Abstract
Selectin-dependent rolling is the earliest observable event in the recruitment of leukocytes to inflamed tissues. Several glycoproteins decorated with sialic acid, fucose, and/or sulfate have been shown to bind the selectins. The best-characterized selectin ligand is P-selectin glycoprotein-1 (PSGL-1) that supports P-selectin– dependent rolling in vitro and in vivo. In vitro studies have suggested that PSGL-1 may also be a ligand for E- and L-selectins. To study the in vivo function of PSGL-1, without the influence of other leukocyte proteins, the authors observed the interaction of PSGL-1–coated microspheres in mouse venules stimulated to express P- and/or E-selectin. Microspheres coated with functional recombinant PSGL-1 rolled in surgically stimulated and tumor necrosis factor alpha (TNFα)-stimulated mouse venules. P-selectin deficiency or inhibition abolished microsphere rolling in surgically and TNFα-stimulated venules, whereas E-selectin deficiency or inhibition increased microsphere rolling velocity in TNFα-stimulated venules. The results suggest that P-selectin–PSGL-1 interaction alone is sufficient to mediate rolling in vivo and that E-selectin–PSGL-1 interaction supports slow rolling.
Introduction
Recruitment of leukocytes to inflamed tissues is critical for defense against pathogens, but also contributes to host injury in conditions such as ischemia-reperfusion injury.1Leukocyte accumulation begins with attachment to and rolling along postcapillary venules.2 Rolling is largely dependent on the selectin family of adhesion molecules3 and is a prerequisite for later arrest and diapedesis. Selectins are differentially expressed with constitutive L-selectin on almost all leukocytes, P-selectin released from storage granules to surfaces of stimulated platelets and endothelial cells, and E-selectin appearing on endothelial cells after cytokine stimulation.3
The selectins share a modular structure with a characteristic lectin domain at the N-terminus, which is largely responsible for ligand recognition. Selectins recognize certain carbohydrate structures such as sialyl Lewisx (sLex) and its topoisomer sialyl Lewisa (sLea).3 Sialic acid and fucose are key elements for all selectins, whereas L-selectin also requires sulfate on the sLex backbone.3,4sLex and variants thereof can bind selectins in vitro,5,6 and cells with proteins decorated by such oligosaccharides roll on selectins under the conditions prevalent in the living microcirculation.7 There is widespread appreciation, however, that real selectin–ligand interaction requires a specific protein backbone decorated with complex, branched carbohydrates. The most characterized selectin ligand is P-selectin glycoprotein ligand-1 (PSGL-1), which was initially identified as a ligand for P-selectin,8,9 but is also reported to bind E-10-13 and L-selectins.14 Gene-targeted mice, genetically deficient for PSGL-1 have been recently described.15 Interestingly, these mice show defects in P-selectin–mediated, but not E-selectin–mediated leukocyte rolling, bringing the relevance of PSGL-1 as an E-selectin ligand into question. The role of PSGL-1 as an L-selectin ligand was not addressed in detail using these mice.
For binding to P-selectin, PSGL-1 requires a posttranslational modification by enzymes to express sialic acid and fucose on polylactosamine extended core-2 O-glycans and sulfate on certain N-terminal tyrosine residues.4 Cells lacking the enzymes for modification of PSGL-1 (or other proteins) do not bind selectins. Some studies suggest that covalent dimerization of PSGL-1 is required for binding to P-selectin,16,17 although this has been recently questioned.18 Physiologic binding of leukocytes to P-selectin is almost entirely dependent on PSGL-1 because a monoclonal antibody (mAb) (PL-1) against PSGL-1 blocks the rolling of neutrophils in vitro8 and in vivo.9 Binding of L-selectin to PSGL-1 supports leukocyte–leukocyte interaction in in vitro models and is blocked by PL-1,14 although the importance of such interaction has not been confirmed in vivo.19 Binding of PSGL-1 to L-selectin requires sulfation of tyrosines in the amino-terminal region and extended O-glycans containing fucose and sialic acid. E-selectin interaction with PSGL-1 apparently differs from that of P- or L-selectin in that sulfation is not required20 and PL-1 barely affects binding.4
Recent investigations revealed that microspheres coated with sLex or sLea21,22 could roll on surfaces expressing E-selectin, whereas microspheres coated with recombinant PSGL-1 grown in COS cells expressing an α(1,3) fucosyltransferase could roll on either E- or P-selectins.13 These results suggest that PSGL-1 alone is sufficient for rolling on either E- or P-selectin.
Because conditions in the living microcirculation cannot be fully reproduced using in vitro methods, our aim was to determine whether PSGL-1 alone could support rolling in vivo. We previously used recombinant PSGL-1 to define the biomechanics of P-selectin/PSGL-1 interaction.23 Here, we use PSGL-1–coated microspheres to study the capacity of PSGL-1 to support rolling interaction with endothelial selectins in vivo.
Materials and methods
Reagents
Murine recombinant tumor necrosis factor alpha (rmTNFα) was from R&D Systems (Abingdon, UK). RB40.34 (rat IgG1), that blocks P-selectin–dependent rolling in mouse cremaster,24phycoerythrin (PE)-conjugated 2PH1 that specifically recognizes and blocks mouse PSGL-1,25 PE-conjugated KPL-1 that specifically recognizes human PSGL-1, the negative control antibody R3-34, RB6-8C5 that selectively depletes granulocytes from the peripheral circulation,26 CellWash and PharmLyse were from Pharmingen (Oxford, UK). Control antibody F8-11-13 was from Serotec (Oxford, UK), 10E6 (rat IgG2b) that blocks E-selectin–dependent rolling,27 10A10 (rat IgG1, nonblocking antimouse–P-selectin) and 14E4 (rat IgG2a, nonblocking antimouse–E-selectin) were gifts from Dr B. A. Wolitzky (Hoffman-La Roche, Nutley, NJ).
Selectin ligand-coated microspheres
PSGL-1 was cloned and a fusion protein to human IgG (PSGL-1/IgG) constructed as described.23 With the exception of fucosyltransferase (required for sLex synthesis), 293 cells express all the enzymes required for production of active PSGL-1.23 PSGL-1/IgG was therefore expressed in 293 cells and in 293 cells transfected with fucosyltransferase III (FTIII) as described.23 Unlike PSGL-1 produced by 293 cells, PSGL-1 produced in 293(FTIII) cells carries sLex and binds to P-selectin.23 It is believed that FTIV in leukocytes (which transfers only to nonsialylated structures) is responsible for internal fucosylation of the extending glycan chains, whereas FTVII transfers the terminal fucose once the terminating sialic acid is in place. We chose to use FTIII because 293 cells transfected with PSGL-1 and this single enzyme generate PSGL-1 decorated with internally and terminally fucosylated extended structures resembling those produced by leukocytes. Although a complicating factor with FTIII is that it can potentially produce sLea, we believe that sLeawould interact with selectins in a similar manner to sLex. PSGL-1/IgG was also expressed in FTIII- transfected Chinese hamster ovary (CHO/FTIII) cells. The CHO/FTIII cell line was constructed by transfecting CHO cells with vector FT3.neo23 using lipofectamine. After G418 (0.5 mg/mL) selection, cells with high sLex were separated by FACS (Becton-Dickinson Immunocytometry Systems, Mountain View, CA), cloned and used for expression of PSGL-1/IgG. CHO cells lack the β1,6 N-acetylglucosaminyltransferase (core 2GlcNAcT) that synthesizes the core-2 structure and, as a result, cannot elaborate extendedO-glycans. PSGL-1/IgG from CHO/FTIII cells and from 293 cells without FTIII were used as inactive controls.
PSGL-1/IgG was purified and biotinylated as described previously.23 Biotinylated sLea-polylysine was also synthesized according to published methods.28Yellow-green fluorescent microspheres (0.07 mL, 1.0-μm diameter) coated with avidin (Fluospheres, Molecular Probes, Eugene, OR) were washed twice by suspension in 5 mL phosphate-buffered saline (PBS), followed by centrifugation (2000g, 15 minutes). Microspheres were then incubated overnight at 4°C with 0.15 mg of biotinylated PSGL-1/IgG or sLea in 0.5 mL PBS. After incubation, microspheres were washed as previously described and resuspended in 0.35 mL PBS to make a final solution of 0.2% microspheres. Microspheres were stored at 4°C and used in rolling experiments within 10 days of coating. For injection into mice, the microsphere solution was diluted 1 to 10.
Density of P-selectin glycoprotein ligand-1 on microspheres and on murine and human neutrophils
Expression of PSGL-1 on mouse peripheral blood neutrophils was determined by whole blood flow cytometry. Peripheral blood was obtained by cardiac puncture from male C57BL/6 mice and 100 μL aliquots incubated for 30 minutes at 4°C with a saturating concentration of PE-conjugated antimouse–PSGL-1 monoclonal antibody (2PH1) or isotype-matched control antibody (R3-34). Cells were washed (2 mL CellWash [Pharmingen], 400g, 10 minutes) and erythrocytes lysed using PharmLyse (Pharmingen). After 2 further washes, cells were resuspended in 0.5 mL CellFix (Pharmingen) and stored in the dark at 4°C until analysis. Expression of PSGL-1 on human neutrophils was determined by the same method, substituting KPL1 (antihuman PSGL-1) for 2PH1.
Because the PSGL-1 on our microspheres was recombinant human material, grown in a transfected cell line, we could not confidently assume that KPL1 would interact with our microspheres with the same efficiency as with native neutrophil PSGL-1. We therefore chose to determine the maximum possible PSGL-1 loading for our microspheres by measuring the number of biotin-binding sites. To do this we produced PE-labeled microspheres according to the method previously described for PSGL-1–coated microspheres, substituting an equimolar concentration of biotinylated PE (Molecular Probes) for biotinylated PSGL-1.
The fluorescent intensity of PE microspheres and PE PSGL-1 antibody staining on peripheral blood neutrophils was determined using a FACScan flow cytometer and CellQuest data acquisition and analysis software (Becton-Dickinson Immunocytometry Systems, Mountain View, CA). Peripheral blood neutrophils were identified on the basis of forward (FSc) and side (SSc) light scatter characteristics and, for mouse cells, on the basis of positive staining with the granulocyte specific antibody RB6-8C5. The fluorescent intensity of gated cells was determined and the median channel of fluorescent intensity (MFI) recorded as a measure of antigen density. Cells incubated with the PE-conjugated control antibodies R3-34 (mouse) or F8-11-13 (human) were studied as controls. PE-conjugated microspheres were identified on the basis of FSc and SSc characteristics and a live analysis gate was placed around this population. The fluorescent intensity of the gated population was determined in logarithmic mode and the MFI recorded. Unstained micropheres acted as a negative control.
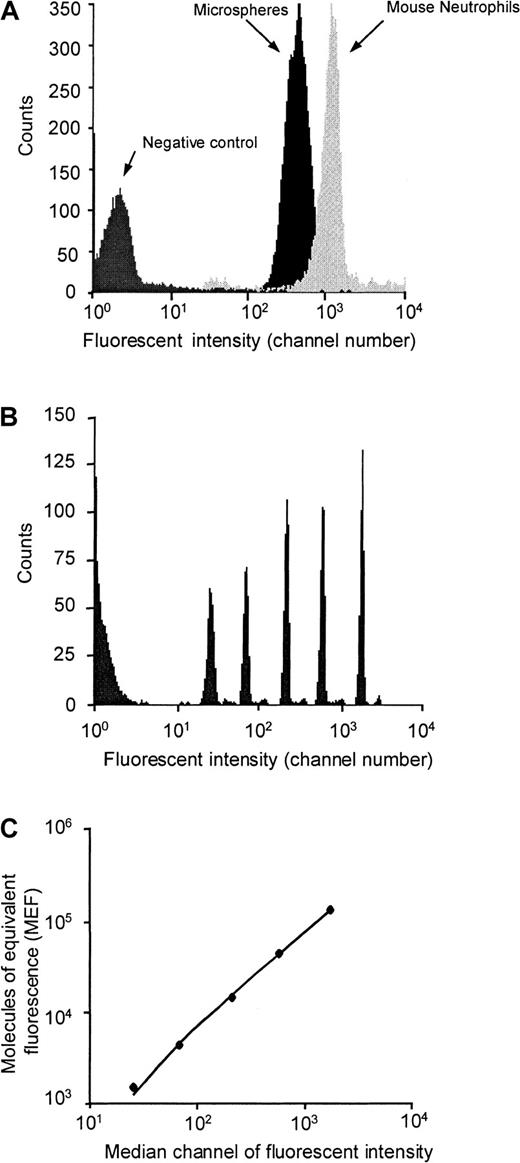
The fluorescence intensities of the microspheres and peripheral blood neutrophils were converted into the number of molecules of equivalent fluorescence (MEF) by reference to a standard regression line generated using calibrated fluorescent microspheres (FluoroSpheres, Dako, Ely, UK). These comprise a mixture of 6 microsphere populations, each of which are labeled with a known number of fluorescent molecules. These microspheres contain a unique combination of fluorochromes, enabling calibration of a number of fluorescent markers, including PE. Microspheres were identified on the basis of light scatter, and the MFI of each population determined. A standard linear regression line of MFI versus the number of molecules of equivalent fluorescence was drawn, from which the MEF of PE-labeled neutrophils and microspheres were derived.
Animals
Male C57BL/6 mice (in-house colony), P-selectin knockout mice (in-house colony derived from C57BL/6J-Selptm1Bay, The Jackson Laboratory, Bar Harbor, ME) and E-selectin knockout mice (in-house colony derived from breeding pairs supplied by Dr B. A. Wolitzky)29weighing between 25 and 35 g were used in these experiments. All procedures were approved by the University of Sheffield ethics committee and by the Home Office Animals (Scientific Procedures) Act 1986 of the UK. Mice were anesthetized with an intraperitoneal injection of 100 mg/kg ketamine hydrochloride after premedication with 30 mg/kg sodium pentobarbital and 0.1 mg/kg atropine sulfate. Some mice received an intrascrotal injection of 500 ng TNFα 2.5 hours before intravital microscopic observation. Other mice were depleted of circulating neutrophils using RB6-8C5 (10 μg, intravenously [iv]).
Intravital microscopy
The cremaster was prepared for intravital microscopy and superfused as described by Ley et al.24 Venules were observed 10 to 30 minutes after surgical stimulation of tissue to study P-selectin–dependent rolling and 2.5 hours after TNFα stimulation of tissue to study combined E/P-selectin–dependent rolling.
Venules were observed using a Leitz diaplan (Leica Microsystems, Cambridge, United Kingdom) equipped with a water immersion objective (20x/0.5W). Images of rolling leukocytes were recorded by a CCD camera (DC330EX, Dage MTI, Michigan City, MI) onto sVHS videocassettes. After recording leukocyte rolling for 1 minute, passage of fluorescent microspheres was observed by dual flash stroboscopic (100-second−1 Strobex 11360, Chadwick Helmuth, Mountain View, CA) epifluorescence illumination. Microspheres injected into the carotid artery in 50 μL boluses could be observed in the cremasteric circulation within 20 seconds and typically circulated for 1 to 2 minutes. Activation of the Strobex lamp (Chadwick Helmuth) was controlled by the video camera. Two flashes were produced for each video frame, giving double images of fast free-flowing microspheres. Distance between such images was used to calculate centerline velocities of observed vessels. Images of fluorescent microspheres were recorded using a silicon-intensified target camera (C2400, Hammamatsu Photonics UK, Enfield, UK).
Data analyses
Sequences of interest were digitized (Miromotion DC30, Pinnacle Systems, Braunschweig, Germany) and analyzed using NIH-Image (National Institutes of Health, Bethesda, MD; available athttp://rsb.info.nih.gov/nih-image). To compare leukocyte rolling before and after different treatments, rolling flux percentage was calculated as described.24 Briefly, the number of leukocytes rolling past a fixed plane perpendicular to the vessel axis was determined. This value was expressed as a percentage of the total number of leukocytes passing through that vessel (calculated as the product of systemic leukocyte count and volumetric flow rate). Because all fluorescent microspheres (free-flowing and interacting) could be visualized, and because relatively small numbers of microspheres rolled continuously for long distances (rolling only short distances in surgically stimulated mice and rolling very slowly in TNFα-stimulated mice), we used a different method to quantify microsphere interaction. Thus, microsphere rolling is quantified as percentage attachment rate per 100 μm vessel length. This value is calculated by dividing the number of new attachments formed in a venule by the total number of microspheres passing through that venule and then correcting for vessel length. Attachment is defined as a rapid deceleration to a velocity clearly lower than that of free-flowing microspheres. Although we did not measure velocities of all observed microspheres, those that were measured uniformly fell below critical velocity calculated as described.9 Rolling of leukocytes and microspheres in surgically stimulated and TNFα-stimulated wild-type mice and in TNFα-stimulated E−/− mice is also depicted in 2 video sequences on the Blood website (see the Supplemental Videos link at the top of the online article). The vessels depicted in these sequences show leukocytes and beads rolling in surgically stimulated venules of wild-type mice, TNFα-stimulated venules of wild-type mice and TNFα-stimulated venules of E-selectin −/− mice and are typical examples of those analyzed in Figures 2 to 5.
Statistics
Differences between groups were compared by one-way analysis of variance (ANOVA), followed by Dunnett's test using INSTAT software (GraphPad Software, San Diego, CA).
All measurements were compared with values given in similarly stimulated wild-type mice receiving no antibodies.
Results
P-selectin glycoprotein ligand-1 expression on microspheres and on murine and human neutrophils
We used flow cytometry to compare the maximum ligand coating density on our microspheres with that of PSGL-1 on murine neutrophils. The fluorescence histograms shown in Figure1 show that microspheres coated with PE-biotin (at concentrations equivalent to concentrations of PSGL-1–biotin used to prepare microspheres for intravital microscopy studies) express fewer PE molecules than murine neutrophils labeled in whole blood with saturating concentrations of PE-conjugated anti–PSGL-1 antibody. Comparing the MFI of neutrophils and microspheres with the regression line generated from the MFI of calibrated microspheres (Figure 1), we calculate that murine neutrophils express approximately 75 000 PSGL-1 molecules per cell, whereas each of our coated microspheres expresses approximately 30 000 molecules. Because our measurement of PSGL-1 density on murine neutrophils is considerably higher than the number of P-selectin binding sites reported for human neutrophils,30 we decided to perform a direct comparison of PSGL-1 density on murine and human neutrophils. Human neutrophils stained with a saturating concentration of KPL1 had MFI of 179, whereas murine neutrophils stained with a saturating concentration of 2PH1 had MFI of 735. Converting the MFI of KPL1-stained neutrophils into molecules of equivalent fluorescence (by comparing with Dako fluorospheres), we estimate that there are approximately 18 000 PSGL-1 molecules on the surface of each human neutrophil. This value is in fairly close agreement with previously reported levels of P-selectin binding sites on neutrophils.30 Taken together, these results indicate that mouse neutrophils express considerably more PSGL-1 molecules on their surface than human neutrophils.
Density of PSGL-1 on murine neutrophils and of PE-biotin on avidin-coated microspheres.
Median fluorescent intensities of appropriately gated neutrophils and microspheres (A) were converted to MEF by comparison to values given by calibration beads containing known numbers of fluorescent molecules (B and C).
Density of PSGL-1 on murine neutrophils and of PE-biotin on avidin-coated microspheres.
Median fluorescent intensities of appropriately gated neutrophils and microspheres (A) were converted to MEF by comparison to values given by calibration beads containing known numbers of fluorescent molecules (B and C).
After correcting for the fact that surface area of a 1-μm microsphere is 1/49th that of a 7-μm neutrophil, it is clear that PSGL-1 density on our microspheres is considerably higher than that on mouse and human neutrophils. Although these differences may be reconciled by the fact that PSGL-1 on neutrophils is selectively targeted to dense clusters on the tips of microvilli, we also accept that the remarkable ability of these microspheres to mimic leukocyte rolling may be in part due to the increased density of PSGL-1.
Hemodynamic variation in observed vessels
Because hemodynamic variation can influence leukocyte rolling,24 diameters and centerline blood flow velocities were recorded for all observed vessels. These data are summarized in Table 1. Although there was some variation in average vessel diameters, no group differed significantly in this respect from equivalently stimulated wild-type mice receiving no antibodies. Surgically stimulated mice receiving P-selectin antibody, TNFα-stimulated wild-type mice receiving combined E- and P-selectin antibodies and TNFα-stimulated P-selectin knockout mice had somewhat lower centerline velocities than similarly stimulated wild-type mice without antibodies. There is a negative relationship between centerline velocity and rolling flux percentage24; however, lower velocities do not explain the reduced leukocyte/microsphere interaction seen.
Hemodynamic variation in observed vessels
| Stimulus . | Surgery . | Surgery . | Surgery . | Surgery . | TNFα . | TNFα . | TNFα . | TNFα . | TNFα . | TNFα . | TNFα . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bead coating | Control | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | Control | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) |
| Mouse genotype | WT | WT | WT | WT | WT | WT | WT | WT | P−/− | E−/− | WT |
| Antibody | None | None | RB40.34 | RB6-8C5 | None | None | 10E6 | 10E6 + RB40.34 | None | None | RB6-8C5 |
| Vessel ∅ (μm) | 32 ± 3 | 33 ± 2 | 35 ± 3 | 35 ± 4 | 44 ± 3 | 40 ± 3 | 39 ± 2 | 39 ± 2 | 34 ± 3 | 36 ± 4 | 44 ± 3 |
| VC1 (mm/s) | 3.2 ± 0.3 | 3.0 ± 0.3 | 2.7 ± 0.4 | 2.1 ± 0.3 | 2.7 ± 0.3 | 2.8 ± 0.4 | 2.8 ± 0.5 | 2.4 ± 0.3 | 2.2 ± 0.3 | 3.2 ± 0.2 | 2.7 ± 0.3 |
| Stimulus . | Surgery . | Surgery . | Surgery . | Surgery . | TNFα . | TNFα . | TNFα . | TNFα . | TNFα . | TNFα . | TNFα . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bead coating | Control | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | Control | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) | PSGL-1 (293/FTIII) |
| Mouse genotype | WT | WT | WT | WT | WT | WT | WT | WT | P−/− | E−/− | WT |
| Antibody | None | None | RB40.34 | RB6-8C5 | None | None | 10E6 | 10E6 + RB40.34 | None | None | RB6-8C5 |
| Vessel ∅ (μm) | 32 ± 3 | 33 ± 2 | 35 ± 3 | 35 ± 4 | 44 ± 3 | 40 ± 3 | 39 ± 2 | 39 ± 2 | 34 ± 3 | 36 ± 4 | 44 ± 3 |
| VC1 (mm/s) | 3.2 ± 0.3 | 3.0 ± 0.3 | 2.7 ± 0.4 | 2.1 ± 0.3 | 2.7 ± 0.3 | 2.8 ± 0.4 | 2.8 ± 0.5 | 2.4 ± 0.3 | 2.2 ± 0.3 | 3.2 ± 0.2 | 2.7 ± 0.3 |
Vessel diameter (∅) and centerline velocity (VC1) were measured for each microsphere coating, mouse genotype (WT = wild-type, E−/− = E-selectin knockout, P−/− = P-selectin knockout) and mAb (RB40.34 = anti–P-selectin, 10E6 = anti–E-selectin, RB6-8C5 = antineutrophil) treatment in surgically stimulated and TNFα-stimulated mice. Data are presented as mean ± SE.
Leukocyte rolling and microsphere interaction in surgically stimulated mice
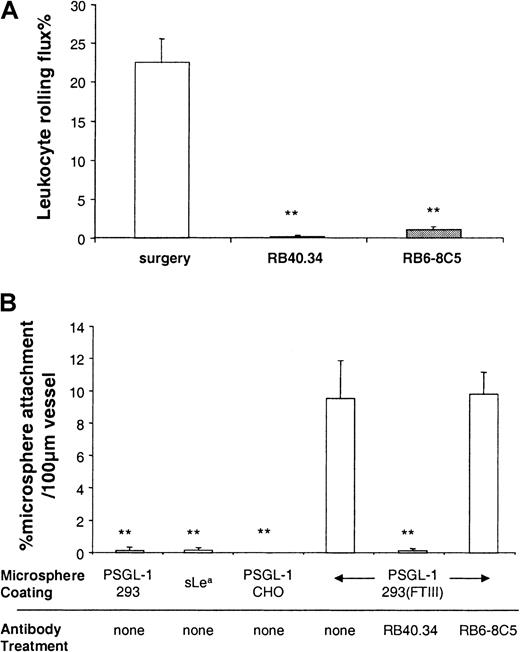
Initially, we investigated whether microspheres coated with PSGL-1 (293/FTIII) could roll in venules with P-selectin–dependent leukocyte rolling induced as described.24 Figure2A and previous studies24 31show that 10 to 30 minutes after surgical stimulation of tissue, more than 20% of leukocytes passing through observed venules were rolling, and that this rolling was abolished by treatment with the P-selectin antibody, RB40.34. Figure 2B shows interaction of coated microspheres in the same vessels. The profile of microsphere interaction in surgically stimulated venules largely mirrors that of leukocyte rolling. Thus, approximately 10% of PSGL-1(293/FTIII)–coated microspheres attached to and rolled for some time (0.5-2 seconds) along observed venules, and this rolling was abolished by treatment with RB40.34. 10A10, a nonblocking anti–P-selectin antibody, did not alter rolling of leukocytes or microspheres (data not shown). Microspheres coated with PSGL-1 (293) PSGL-1(CHO/FTIII) or sLea formed few interactions, emphasizing the importance of both protein backbone and correct glycosylation for function.
Leukocyte rolling and microsphere attachment in surgically stimulated venules.
Leukocyte rolling (A) and attachment of differently coated microspheres (B) was observed before and after P-selectin mAb (RB40.34, 10 μg) or antineutrophil mAb (RB6-8C5, 10 μg). Data are presented as mean ± SE. ** denotes significant difference (P < .01) from surgery in (A) and from PSGL-1(293/FTIII) plus no mAb in (B).
Leukocyte rolling and microsphere attachment in surgically stimulated venules.
Leukocyte rolling (A) and attachment of differently coated microspheres (B) was observed before and after P-selectin mAb (RB40.34, 10 μg) or antineutrophil mAb (RB6-8C5, 10 μg). Data are presented as mean ± SE. ** denotes significant difference (P < .01) from surgery in (A) and from PSGL-1(293/FTIII) plus no mAb in (B).
Because we were concerned that microspheres may be interacting with rolling leukocytes rather than with endothelial P-selectin, we also studied microsphere interaction in mice that had been previously depleted of neutrophils using antibody RB6-8C5. PMN were selectively and completely removed from the peripheral circulation for the duration of our experiments by RB6-8C5 treatment (10 μg, iv) (data not shown). RB6-8C5 also abolished leukocyte rolling in observed venules (Figure2A), a result that was not unexpected because neutrophils comprise more than 90% of the rolling population under the conditions studied herein.32 Removing granulocytes from the circulation with RB6-8C5, and thus rolling neutrophils from observed venules had no effect on microsphere interaction (Figure 2B), suggesting direct binding between human PSGL-1 on the microsphere and murine P-selectin on the endothelium.
Leukocyte rolling and microsphere interaction in TNFα-stimulated mice
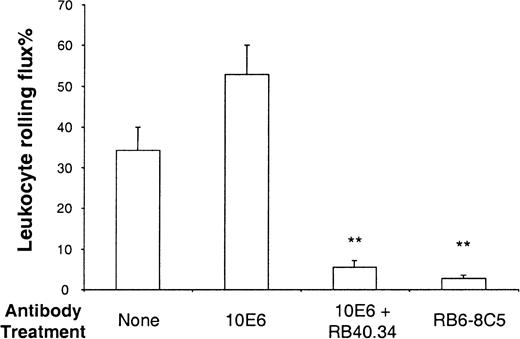
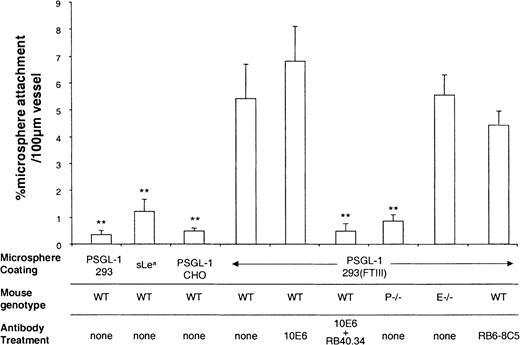
Having demonstrated that PSGL-1(293/FTIII)–coated microspheres rolled on P-selectin in vivo, we then set out to study whether these microspheres could roll on E-selectin. Stimulation of wild-type (C57 BL/6) mice with TNFα produces leukocyte rolling that is dependent on all 3 selectins. Thus, rolling in cremasteric venules of mice stimulated for 2 to 4 hours with TNFα is abolished by a combination of P- and E-selectin antibodies,33 and substantially inhibited when P-selectin deficiency is combined with L-selectin antibody treatment.24 Blocking P-selectin alone24 in TNFα-stimulated mice produces little or no effect, L-selectin deficiency results in a slight reduction of rolling flux percentage,33 and blocking E-selectin alone produces a characteristic increase in rolling velocity,33emphasizing the importance of this molecule for maintenance of slow leukocyte rolling. Figure 3 shows the effect of antibody treatments on the percentage of leukocytes rolling in TNFα stimulated venules of wild-type mice. As described previously,34 blocking E-selectin caused a slight increase in rolling flux percentage, whereas combined blockade of E- and P-selectin markedly reduced leukocyte rolling in observed venules. As seen in surgically stimulated vessels, treatment with RB6-8C5 caused a substantial reduction of leukocyte rolling in TNFα stimulated venules. Figure 4 shows attachment rates of differentially coated microspheres in venules of mice stimulated for 2.5 hours with TNFα. As in surgically stimulated mice, control microspheres (PSGL-1(CHO/FTIII), PSGL-1(293), or sLeacoating) formed few attachments (Figure 4). In contrast, PSGL-1(293/FTIII)–coated microspheres formed a larger number of attachments (Figure 4) and these attachments were generally long lasting (more than 1 minute). Microsphere adhesion was not the result of interaction with rolling leukocytes because their attachment was not significantly altered by RB6-8C5. Blocking E-selectin did not significantly alter PSGL-1(293/FTIII) microsphere attachment, whereas blocking both E- and P-selectins reduced attachment to levels seen with PSGL-1(293) microspheres. A combination of control mAbs 10A10 and 14E4 did not alter microsphere interaction in TNFα-stimulated venules (data not shown). Interestingly, PSGL-1(293/FTIII)–coated microspheres formed few interactions in P-selectin −/− mice (Figure 4) and those that were seen were very brief. This indicates an absolute requirement for P-selectin in the attachment of these beads.
Leukocyte rolling in TNFα-stimulated venules.
Leukocyte rolling was observed in venules stimulated for 2.5 hours with TNFα. Rolling before and after E-selectin mAb (10E6, 10 μg), E-selectin mAb plus P-selectin mAb (RB40.34, 10 μg), or antineutrophil mAb (RB6-8C5) were quantified and data are presented as mean ± SE. **Denotes significant difference (P < .01) from no mAb.
Leukocyte rolling in TNFα-stimulated venules.
Leukocyte rolling was observed in venules stimulated for 2.5 hours with TNFα. Rolling before and after E-selectin mAb (10E6, 10 μg), E-selectin mAb plus P-selectin mAb (RB40.34, 10 μg), or antineutrophil mAb (RB6-8C5) were quantified and data are presented as mean ± SE. **Denotes significant difference (P < .01) from no mAb.
Microsphere attachment in TNFα stimulated venules.
Attachment of differently coated microspheres was observed in venules stimulated with TNFα for 2.5 hours. Attachment efficiencies were quantified for wild-type (WT) mice, P-selectin knockout mice (P−/−), and E-selectin knockout mice (E−/−) and for mice treated with mAb against E-selectin (10E6, 10 μg), P-selectin (RB40.34, 10 μg), and neutrophils (RB6-8C5 10 μg). Data are presented as mean ± SE. **Denotes significant difference (P < .01) from PSGL-1(293/FTIII) in WT mice with no mAb.
Microsphere attachment in TNFα stimulated venules.
Attachment of differently coated microspheres was observed in venules stimulated with TNFα for 2.5 hours. Attachment efficiencies were quantified for wild-type (WT) mice, P-selectin knockout mice (P−/−), and E-selectin knockout mice (E−/−) and for mice treated with mAb against E-selectin (10E6, 10 μg), P-selectin (RB40.34, 10 μg), and neutrophils (RB6-8C5 10 μg). Data are presented as mean ± SE. **Denotes significant difference (P < .01) from PSGL-1(293/FTIII) in WT mice with no mAb.
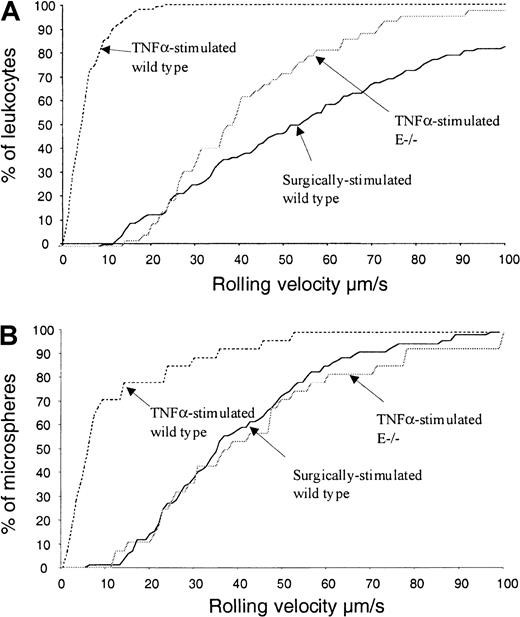
Rolling velocities of leukocytes and microspheres in differentially stimulated mice
A role for E-selectin–PSGL-1 interaction in the maintenance of leukocyte or microsphere rolling is not precluded by the observation that E-selectin inhibition or deficiency does not reduce rolling of PSGL-1(292/FTIII+ve)–coated microspheres (Figure 4). A role for E-selectin in the maintenance of slow leukocyte rolling has been described,33 and we have used leukocyte rolling velocity as a sensitive measure of selective E-selectin inhibition in vivo.31 Because E-selectin–PSGL-1 interaction might support slow rolling in vivo, we compared rolling velocities of leukocytes and PSGL-1(293/FTIII)–coated microspheres in surgically and TNFα-stimulated wild-type mice and in TNFα-stimulated E-selectin −/− mice. Rolling velocities of leukocytes and microspheres under different conditions are compared in Figure5. Leukocytes rolling through TNFα-stimulated venules of wild-type mice travel much more slowly than those rolling through venules of surgically stimulated mice (Figure 5A). In contrast, leukocytes rolling through TNFα-stimulated vessels of E-selectin −/− mice roll at velocities approaching those measured in surgically stimulated mice. Figure 5B shows that, as is the case for leukocytes, the majority of microspheres interacting with observed venules in TNFα-stimulated wild-type mice travel at considerably lower velocities than microspheres rolling in surgically stimulated mice, whereas absence of E-selectin results in a considerable increase in microsphere rolling velocity. Similar increases in leukocyte and microsphere velocities were also given by E-selectin antibody (10E6) treatment (data not shown).
Leukocyte and microsphere rolling velocities in differently stimulated venules.
Leukocyte (A) and microsphere (B) rolling velocities were measured in surgically stimulated wild-type mice, TNFα-stimulated wild-type mice, and TNFα-stimulated E-selectin knockout mice. Data are presented as cumulative velocity histograms.
Leukocyte and microsphere rolling velocities in differently stimulated venules.
Leukocyte (A) and microsphere (B) rolling velocities were measured in surgically stimulated wild-type mice, TNFα-stimulated wild-type mice, and TNFα-stimulated E-selectin knockout mice. Data are presented as cumulative velocity histograms.
Discussion
We have shown that P-selectin–PSGL-1 binding is the only molecular interaction required for rolling of microspheres in living blood vessels with physiologic blood flow. In addition, we find that although binding of E-selectin to PSGL-1 is not sufficient to initiate/support tangible rolling, this interaction can substantially limit rolling velocity when P-selectin is also present.
The discovery that PSGL-1 sustains rolling on P-selectin is entirely consistent with previous in vitro work and with our own intravital microscopy studies using PSGL-1 blocking antibodies. Thus, microspheres coated with PSGL-1 roll on P-selectin in vitro, and blocking PSGL-1 prevents rolling of neutrophils on P-selectin in vitro8 and in vivo.9 The obligate role of PSGL-1 demonstrated by antibody-blocking studies, and reconstitution of rolling behavior by correctly glycosylated recombinant PSGL-1 support the concept that PSGL-1 is the only important P-selectin ligand on leukocytes, although it is possible that other leukocyte molecules might interact with P-selectin subsequent to PSGL-1–dependent adhesion. The PSGL-1 used in our assay was monomerically associated with one heavy chain of IgG, which will spontaneously dimerize with another PSGL-1–bearing heavy chain. Because we do not know about the distances between PSGL-1 molecules on these dimers, we cannot comment on whether they are relevant to dimerization of native PSGL-1.
There is substantial in vitro evidence that PSGL-1 is also an E-selectin ligand.10-13 Our discovery that E-selectin–PSGL-1 interaction limits rolling velocity in TNFα–stimulated venules is, however, the first demonstration that such an interaction contributes to a physiologic response in vivo. This finding does not rule out the possibility that additional E-selectin ligands (not present on our microspheres) might also contribute to slow rolling of leukocytes.
Much of the earlier in vitro evidence for E-selectin–PSGL-1 interaction is based on static assays measuring, for example, binding of CHO cells transfected with E-selectin (CHO-E) to purified immobilized PSGL-1.10 Recent work studying interaction of selectin ligand–coated microspheres with CHO-E suggested that PSGL-1,13 sLex,21,22 and sLea22 are each sufficient to both initiate and support rolling on E-selectin under simulated physiologic flow conditions. Our findings contrast with those from in vitro flow assays on 2 points: first, microspheres coated only with sLea did not form substantial interactions under any of the conditions studied, and secondly, PSGL-1(293/FTIII)–coated microspheres did not interact in TNFα-stimulated P-selectin −/− mice. The reasons for these contrasting results are not clear, but may be due to factors such as higher flow rates that prevail in vivo and the greater complexity of the in vivo situation with factors such as an intact glycocalyx to contend with. Interestingly, because microspheres do not have microvilli, our studies argue against the hypothesis that microvillous concentration of PSGL-1 is required for penetration of the glycocalyx and access to endothelial P-selectin. Our findings in P-selectin −/− mice indicate that P-selectin has an essential role in attachment of PSGL-1–coated microspheres to venular endothelium and that E-selectin–PSGL-1 interaction is unable to initiate substantial rolling on its own. In further support of this view, attachment rates in TNFα-stimulated E-selectin −/− mice were similar to those in wild-type mice. Our results are also entirely consistent with previous intravital microscopy studies of leukocyte rolling. TNFα-stimulated P-selectin −/− mice have substantial numbers of rolling leukocytes and most of this rolling is removed by antibodies against L-selectin.24 This suggests that on its own, E-selectin has a limited capacity to initiate or maintain leukocyte rolling in vivo.
Comparison of the velocity figures for leukocytes and microspheres shown in Figure 5 reveals remarkable similarity between these 2 populations. The 2 populations not only show a similar profile (slow rolling in TNFα-stimulated wild-type mice, faster rolling in surgically stimulated wild-type mice and E −/− mice) but also similar absolute values. This similarity gave us cause for concern when we considered the numerous differences (different ligand densities, human compared with murine PSGL-1, size of microspheres compared with that of leukocytes) between the endogenous mouse leukocytes and the injected microspheres. Our initial worry was that microspheres were in some way associated with rolling leukocytes (surface bound or phagocytosed) rather than rolling independently along venular endothelium and that this caused the microspheres to roll in a very similar manner to leukocytes. Our experiments with RB6-8C5, which removed neutrophils from the circulation and thus rolling leukocytes from observed vessels, negated these concerns, however, demonstrating that microsphere rolling is most likely because of direct interaction with endothelial selectins. The similarity between leukocyte and microsphere rolling velocities that exists, despite the above differences, might be explained, at least in part, by data from in vitro flow assays. Brunk and Hammer22 showed that microsphere rolling velocities were much more sensitive to variations in ligand (E-selectin) density on the substrate (coverslip in flow chamber) than to variations in ligand (sLex) density on the rolling particles. We therefore hypothesize that density of E- and P-selectins on venular endothelium may be a more important determinant of rolling velocity than the density of selectin ligands on the rolling particle.
Our results appear to be in contrast to the findings of Yang et al15 who studied the phenotype of PSGL-1 deficient mice and found no defect of E-selectin–mediated rolling. These results are consistent, however, if one considers the possibility that the genetically modified mice described by Yang et al15 might compensate for the lack of PSGL-1 by overexpressing other E-selectin ligands, or that overall density of E-selectin ligands is so great that removing one of them has no consequence. Taken together, our results and those of Yang et al15 suggest that, although PSGL-1 is able to act as an E-selectin ligand in vivo, it is by no means the only E-selectin ligand on leukocytes and may not even be the major one. Although limiting molecular expression on the beads to PSGL-1 alone has enabled us to reveal the function of this molecule as a ligand for both P- and E-selectins in vivo, we have not addressed the importance of other potential selectin ligands, of which there are a large number of candidates, including ESL-1,35 L-selectin,36and β2 integrins.37,38 A recent study by Crutchfield et al38 provided compelling evidence that CD11b/CD18 integrins are relevant E-selectin ligands, demonstrating that CD11b/CD18-coated microspheres attach to and roll on E-selectin in an in vitro flow chamber assay. We are currently considering experiments to investigate the ability of CD11b/CD18 to support E-selectin–dependent rolling in vivo, although this issue is further complicated by the fact that rolling on E-selectin in vivo is largely dependent on prior attachment via P-, or L-selectin. Although a small amount of leukocyte rolling persists after the blockade of P- and L-selectins in vivo, we do not detect significant attachment of microspheres in our system without P-selectin–PSGL-1 interaction to initiate capture (ie, P-selectin −/− mice have very few bead interactions). One way to circumvent the limited capacity of E-selectin to initiate attachment of particles (leukocytes or microspheres) from flowing blood may be to coexpress putative E-selectin ligands such as CD11b/CD18 on microspheres with L-selectin. L-selectin might then permit capture of microspheres to be followed by slow rolling on E-selectin ligands, although because L-selectin is also a putative E-selectin ligand, we may see slow rolling with this molecule alone.
In summary, we have demonstrated that microspheres coated with recombinant human PSGL-1–IgG chimera can attach to and roll along murine venules differentially activated to express either P-selectin or a combination of P- and E-selectins. In contrast to earlier in vitro work,13,21 22 we find that, in the absence of P-selectin, E-selectin cannot initiate rolling of PSGL-1–coated microspheres. We do show, however, that the rolling velocity of PSGL-1–coated microspheres in TNFα-stimulated venules of the mouse cremaster is increased if E-selectin is either absent or blocked. Whether E-selectin specifically recognizes a distinct subset of glycoproteins, or is happy to interact with any surface presenting sialic acid and fucose in the correct spatial arrangement is a question that requires further investigation.
Acknowledgment
We are grateful to Dr Nicky Brown for helpful discussions and for the loan of the SIT camera.
Supported by British Heart Foundation studentships FS98051 and FS99040 (A.E.H. and M.J.C.). Grants awarded by the Royal Society (RSRG20061) and the Wellcome Trust (057108, 043571) funded purchase of the Dage 330EX camera, intravital microscopy equipment, and flow cytometer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Keith E. Norman, University of Sheffield, Clinical Sciences Centre, Northern General Hospital, Herries Road, Sheffield S5 7AU, UK; e-mail: k.norman@sheffield.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal