Abstract
Analysis of the immunoglobulin receptor (IGR) variable heavy- and light-chain sequences on 17 hepatitis C virus (HCV)-associated non-Hodgkin lymphomas (NHLs) (9 patients also had type II mixed cryoglobulinemia [MC] syndrome and 8 had NHL unrelated to MC) and analysis of intraclonal diversity on 8 of them suggest that such malignant lymphoproliferations derive from an antigen-driven pathologic process, with a selective pressure for the maintenance of a functional IgR and a negative pressure for additional amino acid mutations in the framework regions (FRs). For almost all NHLs, both heavy- and light-chain complementarity-determining regions (CDR3) showed the highest similarity to antibodies with rheumatoid factor (RF) activity that have been found in the MC syndrome, thus suggesting that a common antigenic stimulus is involved in MC syndrome and in HCV-associated lymphomagenesis. Moreover, because HCV is the recognized pathologic agent of MC and the CDR3 amino acid sequences of some HCV-associated NHLs also present a high homology for antibody specific for the E2 protein of HCV, it may be reasonable to speculate that HCV E2 protein is one of the chronic antigenic stimuli involved in the lymphomagenetic process. Finally, the use of specific segments, in particular the D segment, in assembling the IgH chain of IgR seems to confer B-cell disorders with the property to produce antibody with RF activity, which may contribute to the manifestation of an overt MC syndrome.
Introduction
Besides the documented pathogenic involvement of hepatitis C virus (HCV) in hepatic diseases, the virus has been implicated as the etiologic factor for type II mixed cryoglobulinemia (MC), an autoimmune disease that may evolve, several years after diagnosis, into a malignant lymphoma in about 10% of patients.1,2 Moreover, although limited to some countries (ie, Italy, Japan, and the United States), epidemiologic studies also suggest a possible pathogenic role of HCV in a subset of B-cell lymphoid tumors not complicating the course of MC syndrome.3-5 In fact, a higher prevalence of HCV infection (range, 9%-40%) in de novo B-cell non-Hodgkin lymphomas (NHLs) than in other hematologic disorders (ie, T-cell NHLs and Hodgkin disease) or in the normal population was documented. Furthermore, HCV infection preceded NHL onset in some series of B-cell NHLs.3,6,7 A case-control study showed that HCV infection increases by about 50-fold the risk for NHLs involving the liver and major salivary glands (ie, a risk higher than that for hepatocellular carcinoma) and by about 4-fold the risk for NHLs at other sites.6
The identification of the viral genome within prelymphomatous and lymphomatous lesions8-10 also indicates a possible involvement of the virus in the pathogenesis of B-cell NHL. However, in some cases, the localization of the viral genome and viral proteins in the stromal cells but not in the neoplastic lymphoid cells suggests an indirect role of the virus in the pathologic process.8 9
Furthermore, the most frequent histotypes of HCV-associated NHLs are the small lymphocytic/lymphoplasmacytoid lymphoma (immunocytoma), splenic marginal zone lymphoma, lymphoma of mucosa-associated lymphoid tissue, and diffuse large cell lymphoma.2,3 11-13 Because these histotypes are typical of B-lymphoid cells at the germinal center (GC) or post-GC stage of development, it is possible that malignant transformation occurs, or is initiated, in the GC, where the vigorous expansion of B cells consequent to antigenic stimulation may represent a risk for malignant transformation.
The study of the antigen receptor (IgR) variable region genes is a key tool to provide circumstantial evidence for a role of an antigen-driven stimulus in clonal selection and progression of B-cell NHLs.14-18 Using this approach, Ivanovski and coworkers15 supported the presence of an antigen stimulus during B-cell transformation of HCV-associated B-cell NHLs. However, their study was primarily focused on bone marrow lesions, which, although consistent with a pathologic diagnosis of indolent lymphoma, do not always correlate with clinical and molecular features of a B-cell tumor.19 Using similar techniques, we recently analyzed multiple biopsy specimens from an HCV-infected patient with type II MC syndrome and demonstrated that premalignant and overt lymphoma were sequential phases of an antigen (HCV E2 protein)-driven pathologic process.16
To better address the potential role of HCV in the development of different overt malignant lymphomas, we selected 17 NHLs of different histotypes, 9 of them complicating the course of MC syndrome. The variable heavy (VH) and variable light (VL) IgR chains were sequenced, and the amino acid sequences deduced from the complementarity-determining regions (CDR3) were compared with those of antibodies with a known specificity, including anti-HCV antibodies. To ascertain the occurrence of an antigen-driven stimulus in the pathologic process, IgR sequences were compared with their germline counterparts, and the replacement- (R)-to-silent (S) mutation ratio was calculated. Intraclonal variation analysis was also performed to ascertain the persistence of antigenic stimulation after neoplastic transformation. Finally, structural differences in IGR genes between MC syndrome–related and MC syndrome–unrelated NHLs were determined to understand the molecular basis of the lymphoproliferative disorders in different clinical settings.
Materials and methods
B-cell non-Hodgkin lymphomas in individuals infected with hepatitis C virus
Seventeen selected patients (12 women and 5 men; mean age, 65 years; range, 49-76) with overt B-cell NHL of recent onset, all infected with HCV, were studied (Table1). Informed consent was obtained from all patients according to the Helsinki declaration. All were seronegative for human immunodeficiency virus and heterosexual, with no history of intravenous drug abuse. Sera obtained at disease diagnosis were all positive for anti-HCV antibodies (by the enzyme-linked immunosorbent assay [HCV 3.0; Ortho Diagnostic Systems, Raritan, NJ] and the recombinant-based immunoblot assay [Chiron RIBA; Ortho Diagnostic Systems]) and for HCV RNA.3 Overt lymphoma was revealed by the onset of clinical manifestations previously absent and suggesting a neoplastic evolution (B-cell–related symptoms, lymph node enlargement, organomegaly, organ nodular lesions, and others) detected on physical examination or imaging investigation. Bone marrow biopsy was performed in all patients at the time of NHL diagnosis, along with extensive staging procedures, as described previously.3
Histologic parameters in patients with NHL and HCV infection
| Patient no., age, sex . | Histologic diagnosis (REAL classification) . | Site of pathologic diagnosis . | Other sites of pathologic and/or clinical NHL involvement . | Presence of overt MC syndrome . |
|---|---|---|---|---|
| 1. 60, F | Follicle center, follicular | Bone marrow | — | Yes |
| 2. 58, F | Extranodal marginal zone | Submandibular gland, parotid gland | Bone marrow | Yes |
| 3. 63, F | Small lymphocytic | Skin, liver, lymph node | Bone marrow | Yes |
| 4. 73, M | Diffuse large cell | Lymph node | — | Yes |
| 5. 70, F | Diffuse large cell | Lymph node, liver | Bone marrow | Yes |
| 6. 66, F | Extranodal marginal zone | Stomach | — | Yes |
| 7. 49, M | Small lymphocytic | Bone marrow | Liver | Yes |
| 8. 76, F | Diffuse large cell | Parotid gland | — | Yes |
| 9. 61, M | Lymphoplasmacytoid | Bone marrow | Spleen | Yes |
| 10. 57, M | Diffuse large cell | Lymph node | — | No |
| 11. 69, M | Diffuse large cell | Lymph node | Bone marrow | No |
| 12. 72, F | Extranodal marginal zone | Stomach | — | No |
| 13. 66, F | Diffuse large cell | Skin | Bone marrow | No |
| 14. 69, F | Extranodal marginal zone | Submandibular gland | Bone marrow | No |
| 15. 65, F | Diffuse large cell | Lymph node | — | No |
| 16. 71, F | Extranodal marginal zone | Spleen | Bone marrow | No |
| 17. 59, F | Mantle cell | Lymph node | Bone marrow, spleen | No |
| Patient no., age, sex . | Histologic diagnosis (REAL classification) . | Site of pathologic diagnosis . | Other sites of pathologic and/or clinical NHL involvement . | Presence of overt MC syndrome . |
|---|---|---|---|---|
| 1. 60, F | Follicle center, follicular | Bone marrow | — | Yes |
| 2. 58, F | Extranodal marginal zone | Submandibular gland, parotid gland | Bone marrow | Yes |
| 3. 63, F | Small lymphocytic | Skin, liver, lymph node | Bone marrow | Yes |
| 4. 73, M | Diffuse large cell | Lymph node | — | Yes |
| 5. 70, F | Diffuse large cell | Lymph node, liver | Bone marrow | Yes |
| 6. 66, F | Extranodal marginal zone | Stomach | — | Yes |
| 7. 49, M | Small lymphocytic | Bone marrow | Liver | Yes |
| 8. 76, F | Diffuse large cell | Parotid gland | — | Yes |
| 9. 61, M | Lymphoplasmacytoid | Bone marrow | Spleen | Yes |
| 10. 57, M | Diffuse large cell | Lymph node | — | No |
| 11. 69, M | Diffuse large cell | Lymph node | Bone marrow | No |
| 12. 72, F | Extranodal marginal zone | Stomach | — | No |
| 13. 66, F | Diffuse large cell | Skin | Bone marrow | No |
| 14. 69, F | Extranodal marginal zone | Submandibular gland | Bone marrow | No |
| 15. 65, F | Diffuse large cell | Lymph node | — | No |
| 16. 71, F | Extranodal marginal zone | Spleen | Bone marrow | No |
| 17. 59, F | Mantle cell | Lymph node | Bone marrow, spleen | No |
The association between B-cell NHL and previous MC syndrome in these patients was investigated by a clinical expert (S.D.). An accurate clinical history was obtained followed by a complete physical examination. All the laboratory and instrumental tests performed in the years preceding NHL onset were reviewed. Symptoms and signs of MC such as purpura, asthenia, arthralgia, renal involvement, and peripheral neuropathy were investigated, as well as serum cryoglobulin, C3 and C4, and rheumatoid factor (RF) levels. Symptoms and signs of MC were also investigated at the time of onset of NHL.
Nine patients with overt B-cell NHL had a previous history of open MC syndrome (all with type II serum cryoglobulins and positive serum RF); the remaining 8 patients definitely lacked clinical features of MC syndrome both in the previous clinical history and at the time of NHL onset. Serum cryoglobulins were detected in 7 of these latter 8 patients (type II in patient 13; type III in patients 10, 11, 14, 15, 16, 17; not detected in patient 12) and positive serum RF in 7 patients (negative in patient 17).
Histopathology
Tissues were fixed in Bouin solution or neutral buffered formalin. In a few cases, a portion of unfixed tissue was snap frozen in liquid nitrogen and stored at −80°C. Pathologic specimens were classified according to the revised European-American classification of lymphoid neoplasms.20
Deparaffinized and cryostat sections were used for immunophenotyping and lineage assignment of lymphoma cases with monoclonal antibodies (CD3, CD4, CD5, CD8, CD9, CD10, CD15, CD19, CD20, CD21, CD22, CD24, CD30, CD38, CD43, CD45, CD45RA, CD45R0, CD68, CD74, CDw75, LN3, MB2, DDBB42, DBA44, OPD4, DRC-1, Leu8, anti-κ and λ immunoglobulin (Ig) light chains, epithelial membrane antigen [EMA], vimentin, and cytokeratin [MNF116]). Immunohistochemistry was performed with the avidin-biotin-peroxidase complex (ABC-px) or alkaline phosphatase antialkaline phosphatase (APAAP) methods, as previously described.21 22
Polymerase chain reaction (PCR) amplification and sequencing of VH and VK gene regions
Total DNA was isolated from biopsy specimens by phenol-chloroform extraction according to standard protocols. DNA was analyzed for B-cell clonal expansion using the seminested third framework (FR3) protocol of amplification, where the upstream primer is directed to the FR3 variable (V) region and the downstream primer is directed to the joining (J) region of the IGH gene.23 PCR products were analyzed on 10% polyacrylamide gels stained with ethidium bromide. The single-band PCR product from an amplified DNA sample was subjected to direct DNA sequencing on an ABI 310 Genetic Analyzer (Perkin Elmer, Foster City, CA) using the dye-terminator protocol.16
A clonospecific antisense oligoprimer was then synthesized based on the CDR3 DNA sequence and used as a downstream primer paired with each VH family-specific framework 1 primer (FR1 protocol).24 The amplified PCR fragments were purified from the gel and directly sequenced. All of the sequences were confirmed by sequencing twice in both directions.
The VL gene region was amplified using VK family-specific primers and a mixture of JK primers.14 PCR products were analyzed on 3% agarose gel stained with ethidium bromide. The single-band PCR product was then subjected to direct DNA sequencing.
The amino acid sequences deduced from the VH, VK, and CDR3 DNA sequences were analyzed by the NCBI Basic Local Alignment Search Tool (BLAST) program (seehttp://www.ncbi.nlm.nih.gov/blast/blast.cgi?form=0) for homology to multiple protein sequence databases. The protein sequences closely related to the given VH, VK, and CDR3 sequences were scored, starting from the most homologous one, with rigorous statistical analysis to assess the significance of the matches.
The identification of VH, VK, and CDR3 Ig germline sequences was performed by sequence comparison with the International Immunogenetics Database program on the Internet (http://imgt.cnusc.fr:8104/dnaplot/).
Analysis of mutations
Calculation of the number of expected R mutations in IGR gene CDRs or FRs was based on a computer algorithm developed by Chang and Casali.25 This algorithm calculates the inherent susceptibility to amino acid replacement given any single nucleotide change. The number of expected R mutations was calculated as follows: n × (CDR Rf or FR Rf) × (CDRrel or FRrel), where n = total number of observed mutations, Rf = replacement frequency inherent to CDR or FR sequences, and CDRrel or FRrel = relative size of the CDRs or FRs.
The probability (P) that excess or scarcity of R mutations in the IGR gene CDRs or FRs, observed in the predominant clone, was due to chance only was calculated using the binomial distribution model, as reported by Chang and Casali25 as follows:P = {n!/[k!(n-k)!]} × qk × (1 – q)n-k, where n = total number of observed mutations, k = number of observed R mutations in the CDRs or FRs, and q = probability that an R mutation localizes to CDRs or FRs (q = CDRrel × CDR Rf or FRrel × FR Rf).
Intraclonal variation analysis
Electrophoretically purified VHDJH PCR fragment products were ligated into a pGem-T vector (Promega, Madison, WI) and transfected into Escherichia coli DH5α competent cells.16 Clones were picked up at random and DNA was extracted using the Wizard Plus Minipreps DNA Purification System (Promega). Sequence reactions were performed on the ABI 310 Genetic Analyzer (Perkin Elmer) and confirmed by sequencing twice in both directions.
Results
Tumor histopathology
According to the REAL classification, 7 of the 17 HCV-positive patients (41%) presented with diffuse large B-cell lymphoma; extranodal marginal zone lymphoma was observed in 5 patients (29%); small lymphocytic lymphoma occurred in 2 patients (12%); and lymphoplasmocytoid lymphoma/immunocytoma, follicle center lymphoma, and mantle cell lymphoma were seen in 1 patient each (Table 1).
Analysis of R/S mutation ratio in VH and VL genes
The complete nucleotide sequence of the VHDJH rearrangements was obtained for all 17 patients (data not shown). The complete nucleotide sequence of the VKJK rearrangements was obtained in 12 patients (data not shown), whereas the gene family only was determined in patients 8 and 15 due to inefficient sequencing. The inability of sequencing the VKJK gene rearrangement in patients 6, 9, and 17 was due to scarcity of DNA from small biopsy samples.
In the FR regions, the R/S mutation ratio was significantly lower than expected by chance alone (P = .05) in most of the VH genes (13 of 17 patients) (Table2) and VK genes (11 of 12 patients), indicating a selective pressure for maintenance of a functional IgR (Table 3).
Distribution of mutations in VH genes of HCV-associated NHLs
| Patient no. . | VH family∖gene . | R/S mutation ratio in FRs . | R/S mutation ratio in CDRs . | ||||
|---|---|---|---|---|---|---|---|
| Observed (R:S) . | Expected . | P . | Observed (R:S) . | Expected . | P . | ||
| 1 | VH1∖DP-88 | 4.000 (4:1) | 3.297 | .04387 | ∞ (3:0) | 3.028 | .19795 |
| 2 | VH1∖DP-88 | 0.833 (5:6) | 3.297 | .00020 | 0.750 (3:4) | 3.028 | .21336 |
| 3 | VH1∖DP-10 | 1.000 (5:5) | 3.306 | .00074 | 2.000 (4:2) | 3.028 | .20844 |
| 4 | VH3∖DP-47 | 0.667 (2:3) | 3.264 | .00764 | 0.500 (1:2) | 3.792 | .36656 |
| 5 | VH3∖DP-54 | 0.666 (4:6) | 3.264 | .00433 | ∞ (2:0) | 5.091 | .23696 |
| 6 | VH3∖DP-54 | 0.400 (2:5) | 3.226 | .00061 | 1.500 (3:2) | 5.091 | .24162 |
| 7 | VH4∖DP-71 | ∞ (1:0) | 3.000 | 0.29193 | ∞ (1:0) | 3.704 | .34102 |
| 8 | VH3∖DP-51 | 3.000 (6:2) | 3.265 | .00160 | 3.000 (6:2) | 4.875 | .10878 |
| 9 | VH1∖DP-75 | 4.000 (4:1) | 3.686 | 0.10068 | ∞ (1:0) | 3.152 | .31985 |
| 10 | VH3∖DP-54 | 1.154 (15:13) | 3.198 | 9.81 × 10−7 | 5.000 (5:1) | 5.091 | .16141 |
| 11 | VH3∖DP-47 | 2.400 (12:5) | 3.548 | .000204 | ∞ (10:0) | 3.230 | .06237 |
| 12 | VH4∖DP-71 | 1.200 (6:5) | 2.870 | .01760 | ∞ (4:0) | 3.310 | .19803 |
| 13 | VH4∖DP-63 | 1.143 (8:7) | 3.129 | .00333 | ∞ (2:0) | 3.483 | .13081 |
| 14 | VH3∖DP-49 | 1.000 (7:7) | 3.171 | .00202 | ∞ (3:0) | 4.000 | .19038 |
| 15 | VH3∖DP-54 | ∞ (4:0) | 3.226 | .07310 | ∞ (3:0) | 5.091 | .20099 |
| 16 | VH1∖DP-8 | 0.600 (3:5) | 3.713 | .01920 | 0.000 (0:0) | 3.152 | .13103 |
| 17 | VH3∖DP-51 | 1.000 (1:1) | 3.265 | .29014 | 0.000 (0:0) | 4.875 | .54760 |
| Patient no. . | VH family∖gene . | R/S mutation ratio in FRs . | R/S mutation ratio in CDRs . | ||||
|---|---|---|---|---|---|---|---|
| Observed (R:S) . | Expected . | P . | Observed (R:S) . | Expected . | P . | ||
| 1 | VH1∖DP-88 | 4.000 (4:1) | 3.297 | .04387 | ∞ (3:0) | 3.028 | .19795 |
| 2 | VH1∖DP-88 | 0.833 (5:6) | 3.297 | .00020 | 0.750 (3:4) | 3.028 | .21336 |
| 3 | VH1∖DP-10 | 1.000 (5:5) | 3.306 | .00074 | 2.000 (4:2) | 3.028 | .20844 |
| 4 | VH3∖DP-47 | 0.667 (2:3) | 3.264 | .00764 | 0.500 (1:2) | 3.792 | .36656 |
| 5 | VH3∖DP-54 | 0.666 (4:6) | 3.264 | .00433 | ∞ (2:0) | 5.091 | .23696 |
| 6 | VH3∖DP-54 | 0.400 (2:5) | 3.226 | .00061 | 1.500 (3:2) | 5.091 | .24162 |
| 7 | VH4∖DP-71 | ∞ (1:0) | 3.000 | 0.29193 | ∞ (1:0) | 3.704 | .34102 |
| 8 | VH3∖DP-51 | 3.000 (6:2) | 3.265 | .00160 | 3.000 (6:2) | 4.875 | .10878 |
| 9 | VH1∖DP-75 | 4.000 (4:1) | 3.686 | 0.10068 | ∞ (1:0) | 3.152 | .31985 |
| 10 | VH3∖DP-54 | 1.154 (15:13) | 3.198 | 9.81 × 10−7 | 5.000 (5:1) | 5.091 | .16141 |
| 11 | VH3∖DP-47 | 2.400 (12:5) | 3.548 | .000204 | ∞ (10:0) | 3.230 | .06237 |
| 12 | VH4∖DP-71 | 1.200 (6:5) | 2.870 | .01760 | ∞ (4:0) | 3.310 | .19803 |
| 13 | VH4∖DP-63 | 1.143 (8:7) | 3.129 | .00333 | ∞ (2:0) | 3.483 | .13081 |
| 14 | VH3∖DP-49 | 1.000 (7:7) | 3.171 | .00202 | ∞ (3:0) | 4.000 | .19038 |
| 15 | VH3∖DP-54 | ∞ (4:0) | 3.226 | .07310 | ∞ (3:0) | 5.091 | .20099 |
| 16 | VH1∖DP-8 | 0.600 (3:5) | 3.713 | .01920 | 0.000 (0:0) | 3.152 | .13103 |
| 17 | VH3∖DP-51 | 1.000 (1:1) | 3.265 | .29014 | 0.000 (0:0) | 4.875 | .54760 |
P indicates probability that the observed R mutations occurred by chance; the value is reported in bold when statistically significant (P = .05).
Distribution of mutations in VK genes of HCV-associated NHLs
| Patient no. . | VK family∖gene . | R/S mutation ratio in FRs . | R/S mutation ration in CDRs . | ||||
|---|---|---|---|---|---|---|---|
| Observed (R:S) . | Expected . | P . | Observed (R:S) . | Expected . | P . | ||
| 1 | VKIII∖humkv325 | 0.500 (1:2) | 3.407 | .01942 | ∞ (2:0) | 3.833 | .13270 |
| 2 | VKIII∖humkv325 | 0.428 (3:7) | 3.404 | .00030 | 0.000 (0:1) | 3.647 | .16295 |
| 3 | VKIII∖humkv325 | 6.000 (6:1) | 3.404 | .08305 | 0.000 (0:0) | 3.833 | .17392 |
| 4 | VKIII∖humkv325 | 2.000 (2:1) | 3.370 | .04108 | ∞ (2:0) | 3.833 | .14776 |
| 5 | VKIII∖humkv328 | 0.500 (2:4) | 3.280 | .00995 | 0.000 (0:0) | 3.091 | .58344 |
| 7 | VKIII∖humkv325 | 0.500 (1:2) | 3.407 | .01942 | 1.000 (1:1) | 3.833 | .38194 |
| 10 | VKIII∖humkv328 | 0.900 (9:10) | 3.288 | .00000118 | 6.000 (6:1) | 3.706 | .09955 |
| 11 | VKI∖L12a | 2.666 (8:3) | 3.264 | .00474 | ∞ (2:0) | 4.923 | .28112 |
| 12 | VKIII∖humkv325 | 2.000 (4:2) | 3.370 | .03242 | ∞ (1:0) | 3.833 | .37445 |
| 13 | VKI∖A30 | 1.500 (3:2) | 3.292 | .01618 | ∞ (2:0) | 3.158 | .19169 |
| 14 | VKIII∖humkv328 | 0.750 (3:4) | 3.294 | .007609 | ∞ (1:0) | 3.706 | .38134 |
| 16 | VKIII∖humkv325 | 1.000 (4:4) | 3.405 | .00808 | ∞ (1:0) | 3.833 | .34521 |
| Patient no. . | VK family∖gene . | R/S mutation ratio in FRs . | R/S mutation ration in CDRs . | ||||
|---|---|---|---|---|---|---|---|
| Observed (R:S) . | Expected . | P . | Observed (R:S) . | Expected . | P . | ||
| 1 | VKIII∖humkv325 | 0.500 (1:2) | 3.407 | .01942 | ∞ (2:0) | 3.833 | .13270 |
| 2 | VKIII∖humkv325 | 0.428 (3:7) | 3.404 | .00030 | 0.000 (0:1) | 3.647 | .16295 |
| 3 | VKIII∖humkv325 | 6.000 (6:1) | 3.404 | .08305 | 0.000 (0:0) | 3.833 | .17392 |
| 4 | VKIII∖humkv325 | 2.000 (2:1) | 3.370 | .04108 | ∞ (2:0) | 3.833 | .14776 |
| 5 | VKIII∖humkv328 | 0.500 (2:4) | 3.280 | .00995 | 0.000 (0:0) | 3.091 | .58344 |
| 7 | VKIII∖humkv325 | 0.500 (1:2) | 3.407 | .01942 | 1.000 (1:1) | 3.833 | .38194 |
| 10 | VKIII∖humkv328 | 0.900 (9:10) | 3.288 | .00000118 | 6.000 (6:1) | 3.706 | .09955 |
| 11 | VKI∖L12a | 2.666 (8:3) | 3.264 | .00474 | ∞ (2:0) | 4.923 | .28112 |
| 12 | VKIII∖humkv325 | 2.000 (4:2) | 3.370 | .03242 | ∞ (1:0) | 3.833 | .37445 |
| 13 | VKI∖A30 | 1.500 (3:2) | 3.292 | .01618 | ∞ (2:0) | 3.158 | .19169 |
| 14 | VKIII∖humkv328 | 0.750 (3:4) | 3.294 | .007609 | ∞ (1:0) | 3.706 | .38134 |
| 16 | VKIII∖humkv325 | 1.000 (4:4) | 3.405 | .00808 | ∞ (1:0) | 3.833 | .34521 |
P indicates the probability that the observed R mutations occurred by chance; the value is reported in bold when statistically significant (P = .05).
Intraclonal variation in non-Hodgkin lymphomas
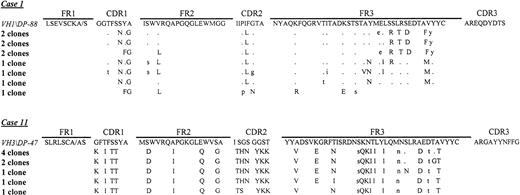
Intraclonal variation was analyzed in 8 NHLs, 5 of which were associated with MC syndrome (patients 1, 2, 6, 7, and 9) and 3 not so associated (patients 11, 12, and 16), by cloning the VHDJH PCR-amplified product. A minimum of 9 randomly selected clones for each NHL were sequenced. Intraclonal variation was observed in all cases analyzed, although with low level of nucleotide substitution between clones (data not shown, except for patients 1 and 11). The number of distinct subclones ranged from 7 of 10 in patient 1 to 5 of 9 in patient 11 (Figure1).
Deduced amino acid sequences of VH genes of a follicular (patient 1) and a diffuse large-cell lymphoma (patient 11).
The amino acid sequences of the most homologous germline VH genes are shown above the lymphoma sequences. Amino acid R mutations are indicated by upper case letters and S mutations by lower case letters.
Deduced amino acid sequences of VH genes of a follicular (patient 1) and a diffuse large-cell lymphoma (patient 11).
The amino acid sequences of the most homologous germline VH genes are shown above the lymphoma sequences. Amino acid R mutations are indicated by upper case letters and S mutations by lower case letters.
Characterization of gene segments involved in IgR rearrangements
Sequencing data showed that the assigned germline counterparts of the VH genes were closely related to the VHIII family (DP 54, DP 51, DP 47, and DP 49) in 9 patients, the VHI family (DP 10, DP 88, DP 8, and DP 75) in 5 patients, and the VHIV family (DP 71 and DP 63) in 3 patients (Table4). The VHI gene family members were more frequently used in MC syndrome–related than in MC syndrome–unrelated NHLs (4 of 9 patients versus 1 of 7), whereas the VHIII and VHIV family genes were almost equally distributed in both groups (Table 4).
VH gene segments and CDR3–deduced amino acid sequences of HCV-associated NHLs and homology to protein sequence databases
| Patient no. . | IgH CDR3 deduced amino acid sequences . | Length of CDR3 AA . | Sequences producing the highest significant similarity . | E value4-150 . | VH family/gene . | DH segment . | JH segment . |
|---|---|---|---|---|---|---|---|
| 1 | CAREQDYDTSSYYYGYWGQGTLV | 13 | RF-WOL | 6e-05 | VHI/DP-88 | D21/9 | J4 |
| 2 | CARGTDSSNYYYFYWGQGTLV | 11 | RF-WOL | 0.002 | VHI/DP-88 | D21/9 | J2 |
| 3 | CARAFSSDTSGYYYYWGQGTLV | 12 | RF-WOL | 0.005 | VHI/DP-10 | D21/9 | J4 |
| 4 | CARGPETSGYYYFYWGQGTLV | 11 | RF-WOL | 0.005 | VHIII/DP-47 | D21/9 | J4 |
| 5 | CARGDYYDTSGYFSDAFDIWGQGTLV | 16 | RF-WA | 1e-06 | VHIII/DP-54 | D21/9 | J3 |
| 6 | CARGDYDNSGDFVDAFDIWGQGTLV | 15 | RF-WA | 1e-05 | VHIII/DP-54 | D21/10 | J3 |
| 7 | CARDRFCSGGSCFDWYFDLWGQGTLV | 19 | RF-MR-20/HCV | 2e-07/1e-04 | VHIV/DP-71 | D2 | J2 |
| 8 | CARDYGGSSGYLNWFDPWGQGTLV | 14 | RF | 4e-05 | VHIII/DP-51 | D21/9 | J5 |
| 9 | CARDLCSGGSCPALGAIDYWGEGTLV | 16 | RF-C93 | 0.017 | VHI/DP-75 | D2 | J4 |
| 10 | CARGDYFDDDGPFIDVFNVWGQGTLV | 17 | RF-WA | 9e-06 | VHIII/DP-54 | D5-24 | J3 |
| 11 | CARGAYYNFFGDSYHYLDYWGQGTLV | 16 | RF-WA | 0.030 | VHIII/DP-47 | DXP1 | J4 |
| 12 | CARDSFCTGGSCFDWYFDWGQG | 15 | RF-MR-20/HCV | 7e-06/0.023 | VHIV/DP-71 | D2 | J5/J2 |
| 13 | CTSTGDRALVYSYDFWGQGTLV | 19 | RF-BOR | 1700 | VHIV/DP-63 | D5-18/DK4 | J4 |
| 14 | CAQGGFSTSWYVPTGGYWGQGTLV | 21 | RF-10-2 | 1000 | VHIII/DP-49 | DN1 | J4 |
| 15 | CARAPTRMGPLWELGYYFDCWGQGTLV | 17 | RF/HCV | 0.030/0.15 | VHIII/DP-54 | DN4 | J4 |
| 16 | CERSKYFDSWSGECVSARESHFDYWGQGTLV | 21 | RF | 0.010 | VHI/DP-8 | DXP4 | J4 |
| 17 | CARANKEQWLVLEYNWFDPWGQGTLV | 16 | RF | 2e-04 | VHIII/DP-51 | D6-19 | J5 |
| Patient no. . | IgH CDR3 deduced amino acid sequences . | Length of CDR3 AA . | Sequences producing the highest significant similarity . | E value4-150 . | VH family/gene . | DH segment . | JH segment . |
|---|---|---|---|---|---|---|---|
| 1 | CAREQDYDTSSYYYGYWGQGTLV | 13 | RF-WOL | 6e-05 | VHI/DP-88 | D21/9 | J4 |
| 2 | CARGTDSSNYYYFYWGQGTLV | 11 | RF-WOL | 0.002 | VHI/DP-88 | D21/9 | J2 |
| 3 | CARAFSSDTSGYYYYWGQGTLV | 12 | RF-WOL | 0.005 | VHI/DP-10 | D21/9 | J4 |
| 4 | CARGPETSGYYYFYWGQGTLV | 11 | RF-WOL | 0.005 | VHIII/DP-47 | D21/9 | J4 |
| 5 | CARGDYYDTSGYFSDAFDIWGQGTLV | 16 | RF-WA | 1e-06 | VHIII/DP-54 | D21/9 | J3 |
| 6 | CARGDYDNSGDFVDAFDIWGQGTLV | 15 | RF-WA | 1e-05 | VHIII/DP-54 | D21/10 | J3 |
| 7 | CARDRFCSGGSCFDWYFDLWGQGTLV | 19 | RF-MR-20/HCV | 2e-07/1e-04 | VHIV/DP-71 | D2 | J2 |
| 8 | CARDYGGSSGYLNWFDPWGQGTLV | 14 | RF | 4e-05 | VHIII/DP-51 | D21/9 | J5 |
| 9 | CARDLCSGGSCPALGAIDYWGEGTLV | 16 | RF-C93 | 0.017 | VHI/DP-75 | D2 | J4 |
| 10 | CARGDYFDDDGPFIDVFNVWGQGTLV | 17 | RF-WA | 9e-06 | VHIII/DP-54 | D5-24 | J3 |
| 11 | CARGAYYNFFGDSYHYLDYWGQGTLV | 16 | RF-WA | 0.030 | VHIII/DP-47 | DXP1 | J4 |
| 12 | CARDSFCTGGSCFDWYFDWGQG | 15 | RF-MR-20/HCV | 7e-06/0.023 | VHIV/DP-71 | D2 | J5/J2 |
| 13 | CTSTGDRALVYSYDFWGQGTLV | 19 | RF-BOR | 1700 | VHIV/DP-63 | D5-18/DK4 | J4 |
| 14 | CAQGGFSTSWYVPTGGYWGQGTLV | 21 | RF-10-2 | 1000 | VHIII/DP-49 | DN1 | J4 |
| 15 | CARAPTRMGPLWELGYYFDCWGQGTLV | 17 | RF/HCV | 0.030/0.15 | VHIII/DP-54 | DN4 | J4 |
| 16 | CERSKYFDSWSGECVSARESHFDYWGQGTLV | 21 | RF | 0.010 | VHI/DP-8 | DXP4 | J4 |
| 17 | CARANKEQWLVLEYNWFDPWGQGTLV | 16 | RF | 2e-04 | VHIII/DP-51 | D6-19 | J5 |
Smallest sum probability according to the BLAST similarity search program. The E value is inversely related to statistical significance and is considered significant for a value below 0.05.
Genbank accession numbers for RF-WOL: prf0707281c; RF-WA: U03400; RF-MR-20: U85234; anti E2 protein of HCV: AJ236544 for patient 7 and 12 and AJ236543 for patient 15; RF: AF021950 for patient 8, AF021978 for patient 15, AF021967 for patient 16 and AF022007 for patient 17; RF-C93: 573143; RF-BOR: prf1313976A; RF-10-2: S74542.
Concerning the DH segment, 7 of 9 MC syndrome–associated NHLs used the D21/9 or D21/10 gene (D21/9 and D21/10 differ by only one amino acid) and the other 2 used the D2 gene. This was also used by one NHL not associated with the MC syndrome but associated with another autoimmune disease (ie, Sjögren syndrome [SS]). The remaining NHLs that were not associated with autoimmune syndromes did not use the previously mentioned DH segments, but each of them used a different DH segment (Table 4).
No specificity in the use of JH fragments was noted (JH2 to JH5 gene segments were used), although the JH4 segment was the most represented one in both groups of NHLs (Table 4).
Data for the light-chain sequences were obtained in 14 patients only. Seven NHLs used a mutated VKIII kv325 gene, 3 a segment similar to the VKIII kv328h5 gene, and 2 each aVKII and a VKI gene (Table5). The VKIII kv325gene was more frequently used in the MC syndrome–associated NHL group (5 of 7) than in the MC syndrome–unrelated group (2 of 7, 1 of them associated with SS) (Table 5).
VK gene segments and CDR3-deduced amino acid sequences of HCV-associated NHLs and homology to protein sequence databases
| Patient no. . | IgK CDR3–deduced amino acid sequences . | Length of CDR3 AA . | Sequences producing the highest significant similarity . | E value5-150 . | Vkfamily/gene . | Jk segment . |
|---|---|---|---|---|---|---|
| 1 | CQQYGSSRTFGQGTKLEIK | 19 | anti-HCV/RF-CUR | 0.003/0.011 | VKIII-kv325 | JKI |
| 2 | CQQYGSSPYTFGQGTKLEIKR | 21 | RF-FLO/anti-HCV | 4e-06/2e-04 | VKIII-kv325 | JK2 |
| 3 | CQQYGSSPYTFGQGTKL | 17 | RF/anti-HCV | 7e-04/0.006 | VKIII-kv325 | JK2 |
| 4 | CQQYGSSPYTFGQGTKVEI | 19 | RF-FLO/anti-HCV | 1e-04/2e-04 | VKIII-kv325 | JK2 |
| 5 | CQHYNNWPPWTFGQGTKLEIK | 21 | RF-WA | 6e-07 | VKIII-kv328h5 | JKI |
| 6 | ND | ND | ||||
| 7 | CQQYGSSPRTFGQGTKL | 17 | anti-HCV/RF-CUR | 0.001/0.003 | VKIII-kv325 | JKI |
| 8 | ND | ND | VKII | |||
| 9 | ND | ND | ||||
| 10 | CQQYNNWPPWTFGPGTKVKSNR | 22 | RF-4C9 | 5e-06 | VKIII-kv328h5 | JKI |
| 11 | CQQYNIYPYTFGQGTKLEIKREY | 23 | RF-FLO | 5e-04 | VKI-L12a | JK2 |
| 12 | CQQYGSSPTFGQGTKL | 16 | anti-HBV/anti-HCV/RF-FLO | 0.073/0.12/0.12 | VKIII-kv325 | JKI |
| 13 | CLQYNSYPRTFGQGTKVEIK | 20 | anti-GPIIb/anti-CMV | 5e-04/7e-04 | VKI-A30 | JKI |
| 14 | CQQYNNWPPWTFGQGTKVEIK | 21 | RF-WA | 7e-08 | VKIII-kv328h5 | JKI |
| 15 | ND | ND | VKII | |||
| 16 | CQQYGSSPRTFGQGTKVEI | 19 | RF-CUR/anti-HCV | 1e-04/2e-04 | VKIII-kv325 | JKI |
| 17 | ND | ND |
| Patient no. . | IgK CDR3–deduced amino acid sequences . | Length of CDR3 AA . | Sequences producing the highest significant similarity . | E value5-150 . | Vkfamily/gene . | Jk segment . |
|---|---|---|---|---|---|---|
| 1 | CQQYGSSRTFGQGTKLEIK | 19 | anti-HCV/RF-CUR | 0.003/0.011 | VKIII-kv325 | JKI |
| 2 | CQQYGSSPYTFGQGTKLEIKR | 21 | RF-FLO/anti-HCV | 4e-06/2e-04 | VKIII-kv325 | JK2 |
| 3 | CQQYGSSPYTFGQGTKL | 17 | RF/anti-HCV | 7e-04/0.006 | VKIII-kv325 | JK2 |
| 4 | CQQYGSSPYTFGQGTKVEI | 19 | RF-FLO/anti-HCV | 1e-04/2e-04 | VKIII-kv325 | JK2 |
| 5 | CQHYNNWPPWTFGQGTKLEIK | 21 | RF-WA | 6e-07 | VKIII-kv328h5 | JKI |
| 6 | ND | ND | ||||
| 7 | CQQYGSSPRTFGQGTKL | 17 | anti-HCV/RF-CUR | 0.001/0.003 | VKIII-kv325 | JKI |
| 8 | ND | ND | VKII | |||
| 9 | ND | ND | ||||
| 10 | CQQYNNWPPWTFGPGTKVKSNR | 22 | RF-4C9 | 5e-06 | VKIII-kv328h5 | JKI |
| 11 | CQQYNIYPYTFGQGTKLEIKREY | 23 | RF-FLO | 5e-04 | VKI-L12a | JK2 |
| 12 | CQQYGSSPTFGQGTKL | 16 | anti-HBV/anti-HCV/RF-FLO | 0.073/0.12/0.12 | VKIII-kv325 | JKI |
| 13 | CLQYNSYPRTFGQGTKVEIK | 20 | anti-GPIIb/anti-CMV | 5e-04/7e-04 | VKI-A30 | JKI |
| 14 | CQQYNNWPPWTFGQGTKVEIK | 21 | RF-WA | 7e-08 | VKIII-kv328h5 | JKI |
| 15 | ND | ND | VKII | |||
| 16 | CQQYGSSPRTFGQGTKVEI | 19 | RF-CUR/anti-HCV | 1e-04/2e-04 | VKIII-kv325 | JKI |
| 17 | ND | ND |
Smallest sum probability according to the BLAST similarity search program. The E value is inversely related to statistical significance and is considered significant for a value below 0.05.
ND indicates not determined.
Genbank accession numbers for anti E2 protein of HCV: AJ236554 for patients 1 and 12, AJ236552 for patients 2, 3, and 4, and AJ236551 for patients 7 and 16; RF-CUR: prf1206991A; RF-FLO: prf1714186A for patient 2 and prf1206991B for patients 4, 11, and 12; RF: prf1714186A; RF-WA:U03401; RF-4C9: L48238; anti-HBV: M88319; anti-GPIIIb: S73911; anti-CMV: L26893.
The Jk1 or Jk2 gene fragments were used in both groups of NHLs, with a predominance of the Jk1 fragment in the MC syndrome-unrelated group (Table 5).
VH-CDR3– and VK-CDR3–deduced amino acid sequences and homology to antibodies with known specificity
The amino acid sequences deduced from the VH-CDR3 and VK-CDR3 sequences are reported in Tables 4 and 5, respectively. Because the antibody specificity is mainly based on the amino acid sequence of the CDR3 IgR region,26 we compared by the BLAST program the deduced CDR3 amino acid sequences with those of antibodies with defined specificity. The significance of the rigorous statistical comparison is indicated by the E value, which is inversely proportional to the similarity between antibody and CDR3.
VH-CDR3–deduced amino acid sequences showed the highest significant similarity (Table 4) to human antibodies with RF specificity. In particular, 4 NHLs had the highest homology with RF-WOL, 4 with RF-WA, 2 with RF-MR-20, and the remaining 7 with other RFs. Homology to RF-WOL was only associated with MC syndrome-related NHLs. Moreover, higher similarity values between VH-CDR3 and antibodies with RF activity were found in the MC syndrome–associated NHL group (Table 4). Three NHL cases (patients 7, 12, and 15) also exhibited a significant homology to an antibody specific for an epitope expressed by HCV E2 protein (Table 4).
The VK-CDR3–deduced amino acid sequences also showed the highest similarity to antibodies with RF specificity in 9 of 12 patients. The remaining 3 patients (1, 12, and 13) had similarity to an antibody with RF activity but lower than that to antibodies with other specificities (Table 5). Interestingly, 7 of 12 patients (5 MC syndrome–associated and 2 MC syndrome–unrelated, but 1 of them with SS; patients 1, 2, 3, 4, 7, 12, and 16) also exhibited a high homology (> 90%) to an antibody specific for HCV E2 protein (accession numberAJ236551; data not shown) and all used the VKIII kv325 gene. Furthermore, all of the VK-CDR3–deduced amino acid sequences presented a very similar motif (Table6). GenBank accession numbers for the mentioned antibodies with defined specificity are reported in Tables 4and 5.
VKIII-CDR3 multiple sequence alignment
| Patient no. . | VK-CDR3 sequence from kv325 gene . |
|---|---|
| 4 | CQQYGSSPYTFGQGTKVEI– |
| 12 | CQQYGSSPRTFGQGTKVEIK- |
| 6 | CQQYGSSPRTFGQGTKL—- |
| 2 | CQQYGSSPYTFGQGTKLEIKR |
| 3 | CQQYGSSPYTFGQGTKL—- |
| 1 | CQQYGSS-RTFGQGTKLEIK |
| 9 | CQQYGSS-PTFGQGTKL—- |
| ******6-150******6-150: | |
| Patient no. | VK-CDR3 sequence from kv328 gene |
| 5 | CQHYNNWPPWTFGQGTKLEIK- |
| 14 | CQQYNNWPPWTFGQGTKVEIK- |
| 10 | CQQYNNWPPWTFGPGTKVKSNR |
| **:*********6-150***:: : |
| Patient no. . | VK-CDR3 sequence from kv325 gene . |
|---|---|
| 4 | CQQYGSSPYTFGQGTKVEI– |
| 12 | CQQYGSSPRTFGQGTKVEIK- |
| 6 | CQQYGSSPRTFGQGTKL—- |
| 2 | CQQYGSSPYTFGQGTKLEIKR |
| 3 | CQQYGSSPYTFGQGTKL—- |
| 1 | CQQYGSS-RTFGQGTKLEIK |
| 9 | CQQYGSS-PTFGQGTKL—- |
| ******6-150******6-150: | |
| Patient no. | VK-CDR3 sequence from kv328 gene |
| 5 | CQHYNNWPPWTFGQGTKLEIK- |
| 14 | CQQYNNWPPWTFGQGTKVEIK- |
| 10 | CQQYNNWPPWTFGPGTKVKSNR |
| **:*********6-150***:: : |
Presence of the same amino acid in the sequences.
: Presence of amino acids with conservative change.
Discussion
Epidemiologic and experimental data strongly suggest an etiopathogenic role for HCV in the development of a subset of NHLs, particularly those complicating the course of some autoimmune diseases, mainly the MC syndrome.3,5,6,11-13,16,19,27,28 At present, the most plausible pathogenic hypothesis supposes that specific B-cell clones proliferate, mainly in the bone marrow and liver tissue, as a consequence of a chronic antigenic stimulation exerted by HCV-associated antigen(s).19,29 30 The continuous expansion of such chronically stimulated B-cells may then represent a risk for malignant transformation.
Our recent report16 supports such a pathogenic hypothesis, demonstrating that premalignant and malignant lymphoproliferations in an HCV-infected patient with MC syndrome were sequential phases of an antigen (HCV E2 protein)-driven pathologic process. The aim of the present work was to ascertain whether such a pathogenic mechanism may involve a higher proportion of NHLs arising in HCV-infected patients with or without MC syndrome.
Sequencing of the IgR expressed by 17 HCV-associated NHLs and analysis of intraclonal variability in 8 of them suggest that the malignant lymphoproliferations derive from an antigen-driven pathologic process. In fact, the NHLs presented a significantly lower R/S mutation ratio than expected by chance only in both the heavy-chain (14 of 17) and light-chain (11 of 12) FR sequences, consistent with a selective pressure to conserve such structure to provide the scaffolding for the antigen-contracting CDRs. Moreover, the neoplastic populations presented intraclonal variation, a phenomenon highly indicative of an ongoing antigen-dependent proliferation. The absence of significant R mutations in the CDRs argues against the selection for variants with higher antigen-binding specificity; alternatively, it may derive from elimination of variants too autoreactive and, therefore, dangerous for the host.16 31-35
The sequencing of IgR expressed by the HCV-associated NHLs showed a highly restricted use of gene segments. All VH chains used exclusively the VH3, VH1, and VH4 family genes. Seven of the 12 sequenced VK chains (58%) used the kv325 gene and 3 (25%) the kv328gene, which present very similar deduced amino acid sequences (Table6). Both these VH and VK genes are known to be preferably used by B cells secreting RF in patients with type II MC, and they are associated with WA cross-idiotype (XId), the major cross-idiotype among human monoclonal RF.15,31,36-38 Such a restricted use of specific gene segments in many different lymphomas is unlikely to occur by chance only, thus suggesting that the antigen-binding specificity of the IgR must be causally associated with events predisposing B cells to neoplastic transformation.39 The picture that emerges from these experimental data is, therefore, compatible with the pathogenic hypothesis that a chronic, and possibly common, antigenic stimulus drives the expansion of specific B-cell clones in a GC-like reaction where the vigorous expansion of the B cells makes them susceptible to mutation events responsible for neoplastic transformation.
Concerning the antigen responsible for the chronic B-cell stimulation, a clue derives from the comparison of the IgR CDR3– deduced amino acid sequences with those of antibodies with defined specificity. For almost all NHLs, both heavy- and light-chain CDR3 showed the highest similarity to antibodies with RF activity that have been found in MC syndrome.15,28,31,36-38,40 41 These data suggest, therefore, that a common antigenic stimulus is involved in MC syndrome and in HCV-associated lymphomagenesis also in patients without an overt autoimmune disease.
Because HCV is the recognized pathologic agent of MC and the CDR3 amino acid sequences of some HCV-associated NHLs also present a high homology for an antibody specific for the E2 protein of HCV, it may be reasonable to speculate that the HCV E2 protein is one of the chronic antigenic stimuli involved both in the lymphomagenetic process and in the production of antibody with RF activity. Further studies are, however, required to directly demonstrate such a possibility.
The 17 patients presented, at the time of NHL diagnosis, a dichotomic clinical behavior: 9 had a history of overt MC syndrome and 8 did not, although serum cryoglobulins were detected in 7 of them. Interestingly, the IgR-VH CDR3 sequencing data seem to offer a plausible molecular interpretation of such differential clinical behavior. The patients with MC syndrome used only the D21 (7 of 9) or D2(2 of 9) gene segments to assemble the IgH chain, but none of the patients without overt MC syndrome used such segments, with the only exception being patient 12. Patient 12, however, had SS, an autoimmune syndrome, which may be complicated by NHL development in patients with consistent levels of serum cryoglobulin.42,43 Such findings suggest that specific D segments may confer to the Ig expressed and possibly secreted by the lymphoma cells (and their precursors) high affinity for antigens involved in the MC syndrome or other cryoglobulinemic-associated autoimmune syndromes. This is in agreement with previous reports suggesting that a discrete-sized D region in WA Xid-positive IgM33 is strictly required for RF activity. Moreover, the D21-9 gene segment is frequently expressed by B-cell clones from myoepithelial sialadenitis lesions occurring in patients with SS,44 B-cell clones from patients with HCV-associated immunocytomas and MC syndrome,15 and B-cell clones producing IgM with RF activity from healthy donors45 than by the totality of VDJ rearrangements occurring in normal individuals.46
In conclusion, our data provide evidence that a large proportion of HCV-associated NHLs derive from B-cell clones chronically stimulated by a common agent. The high homology of the VH and VK IgR chains with antibodies with RF activity, which are mainly found in MC syndrome etiologically related to HCV infection, and with antibodies specific for E2 protein of HCV (particularly evident for the VK chain) suggests a possible involvement of viral antigen(s) (E2) in driving B-cell clonal expansion. Finally, the use of specific D segments in assembling IgR possibly confers on B-cell disorders the property to produce antibodies that may contribute to the development of an overt MC syndrome. In this context, HCV eradication may lead to reduction of the HCV-driven lymphoproliferative expansion, with mitigation of the MC syndrome, if present, and to a decreased probability of NHL occurrence.
Acknowledgment
The authors are grateful to Dr P. Tonel for help with the manuscript.
Supported in part by the Italian Association for Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mauro Boiocchi, Experimental Oncology 1, Centro di Riferimento Oncologico, via Pedemontana Occidentale 12, 33081 Aviano (PN), Italy; e-mail: mboiocchi@ets.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal