULffhhUc4EPIRxLwVYMbhQcCxr3~pen6fYlb0bh08dl9ofRy74h-2BkOsBc5e0QJ0tTfAjdPC5X05XTnyO8k4Y9og__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Morphology and immunophenotype of the small cell variant and the lymphohistiocytic variant of ALCL. / (A) Small cell variant of ALCL. Most of the cells have a small irregular nucleus, the cytoplasm is light, and larger cells are located in close vicinity to small vessels (Giemsa). (B) Immunoreactivity of a small cell variant of ALCL for CD30. The larger tumor cells are strongly positive, whereas the small tumor cells are only weakly positive (alkaline phosphatase–antialkaline phosphatase [APAAP]). (C) Immunoreactivity of a small cell variant of ALCL for ALK. The larger cells (often located in the vicinity of small vessels) are positive in the cytoplasm and the nucleus, whereas in the smaller cells, the labeling is restricted mainly to the nucleus (APAAP). (D) Lymphohistiocytic variant of ALCL. Note the broad cytoplasm and the frequently slightly eccentrically located nuclei, which are revealed immunohistologically as belonging to reactive histiocytes (see panel E) (hematoxylin and eosin). (E) Immunoreactivity of the same case as in (D) with the CD68 antibody clone PG-M1. Nearly all of the larger cells are positive. These PG-M1–positive cells, representing nonneoplastic bystander macrophages, obscure the few CD30+ tumor cells (see Figure 2F) (APAAP). (F) Immunoreactivity of the same case as in (D) for CD30. Only a minority of the large cells are positive and represent the tumor cell population (APAAP). (G) Immunoreactivity of the same case as in (D) and (E) for ALK. The labeled cells vary greatly in size, with a predominance of smaller cells. The smaller the cells, the more the labeling is restricted to the nucleus. The negative cells are the bystander macrophages (antibody clone ALK1; APAAP). (H) Immunoreactivity of the same case as in (D), (E), and (G) for perforin. The tumor cells are strongly positive, whereas the bystander macrophages are negative (antibody clone P1-8;APAAP).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3681/5/m_h82300432002.jpeg?Expires=1769081162&Signature=WjAULmWTbJmoP7chqbyVkh~ZAB-K0d8~oSHLefhov-qh3h6A-J0~9iJajNigUHdil4XlXQ1m3DXB1mww13myVYJ7Ky35JxiWpt9GeLHV18GiozfbWBELaG4ECgKeMcKT5UcrWOO3eYg-vKqZGrueNS1xDnGNUit6BoTG41y9MyODcYJihfEZxOe5dtg0YxeEsbgK6jUcVjIxKMHXbRLC3K6KAZylv2anTMeosfN7zjwrNE20JZh5JPgDA3oWbvqpYDMdb48g3L87EnqqbmDnU5z~JVMl892UqilVkQI28o-LzDlxyu0M~1yIAURS7rihWAGgOKA4aG6rEH~Htbftow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
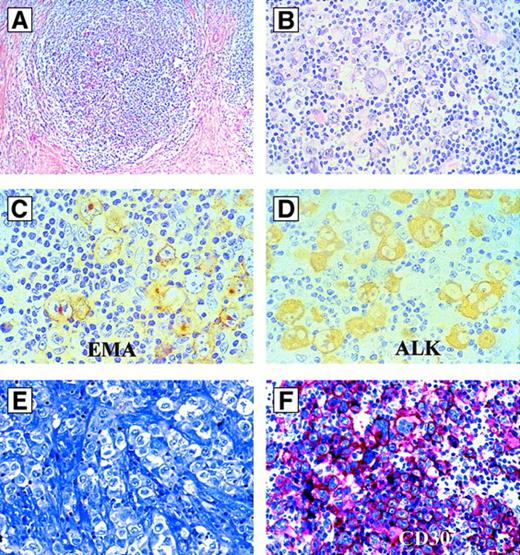
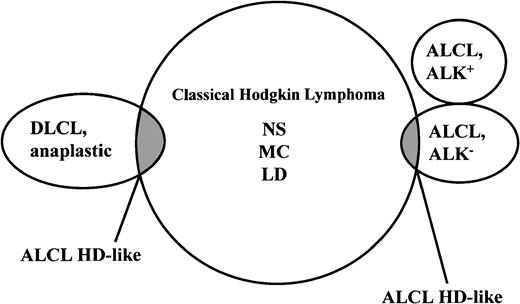
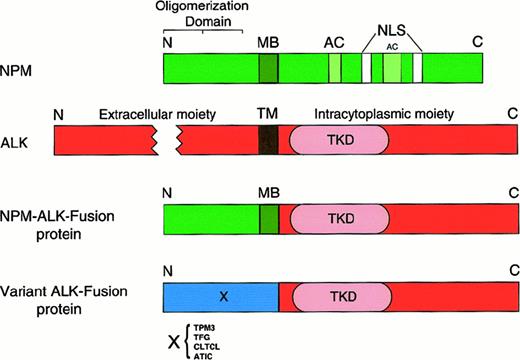
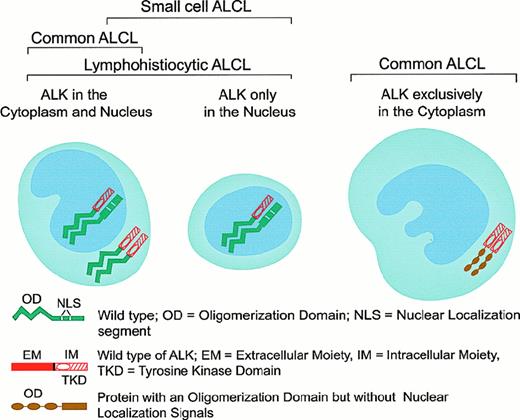
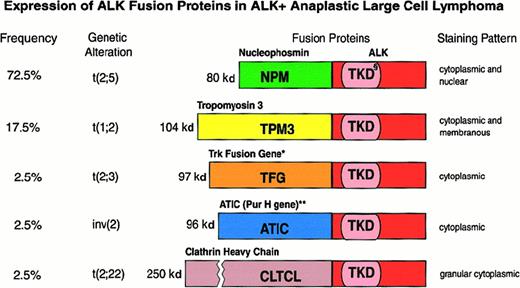
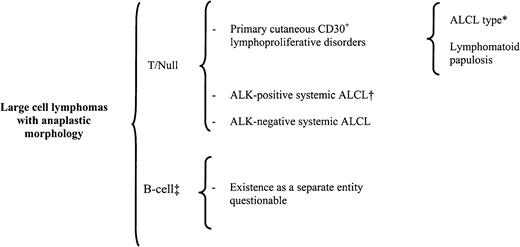
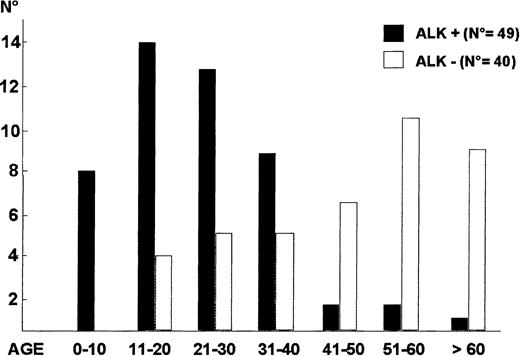
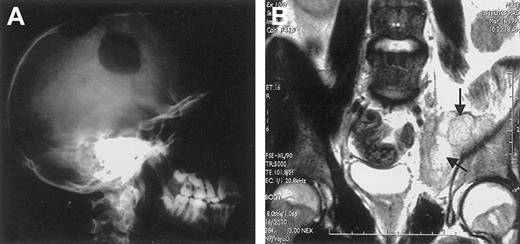
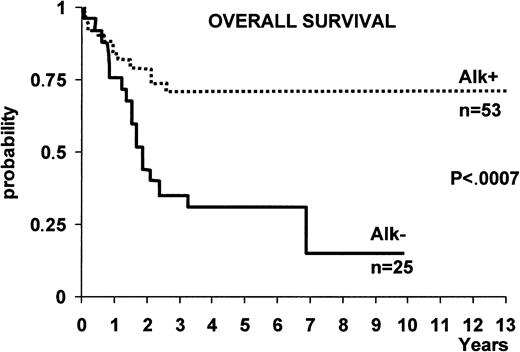
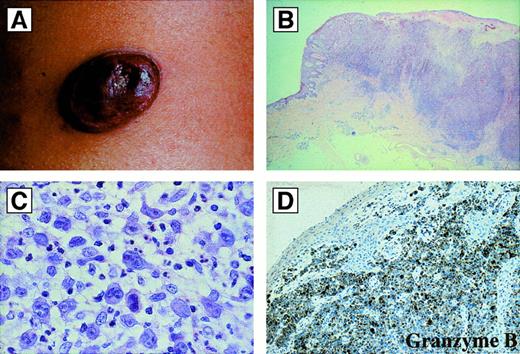
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal