Abstract
Fizzy-related (fzr) is a recently identified 7WD domain family member implicated in cell cycle regulation of Drosophila and yeast. In this study, the murine homologue of fzr was isolated by suppression subtractive hybridization as a gene with decreased expression during malignant progression of a murine B-lymphoma cell line. Retroviral overexpression of fzr in B-lymphoma cells reduced tumor formation. Those tumors that did arise had diminished or extinguished retroviral Fzr. Surprisingly, fzr overexpression dramatically increased B-lymphoma cell susceptibility to natural killer cell (NK) cytotoxicity, a host-resistant mechanism for tumor formation in this model system. These findings implicate fzr as a new category of genes suppressing B-cell tumorigenesis and suggest a novel role for fzr in the target cell interaction with NK cells.
The molecular basis of tumor progression is an important issue in B lymphomagenesis. Although a number of proto-oncogenes or tumor suppressors have been identified as early molecular events, in vivo and in vitro experimentation has shown that inappropriate expression of such genes alone is insufficient for efficient tumorigenicity.1,2 In the case of c-myc, certain collaborating genes have been identified by a candidate approach, including p53 and bcl-2.3-6 Other novel genes (pim-1, pal-1, and bmi-1) have been isolated by retroviral insertional mutagenesis.7-9 However, with the exception of p53, these genes are not associated with natural human or murine c-myc B lymphomas.
Our laboratory has previously established a murine model system for the study of the progression of c-myc–dependent lym-phomagenesis.10 11 This model system is based on an in vitro–derived premalignant B-cell line (DAC), which bears rearranged c-myc but is nontumorigenic in wild-type immunocompetent mice, in part because of their susceptibility to host natural killer cell (NK) cytolytic activity. A malignant variant (MV) of these cell lines was isolated as forming tumors in immunocompetent mice and was found to have acquired resistance to NK cytolysis. These findings suggest that differentially expressed genes in these 2 populations account for tumor progression in this model system.
In the present study, we used suppression subtractive hybridization to identify the differentially expressed genes in DAC and MV cells.12 A novel gene revealed by this screen was murinefizzy-related (fzr), a recently identified 7WD domain family member involved in Drosophila cell cycle regulation.13Expression of murine fzr was reduced in fully malignant MV cells compared with premalignant DAC cells. Forced overexpression offzr in B-lymphoma cell lines increased cell susceptibility to NK cytotoxicity and suppressed tumor formation. These findings reveal a novel role for fzr in the NK-mediated cell death pathway and host-tumor interaction.
Materials and methods
Cell culture
The isolation of DAC and MV cell lines was described previously.10 14 These cells were passaged every 3 days in RPMI 1640 medium supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 5 × 10−5 mol/L 2-mercaptoethanol (Sigma, St. Louis, MO), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO BRL, Grand Island, NY).
Suppression subtractive hybridization
Messenger RNA was prepared from DAC and MV cells using the Fast Track kit (Invitrogen Corp., San Diego, CA). The PCR-select cDNA subtraction kit (Clontech Laboratories, Palo Alto, CA) was employed to isolate differentially expressed genes according to the manufacturer's procedures.12 The amplified subtracted cDNAs were then cloned into a T/A vector (Invitrogen).
Recombinant murine fzr expression
The full-length sequence of murine fzr cDNA was obtained by RACE, a PCR-based extension methodology.15 Murinefzr cDNA containing the entire open reading frame with a flag tag (DYKDDDDK) at the 5′ end was amplified by polymerase chain reaction from a mouse spleen cDNA library using the primers 5′-CCGGAATTCCACCATGGACTACAAGGACGACGATGACAAGGACCAGGACTATGAGCGAAGG-3′ and 5′-GCCGGAATTCG TGGGCTTCACATCCCGCCTG-3′. The retroviral expression vector pMSCV IRES NEO (a gift from Dr. Tony Koleske, University of Toronto, Canada) was used for gene transfer. Human fibroblast 293T cells were cotransfected with pMSCV IRES NEO constructs and the viral helper ψ to produce virus using a standard transfection procedure.16 The B-lymphoma cell lines DAC and MV were infected with virus. Stably infected cells were produced by Geneticin selection (500 μg/mL; GIBCO BRL).
Antibodies
Anti-Fzr polyclonal rabbit serum was produced against the N terminal fragment (1-173 amino acid) of murine Fzr-glutathione-s-transferase (GST) fusion protein. Fzr expression was detected by Western blot analysis using the anti-Fzr rabbit serum or an anti-flag M2 monoclonal antibody (Eastman Kodak, New Haven, CT). The rabbit serum was used at a final concentration of 1:10 000 in Western blot analysis. Both antibodies recognized a single band with an apparent molecular mass of 55 kd (expected for murine Fzr) in the infected cells containing the murine Flag-Fzr construct. Secondary antibodies, goat anti-rabbit IgG and goat anti-mouse IgG labeled with horseradish peroxidase, were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Northern blot analysis
Total RNA from DAC and MV cells was prepared by using an RNA purification kit (QIAGEN, Valencia, CA). Total RNA (10 μg) was loaded onto a formaldehyde agarose gel and subjected to electrophoresis. Separated RNA was transferred to a nylon membrane by capillary action and cross-linked to the membrane by exposure to UV light (Stratalinker; Stratagene, San Diego, CA). Full-length Fzr cDNA was labeled by random primer synthesis (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were prehybridized for 30 minutes and hybridized with the cDNA probe for at least 2 hours in Rapid-Hyb buffer (Amersham Pharmacia Biotech) at 65°C. Blots were then washed with 0.1× SSC, 0.1% sodium dodecyl sulfate and exposed to x-ray film.
NK cytotoxicity assay
Target cells were labeled with 100 μCi of Na2[51Cr]CrO4 (Amersham Pharmacia Biotech) for 1.5 hours at 37°C. Cells were washed twice, resuspended in RPMI 1640, and plated at 2 × 104cells per well in V-bottom 96-well plates. Effector cells were prepared from Balb/c mice (Jackson Laboratory, Bar Harbor, ME) injected intraperitoneally (IP) with poly(IC) (Sigma) 12 to 16 hours before sacrifice. A single cell suspension of splenocytes was obtained, and erythrocytes were lysed by treatment with 0.83% NH4Cl. The nucleated cells were counted and used as effector cells. Effector cells were added at various concentrations and incubated with the target cells at 37°C with 5% CO2. After 6 hours, the supernatant was harvested and counted with a gamma counter. Data were analyzed by regression analysis to determine lytic units, expressed as LU20, as described previously.11 No significant difference was observed in chromium loading by DAC, MV, and their infectant sublines.
Tumorigenicity
Exponentially growing DAC and MV cells were washed and resuspended in sterile phosphate-buffered saline (PBS) at a concentration of 5 × 105 cells/mL. Cells (5 × 105) were injected IP into Balb/c mice (Jackson Laboratory) at 8 to 15 weeks of age. Animals were monitored for 2 months. Mice with evidence of disease were killed and autopsied to confirm tumor formation and to obtain tumor tissue for Western blot analysis of fzr expression.
Flow cytometry of major histocompatibility complex (MHC) class I surface expression and DNA content
Murine H-2Dd was detected using a murine IgG2a, kappa monoclonal alloantibody as a purified biotin conjugate (06135; Pharmingen, San Diego, CA). A total of 5 × 105cells were stained with anti-Dd or an isotype control and phycoerythrin-streptavidin (Pharmingen). To measure DNA content, exponentially growing cultures were rinsed with Ca2+/Mg2+–free PBS and then fixed in cold 70% ethanol at 4°C overnight. Cellular DNA was stained with 50 μg/mL propidium iodine and 5 μg/mL RNase A and incubated at 37°C for 15 minutes. Stained cells were analyzed with a FACSTAR instrument (Becton Dickinson Immunocytometry System, Mountain View, CA) and CellQuest software for the Macintosh.
Results
Isolation of murine homologue of fzr from murine B-lymphoma cell lines
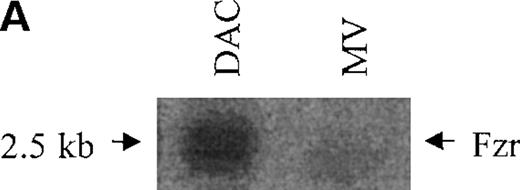
DAC and MV cells are the parent and daughter cell lines, distinguished by their differential tumor formation in immunocompetent mice. A set of differentially expressed genes in DAC and MV cells was isolated by suppression subtractive hybridization. This study characterizes one of the clones chosen for highly differential expression in DAC and MV cells. Expression of this gene was decreased in MV cells compared with DAC cells (5- to 10-fold by Northern blot; 10- to 20-fold by Western blot analysis; Figure1A-B).
Murine fzr in DAC and MV B-lymphoma cells.
(A) Northern blot was performed by using 32P-labeled murinefzr cDNA. Equal loading was verified by ethidium bromide staining. (B) Western blot analysis was performed using anti-Fzr rabbit serum. Samples were normalized based on cell number. (C) The full-length sequence of murine fzr cDNA. The DNA sequence contains 2140 nucleotides predicting a protein of 493 amino acids. The 7WD domains are shaded.
Murine fzr in DAC and MV B-lymphoma cells.
(A) Northern blot was performed by using 32P-labeled murinefzr cDNA. Equal loading was verified by ethidium bromide staining. (B) Western blot analysis was performed using anti-Fzr rabbit serum. Samples were normalized based on cell number. (C) The full-length sequence of murine fzr cDNA. The DNA sequence contains 2140 nucleotides predicting a protein of 493 amino acids. The 7WD domains are shaded.
A full length of this cDNA clone was obtained by RACE extension methodology, yielding a 2140-bp cDNA fragment from a mouse spleen cDNA library (Figure 1C).15 The cDNA contained an open reading frame of 1479 nucleotides, predicting a polypeptide of 493 amino acids with an estimated molecular weight of 55 kd. BLAST-P analysis revealed that this clone is the murine homologue offizzy-related (fzr) with 97%, 96%, and 73% amino acid identity to human, Xenopus, and Drosophila fzr, respectively.17Fzr is closely homologous to the Drosophila fizzy gene (CDC20 and p55CDC in yeast and mammal, respectively). These proteins are cell cycle regulatory proteins, controlling mitotic progress by mediating cyclin degradation by the anaphase-promoting complex (APC; a specialized mitotic proteasome component).18-20
Overexpression of murine fzr suppresses tumor growth
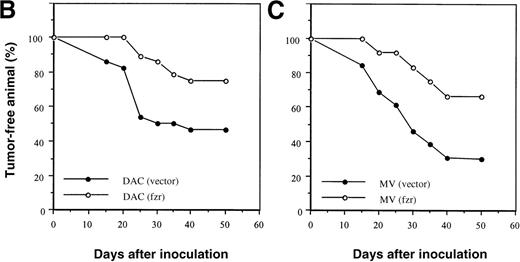
Because reduced expression of fzr was associated with malignant progression, we overexpressed murine fzr in B-lymphoma cells via a retroviral vector. Western blot analysis showed that both DAC and MV cells infected with the fzr construct [DAC(fzr) and MV(fzr)] had much higher levels of fzr protein expression than cells infected with the vector alone [DAC(vector) and MV(vector)] (Figure 2A). A total of 5 × 105 B-lymphoma cells were injected into Balb/c mice, which were monitored for up to 50 days. This relatively high number of transferred cells was chosen to permit tumor formation by both MV and DAC cells. Figures 2B and 2C show that overexpression offzr substantially reduced tumor frequency in DAC and MV cells, respectively. Notably, the expression of fzr was typically silenced in MV(fzr) and DAC(vector) tumors (Figure 2A).
Effect of fzr overexpression on tumor formation.
(A) Western blot analysis of DAC and MV cells retrovirally infected with murine stem cell virus (MSCV) retrovirus produced from empty vector control (“vector”) or fzr constructs (“fzr”). Expression was tested in the in vitro cell lines (“cells”) and in cells obtained from tumors (“tumors”) using anti-Fzr rabbit serum. Equal protein loading was verified by Ponceau S staining. (B, C) Balb/c mice were injected IP with vector-only or fzr infectants of DAC (B) or MV (C) at 5 × 105. Thirteen mice were injected for each of the 4 groups and monitored for 50 days. Mice were killed upon onset of disease or at the end of the monitoring period to confirm tumor formation and collect tissues, and all mice were killed after the monitoring period.
Effect of fzr overexpression on tumor formation.
(A) Western blot analysis of DAC and MV cells retrovirally infected with murine stem cell virus (MSCV) retrovirus produced from empty vector control (“vector”) or fzr constructs (“fzr”). Expression was tested in the in vitro cell lines (“cells”) and in cells obtained from tumors (“tumors”) using anti-Fzr rabbit serum. Equal protein loading was verified by Ponceau S staining. (B, C) Balb/c mice were injected IP with vector-only or fzr infectants of DAC (B) or MV (C) at 5 × 105. Thirteen mice were injected for each of the 4 groups and monitored for 50 days. Mice were killed upon onset of disease or at the end of the monitoring period to confirm tumor formation and collect tissues, and all mice were killed after the monitoring period.
Overexpression of murine fzr increases NK-mediated cell death
Our previous studies demonstrated that MV cells were more resistant than DAC cells to NK killing.11 Because DAC cells differed from MV cells by increased fzr expression, we wondered whether higher levels of fzr expression might increase cell susceptibility to NK killing.
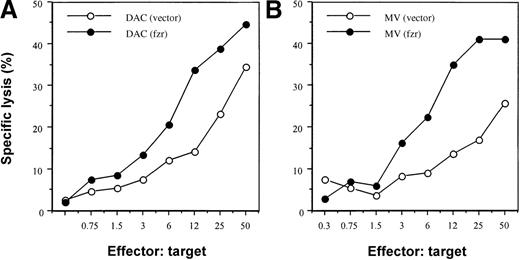
We evaluated the effect of fzr overexpression on susceptibility to NK killing using a standard chromium-release assay and an in vivo activated Balb/c splenic effector population (Figure3). As expected, DAC(vector)–only control infectants were more sensitive than the corresponding MV population (2- to 5-fold difference in LU20; Figure 3 and data not shown). Overexpression of fzr markedly increased NK sensitivity of both DAC and MV cells (5- and 10-fold increase in LU20, respectively; Figure 3), raising the 2 cell lines to the same level of NK susceptibility.
Effect of fzr overexpression on susceptibility to NK cytotoxicity.
DAC (A) or MV (B) cells (2 × 104) were plated into each well in V-bottom 96-well plates. Effector cells were added and cocultured for 6 hours. Chromium release was measured by a gamma counter. Data shown are representative of 3 experiments.
Effect of fzr overexpression on susceptibility to NK cytotoxicity.
DAC (A) or MV (B) cells (2 × 104) were plated into each well in V-bottom 96-well plates. Effector cells were added and cocultured for 6 hours. Chromium release was measured by a gamma counter. Data shown are representative of 3 experiments.
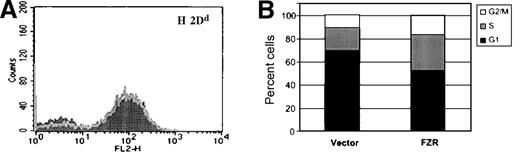
MHC class I expression by target cells inhibits activation and cytotoxicity of NK cells through inhibitory subsets of the Ly-49 and killer inhibitory receptor (KIR) receptor families. We therefore considered the possibility that fzr overexpression might affect NK susceptibility by increasing MHC class I expression. Accordingly, MV(vector) and MV(fzr) cells were compared by flow cytometry for surface levels of H-2D (the major inhibitory MHC class I target in the H-2d haplotype). As shown in Figure4A, fzr overexpression did not alter the surface expression of H-2D. In addition, no difference in MHC class I expression was observed in MV or DAC cells analyzed from tumors (data not shown). Thus, the effect of fzr on NK susceptibility does not appear to act by modulating the expression of MHC class I.
Phenotype of fzr-overexpressing cells.
(A) MV(vector) and MV(fzr) were stained with biotin–anti-H-2Dd monoclonal antibody and phycoerythrin-streptavidin and analyzed by flow cytometry. The MV(vector) and MV(fzr) histograms are shown as the dark and light traces in the foreground and background, respectively. (B) The DNA content of exponentially growing cells was evaluated by propidium iodide staining and flow cytometry and calculated to determine the proportion of cells in G1, S, and G2/M.
Phenotype of fzr-overexpressing cells.
(A) MV(vector) and MV(fzr) were stained with biotin–anti-H-2Dd monoclonal antibody and phycoerythrin-streptavidin and analyzed by flow cytometry. The MV(vector) and MV(fzr) histograms are shown as the dark and light traces in the foreground and background, respectively. (B) The DNA content of exponentially growing cells was evaluated by propidium iodide staining and flow cytometry and calculated to determine the proportion of cells in G1, S, and G2/M.
NK susceptibility can be affected by cell cycle stage, and fzris believed to regulate M to G1 transition and G1 progression in yeast and Drosophila. In DAC and MV cells, overexpression of fzrincreased the rate of cell growth (133% to 145% after 3 days of exponential growth). Cell cycle analysis of exponentially growing cultures revealed that fzr-overexpressing cells had a reduced fraction of cells in G1 (Figure 4B). The ratio of cycling cells (S/S+G2, M) was unchanged between control andfzr-overexpressing cells (65% and 67%). Taken together, these findings suggest that fzr overexpression accelerates the growth rate by shortening the duration of G1.
Discussion
In the present study, a murine homologue of the Drosophilafzr was isolated from a screen for genes with reduced expression upon malignant progression in B lymphomagenesis. Retroviral overexpression of fzr reduced tumorigenicity in B-lymphoma cell lines and strikingly increased their susceptibility to NK cytolysis. Fzr is highly conserved phylogenetically and is implicated in mitotic cell cycle regulation. The present findings suggest that fzr may also play a role in tumorigenesis and target–NK cell interaction.
Fzr is a member of the large family of WD domain proteins, which mediate protein–protein interactions for diverse regulatory and signaling functions (eg, the trimeric G-protein beta subunits and various cell cycle regulatory proteins21,22). The present study introduces the murine fzr homologue to the known human, Xenopus, and Drosophila genes (97%, 96%, and 73% amino acid identity) and their probable homologues in budding and fission yeast (hct1/cdh1 and ste9, respectively23,24). Fzr was first reported in Drosophila, where it promotes the degradation of cyclins A, B, and B3, thereby allowing mitotic exit and G1 arrest.13 Recent studies in yeast have demonstrated that cdh1/hct1 directly mediates a ternary complex between specific substrate proteins and the APC (the mitosis-specific 9S ubiquitin-conjugating subcomplex of the proteasome).24,25Among the WD domain family members, fzr is most related to the Drosophila fizzy (p55Cdc and cdc20 in human and yeast).26-29 As with cdh1/hct1, cdc20 undergoes regulated association with the APC, targeting distinct substrates to the APC at an earlier phase of mitosis.30 Accordingly, these 2 proteins sequentially serve as limiting, substrate-specific activators of APC-dependent proteolysis.
Retroviral overexpression of fzr increased the growth rate, and this was related to a change in cell cycle distribution (reduced fraction of G1 cells and a balanced increase of S and G2/M cells). These findings are, to our knowledge, the first describing the cell cycle effect of fzr overexpression in mammalian cells. The simplest interpretation is that fzr accelerates progression through G1, resulting in a reduced fraction of G1 cells and an increased rate of cell growth. This phenotype is consistent with the role of fzr for G1 progression demonstrated by genetic and biochemical studies in yeast and Drosophila.
The phenotype of fzr overexpression with respect to growth and cell cycle is unlikely to account directly for its effect on tumorigenicity and NK susceptibility. First, the increased growth rate with fzr overexpression would be expected to enhance, not reduce, in vivo growth and tumor formation. Second, proliferating versus quiescent cells typically are more sensitive to NK cytotoxicity, but the mechanism of this sensitivity is not well defined. Specifically, cell cycle stage has a minimal effect on NK susceptibility.31 We note 2 mechanisms that may play a role. First, proteasome function has been implicated in certain models of JNK, stress protein, and NFκB cell death pathways.32-35 Target cells initiate apoptotic and cytolytic death upon NK interaction, notably from perforin/granule and Fas-mediated pathways. The distal events of these pathways are incompletely defined for NK killing and may include proteasomal functions targeted by fzr. It is unlikely that cell cycle regulation by fzr accounts for its effect on B-cell tumorigenicity and NK susceptibility.
Second, fzr may affect MHC class I peptide decoration. The Ly-49, Cd94/NKG2, and other KIR receptor families of the NK cell use various MHC class I molecules as their ligand and mediate inhibitory or activation signals for the NK cytolytic pathway through an immunoreceptor tyrosine-based inhibition or activation motif (ITIM or ITAM)-bearing cytoplasmic domain.36Mutational and biochemical evidence indicates that class I recognition by these receptors is peptide discriminant.37-43 It is intriguing to consider that fzr may influence the distribution of class I–associated peptides by its capacity for substrate-selective delivery of proteins to ubiquitin-conjugating 9S proteasome subunits.44 45
In summary, fzr represents a new category of genes affecting tumor formation, a phenotype in this lymphoma model probably due to augmentation of target–NK cell interaction. Recent studies of cell cycle regulation indicate that fzr expression is a limiting factor for mitotic proteasome function, and the present study surprisingly implicates fzr in the targeting or death process mediated by target-NK interaction.
Acknowledgments
We thank Drs. Benjamin Bonavida, David Rawlings, Ramaswamy Iyer, and Tony Koleske for gifts of reagents; and Drs. Owen Witte, Harvey Herschman, and Lee Goodglick for advice.
Supported by NIH grants AI38545, CA12800, the Jonsson Comprehensive Cancer Center, the Gustavus and Louise Pfeiffer Foundation, and the Lymphoma Research Foundation of America.
Reprints:Jonathan Braun, Department of Pathology and Laboratory Medicine, UCLA School of Medicine, CHS 13-222, 10833 Le Conte Ave, Los Angeles, CA 90095-1732; e-mail: jbraun@mednet.ucla.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal