Abstract
Stem cell factor (SCF) is a potent costimulatory molecule for many cytokines. Its synergy with granulocyte colony-stimulating factor (G-CSF) results in important biologic and clinical effects, although the mechanism by which this occurs remains poorly understood. To investigate this interaction, this study used a retroviral vector to transduce the G-CSF receptor into MO7e cells, which are known to express the SCF receptor. The transduced G-CSF receptor is functionally active, and the resultant MO7e-G cells recapitulate the proliferative synergy between SCF and G-CSF. When treated with both cytokines, a marked shortening of the G0/G1 phase of the cell cycle occurs, associated with a suppression of the cyclin-dependent kinase inhibitor p27kip-1. In addition, SCF and G-CSF induce the synergistic activation of c-fos, a proto-oncogene involved in propagation of mitogenic signals in hematopoietic cells. G-CSF, but not SCF, induces the tyrosine phosphorylation of STAT1 and STAT3, transcription factors that can mediate the induction of c-fos. However, SCF induces phosphorylation of STAT3 on serine727 (ser727), which is necessary for maximal STAT transcriptional activity, and the combination of SCF and G-CSF leads to complete STAT3 phosphorylation on ser727. The pathways by which SCF and G-CSF lead to serine phosphorylation of STAT3 are distinct and are partially dependent on phosphatidylinositol-3 kinase and ERKs, pathways that are also necessary for the synergistic effects of SCF and G-CSF on proliferation and c-fos induction. Thus, MO7e-G cells provide a powerful system in which the molecular basis of the synergy between SCF and G-CSF can be dissected.
Introduction
Stem cell factor (SCF) is a potent costimulatory growth factor in hematopoiesis.1-3 In combination with cytokines, SCF results in a synergistic enhancement of the proliferation, differentiation, and survival of hematopoietic cells, which is critical for an adequate expansion and development of the hematologic lineages.4-13 In particular, its synergy with granulocyte colony-stimulating factor (G-CSF) has important biologic and clinical implications. G-CSF and SCF are crucial factors in ex vivo long-term culture of human primitive hematopoietic cells.14 Furthermore, the combination of SCF and G-CSF in vivo increases the mobilization of peripheral blood progenitor cells (PBPC) over that seen with G-CSF alone15-17 and enhances the antileukemic effect mediated by these PBPC.18 In addition, several clinical trials have reported that the combination of SCF and G-CSF improves yield of CD34+ cells in patients at risk of poor mobilization.19-21 However, the mechanism by which this biologically and clinically important interaction between SCF and G-CSF occurs remains poorly understood.
The G-CSF receptor is a single-chain member of the cytokine receptor superfamily, which lacks tyrosine kinase activity.22Binding of G-CSF to its receptor induces the tyrosine phosphorylation of a number of cellular proteins and activates signaling cascades including the signal transducer and activator of transcription (STAT) and mitogen-activated protein kinase (MAPK) pathways.23 In contrast to the G-CSF receptor, the receptor for SCF, encoded by the proto-oncogene c-kit,24-26 possesses intrinsic tyrosine kinase activity. Binding of SCF to c-kit induces kinase activation and transphosphorylation of the receptor chains. Phosphotyrosine residues in the receptor then function as docking sites for proteins that link c-kit activation to various signaling pathways.27 28 Despite the biologic and clinical importance of the combination of G-CSF and SCF, no system has existed in which to study the interaction of these receptor pathways. To overcome this impediment, we generated a cell line that recapitulates the biologic synergy between SCF and G-CSF and has allowed analysis of the molecular events that underlie it.
Material and methods
Cytokines, antibodies, and other reagents
Recombinant human (rh) granulocyte-macrophage colony-stimulating factor (GM-CSF) (sargramostin) was obtained from Immunex Corporation (Seattle, WA), rhSCF from Biosource International (Camarillo, CA), and rhG-CSF from Amgen (Thousand Oaks, CA). H7, puromycin, tetracycline, polybrene, and fetal calf serum (FCS) were purchased from Sigma Chemical Company (St Louis, MO). Antibodies to STAT1 (E-23) and STAT3 (C-20) were purchased from Santa Cruz Biochemicals (Santa Cruz, CA). PD98059 and antibodies to ERK1 were purchased from New England Biolabs (Beverly, MA). Phycoerythrin (PE)-conjugated antibody to c-kit and antibodies to the retinoblastoma protein (Rb) and G-CSF receptor were purchased from Becton Dickinson (San Jose, CA), to p27kip-1 from Transduction Laboratories (Lexington, KY), and to p21cip-1 from Upstate Biotechnology (Lake Placid, NY). Antibodies to the tyrosine phosphorylated forms of STAT129 and STAT330 and to the ser727-phosphorylated forms of STAT1 and STAT331 were generated as described. RPMI 1640, Dulbecco modified Eagle medium (DMEM), l-glutamine, HEPES, penicillin/streptomycin, and G418 were purchased from Gibco/BRL-Life Technologies (Rockville, MD). Horseradish peroxidase-conjugated antirabbit and antimouse antibodies were purchased from Calbiochem (La Jolla, CA).
Cells
The human factor–dependent myeloid cell line MO7e was obtained from Dr J. Griffin (Dana-Farber Cancer Institute, Boston, MA).8,32 Cells were maintained in RPMI 1640 supplemented with 20% (vol/vol) heat-inactivated fetal calf serum (FCS) and 10 ng/mL GM-CSF. The human 293-derived retroviral packaging cell line 293GPG was obtained from Dr G. Dranoff (Dana-Farber Cancer Institute). This cell line is capable of producing high titers of recombinant Moloney murine leukemia virus particles that incorporate, in a tetracycline-regulated fashion, the vesicular stomatitis virus G (VSV-G) protein.33 The 293GPG cells were grown in DMEM supplemented with 10% (vol/vol) FCS, 2 mmol/L l-glutamine, 10 mmol/L HEPES, 250 U/mL penicillin and streptomycin, 2 μg/mL puromycin, 0.3 mg/mL G418, and 1 μg/mL tetracycline. All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2, and cell counts were obtained with a Z2 cell counter (Beckman-Coulter, Miami, FL). Flow cytometric analysis was performed using an Epics MCL-XL (Coulter).
Whole cell and nuclear extracts
To prepare whole cell extracts, cells were placed on ice, washed once with ice-cold phosphate-buffered saline (PBS; 137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, pH 7.4), and extracted at 4°C for 30 minutes in lysis buffer (10 mmol/L Tris, pH 8.0, 0.5% NP-40, 250 mmol/L NaCl, 10 mmol/L sodium orthovanadate, 100 μmol/L phenylmethylsulfonyl fluoride [PMSF], 1 μg/mL leupeptin, 1 μg/mL pepstatin, and 1 μg/mL aprotinin). Insoluble material was removed by centrifugation at 12 000g for 5 minutes. To generate nuclear extracts, cells were washed once with ice-cold PBS, then resuspended in 5 mL hypotonic buffer (10 mmol/L Tris, pH 7.4, 10 mmol/L NaCl, and 6 mmol/L MgCl2) and incubated on ice for 5 minutes. The cells were then centrifuged and resuspended in 0.8 mL hypotonic buffer containing 1 mmol/L β-mercaptoethanol, 10 μg/mL PMSF, and 1 mmol/L sodium orthovanadate. Cells were disrupted by shearing in a Dounce homogenizer (type b pestle, 25 strokes), then nuclei were collected by a 10-second centrifugation at 12 000g. The supernatant was removed and the nuclear pellet was washed once with hypotonic buffer, then resuspended in 3 volumes of high-salt buffer (20 mmol/L HEPES, pH 7.9, 420 mmol/L NaCl, 25% glycerol, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 1 mmol/L β-mercaptoethanol, 1 mmol/L sodium orthovanadate, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 μg/mL aprotinin, and 100 μmol/L PMSF), and rocked at 4°C for 30 minutes. Insoluble material and intact nuclei were removed by centrifugation at 12 000g for 3 minutes at 4°C, and the supernatant was recovered and designated the nuclear extract.
Western blotting
Whole cell extracts (100 μg/lane) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and blocked with 5% nonfat dry milk (for anti-STAT, antiphosphotyrosine-STAT, and antiphosphoser727-STAT blots) or 5% bovine serum albumin (BSA) (for other blots) in TBST (100 mmol/L Tris, pH 8.0,150 mmol/L NaCl, and 0.05% Tween-20). The primary antibodies were diluted in TBST containing 3% BSA at a dilution of 1:20 000 (anti-STAT, antiphosphotyrosine-STAT, and antiphosphoser727-STAT) or 1:500 (others) and incubated with the blots for 1 hour at room temperature. After being washed with TBST extensively, membranes were incubated for 1 hour at room temperature with horseradish peroxidase–conjugated secondary antibody diluted 1:20 000 in TBST containing 1% BSA. Membranes were then washed extensively, and bound antibody was detected by chemiluminescence (Renaissance Kit; NEN; Boston, MA). Band intensity was quantitated by densitometry. Membranes were stripped by incubation in 62.5 mmol/L Tris, pH 6.9, containing 2% SDS and 100 mmol/L β-mercaptoethanol for 1 hour at 65°C and then washed in TBST at 4°C overnight.
DNA binding assay
Nuclear extract (2 μL) was mixed with 1 ng32P-labeled oligonucleotide (5′-AGCCTGATTTCCCCAAATGACGGC-3′ and its complement34 in 10 μL binding buffer (25 mmol/L HEPES, pH 7.9, 100 μmol/L EGTA, 200 μmol/L MgCl2, 500 μmol/L dithiothreitol, 1 μg/μL BSA, 0.2 μg/μL poly dI:dC, 1% Ficoll, and 0.1 μg/μL herring testis DNA). The incubation was performed at room temperature for 15 minutes. For antibody competition, antiserum (1:200 dilution) was added at the end of the binding reaction and incubated at 4°C for an additional 15 minutes. The products of the binding reaction were separated on a 4% acrylamide gel in 0.2 × Tris-borate/EDTA, after which the gel was dried and exposed to film.
Semiquantitative reverse transcription–polymerase chain reaction (RT-PCR)
RNA was collected (RNeasy Kit; Qiagen; Valencia, CA) and reverse-transcribed using Superscript IIRT (Life Technologies). PCR to amplify c-fos used primers for a 482-bp fragment (sense: 5′-CTACGAGGCGTCATCCT-3′; antisense: 5′-TCTGTCTCCGCTTGGAGTGTA-3′), and PCR to amplify GAPDH used primers for a 226-bp fragment (Perkin Elmer Applied Biosystems; Branchburg, NJ). Minimal cycle numbers were used to allow quantitative comparisons.
Construction of granulocyte colony-stimulating factor receptor retroviral plasmid
The retroviral vector pMFG was originally constructed in the laboratory of Dr R. C. Mulligan (Harvard Medical School, Boston, MA) and was kindly provided by Dr G. Dranoff. The wild-type hG-CSF receptor complementary DNA (cDNA), a gift from Dr Seth Corey35 (University of Pittsburgh School of Medicine, Pittsburgh, PA), was amplified by PCR using PfuTurbo DNA polymerase (Stratagene; La Jolla, CA) and a set of primers with flanking Xba I and Bgl II restriction site sequences (forward, 5′-ATCCTCTAGACTGCCATGGCAAGGCTGGGAAACTGC-3′; reverse, 5′-TAATCCAGATCTGCCCTAGAAGCTCCCCAGCGCCTC-3′), and then subcloned in frame into pMFG after an intermediate cloning step of the blunt-ended PCR product into the pCR-BluntII-TOPO plasmid (Invitrogen; Carlsbad, CA). Sequence analysis was performed to confirm the fidelity of the pMFG-G-CSF receptor construct.
Retroviral generation and infection of MO7e cells
The pMFG-G-CSF receptor (10 μg) was transfected into 293GPG packaging cells that were at 80% confluence in 10-cm dishes using LipofectAMINE (Gibco/BRL) according to the manufacturer's recommendations. The day after transfection, the media was replaced with fresh media without antibiotics (Tet-Off media). This allowed the expression of the tetracycline-dependent VSV-G gene, and the subsequent release into the supernatant of infectious replication-incompetent retroviruses containing the G-CSF receptor gene. The retrovirus-containing supernatant was collected, filtered, stored at −70°C, and replaced with fresh Tet-Off media for 7 days, after which the supernatants were thawed, pooled, and centrifuged at 50 000g for 90 minutes at 4°C to concentrate the retroviral particles. After centrifugation the supernatant was poured off, and the retroviral pellet was resuspended in 2.5 mL of 10% Hank's balanced salt solution in PBS, aliquoted, and either used fresh for infection of MO7e cells or stored at −70°C. MO7e cells growing in log phase were plated in 4 mL media at 3 × 105cells/mL, incubated overnight with 250 μL pMFG-G-CSF receptor retroviral concentrate in the presence of 8 μg/mL polybrene, and then centrifuged and resuspended in fresh media.
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry after staining DNA with propidium iodide (50 μg/mL) in 0.1% (vol/vol) NP-40 and 0.1% (wt/vol) sodium citrate as described previously.36 Data analysis was performed using Multi-Cycle software (Phoenix Flow, San Diego, CA).
Results
High-efficiency transduction of MO7e cells with granulocyte colony-stimulating factor receptor expressing retrovirus
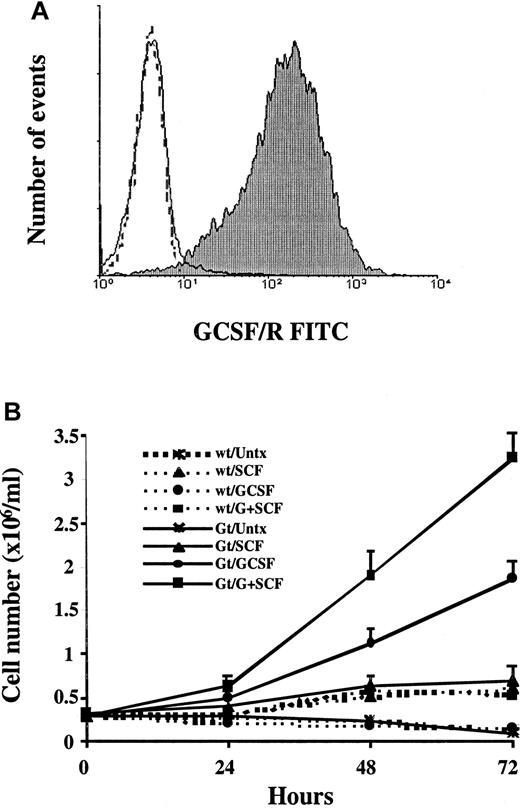
Given the clinical importance of the synergistic interaction between G-CSF and SCF, we generated a model system in which the intracellular signaling events induced by these cytokines could be studied. The hG-CSF receptor was cloned into the retroviral vector pMFG and transduced into MO7e cells, which constitutively express c-kit. After 72 hours, more than 90% of the infected cells were positive for G-CSF receptor surface expression (MO7e-G cells) in 4 independent infection experiments (Figure1A). The level of expression of the G-CSF receptor in MO7e-G cells was comparable to that in neutrophils isolated from the peripheral blood of healthy donors, indicating that expression of the G-CSF receptor was at physiologic levels (data not shown). Given the level of expression achieved by direct transduction, no further selection or purification steps were necessary. The expression of the G-CSF receptor in MO7e-G cells has remained stable for more than 3 months in culture and had no effect on c-kit expression, because 100% of both MO7e-G and MO7e cells expressed c-kitas measured by direct immunostaining with a PE-conjugated antic-kit monoclonal antibody (data not shown).
Transduced MO7e-G cells express the G-CSF receptor and show synergistic proliferation in response to SCF and G-CSF.
(A) Three days after infection, MO7e cells transduced with pMFG-G-CSF receptor retroviral concentrate (MO7e-G cells) were stained with either antihuman G-CSF receptor antibody (filled) or an idiotype control (unfilled), and fluorescein isothiocyanate–conjugated secondary antimouse antibody, and analyzed by flow cytometry. One experiment representative of 4 is presented, in which 94% of the cells express the receptor. Untransfected wild-type MO7e cells did not express the G-CSF receptor (dotted line). (B) Parental MO7e cells and MO7e-G cells were factor starved for 18 hours and then incubated in medium containing no growth factor, 100 ng/mL SCF, 10 ng/mL G-CSF, or both. The data presented represent the means and SD of 4 independent experiments.
Transduced MO7e-G cells express the G-CSF receptor and show synergistic proliferation in response to SCF and G-CSF.
(A) Three days after infection, MO7e cells transduced with pMFG-G-CSF receptor retroviral concentrate (MO7e-G cells) were stained with either antihuman G-CSF receptor antibody (filled) or an idiotype control (unfilled), and fluorescein isothiocyanate–conjugated secondary antimouse antibody, and analyzed by flow cytometry. One experiment representative of 4 is presented, in which 94% of the cells express the receptor. Untransfected wild-type MO7e cells did not express the G-CSF receptor (dotted line). (B) Parental MO7e cells and MO7e-G cells were factor starved for 18 hours and then incubated in medium containing no growth factor, 100 ng/mL SCF, 10 ng/mL G-CSF, or both. The data presented represent the means and SD of 4 independent experiments.
MO7e-G cells recapitulate the biologic synergy between stem cell factor and granulocyte colony-stimulating factor
To validate MO7e-G cells as a model system for the synergy between SCF and G-CSF, we first assessed the biologic characteristics of these cells. Experimental data suggest that the combination of these cytokines enhances myeloid cell proliferation.37 38 To assess the cytokine-dependent growth of these cells, wild-type MO7e and transduced MO7e-G cells were starved for 18 hours and then cultured in the presence or absence of growth factors (Figure 1B). Parental MO7e cells are factor dependent and neither proliferate nor survive without GM-CSF. The expression of the transduced G-CSF receptor enables MO7e-G cells to proliferate in response to G-CSF in the absence of GM-CSF. This was not due to an interaction between G-CSF and the GM-CSF receptor, because wild-type MO7e cells neither proliferated nor survived in response to G-CSF. Moreover, although SCF had a minimal effect on the proliferation of either MO7e or MO7e-G, the combination of SCF and G-CSF led to a greater-than-additive enhancement of proliferation over that seen with G-CSF alone in MO7e-G cells.
Cotreatment of MO7e-G cells with stem cell factor and granulocyte colony-stimulating factor shortens the G1/G0 phase of the cell cycle
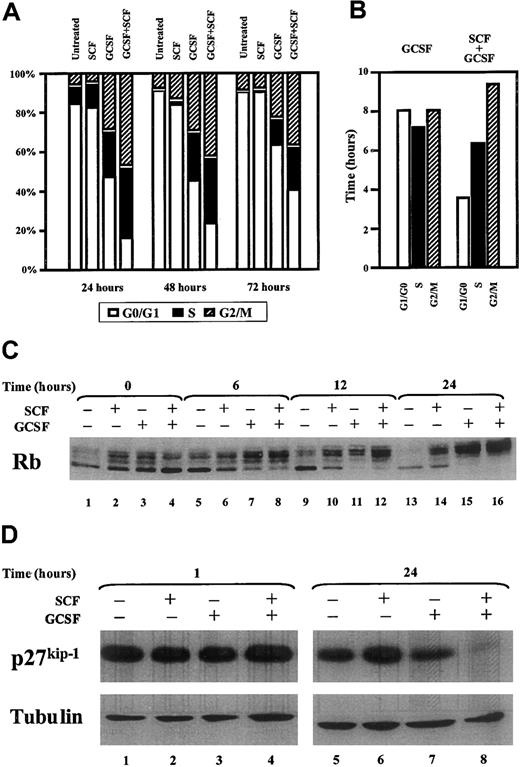
Given the likelihood that the enhanced proliferative state induced by cotreatment with SCF and G-CSF of MO7e-G cells resulted from modifications in cell cycle control, we analyzed the cell cycle distribution induced by these cytokines. Cells were cultured as above, and stained with propidium iodide, after which cell cycle distribution was determined by measuring DNA content with flow cytometry (Figure2A). SCF induced no significant changes in cell cycle distribution compared to untreated samples. G-CSF induced a significant reduction in the percentage of cells in G0/G1 and a parallel increase in the percentages of cells in S and G2/M. However, the combination of G-CSF and SCF led to a significant reduction in the percentage of cells in G1/G0 compared to cells treated with G-CSF alone at all time points (24 hours,P < .01; 48 and 72 hours, P < .05). The duration of each phase was calculated using the doubling times of the cultures and the percentage of cells in each phase of the cell cycle 48 hours after initiation of the culture.39 No significant difference was found in the duration of S and G2/M in cells cultured in G-CSF alone or in combination with SCF. However, a marked shortening of the duration of the G0/G1 was seen in cells cultured with G-CSF and SCF compared to those cultured in G-CSF alone (3.5 versus 8.0 hours; Figure 2B).
Cytokine treatment of MO7e-G cells with G-CSF and SCF leads to a shortening of the G1/G0 phase of the cell cycle and a decrease in p27kip-1.
MO7e-G cells were starved overnight and then cultured with cytokines as indicated. At each time point, cells were stained with propidium iodide, and DNA content was determined by flow cytometry. (A) The percentage of cells in each phase of the cell cycle was determined (1 representative experiment of 4 is presented). (B) The duration of each phase was derived from the cell cycle distribution at 48 hours (steady-state exponential growth) and the doubling time of the population. (C,D) MO7e-G cells were cultured as described for the indicated times, after which whole cell extracts were prepared and immunoblots were performed to Rb, p27kip-1, and tubulin.
Cytokine treatment of MO7e-G cells with G-CSF and SCF leads to a shortening of the G1/G0 phase of the cell cycle and a decrease in p27kip-1.
MO7e-G cells were starved overnight and then cultured with cytokines as indicated. At each time point, cells were stained with propidium iodide, and DNA content was determined by flow cytometry. (A) The percentage of cells in each phase of the cell cycle was determined (1 representative experiment of 4 is presented). (B) The duration of each phase was derived from the cell cycle distribution at 48 hours (steady-state exponential growth) and the doubling time of the population. (C,D) MO7e-G cells were cultured as described for the indicated times, after which whole cell extracts were prepared and immunoblots were performed to Rb, p27kip-1, and tubulin.
Stem cell factor/granulocyte colony-stimulating factor–induced shortening of G0/G1 is associated with a loss of expression of the cyclin-dependent kinase inhibitor p27kip-1
Because the proliferative synergy between SCF and G-CSF is mediated through a shortening of G0/G1, we investigated the effect of G-CSF and SCF cotreatment on several key regulatory components of the G1-S transition. Rb, a critical mediator of the progression from G1 to S phase,40,41 is modulated through phosphorylation by cyclin-dependent kinases (cdks).42 The regulation of the activity of these cdks is critical for progression through the cell cycle.43 In particular, cdk inhibitors (cdkis) such as p27kip-1 or p21cip-1 block cdk activity and prevent progression from G1 to S phase.44-46To analyze the effect of SCF and G-CSF on Rb phosphorylation, MO7e-G cells were starved and then cultured in the presence or absence of cytokines. Both SCF and G-CSF induced hyperphosphorylation of Rb, as measured by decreased gel mobility of Rb as seen in a Western blot (Figure 2C). However, the hyperphosphorylation induced by SCF alone was not complete at any of the time points, because the fastest-migrating hypophosphorylated band was always present. By contrast, the combination of SCF and G-CSF, or G-CSF alone, induced complete hyperphosphorylation of Rb. To investigate the role that cdkis play in this cytokine-induced Rb-phosphorylation, p27kip-1 levels were analyzed by Western blot (Figure 2D). SCF alone did not induce a decrease in p27kip-1 protein level (lanes 1, 2, 5, and 6). Although G-CSF induced a 22% decrease of p27kip-1 protein level at 24 hours compared to untreated cells (lane 7), the combination of SCF and G-CSF induced a 90% reduction in p27kip-1protein level (lane 8). No differences were found in the expression of p21cip-1 among the different treatments at any time point (data not shown). Thus, the combination of G-CSF and SCF leads to specific suppression of p27kip-1 in MO7e-G cells.
Costimulation of MO7e-G cells with stem cell factor and granuloctye colony-stimulating factor leads to an increased expression of c-fos
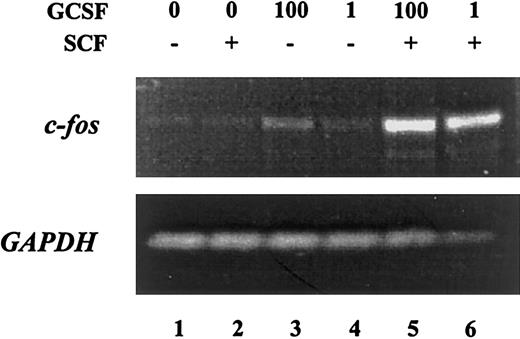
Because cellular function is dictated by the pattern of gene expression, we analyzed the activation of c-fos, a proto-oncogene involved in cell cycle progression47 48 in response to SCF and G-CSF. MO7e-G cells were starved overnight and then treated with SCF or G-CSF alone or in combination for 30 minutes. Total RNA was harvested and analyzed by semiquantitative RT-PCR (Figure 3). Although SCF was unable to induce c-fos expression, G-CSF at high concentrations (1 ng/mL) but not at low concentrations induced modest expression of this proto-oncogene. Costimulation of MO7e-G cells with SCF and G-CSF led to a marked increase in the expression of c-fos compared with cells treated with G-CSF alone. Thus, SCF and G-CSF, which show synergistic effects in inducing cellular proliferation, also lead to marked enhancement in inducing the expression of a proto-oncogene involved in cell cycle progression.
Treatment of MO7e-G cells with the combination of G-CSF and SCF leads to synergistic induction of c-
fos. MO7e-G cells were factor starved overnight, then treated for 30 minutes with SCF (100 ng/mL), G-CSF (1 or 100 ng/mL) or both. RNA was harvested and analyzed by semiquantitative RT-PCR with primers specific for c-fos (upper) andGAPDH (lower).
Treatment of MO7e-G cells with the combination of G-CSF and SCF leads to synergistic induction of c-
fos. MO7e-G cells were factor starved overnight, then treated for 30 minutes with SCF (100 ng/mL), G-CSF (1 or 100 ng/mL) or both. RNA was harvested and analyzed by semiquantitative RT-PCR with primers specific for c-fos (upper) andGAPDH (lower).
Granulocyte colony-stimulating factor–induced activation of STAT1 and STAT3 is unaffected by stem cell factor
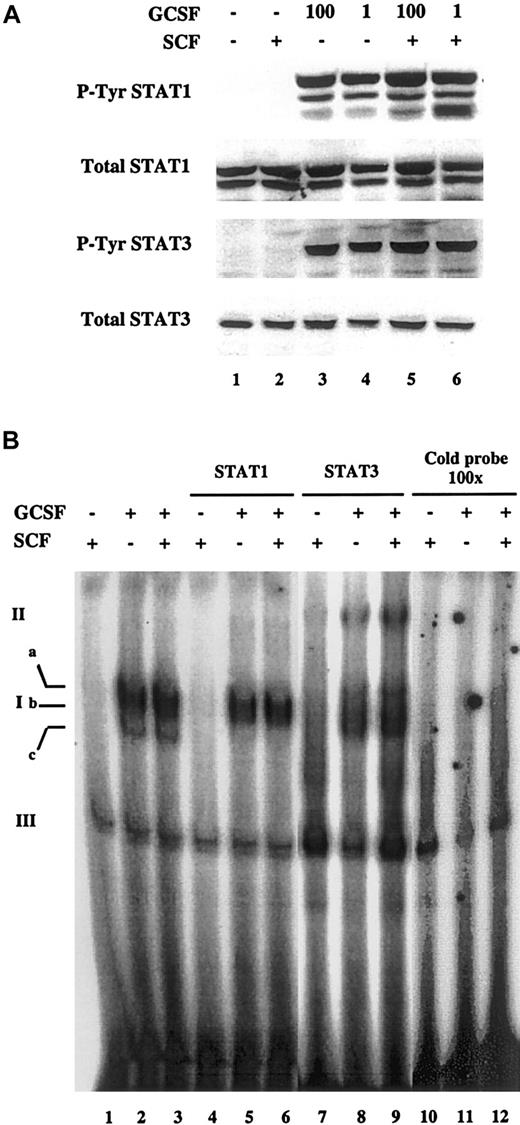
The enhanced activation of c-fos by cotreatment with SCF and G-CSF suggested that one or more signaling pathways activated by these factors was amplified when the cytokines were administered together.23,27,28 In particular, STATs are known to mediate the effects of many cytokines in hematopoietic cells, including G-CSF,49-52 and have been found to control cellular proliferation by regulating p27kip-1expression.53 Therefore, we explored the possibility that enhanced STAT activation mediated the synergistic effects of SCF and G-CSF on proliferation, p27kip-1 regulation, and c-fos induction. To analyze this, MO7e-G cells were factor starved overnight and treated with SCF, G-CSF, or their combination. Whole cell extracts were obtained and Western blots were performed to detect the activated tyrosine-phosphorylated forms of STAT1 and STAT3. G-CSF alone led to phosphorylation of STAT1 and STAT3 (Figure4A; lanes 3 and 4), whereas SCF did not induce any phosphorylation of these STATs (lane 2). The addition of SCF to G-CSF failed to induce any detectable change in the level of tyrosine phosphorylation of either STAT1 or STAT3 compared to that induced by G-CSF alone (lanes 5 and 6). This suggests that the synergy between SCF and G-CSF is not mediated at the level of STAT tyrosine phosphorylation. This is further supported by the finding that SCF did not enhance the phosphorylation of JAK1 and JAK2, the tyrosine kinases activated by G-CSF that mediate STAT tyrosine phosphorylation (data not shown).
Tyrosine phosphorylation and DNA binding of STAT1 and STAT3 induced by G-CSF is not affected by SCF.
MO7e-G cells were factor starved overnight, then treated for 15 minutes with SCF (100 ng/mL), G-CSF (1 or 100 ng/mL), or both. (A) Total cell extracts were prepared and Western blots were performed to detect total and tyrosine-phosphorylated forms of STAT1 and STAT3. The same membrane was stripped and reprobed for each blot. (B) Nuclear extracts were prepared and binding was assessed to a 32P-labeled double-stranded oligonucleotide containing a STAT-binding high-affinity sequence. G-CSF was used at a concentration of 1 ng/mL. Ia, Ib, and Ic indicate specific STAT-DNA complexes; II, “super-shifted” complexes; III, nonspecific complexes.
Tyrosine phosphorylation and DNA binding of STAT1 and STAT3 induced by G-CSF is not affected by SCF.
MO7e-G cells were factor starved overnight, then treated for 15 minutes with SCF (100 ng/mL), G-CSF (1 or 100 ng/mL), or both. (A) Total cell extracts were prepared and Western blots were performed to detect total and tyrosine-phosphorylated forms of STAT1 and STAT3. The same membrane was stripped and reprobed for each blot. (B) Nuclear extracts were prepared and binding was assessed to a 32P-labeled double-stranded oligonucleotide containing a STAT-binding high-affinity sequence. G-CSF was used at a concentration of 1 ng/mL. Ia, Ib, and Ic indicate specific STAT-DNA complexes; II, “super-shifted” complexes; III, nonspecific complexes.
Although SCF did not enhance the tyrosine phosphorylation of STAT1 and STAT3 induced by G-CSF, we considered the possibility that SCF might amplify the actions of G-CSF by increasing STAT translocation or binding to DNA. To address this possibility, nuclear extracts were prepared from cells treated with SCF and G-CSF alone and in combination. The nuclear extracts were incubated with32P-double-stranded oligonucleotide containing a high-affinity STAT-binding sequence,34 and protein-DNA complexes were analyzed in an electrophoretic mobility shift assay (Figure 4B). G-CSF treatment led to the induction of 3 protein-DNA complexes (lane 2; complexes Ia, Ib, and Ic) whose specificity was demonstrated by their abolition by inclusion of a 100-fold excess of unlabeled probe in the binding reaction (lane 11). By contrast, a higher mobility nonspecific complex (complex III) was unaffected by excess unlabeled probe. That these complexes contain STAT1 and STAT3 was demonstrated by including antibodies to each of these proteins in the binding reaction. Antibody to STAT1 abolished the high-mobility complex Ic, and antibody to STAT3 diminished complex Ib while inducing formation of a low-mobility band (complex II, lane 8). It is likely that other proteins are participating in the formation of at least some of these complexes because antibodies to STAT1 and STAT3 do not completely abolish all of the protein-DNA complexes. However, antibodies to other STATs did not affect these complexes (data not shown). In contrast to G-CSF, SCF failed to induce formation of any specific protein-DNA complexes (lane 1), consistent with its inability to induce tyrosine phosphorylation of STAT1 or STAT3. Cotreatment with SCF and G-CSF did not lead to any qualitative or quantitative differences in the protein-DNA complexes induced compared to those seen with G-CSF alone (lane 3). Thus, the synergy between SCF and G-CSF is not mediated by increased STAT tyrosine phosphorylation or DNA binding.
Stem cell factor enhances ser727 phosphorylation of STAT3
The STAT transcription factors can be phosphorylated not only on tyrosine residues, but also on specific serine residues within their transactivation domain (ser727 in STAT1 and STAT3). Although tyrosine phosphorylation directly activates a STAT, ser727 phosphorylation increases the magnitude of the gene transcription mediated by the tyrosine-phosphorylated STATs and is necessary for maximal transcriptional activity.54 Thus, we tested the possibility that SCF enhanced STAT-dependent gene activation by enhancing phosphorylation of the ser727 residue. MO7e-G cells were factor starved and treated with SCF and G-CSF alone and in combination. Whole cell extracts were prepared and immunoblots were performed with antibodies specific for the tyrosine-phosphorylated and the ser727-phosphorylated form of STAT3 (Figure5A). Treatment of MO7e-G cells with SCF led to the prominent phosphorylation of STAT3 on ser727, but not on the activating tyrosine705 (lane 2). A Western blot using an antibody that recognizes all forms of STAT3 revealed that ser727-phosphorylated STAT3 migrated slightly slower than the unphosphorylated form (total-STAT3 blot; lane 2). G-CSF treatment led to both tyrosine and ser727 phosphorylation of STAT3, which added a third band in the pattern of migration of STAT3 (lane 3). Combined treatment of MO7e-G cells with SCF and G-CSF induced an increase in ser727 phosphorylation of STAT3 (lane 4), which was greater than that induced by SCF or G-CSF alone. In 4 independent experiments, the phosphorylation of STAT3 on ser727 induced by the combination was 54% greater than that induced by SCF alone and 67% greater than that induced by G-CSF alone. Cotreatment with SCF and G-CSF also abolished the fastest-migrating unphosphorylated STAT3 band, which neither G-CSF nor SCF did alone (total-STAT3 blot; lanes 1-4). SCF had no effect on the phosphorylation of STAT1 on ser727 (data not shown).
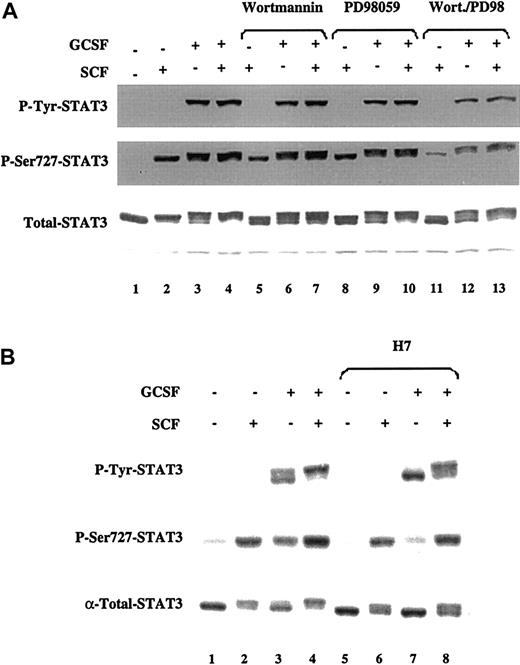
SCF and G-CSF induce ser727 phosphorylation of STAT3 through activation of distinct serine/threonine kinases.
MO7e-G cells were factor starved for 18 hours and then either left untreated or treated with SCF (100 ng/mL), G-CSF (10 ng/mL), or both. Where indicated, cells were incubated for 1 hour prior to cytokine treatment with PD98059 (100 μmol/L), wortmannin (500 nmol/L), or H7 (50 μmol/L). Western blots were performed with the indicated antibodies. The same membrane was stripped and reprobed for each blot.
SCF and G-CSF induce ser727 phosphorylation of STAT3 through activation of distinct serine/threonine kinases.
MO7e-G cells were factor starved for 18 hours and then either left untreated or treated with SCF (100 ng/mL), G-CSF (10 ng/mL), or both. Where indicated, cells were incubated for 1 hour prior to cytokine treatment with PD98059 (100 μmol/L), wortmannin (500 nmol/L), or H7 (50 μmol/L). Western blots were performed with the indicated antibodies. The same membrane was stripped and reprobed for each blot.
Stem cell factor–induced ser727 phosphorylation of STAT3 is partially mediated by PI3 kinase and mitogen-activated protein kinase
Ser727 of STAT3 is contained in the sequence leu-pro-met-ser-pro, a motif found in substrates of proline-directed serine/threonine kinases.55 56 However, the identity of the serine/threonine kinase(s) that phosphorylate this conserved carboxy-terminus serine in STAT family members has remained elusive. To determine the upstream signaling events necessary for the serine phosphorylation of STAT3 induced by these cytokines, MO7e-G cells were factor starved and then incubated for 1 hour with inhibitors of PI3K (wortmannin), MEK1/ERK (PD98059), or p38-MAPK (SB203580). The cells were then treated with SCF and G-CSF alone and in combination. Both wortmannin and PD98059 induced a modest reduction in the level of ser727 phosphorylation of STAT3 induced by SCF, G-CSF, or SCF and G-CSF (Figure 5A). However, the combination of wortmannin and PD98059 led to a much greater inhibition of this phosphorylation for all stimuli. These data suggest that both PI3K and ERK-MAPK pathways are involved in ser727 phosphorylation of STAT3 in response to SCF and G-CSF.
Although it has been suggested that ERKs can phosphorylate STAT serine residues, the fact that PD98059 completely suppresses ERK activity (data not shown) without abolishing ser727 phosphorylation of STAT3 indicates that other kinases must be involved. Several lines of evidence indicate that p38-MAPK can mediate ser727 phosphorylation of STATs.57 58 Inhibition of p38-MAPK with 20 μmol/L SB203580 for 1 hour did not affect the induction of ser727 phosphorylation of STAT3 by SCF and G-CSF, either alone or in combination (data not shown), indicating that this kinase is not involved in ser727 phosphorylation of STAT3 in response to these cytokines.
To further examine the kinase(s) mediating the phosphorylation of STAT3 on ser727, MO7e-G cells were incubated with the serine/threonine kinase inhibitor H7,31 and then left untreated or treated with SCF and G-CSF alone or in combination. Incubation of cells with H7 for 1 hour prior to cytokine treatment led to a marked reduction in the phosphorylation of ser727-STAT3 by G-CSF (Figure 5B, lanes 3 and 7). However, H7 had a minimal effect on the induction of phosphorylation of ser727-STAT3 by SCF (lanes 2 and 6) or by the combination of both cytokines (lanes 4 and 8). These data suggest that SCF and G-CSF induce ser727 phosphorylation of STAT3 through different pathways.
Gene activation and proliferation of MO7e-G cells in response to cotreatment with stem cell factor and granulocyte colony-stimulating factor are dependent on PI3K and ERK
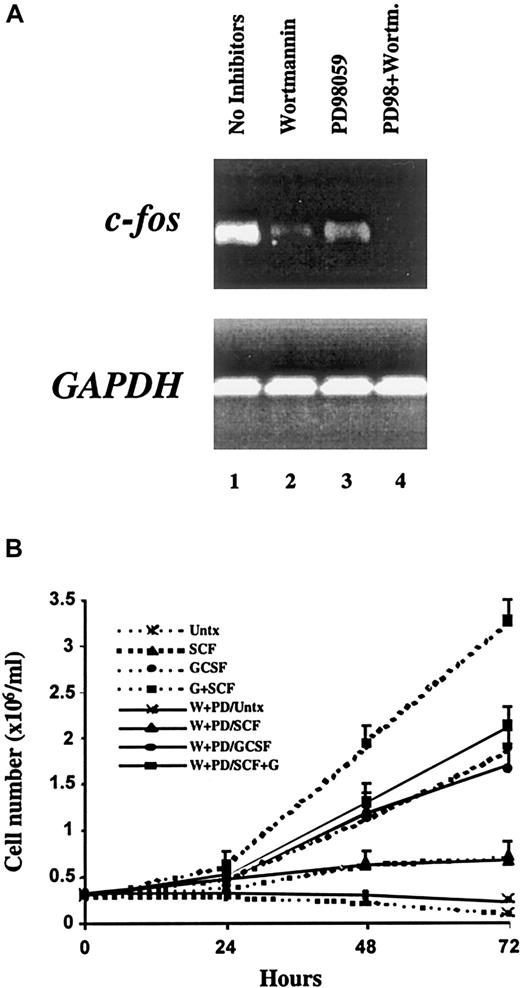
Given that PI3K and ERK-dependent pathways are necessary for the synergistic phosphorylation of STAT3 on ser727 in response to G-CSF and SCF, we directly tested whether inhibition of these pathways would alter gene activation or proliferation induced by this combination of cytokines. To examine the induction of the c-fosproto-oncogene, semiquantitative RT-PCR was performed on total RNA from MO7e-G cells cotreated with SCF and G-CSF for 30 minutes with or without previous incubation with wortmannin, PD98059, or the combination thereof. Treatment with either wortmannin or PD98059 partially reduced the expression of c-fos elicited by the combination of SCF and G-CSF. However, simultaneous inhibition of PI3K and ERK led to a complete abolition of this synergistic effect (Figure6A). Thus, both of these signaling pathways contribute not only to ser727 phosphorylation of STAT3, but also to the activation of a key target gene.
Proliferation and c-
fos activation induced by G-CSF and SCF require PI3K and ERK activity. (A) MO7e-G cells were factor starved overnight, incubated with the indicated compounds, then treated for 30 minutes with SCF (100 ng/mL) and G-CSF (10 ng/mL). RNA was extracted and analyzed by semiquantitative RT-PCR with primers specific for c-fos (upper) and GAPDH (lower). (B) MO7e-G cells were factor starved for 18 hours, incubated with or without the combination of PD98059 (P) and wortmannin (W), then cultured in the presence of no growth factor, SCF (100 ng/mL), G-CSF (10 ng/mL), or both. Cell counts were obtained daily and are presented as the means and SD of 4 independent experiments.
Proliferation and c-
fos activation induced by G-CSF and SCF require PI3K and ERK activity. (A) MO7e-G cells were factor starved overnight, incubated with the indicated compounds, then treated for 30 minutes with SCF (100 ng/mL) and G-CSF (10 ng/mL). RNA was extracted and analyzed by semiquantitative RT-PCR with primers specific for c-fos (upper) and GAPDH (lower). (B) MO7e-G cells were factor starved for 18 hours, incubated with or without the combination of PD98059 (P) and wortmannin (W), then cultured in the presence of no growth factor, SCF (100 ng/mL), G-CSF (10 ng/mL), or both. Cell counts were obtained daily and are presented as the means and SD of 4 independent experiments.
To examine whether these pathways contribute to the synergistic effects on proliferation induced by SCF and G-CSF, MO7e-G cells were incubated in the presence or absence of wortmannin and PD98059, and population growth in response to SCF and G-CSF, alone and in combination, was assessed (Figure 6B). The enhanced growth seen with the combination of SCF and G-CSF was lost in the presence of these inhibitors, and population growth was reduced to that seen with G-CSF alone. Furthermore, the growth of cells cultured in G-CSF alone was not reduced further by these inhibitors. These findings indicate that the added component of growth provided by SCF requires these signaling pathways, whereas growth in the presence of G-CSF alone, or the minimal growth seen with SCF alone, is independent of PI3K and ERKs.
Discussion
Although the synergy between SCF and G-CSF is of great biologic and clinical importance, the absence of a model system to explore this interaction at the cellular level has impaired the ability to understand its mechanism. In this study, we have developed a system to examine the intracellular events induced by these molecules alone and in combination by using retroviral transduction to introduce the hG-CSF receptor into the SCF-responsive human hematologic cell line, MO7e. This has demonstrated that the use of VSV-G glycoprotein-pseudotyped retroviruses is a feasible approach toward high efficiency and stable transduction of this human acute megakaryocytic leukemia-derived cell line. This may be an important strategy for the introduction of genes into other human hematologic cells and cell lines, which are difficult to transfect by other means.
The transduced G-CSF receptor is functionally active, in that it not only supports G-CSF–dependent proliferation of MO7e-G cells, but also recapitulates the proliferative synergy between SCF and G-CSF (Figure1B). Cell cycle analysis revealed that the enhanced proliferative state induced by SCF and G-CSF cotreatment was associated with a direct effect of these cytokines on cell cycle distribution, specifically a marked shortening of the duration of G0/G1. This is mediated, at least in part, by a marked decrease of expression of the cdki p27kip-1, which is known to set a stoichiometric inhibitory threshold of cdk activity that prevents cdk-induced phosphorylation of Rb and cell cycle progression.45 59 Therefore, the loss of p27kip-1 following treatment with SCF and G-CSF may facilitate cell proliferation.
Mitogenic cytokines induce the activation of immediate-early genes, which are necessary for cellular proliferation. These genes are regulated by transcription factors that are activated by cytokine-induced signaling pathways. SCF and G-CSF, which lead to a marked enhancement of proliferation in MO7e-G cells, also lead to synergistic induction of c-fos, a proto-oncogene necessary for cell cycle progression in many systems. The promoter for c-fos contains elements responsive to a number of transcription factors. Among these, we focused on STATs, which are known to be key mediators of cytokine signaling in hematopoietic cells,49-52 and have been shown to control cellular proliferation by regulating p27kip-1expression.53 As has been reported in other systems, we found that G-CSF induces tyrosine phosphorylation of STAT1 and STAT3 and their subsequent binding to specific DNA elements.60-65 By contrast, SCF failed to induce activation of these STATs in MO7e-G cells. The ability of SCF to activate STATs remains controversial.66-68 In MO7e cells, SCF has been shown to induce activation of STAT1 in some reports,69,70 but not in others.71,72 SCF has not been found to activate STAT3 in any cell type, including MO7e.71 No evidence was found that the synergy between SCF and G-CSF occurs at the level of STAT1 or STAT3 tyrosine phosphorylation, nuclear translocation, or DNA binding (Figure 4).
Although tyrosine phosphorylation is required for STAT dimerization and nuclear translocation, the magnitude of STAT1- or STAT3-mediated (or both) gene induction is modulated by phosphorylation on ser727 within the transactivation domain of both STATs.73,74 We found that SCF induces ser727 phosphorylation of STAT3 in MO7e-G cells, as has been reported by others.71 Furthermore, the combination of SCF and G-CSF maximized the induction of ser727 phosphorylation of STAT3. In fact, all of the STAT3 detectable by Western blot is completely phosphorylated on this residue after treatment with SCF and G-CSF, whereas neither G-CSF nor SCF alone induce this complete shift to the slow-migrating form of STAT3 (Figure 5A). Ser727 is located within a proline-directed serine-threonine kinase consensus site,55,56 which can be phosphorylated by MAPK family members including ERKs75-79 and p38MAPK.57,58,80 81 We found that both PI3K and ERK are upstream of ser727 phosphorylation of STAT3 in MO7e-G cells, but that SCF and G-CSF induce phosphorylation of this residue through different pathways. SCF-induced phosphorylation of STAT3 on ser727 is insensitive to H7, whereas G-CSF is highly sensitive to this kinase inhibitor. Moreover, the fact that complete ERK inhibition does not fully block STAT3 ser727 phosphorylation is additional evidence that even if ERK can phosphorylate ser727, other kinases must be involved in this phosphorylation.
Because PI3K and ERK-dependent pathways are necessary for the synergistic phosphorylation of STAT3 on ser727, we explored whether inhibition of these pathways would affect gene activation or proliferation elicited by cotreatment with SCF and G-CSF. Simultaneous inhibition of these 2 pathways led to complete abolition of the synergistic induction of c-fos. Furthermore, the proliferative synergy between SCF and G-CSF was also completely dependent on PI3K and ERK, because the growth of MO7e-G cells in response to SCF and G-CSF was reduced to that seen with G-CSF alone in the presence of inhibitors of these pathways (Figure 6B).
We have described a novel system to explore the signaling events underlying the important biologic interaction between SCF and G-CSF. MO7e-G cells recapitulate the proliferative synergy between SCF and G-CSF and have allowed us to identify a number of intracellular pathways that may mediate their biologic actions. It remains to be determined whether other pathways play a role in the biologic and clinical effects of SCF and G-CSF.
Acknowledgments
We thank Drs J. Griffin, G. Dranoff, V. Boussiotis, and S. Corey for generously sharing reagents, and J. Daley and S. Lazo for assistance with flow cytometry.
Department of Adult Oncology, Dana-Farber Cancer Institute; and Departments of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA.
Submitted May 24, 2000; accepted July 26, 2000.
Supported by a grant from the NIH (CA79547) and the Brent Leahey Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David A. Frank, Department of Adult Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: david_frank@dfci.harvard.edu

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal