Abstract
Breast cancer resistance protein (BCRP) is a novel member of the adenosine triphosphate–binding cassette superfamily of transport proteins. Transfection and enforced expression of BCRP in drug-sensitive cells confer resistance to mitoxantrone, doxorubicin, daunorubicin, and topotecan. We studied blast cells from 21 acute leukemia patients (20 acute myeloid leukemia, 1 acute lymphocytic leukemia) for the expression of BCRP mRNA using a quantitative reverse-transcription polymerase chain reaction assay. BCRP mRNA expression varied more than 1000-fold among the samples tested, with low or barely detectable expression in half of the samples. Seven samples (33%) had relatively high expression of BCRP mRNA. High expression of BCRP did not correlate strongly with high expression of P-glycoprotein, suggesting that BCRP may cause resistance to certain antileukemic drugs in P-glycoprotein–negative cases. High expression of BCRP mRNA is sufficiently frequent in AML to warrant more extensive investigations to determine the relation of disease subtype and treatment outcome to BCRP expression and function.
To improve treatment outcome, intensive research has focused on clinically relevant mechanisms of chemotherapeutic drug resistance in acute myeloid leukemia (AML). Among the potential causes of drug resistance identified is increased expression of the multidrug resistance transporters P-glycoprotein (product of the MDR1 gene), the multidrug resistance protein (MRP1), the major vault protein (also known as lung cancer resistance protein, LRP), and the anti-apoptosis protein Bcl-2 and related family members in refractory or relapsed AML.1,2 A recent study of the expression of novel drug resistance–associated proteins in blast cells from 352 newly diagnosed AML patients by the Southwest Oncology Group3confirmed the importance of P-glycoprotein as a negative prognostic indicator. Interestingly, in this study a “distinct and nonoverlapping phenotype was detected in 18% of these cases: cyclosporine-resistant efflux not associated with MDR1, MRP1, or LRP expression, implying the existence of other as yet undefined efflux mechanisms in AML.” 3
Not long ago, our laboratory isolated a novel adenosine triphosphate (ATP)-binding cassette transporter protein from multidrug-resistant human breast cancer MCF-7/AdrVp cells.4 Transfection and overexpression of this transporter in drug-sensitive MCF-7 cells reproduced the drug-resistance phenotype of MCF-7/AdrVp cells, including resistance to daunorubicin, topotecan, and mitoxantrone.4 We designated the new transporter “breast cancer resistance protein” (BCRP) because it caused drug resistance when transfected into drug-sensitive cells and was isolated from human breast cancer cells. The GenBank accession number for BCRP is AF098951. Others have termed BCRP protein “mitoxantrone resistance protein”5 or “placental ABC transporter.” 6Marked overexpression of BCRP has been observed in human cancer cell lines selected for resistance with mitoxantrone7 or topotecan.8
Daunorubicin, mitoxantrone, and topotecan are drugs that have considerable activity against AML and are frequently incorporated into AML treatment regimens. Because BCRP can confer cellular resistance to these drugs, it is reasonable to hypothesize that BCRP may play a part in the drug resistance manifested in AML. As an initial step in testing this hypothesis, we sought to determine the range (depth) and prevalence of relatively high expression of BCRP in blast cells from AML patients.
Study design
Cell culture and isolation of marrow or peripheral blood mononuclear cells
MCF-7 cells were cultivated as described previously.4HL-60/Vinc cells, which overexpress P-glycoprotein, were grown as described previously.9 Bone marrow or peripheral blood samples were obtained after informed consent, according to a protocol approved by the institutional review board of the University of Maryland, Baltimore, MD. Mononuclear cells were isolated by Ficoll density gradient separation as described previously.9 Blast cells represented greater than 80% of the purified mononuclear cells (median 92%).
RNA preparation and reverse-transcription polymerase chain reaction (RT-PCR)
Total cellular RNA was obtained from the blast cells of 20 patients with AML and 1 patient with acute lymphocytic leukemia (ALL). One patient (patient 14) was studied twice, once at presentation before treatment and again at the time of relapse. Copy DNA (cDNA) was prepared as described previously,9 except that oligo dT primers were used for BCRP and β-actin. The PCR assays were performed with a modification of those described previously using primers specific for BCRP: 5′-TTA GGA TTG AAG CCA AAG G-3′ (sense) and 5′-TAG GCA ATT GTG AGG AAA ATA-3′ (antisense). The expected PCR product length for BCRP is 446 bp. For BCRP, MDR1, or β-actin, respectively, 35, 37, or 23 cycles of denaturation (94°C, 40 seconds), annealing (50°C, 1 minute), and elongation (72°C, 1 minute) were performed. Gel electrophoresis and Southern blotting were done as described previously.9 Under the assay conditions used, a linear relation was noted for log amount of cDNA from MCF-7 or HL-60/Vinc cells added to the PCR reaction and the amount of BCRP-, MDR1-, or β-actin–specific PCR product produced. Patient-sample PCR product formation relative to MCF-7 or HL-60/Vinc cells was determined by extrapolation from regression analysis of standard curves run concurrently. BCRP or MDR1 expression was then normalized for the expression of β-actin in that sample. The final expression of BCRP or MDR1 mRNA is given relative to the expression of BCRP mRNA in 1 μg of total cellular RNA from MCF-7 cells or to the expression of MDR1 mRNA in 1 μg of total cellular RNA from HL-60/Vinc cells (× 10−7).
Results and discussion
Table 1 summarizes individual patient characteristics at the time the blast cell samples were obtained. Treatment of these patients varied, but consisted of dose-intensive regimens containing daunorubicin or idarubicin, etoposide, and cytosine arabinoside. As can be seen from Table 1, the blast cells studied were from a patient population characterized predominantly by drug-resistant disease.10 11 In fact, after marrow sampling, only 4 of the 20 AML patients achieved a durable complete remission (CR) in response to treatment.
Patient characteristics and BCRP and MDR1 mRNA expression, listed in order of BCRP expression
| Patient . | Age, y* . | Sex . | Ethnicity . | Marrow or PB . | FAB . | Cytogenetics . | Immunophenotype . | Disease status† . | Response to Rx‡ . | % Blasts cytospin1-153 . | mRNA expression relative to MCF-7 or HL-60 . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCRP1-155 . | MDR11-154 . | |||||||||||
| 17 | 61 | F | W | PB | M5 | 46,XX,−5,−7, +8, 11q23 | CD2, 7, 11c, 14 (partial), 33, 38, 45 | New | NR | 95 | 0.002 | 0.702 |
| 1 | 40 | M | W | BM | M5 | 46,XY | CD11c, 13, 33, 34, 36, DR | Rel | NR | 98 | 0.01 | — |
| 6 | 43 | M | W | BM | M2 | 46,XY | CD13, 33, 34, DR | New | CR | 88 | 0.01 | — |
| 8 | 33 | M | W | BM | M2 | 45X, −Y, t(8;21) | CD33, 34, DR | New | CR-S | 98 | 0.01 | — |
| 9 | 55 | F | A | PB | M1 | 46,XX | CD13, 33, 45, DR | New | CR-S | 95 | 0.01 | — |
| 14b | 27 | M | W | BM | M4eos | 46,XY, (inv)16 (p13q22) | CD13, 33, 34 | Rel | CR | 98 | 0.01 | 0.142 |
| 19 | 77 | F | W | BM | M1 | 46,XX | CD7, 11c, 13, 33, 34 (partial), 38, 45, DR | Rel | NE | 91 | 0.017 | 0.413 |
| 10 | 55 | M | W | PB | M1 | 46,XY, −13q12-22, −1q32 | CD13, 33, 38 | New | CR-S | 98 | 0.02 | — |
| 13 | 29 | M | W | BM | M2 | 47,XY, +8 | CD13, 33 | New | NR | 90 | 0.02 | — |
| 11 | 67 | M | W | BM | M1 (2°) | 47,XY, +13 | CD13, 33, 34, DR | Rel | NR | 81 | 0.04 | — |
| 14a | 23 | M | W | BM | M4eos | 46,XY, (inv)16 (p13q22) | CD13, 33, 34 | New | CR | 98 | 0.05 | — |
| 2 | 62 | M | W | PB | M5 | 46,XY, t(3;5) | CD11c, 13, 14, 33, 38, DR | New | NR | 98 | 0.06 | 0.322 |
| 12 | 77 | F | Other | BM | M1 | Not done | Not done | New | CR-S | 80 | 0.08 | — |
| 18 | 67 | M | W | BM | M1 (MDS) | 46,XY | CD13, 33 (dim), 34, 35, DR | Rel | NR | 91 | 0.168 | 3.123 |
| 20 | 49 | M | W | BM | M1 | 46,XY | CD13, 33, 34, 38, 45, DR | Rel | CR | 88 | 0.198 | 3.291 |
| 21 | 21 | M | W | PB | ALL (L2) | Not done | Not done | Rel | NR | 97 | 0.315 | 3.617 |
| 4 | 30 | M | B | BM | M4 | 45,XY, −7 | CD11C, 13, 14, 33, 38, DR | New | CR-S | 80 | 0.33 | — |
| 7 | 56 | F | W | BM | M0 (MDS) | 46,XX t(X;12) | CD7, 33, 34, 45(dim), DR | New | NR | 93 | 0.35 | — |
| 5 | 63 | M | W | BM | M1 | 46,XY, −2p16 | CD13, 33, 34, 38, DR | New | NR | 94 | 0.36 | — |
| 15 | 59 | M | W | BM | M0 | 46,XY | CD2, 16/56, 7, 33, 38, 45, DR | Partial response | CR | 80 | 0.616 | 2.432 |
| 16 | 72 | F | W | BM | M4 (2°) | 46,XX | CD13 (dim), 16/56, 33, 38, DR | New | NR | 88 | 0.675 | 0.955 |
| 3 | 72 | F | B | BM | M6 | 45,XX, −3, −4, −5, −7, +8, −11, −17, −18, −19, 3 markers | Not done | New | NR | 82 | 2.59 | — |
| Patient . | Age, y* . | Sex . | Ethnicity . | Marrow or PB . | FAB . | Cytogenetics . | Immunophenotype . | Disease status† . | Response to Rx‡ . | % Blasts cytospin1-153 . | mRNA expression relative to MCF-7 or HL-60 . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCRP1-155 . | MDR11-154 . | |||||||||||
| 17 | 61 | F | W | PB | M5 | 46,XX,−5,−7, +8, 11q23 | CD2, 7, 11c, 14 (partial), 33, 38, 45 | New | NR | 95 | 0.002 | 0.702 |
| 1 | 40 | M | W | BM | M5 | 46,XY | CD11c, 13, 33, 34, 36, DR | Rel | NR | 98 | 0.01 | — |
| 6 | 43 | M | W | BM | M2 | 46,XY | CD13, 33, 34, DR | New | CR | 88 | 0.01 | — |
| 8 | 33 | M | W | BM | M2 | 45X, −Y, t(8;21) | CD33, 34, DR | New | CR-S | 98 | 0.01 | — |
| 9 | 55 | F | A | PB | M1 | 46,XX | CD13, 33, 45, DR | New | CR-S | 95 | 0.01 | — |
| 14b | 27 | M | W | BM | M4eos | 46,XY, (inv)16 (p13q22) | CD13, 33, 34 | Rel | CR | 98 | 0.01 | 0.142 |
| 19 | 77 | F | W | BM | M1 | 46,XX | CD7, 11c, 13, 33, 34 (partial), 38, 45, DR | Rel | NE | 91 | 0.017 | 0.413 |
| 10 | 55 | M | W | PB | M1 | 46,XY, −13q12-22, −1q32 | CD13, 33, 38 | New | CR-S | 98 | 0.02 | — |
| 13 | 29 | M | W | BM | M2 | 47,XY, +8 | CD13, 33 | New | NR | 90 | 0.02 | — |
| 11 | 67 | M | W | BM | M1 (2°) | 47,XY, +13 | CD13, 33, 34, DR | Rel | NR | 81 | 0.04 | — |
| 14a | 23 | M | W | BM | M4eos | 46,XY, (inv)16 (p13q22) | CD13, 33, 34 | New | CR | 98 | 0.05 | — |
| 2 | 62 | M | W | PB | M5 | 46,XY, t(3;5) | CD11c, 13, 14, 33, 38, DR | New | NR | 98 | 0.06 | 0.322 |
| 12 | 77 | F | Other | BM | M1 | Not done | Not done | New | CR-S | 80 | 0.08 | — |
| 18 | 67 | M | W | BM | M1 (MDS) | 46,XY | CD13, 33 (dim), 34, 35, DR | Rel | NR | 91 | 0.168 | 3.123 |
| 20 | 49 | M | W | BM | M1 | 46,XY | CD13, 33, 34, 38, 45, DR | Rel | CR | 88 | 0.198 | 3.291 |
| 21 | 21 | M | W | PB | ALL (L2) | Not done | Not done | Rel | NR | 97 | 0.315 | 3.617 |
| 4 | 30 | M | B | BM | M4 | 45,XY, −7 | CD11C, 13, 14, 33, 38, DR | New | CR-S | 80 | 0.33 | — |
| 7 | 56 | F | W | BM | M0 (MDS) | 46,XX t(X;12) | CD7, 33, 34, 45(dim), DR | New | NR | 93 | 0.35 | — |
| 5 | 63 | M | W | BM | M1 | 46,XY, −2p16 | CD13, 33, 34, 38, DR | New | NR | 94 | 0.36 | — |
| 15 | 59 | M | W | BM | M0 | 46,XY | CD2, 16/56, 7, 33, 38, 45, DR | Partial response | CR | 80 | 0.616 | 2.432 |
| 16 | 72 | F | W | BM | M4 (2°) | 46,XX | CD13 (dim), 16/56, 33, 38, DR | New | NR | 88 | 0.675 | 0.955 |
| 3 | 72 | F | B | BM | M6 | 45,XX, −3, −4, −5, −7, +8, −11, −17, −18, −19, 3 markers | Not done | New | NR | 82 | 2.59 | — |
PB indicates peripheral blood; FAB, French, American, and British classification of acute leukemia; F, female; W, white; new, newly diagnosed, untreated; NR, no response; M, male; BM, bone marrow; Rel, in relapse; CR, durable complete remission (>8 mo), CR-S, short remission (<8 mo); A, Asian; NE, not evaluable; B, black.
Median = 55.5 y.
At time of blast cell sampling for BCRP studies.
Response to subsequent treatment.
Median = 92.
Expression of BCRP mRNA relative to that of MCF-7 cells, after correction for the expression of β-actin.
Expression of MDR1 mRNA relative to that of HL-60/Vinc cells (×10−7) after correction for the expression of β-actin.
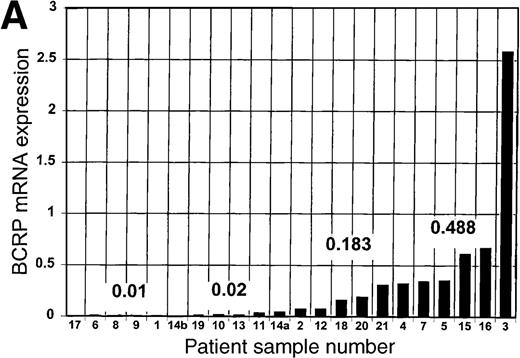
The expression of BCRP mRNA in blast cells from the 21 patients is shown in Table 1 and Figure 1A. MCF-7 cells were used as a reference standard for BCRP because we found that among the 60 human cancer cell lines used in the National Cancer Institute drug screen,12 the endogenous expression of BCRP mRNA by MCF-7 cells is relatively high (D.D.R., unpublished observations) and quantitatively measurable by the RT-PCR method used. Furthermore, because selective pressure is restricted in the clinical setting, the expression of a given putative resistance factor by an unselected cell line such as MCF-7 should more closely approximate the range of expression of that resistance factor in clinical blast cell samples than would the expression of the same resistance factor in highly resistant cell lines selected in vitro.
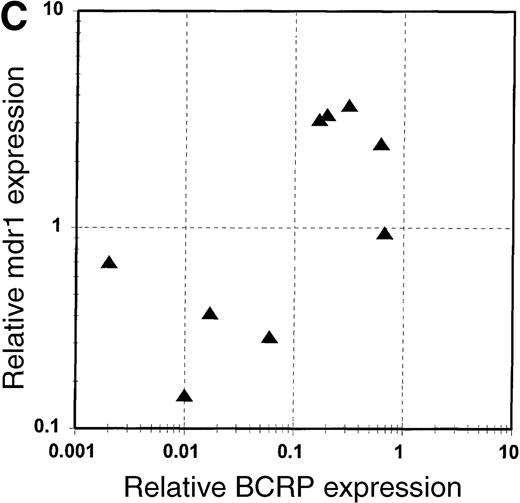
BCRP and MDR1 mRNA expression in blast cells.
(A) Expression of BCRP mRNA in blast cell samples from 21 patients with acute leukemia, determined by RT-PCR, as described in “Study design.” Data are expressed relative to the expression of BCRP mRNA in MCF-7 cells, after correction for the expression of β-actin mRNA in the sample, and are arranged in the figure in the order of magnitude of BCRP expression. The numbers given in the figure refer to the median values of BCRP expression for each quartile. Patient sample number is a unique identifier for each patient in the study. (B) Expression of MDR1 mRNA in blast cell samples from 9 patients with acute leukemia, determined by RT-PCR, as described in “Study design.” Data are expressed relative to the expression of MDR1 mRNA in HL-60/Vinc cells (× 10−7), after correction for the expression of β-actin mRNA in the sample. (C) Correlation of the expression of MDR1 mRNA with the expression of BCRP mRNA in blast cells from 9 acute leukemia patients. BCRP mRNA expression is reported relative to MCF-7 cells. MDR1 mRNA expression is reported relative to HL-60/Vinc cells (× 10−7).
BCRP and MDR1 mRNA expression in blast cells.
(A) Expression of BCRP mRNA in blast cell samples from 21 patients with acute leukemia, determined by RT-PCR, as described in “Study design.” Data are expressed relative to the expression of BCRP mRNA in MCF-7 cells, after correction for the expression of β-actin mRNA in the sample, and are arranged in the figure in the order of magnitude of BCRP expression. The numbers given in the figure refer to the median values of BCRP expression for each quartile. Patient sample number is a unique identifier for each patient in the study. (B) Expression of MDR1 mRNA in blast cell samples from 9 patients with acute leukemia, determined by RT-PCR, as described in “Study design.” Data are expressed relative to the expression of MDR1 mRNA in HL-60/Vinc cells (× 10−7), after correction for the expression of β-actin mRNA in the sample. (C) Correlation of the expression of MDR1 mRNA with the expression of BCRP mRNA in blast cells from 9 acute leukemia patients. BCRP mRNA expression is reported relative to MCF-7 cells. MDR1 mRNA expression is reported relative to HL-60/Vinc cells (× 10−7).
BCRP mRNA expression arranged in order of magnitude is given in Figure1A, along with the median of each quartile. Among the patient blast cell samples, BCRP mRNA expression varied more than 1000-fold overall and more than 48-fold from the median of the highest quartile to the median of the lowest quartile. In half of the patient samples (11), the relative expression of blast cell BCRP mRNA was very low (< 0.05 relative to MCF-7 cells). Four patients had blast cells with relative BCRP mRNA expression greater than 0.05 but less than 0.2 relative to MCF-7 cells. The 7 leukemia blast cell samples (33%) with the highest BCRP mRNA expression exceeded 0.3 relative to MCF-7 cells. Of these high BCRP expressers, 5 of the patients (71%) never achieved a CR with subsequent treatment, 1 had a CR of less than 8 months, and 1 achieved a CR of 10 months following autologous stem cell transplantation in second remission. Of the 15 blast cell samples with relatively lower BCRP mRNA expression, 6 (40%) were from patients who did not achieve a CR, 4 (27%) were from patients who did achieve a CR, and 4 (27%) were obtained from patients who attained a brief CR; 1 patient was not evaluable for response.
The multidrug resistance transporter P-glycoprotein is the product of the MDR1 gene. MDR1 mRNA expression was evaluated in blast cell samples from 9 patients. Four of the 9 patients (44%) displayed relatively high levels of MDR1 mRNA (> 1 × 10−7relative to HL-60/Vinc cells; Figure 1B and Table 1). This percentage is consistent with the reported frequency of high P-glycoprotein expression in an acute leukemia population such as the one we studied.1 There was a weak correlation (R2 = 0.44) between the expression of MDR1 mRNA and that of BCRP in the 9 samples tested (Figure 1C). If BCRP expression is found clinically to affect drug resistance in leukemia, coexpression of MDR1 and BCRP may explain, at least in part, why long-term survival was not enhanced in clinical trials of P-glycoprotein inhibitors in AML.13
This exploratory study shows clearly that a wide range of expression of BCRP mRNA is found among blast cell samples obtained from AML patients. This observation is consistent with the hypothesis that BCRP expression may be of prognostic or diagnostic significance in the patients whose blast cells manifest the higher expression levels of BCRP. The development of flow cytometric assays for BCRP protein and function will serve to validate measurements of BCRP mRNA levels. Production of antibodies to BCRP protein will be useful in quantitative assays for BCRP protein. The recent development of a potent and specific inhibitor of BCRP, fumitremorgin C,14 will greatly aid the development of a valid and specific assay for BCRP function. Functional evidence of BCRP transport in AML blast cells may stimulate the development of BCRP inhibitors for clinical use. Hence, these studies indicate that AML should be targeted for more extensive studies to determine the relation between functional BCRP expression and both disease subtype and treatment outcome. It is possible that BCRP may account, at least in part, for the subset of AML patients whose blast cells displayed enhanced drug efflux without overexpression of P-glycoprotein, MRP, or LRP observed in the recent Southwest Oncology Group trial.3
Acknowledgments
We thank Ms Yongming Gao, Mr Weidong Yang, and Mr Yuetong David Wei for their superb help in performing technical aspects of this work.
Supported in part by a Translational Research Award from the Leukemia Society of America (L.A.D. and D.D.R.); by grants RO1-CA77545 (L.A.D. and D.D.R.), RO1-CA52178 (D.D.R.), and UO1-CA69854 (J.E.K.) from the National Cancer Institute, National Institutes of Health; and by a Department of Veterans Affairs Merit Review Grant (D.D.R.).
Reprints:Douglas D. Ross, University of Maryland Greenebaum Cancer Center, University of Maryland School of Medicine, Room 9-015 Bressler Research Building, 655 W Baltimore St, Baltimore, MD 21201; e-mail: dross@umgcc.umaryland.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal