Abstract
To isolate soluble factors expressed in early phases of hematopoietic differentiation, we applied the signal sequence trap method to the in vitro murine hematopoietic differentiation system, in which ES cells are cocultured with OP-9 stroma cells. This strategy allowed us to isolate cDNA for a secreted protein, ESOP-1, of 160 amino acids, the sequence of which shows 64% identity with human ESOP-1/MD-2. ESOP-1 mRNA was highly expressed in the mouse embryos at 7.5 days after coitus. Expression of the ESOP-1 mRNA and protein was shown in the embryonic and adult hematopoietic system. In addition, the ESOP-1 protein was found in the yolk sac–blood islands, the developing nervous system, and the adult reproductive system. These results suggest that ESOP-1 may play some roles in the development or maintenance of hematopoietic, nervous, and reproductive systems.
To understand the complex molecular mechanisms for mesoderm induction and early hematopoiesis of mouse embryos, several attempts were performed, and some genes were isolated,1-3 from in vitro differentiation systems that form embryoid bodies and generate hematopoietic cells from embryonic stem (ES) cells. More recently, the in vitro system to yield hematopoietic cells was further refined to avoid the formation of embryoid bodies by coculturing ES cells with the OP9 stromal cell line4 or by plating ES cells on collagen IV plates with the enrichment of differentiated cells by cell sorting.5Such techniques allowed the identification and characterization of the hemangioblasts, the common precursor of both endothelial cells and hematopoietic cells.6,7 In addition, several membrane receptors and their ligands have been shown to be involved in the regulation of hematopoietic stem cell differentiation. The best examples are c-Kit and its ligand stem cell factor (SCF), Flk-1/vascular endothelial growth factor (VEGF),8,9 and Tie2/angiopoietin-1.10 11
Because these factors are too limited to explain the complex regulation of the in vivo hematopoietic pathway(s), it is likely that there are other soluble factors involved in the early phases of hematopoiesis.
By combining the ES-OP9 hematopoietic differentiation system4 and the signal sequence trap method,13we attempted to isolate cDNA for secreted proteins and membrane proteins expressed when the hematopoietic progenitor cells appear, and we isolated a candidate cDNA.14 Here we report the structure and expression of the cDNA-encoded secreted protein, named ESOP-1 (ES-OP9 coculture clone-1).
Study design
Isolation of ESOP-1 cDNA
As described previously,4 mRNA was extracted from 5-day differentiation-inducted ES cells. A signal sequence trap cDNA library was constructed as described,13 with modifications described below. First-strand cDNA was synthesized with a random nonamer-SalI-linker primer as follows.
5′-GAGACGGTAATACGATCGACAGTAGGTCGACNNNNNNN- NN-3′. The second strand was synthesized according to Gublerr and Hoffman's method. A double-stranded EcoRI adaptor was ligated to both ends of the double-stranded cDNA, as follows:
5′-CCGCGAATTCTGACTAACTGATT-3′
3′-CAGGCGCTTAAGACTGATTGACTAA-5′
After the cDNA was size-fractionated from 400 to 600 bp in length, 25 cycles of polymerase chain reaction (95°C for 30 seconds, 55°C for 1 minute, 72°C for 1 minute) was performed with the primers 5′-CCGCGAATTCTGACTAACTGATT-3′ and 5′-GACGGTAATACGATCGACAGTAGG-3′. To obtain the full-length ESOP-1 cDNA, the 3′ RACE system (GIBCO-BRL) was used.
Anti-mESOP-1 antibody preparation
Two polypeptides, ESEKQQWFCNSSD and AEAIAGDTEEKLF, were synthesized and used as antigens for production of the antibodies AK612 and AK641, respectively (Biologica, Nagoya, Japan). Antibodies were then affinity purified with each polypeptide.
Whole-mount immunohistochemistry
Specimens were fixed with 2% paraformaldehyde. Immunostaining was performed on whole embryos or after they were sliced into 0.5-mm thick sections, and were visualized using 0.025%p-dimethylaminoazobenzene (Dojin, Tokyo, Japan), 0.08% NiCl2.6H2O, and 0.0075% H2O2.
Results and discussion
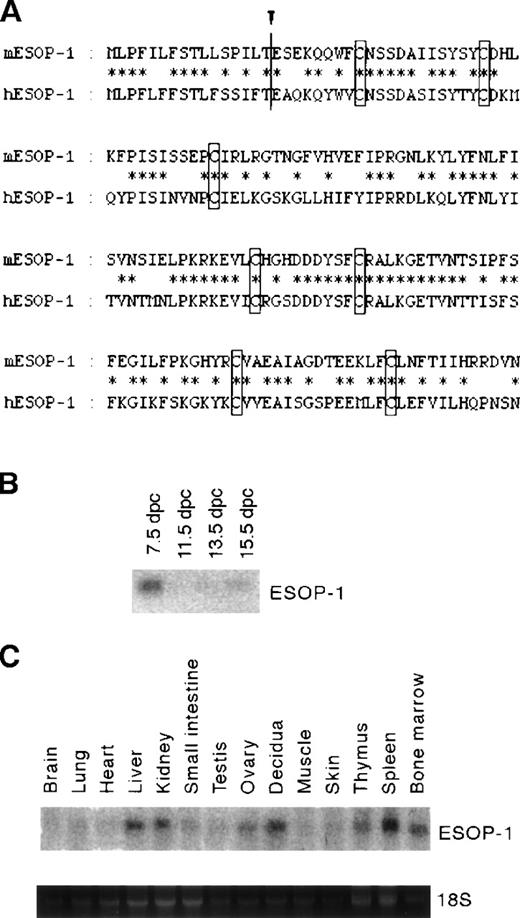
Murine ESOP-1 (mESOP-1) cDNA encodes a protein of 160 residues with an N-terminal hydrophobic region (16 residues) that was predicted to be the signal sequence for secretion.15 In fact Western blot analysis showed that 5% to 10% of the total ESOP-1 produced was secreted into the culture supernatant of 293T cells transfected by ESOP-1 cDNA (data not shown). The human ESOP-1 (hESOP-1) cDNA was also isolated using mESOP-1 cDNA as probe14; hESOP-1 is composed of 160 residues and is 64% identical to mESOP-1 (Figure1A). A database search indicated that hESOP-1 is identical to MD-2.16
Alignment of mouse and human ESOP-1 and mESOP-1 expression.
(A) Alignment of amino acid sequences of murine and human ESOP-1. Identical residues are indicated by asterisks. Putative cleavage site by the signal peptidase is indicated by an arrowhead.17 The 7 cysteine residues conserved are boxed. (B) mESOP-1 mRNA expression during mouse embryogenesis. Clontech mouse embryo multiple-tissue Northern blot was probed with mESOP-1 cDNA. (C) mESOP-1 expression among adult mouse organs; 20 μg total RNA was isolated from mouse tissues and blotted onto the nylon membrane, then hybridized with labeled mESOP-1 cDNA. The expression of mESOP-1 mRNA in adult tissues was detected strongly in spleen, bone marrow, decidua of 8.5 dpc, liver, kidney, and weakly in thymus, ovary, testis, small intestine, and skin.
Alignment of mouse and human ESOP-1 and mESOP-1 expression.
(A) Alignment of amino acid sequences of murine and human ESOP-1. Identical residues are indicated by asterisks. Putative cleavage site by the signal peptidase is indicated by an arrowhead.17 The 7 cysteine residues conserved are boxed. (B) mESOP-1 mRNA expression during mouse embryogenesis. Clontech mouse embryo multiple-tissue Northern blot was probed with mESOP-1 cDNA. (C) mESOP-1 expression among adult mouse organs; 20 μg total RNA was isolated from mouse tissues and blotted onto the nylon membrane, then hybridized with labeled mESOP-1 cDNA. The expression of mESOP-1 mRNA in adult tissues was detected strongly in spleen, bone marrow, decidua of 8.5 dpc, liver, kidney, and weakly in thymus, ovary, testis, small intestine, and skin.
By Northern blot analysis, mESOP-1 mRNA expression was induced 5 days after the differentiation induction of ES cells in vitro (data not shown). During embryogenesis, mESOP-1 mRNA expression was detected strongly on 7.5 dpc embryos (Figure 1B). The expression of mESOP-1 mRNA in adult tissues was detected in hematolymphoid tissues (spleen, bone marrow, and thymus), reproductive organs (ovary, decidua of 8.5 dpc, and testis), liver, kidney, small intestine, and skin (Figure1C) at variable expression levels.
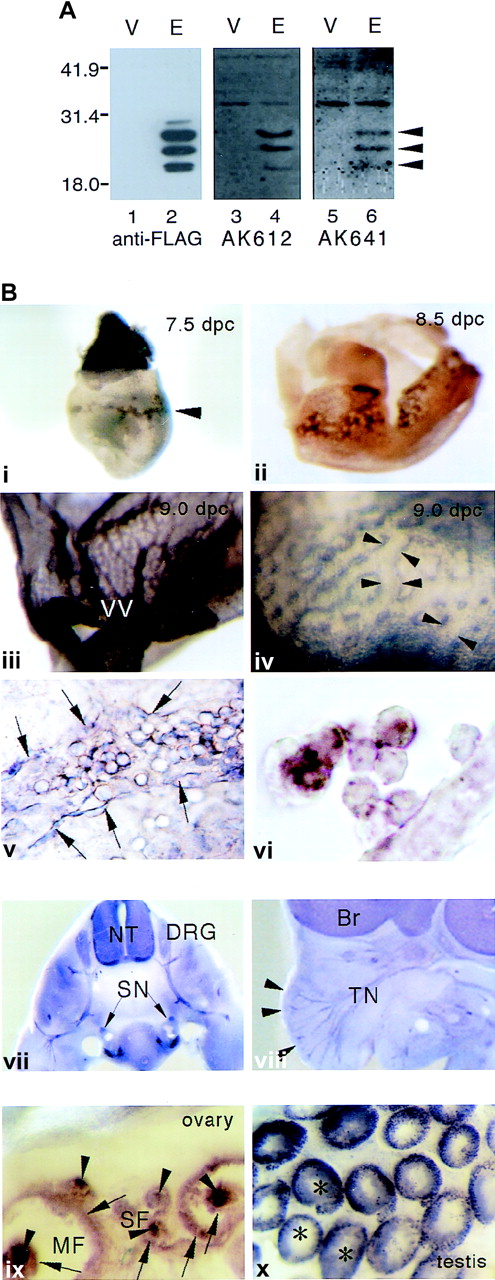
To know the distribution of mESOP-1 during embryonic development, whole-mount immunohistochemical analysis was performed. Both anti-mESOP-1 polyclonal antibodies, AK612 and AK641, always gave the same results in Western blot and immunostaining analyses (Figure2A,B). In 7.5 dpc embryos, mESOP-1 was expressed in the yolk sac–blood islands (Figure 2Bi). Expression of mESOP-1 was seen in the spreading vascular network derived from blood islands (Figure 2Bii, 2Biii). By high-power magnification of the 9.0 dpc yolk sac, endothelial cells of the vascular plexus was found to be ESOP-1 positive (Figure 2Biv). Flat-mount view of the 9.0 dpc yolk sac and a cross-section of 8.5 dpc yolk sac showed that mESOP-1 was expressed in blood cells and endothelial cells (Figure 2Bv, 2Bvi). This immunostaining pattern in the yolk sac partially overlapped those of c-Kit, Flk-1, and Tie2.12 17 Because ESOP-1 was expressed in both blood and endothelial cells in blood islands, ESOP-1 may be also expressed in their common progenitor cells, hemangioblasts, and may regulate their differentiation. These remain to be tested.
Anti-mESOP-1 antibodies and mESOP-1 protein expression.
(A) Analysis of the specificity of anti-mESOP-1 antibodies. The coding region of mESOP-1 with FLAG tag at the 3′ end was recloned into the expression vector pEF-BOS (pEF-BOS-mESOP-1-FL). 293T cells were transfected with pEF-BOS-mESOP-1-FL (lanes 2, 4, 6) or vacant pEF-BOS vector (lanes 1, 3, 5) as a negative control using lipofectamine. Lysates of 293T cells were probed by either anti-FLAG antibodies (lanes 1, 2) or anti-mESOP-1 antibodies AK612 (lanes 3, 4) or AK641 (lanes 5, 6). (B) Immunohistochemical detection of mESOP-1 protein expression in yolk sac (i-vi), developing nervous system (vii, viii), and adult genital organs (ix, x). Both AK612 and AK641 gave the same results. Whole-mount staining of 7.5 dpc (i, ×30), 8.5 dpc (ii, ×25) embryo, and 9.0 dpc yolk sac (iii, ×12) . Magnified view (iv, ×80) and flat-mount view (v, ×200) of 9.0 dpc yolk sac, Cross-section of ii (vi, ×600). Arrowheads in a show blood islands. Arrowheads in iv show ESOP-1–positive endothelial cells in yolk sac. Spaces between the pairs of facing arrowheads indicate lumen of vascular plexus. Arrows in e show endothelial cells that expressed ESOP-1 protein. Transverse (vii, ×45) and parasagittal (viii, ×12) slices of 12.5 dpc embryos show ESOP-1 expression in the nervous system. Arrowheads show the hair follicles of vibrissae. Staining of adult ovary (ix, ×45) and testis (x, ×30) shows that all follicles, from small follicles to large, maturated follicles, oocytes (arrowheads), and granulosa cells (arrows) are ESOP-1 positive, and the signals become stronger as follicles develop. In testis, the cells in seminiferous tubules (asterisks) are stained. They may be spermatogonia or spermatocytes. VV, vitelline vessel; Br, brain; NT, neural tube; DRG, dorsal root ganglia; SN, sympathetic nerve; TN, trigeminal nerve; SF, small follicles; MF, mature follicles.
Anti-mESOP-1 antibodies and mESOP-1 protein expression.
(A) Analysis of the specificity of anti-mESOP-1 antibodies. The coding region of mESOP-1 with FLAG tag at the 3′ end was recloned into the expression vector pEF-BOS (pEF-BOS-mESOP-1-FL). 293T cells were transfected with pEF-BOS-mESOP-1-FL (lanes 2, 4, 6) or vacant pEF-BOS vector (lanes 1, 3, 5) as a negative control using lipofectamine. Lysates of 293T cells were probed by either anti-FLAG antibodies (lanes 1, 2) or anti-mESOP-1 antibodies AK612 (lanes 3, 4) or AK641 (lanes 5, 6). (B) Immunohistochemical detection of mESOP-1 protein expression in yolk sac (i-vi), developing nervous system (vii, viii), and adult genital organs (ix, x). Both AK612 and AK641 gave the same results. Whole-mount staining of 7.5 dpc (i, ×30), 8.5 dpc (ii, ×25) embryo, and 9.0 dpc yolk sac (iii, ×12) . Magnified view (iv, ×80) and flat-mount view (v, ×200) of 9.0 dpc yolk sac, Cross-section of ii (vi, ×600). Arrowheads in a show blood islands. Arrowheads in iv show ESOP-1–positive endothelial cells in yolk sac. Spaces between the pairs of facing arrowheads indicate lumen of vascular plexus. Arrows in e show endothelial cells that expressed ESOP-1 protein. Transverse (vii, ×45) and parasagittal (viii, ×12) slices of 12.5 dpc embryos show ESOP-1 expression in the nervous system. Arrowheads show the hair follicles of vibrissae. Staining of adult ovary (ix, ×45) and testis (x, ×30) shows that all follicles, from small follicles to large, maturated follicles, oocytes (arrowheads), and granulosa cells (arrows) are ESOP-1 positive, and the signals become stronger as follicles develop. In testis, the cells in seminiferous tubules (asterisks) are stained. They may be spermatogonia or spermatocytes. VV, vitelline vessel; Br, brain; NT, neural tube; DRG, dorsal root ganglia; SN, sympathetic nerve; TN, trigeminal nerve; SF, small follicles; MF, mature follicles.
In the nervous system, mESOP-1 was found in 12.5 dpc embryos (Figure2Bvii, 2Bviii). Neural tube, sympathetic nerves, dorsal root ganglia, brain, trigeminal nerves, and hair follicles of vibrissae were clearly stained. It was also found in olfactory bulb, optic nerve, and nipples (data not shown). Immunostaining also revealed that mESOP-1 was expressed in female and male genital organs of adult mice (Figure 2Bix, 2Bx). In ovary, mESOP-1 was present in oocytes and granulosa cells. In testis, mESOP-1 was expressed in the cells of seminiferous tubules. In these systems, ESOP-1 expression also overlaps with c-Kit expression.17,18 c-Kit/SCF is thought to act in synaptic connection, neuritis elongation, and maintenance of DRG cell differentiation.19 c-Kit/SCF also regulates the oocyte maturation and the development of follicles20and spermatocytes.21 These results, taken together suggest that ESOP-1 may play some roles in the development, maintenance, or both of the hematopoietic, nervous, and reproductive systems.
Recently, it was reported that MD-2, the human counterpart of mESOP-1, confers lipopolysaccharide responsiveness by physical association with Toll-like receptor 4 (TLR4).16 Toll was originally isolated as a gene for dorsoventral patterning of the Drosophilaembryo,22 and it was reported to be needed for proper motoneuron and muscle development, larval hematopoiesis, and oocyte development.23,24 Although the tissue distribution of ESOP-1 in embryo and adult mice does not contradict the hypothesis that ESOP-1/MD-2 collaborates with a member of the TLR family during embryogenesis, it is an open question whether ESOP-1 acts in association with TLR4 in embryonic development because TLR4-deficient mice showed the hyporesponsiveness to lipopolysaccharide but have no embryonic abnormality,25 and embryonic TLR4 expression is unknown.
Acknowledgments
We thank Dr S.-I. Nishikawa and Dr H. Yoshida for their kind gift of the anti-c-Kit and anti-Flk-1 monoclonal antibodies, their teaching on whole-mount immunohistochemistry, and the helpful discussions. We thank N. Tomikawa, M. Tanaka and M. Yamaguchi for their assistance in the experiments and in the preparation of the manuscript.
Supported in part by grants from the Ministry of Education, Science, and Culture of Japan (Monbusho) and by a fellowship from the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Reprints:Tasuku Honjo, Department of Medical Chemistry, Faculty of Medicine, Yoshida, Konoe-cho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: honjo@mfour.med.kyoto-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal