Abstract
Myeloma tumor cells, both freshly excised and cultured, are extremely resistant to cell-mediated cytolysis. As evidence suggests that B-cell susceptibility to lysis is dependent upon its state of differentiation and activation, we tested the ability of a variety of B-cell proliferation and differentiation agents, including pokeweed mitogen (PWM), to enhance the sensitivity of myeloma cells to cell-mediated lysis. PWM was found to significantly enhance the susceptibility of myeloma cell lines and freshly isolated myeloma cells to interleukin-2 (IL-2)–activated cell-mediated cytolysis. This effect was seen with the use of both IL-2–stimulated natural killer (NK) cells and T cells as effectors. The enhanced sensitivity of myeloma cells to cytolysis correlated with an increase in their cell surface expression of CD9, a pre-B cell marker and member of the transmembrane 4 superfamily. Incubation of PWM-stimulated myeloma cells with either monoclonal antibodies or antisense oligonucleotides directed against CD9 abrogated the effect of PWM. In order to determine whether there was a direct relationship between the expression of CD9 and enhanced sensitivity to cytolysis, myeloma cell lines that lacked CD9 expression were transfected with the CD9 gene. The level of cell surface CD9 expression correlates with enhanced susceptibility to lysis. Therefore, CD9 appears to be an important component in enhancing the sensitivity of myeloma cells to lysis mediated by IL-2–activated T cells and NK cells.
Peripheral blood mononuclear cells (PBMCs) treated with interleukin (IL)-2 acquire the ability to lyse both fresh and cultured tumor cells in a non–major histocompatibility complex (MHC) specific manner1-3; this is the lymphokine activated killer (LAK) phenomenon. Techniques that exploit this phenomenon include the adoptive transfer of autologous patient-derived IL-2–activated PBMCs for the treatment of neoplasias, such as metastatic renal cell carcinoma and melanoma.4 5 However, the mechanism by which IL-2–activated PBMCs are able to mediate this cytolytic activity has not been completely defined.
Multiple myeloma is characterized by the presence of neoplastic plasma cells in the bone marrow, bone lesions, and the presence of monoclonal immunoglobulin in serum and urine. Treatment entails the use of alkylating agents to relieve pain and reduce plasma cell proliferation in the bone marrow. The successful management of this disease is limited owing to the development of resistance to chemotherapeutic agents by tumor cells.6 We have observed that myeloma cells are extremely resistant to lysis by IL-2–activated lymphocytes, independently of whether these effectors were derived from normal individuals or myeloma patients.7 We were, therefore, interested in defining the characteristics of myeloma cells that enable them to resist IL-2–activated PBMC-mediated lysis. As part of this assessment, we looked at agents that induce proliferation and differentiation in B cells for their ability to alter the susceptibility of myeloma cells to lysis. One such agent is pokeweed mitogen (PWM),8,9 which activates B cells by cross-linking cell surface molecules and, through a signal-transduction pathway, induces B cells to proliferate and/or differentiate. PWM may also, by activating B cells, induce the expression of various cell surface molecules. We have found that myeloma cells treated with PWM become significantly more susceptible to lysis by IL-2–activated PBMCs, T cells, and natural killer (NK) cells. We also determined that the enhanced susceptibility to lysis by IL-2–activated PBMCs, T cells, and NK cells correlated with an increase in expression of CD9. CD9, a member of the transmembrane 4 superfamily,10 is structurally reminiscent of some receptors and transport molecules.11-13 CD9, in addition to other members of this tetraspan family of proteins, has been implicated in signal transduction and/or activation events.14-16 CD9 has been shown to play a role in Ca++ influx in platelets,15,17-20 induce homotypic aggregation of pre-B cells,21 inhibit cell migration, and suppress motility and metastasis in breast cancer and melanoma.22-25
Initial studies using antibodies to CD9 or CD9-antisense oligonucleotides indicated that blocking CD9 expression on PWM-stimulated myeloma cells reversed their PWM-induced sensitivity to cell-mediated cytolysis. In order to more definitively assess the role of CD9 in cytotoxicity, we transfected resistant myeloma cell lines that expressed little or no CD9 with a plasmid containing the CD9 gene. Expression of CD9 correlated with enhanced susceptibility to lysis, confirming the role of CD9 in cytolysis of myeloma cells by IL-2–activated effectors.
Materials and methods
Isolation and generation of effector cells
Peripheral blood was obtained from normal donors, and mononuclear cell fractions were isolated by centrifugation over a Ficoll-Hypaque gradient (Sigma, St. Louis, MO). PBMCs were cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS) and 2 mmol/L L-glutamine (Gibco BRL, Gaithersburg, MD). Cytokine-activated killer cell activity was generated by addition of 100 U/mL recombinant IL-2 (Roche, Nutley, NJ) to these cultures for 3 days. For the isolation of T cells and NK cells, buffy coats were obtained from the local chapter of the American Red Cross (Little Rock, AR). PBMCs were separated by Ficoll centrifugation; monocytes and macrophages were then depleted by adhesion to plastic. T cells were generated with the use of 2-aminoethylisothiouronium bromide–treated sheep red blood cells (SRBCs) by a T-cell rosetting technique.26 These cells were then subjected to a second Ficoll centrifugation. The SRBC–T-cell pellet was collected, and SRBCs were lysed by treatment with ACK solution (0.15 mol/L NH4Cl, 1.0 mmol/L KHCO3, 0.1 mmol/L EDTA, pH 7.3). After lysis of SRBCs, the remaining cells were washed thoroughly. After staining with dual fluorescence antibodies (fluorescein isothiocyanate [FITC]–conjugated CD3, phycoerythrin [PE]–conjugated CD19; and FITC-conjugated CD3, PE-conjugated CD16/56), aliquots of cells were analyzed by flow cytometry to ascertain their purity as T cells (CD3+, CD19−, CD16−, CD56−). Antibodies were purchased from Becton Dickinson (Mountain View, CA). T cells used as effectors were more than 97% pure. Purified NK cells were generated as previously described.27 The interface from the Ficoll centrifugation of SRBC–T-cell rosettes was collected, washed twice with phosphate buffered saline (PBS), and resuspended at 1 × 107cells per mL in complete RPMI. These cells were then incubated on ice with 400 ng/mL each of anti-CD14, anti-CD19, and anti-CD3 monoclonal antibodies (mAbs) for 1 hour. Magnetic beads coated with anti–immunoglobulin (Ig)G (Dynal Inc, Lake Success, NY) were then added and incubated at room temperature for 30 minutes with gentle rocking. This mixture was placed adjacent to a strong magnet to allow the adherence of magnetic-bead–cell complexes; all cells remaining in solution were collected. Aliquots of cells were analyzed by flow cytometry to determine their purity as NK cells (CD56+, CD16+, CD3−, CD19−). NK cells used in subsequent assays were more than 94% pure.
Tumor cell lines
Myeloma cell lines used as targets in these assays included MER, COL, RAM, RLO, ARK, and ARD-2.28All of these cell lines express the IgGκ isotype, except ARD-2, which expresses IgA on its surface. These cell lines were generated from peripheral blood or bone marrow from multiple myeloma patients at the Arkansas Cancer Research Center. Bone marrow from myeloma patients was also used as a source of fresh myeloma tumor cells. The NK-cell–sensitive erythroleukemia cell line K562 and the NK- and LAK-susceptible T-cell lines Jurkat, MOLT-4, CEM, HSB2, and 8402 were also used as targets and are available from the American Type Culture Collection (Manassas, VA). All targets were maintained in culture media consisting of RPMI 1640 supplemented with 10% FBS and 2 mmol/L glutamine at a concentration of 3 × 105 cells per mL.
Reagents
RPMI 1640, L-glutamine, FBS, PWM, and phytohemagglutinin (PHA) were purchased from Gibco BRL. Concanavalin A (ConA) and Protein A–Sepharose CL were purchased from Sigma. Interferon (IFN) γ was purchased from Boehringer Mannhein (Indianapolis, IN). Anti-CD9 antibody MM2/57 was purchased from Biosource (Camarillo, CA), and ALB6 was a kind gift from Dr Claude Boucheix, Hôpital Paul-Brousse, Villejuif, France. Anti-CD38 antibody was purchased from Becton Dickinson.
Cytotoxicity assays
IL-2–stimulated PBMCs, T cells, and NK cells were used as effectors in 51Cr release assays as described previously.27 29 The effector:target (E:T) cell ratios used were 30:1, 15:1, 7.5:1, and 3.75:1, unless otherwise stated. Aliquots of the appropriate number of effector cells were added in a total volume of 0.1 mL per well. All E:T ratios were performed in triplicate for each target tested. Aliquots of 3 × 10351Cr-labeled target cells in 0.1 mL were added to each well of 96-well U-bottom tissue culture plates. As controls,51Cr-labeled targets (3 × 103 cells) were incubated with 0.1 mL of media alone (spontaneous release) or 0.1 mL of 0.1 mol/L hexadecyltriammonium bromide detergent (maximum release). Plates were centrifuged to a maximum of 2000 rpm and incubated at 37°C for 4 hours. After incubation, aliquots of 0.1 mL were removed from each well, and 51Cr release was detected by means of a gamma counter. The percent-specific 51Cr release was calculated for each E:T ratio by the formula:
Cold-target inhibition assays were performed as described previously.29 Effector cells were kept at a constant concentration of 9 × 104 cells per well.51Cr-labeled targets were prepared, and aliquots of 3 × 103 cells per well were added. Unlabeled targets (competitors) were added in varying ratios of unlabeled to labeled targets (0:1, 10:1, 50:1, and 100:1). All conditions were repeated in triplicate in 96-well U-bottom tissue culture plates. The percent-specific lysis was determined as described earlier.
Conjugate binding assays
The assay was performed as described previously.30Effectors were generated as described above and labeled with PKH26 (Zynaxis, Malvern, PA), a lipophilic fluorescent dye, immediately prior to use. Targets were myeloma cell lines MER and COL, both untreated and treated with PWM. An equal number of targets and effectors (5 × 105 cells per 0.5 mL each) were incubated at 37°C and allowed to bind for 5 to 10 minutes prior to counting. Total effectors, bound and unbound to target cells, were counted with the use of a fluorescence microscope. Each condition was repeated 3 times, and a minimum of 1000 effectors were counted for each E:T combination. The percentage of bound effectors was then determined for each experiment, with a mean overall percentage ± SEM calculated for each combination.
Flow cytometry analysis
Myeloma cells (4 × 105) were washed with PBS and then resuspended in 1 mL of cold staining buffer (PBS + 2% FBS + 0.02% sodium azide). After centrifugation, the supernatant was removed by vacuum aspiration, and 10 μg/mL of primary antibody was added to the cells. After mixing, the cells were incubated for 30 minutes at 4°C. The cells were washed 3 times with ice-cold staining buffer. Fluorescence-labeled secondary antibody (PE- or FITC-conjugated goat antimouse immunoglobulin) was then added. After mixing, the cells were incubated for an additional 30 minutes at 4°C in the dark. After incubation, the pellet was washed 3 times with staining buffer and then resuspended in 0.5 mL of PBS. The analysis was carried out by means of a flow cytometer (FACStarPLUS; Becton Dickinson). Purified NK- and T-cell populations were stained in a 1-step procedure with the use of labeled primary antibodies as described earlier.
mAb treatment
An aliquot of 5 × 105 myeloma tumor cells was washed and incubated in RPMI 1640 media containing 10% heat-inactivated FBS (65°C, 45 minutes) and 1% glutamine for 24 hours. Anti-CD9 mAb (10 μg/mL ALB6, IgG1 isotype) or an equal amount of isotype-matched anti–HLA-DR mAb was added to the cells. The cells were then incubated for a further 24-hour period. Prior to being labeled with 51Cr, these cells were either left untreated or treated with 1% PWM. These cells were then used as targets in standard 4-hour 51Cr release assays.
Antisense oligonucleotide treatment
An antisense 18-mer oligonucleotide (5′ CAGCAGGTATTTGATCGA 3′), which corresponds to an area near the start site of the CD9 gene, was synthesized. A complementary sense oligonucleotide (5′ TCGATCAAATACCTGCTG 3′) was also synthesized and used as a control sequence in these experiments. Myeloma cells were washed and incubated as described previously. Oligonucleotides were then added at a concentration of 25 to 150 μmol/L and incubated for a period of 2 hours. These cells were then treated with 1% PWM where appropriate and incubated for an additional hour. All cells were then labeled with 51Cr and used in standard 4-hour51Cr release assays.
Transfection of cell lines
The vector pcDNA3 (Invitrogen, San Diego, CA) containing the full-length CD9 gene was kindly provided by Dr Claude Boucheix.31 Myeloma cell lines MER, COL, and RLO were used for transfection assays because they expressed the lowest levels of endogenous CD9. For each transfection, 8 to 10 × 106 cells and 7 to 8 μg of linearized pcDNA3 or pcDNA3-CD9 DNA were used. Electroporation was carried out in 0.4-cm–gap electroporation chambers in 0.5 mL of RPMI 1640 containing 10% FBS. Myeloma cells were electroporated at 250 to 300 V and 1180 μF (Cell-Porator, Gibco BRL). Stable transfectants were generated in 5 to 6 weeks after G418 selection. G418 was used at concentrations of 350 to 400 μg/mL.
Calcium influx assays
A 2-mmol/L stock solution of fluo-3/AM was prepared in dimethylsulphoxide, containing 37.5 mg/mL Pluronic F-127 as previously described.32 Cells (106/mL) were placed in 8-well chamber slides coated with poly-L-lysine and loaded with fluo-3/AM at a final concentration of 4 μmol/L in Hank's balanced salt solution (HBSS) at 37°C for 20 minutes. Cells were then diluted 1/5 in HBSS containing 1% FBS and incubated for an additional 40 minutes at 37°C. Cells were then washed 3 times and resuspended in 0.2 mL of HBSS containing 1 mmol/L CaCl2, 0.5 mmol/L MgCl2, and 1 mg/mL bovine serum albumin. Anti-CD9 antibodies (ALB6 and MM2/57) were added at concentrations of 20 μg/mL to elicit a maximal calcium flux. An unrelated antibody, anti-CD38, was used as a negative control. All detection and analysis were performed on a Meridian ACAS 570 image analyzer (Okemos, MI).
Statistical analyses
All statistical analyses were performed with the use of the Student t test.
Results
Effects of differentiating agents on the susceptibility of myeloma cells to cytolysis
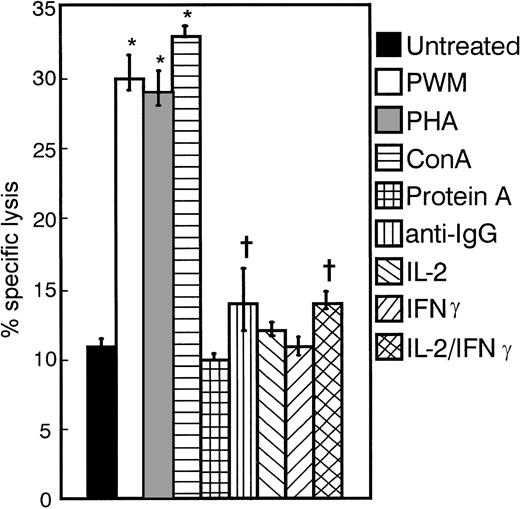
The myeloma cell line MER was treated with various mitogens, cytokines, and chemical agents for a period of 3 days. Treated and untreated cells were then employed as targets in standard 4-hour51Cr release assays with the use of IL-2–activated PBMCs as effectors (Figure 1). PWM, PHA, and ConA were able to significantly enhance the susceptibility of these cells to cytolysis (P < .005). The enhanced susceptibility to lysis induced by anti-IgG and the combination of IL-2 and IFNγ was also significant (P < .05), but less consistent than that seen with the use of PWM. Such agents as Protein A, IL-2, and IFNγ did not alter the sensitivity of these targets to lysis by IL-2–activated PBMCs. Similar results were obtained for other cell lines and fresh tumor cells tested (data not shown). We chose to focus our efforts on one specific B-cell differentiating agent, PWM, to better understand its effects on myeloma tumor cells.
Effect of various differentiating agents on the susceptibility of MER cells to lysis by IL-2–activated PBMCs.
MER cells were treated with PWM (1%), PHA (1μg/mL), ConA (1μg/mL), Protein A (.05 μg/mL), anti-IgG (10 μg/mL), IL-2 (100 U/mL), and IFNγ (10 U/mL)—or the combination of IL-2 and IFNγ—for 3 days. These cells were then washed extensively and used as targets in 4-hour 51Cr release assays. The E:T ratio was 30:1. The data represent the mean values ± SEM of 4 individual experiments. *P < .005; +P < .05
Effect of various differentiating agents on the susceptibility of MER cells to lysis by IL-2–activated PBMCs.
MER cells were treated with PWM (1%), PHA (1μg/mL), ConA (1μg/mL), Protein A (.05 μg/mL), anti-IgG (10 μg/mL), IL-2 (100 U/mL), and IFNγ (10 U/mL)—or the combination of IL-2 and IFNγ—for 3 days. These cells were then washed extensively and used as targets in 4-hour 51Cr release assays. The E:T ratio was 30:1. The data represent the mean values ± SEM of 4 individual experiments. *P < .005; +P < .05
Effects of variable concentrations of PWM on myeloma cell sensitivity to cytolysis
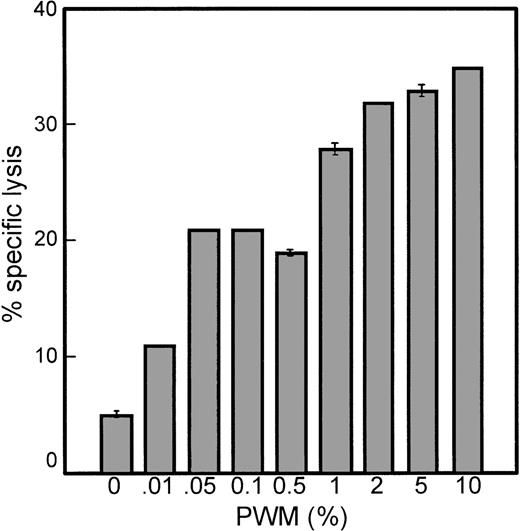
The myeloma cell line MER was cultured in 0.01% to 10% PWM for 3 days. After extensive washing to remove PWM, MER cells were used as targets in standard 4-hour 51Cr release assays. As little as 0.01% PWM enhanced the susceptibility of MER cells to lysis mediated by IL-2–activated PBMCs by twofold (Figure2). A concentration of 1% PWM induced a maximum level of susceptibility to lysis (5.5-fold) without having a cytotoxic effect on the myeloma cells. It was observed that PWM did not affect the rate of proliferation of these cells. However, at concentrations higher than 1% PWM, although increased sensitivity to lysis was achieved, cells were not able to survive longer than 5 to 7 days in culture. At 3 days after culture in the presence of 2% or greater PWM, cells were only 50% to 60% viable. Therefore, we continued our studies using 1% PWM, which produced the maximum level of sensitivity to lysis without producing any toxic effects.
PWM enhances the susceptibility of myeloma cells to lysis mediated by IL-2–activated PBMCs in a dose-dependent manner.
MER cells were cultured with varying amounts of PWM for 3 days and then used as targets in standard 4-hour 51Cr release assays. The E:T ratio was 30:1. The data represent the mean ± SD of triplicate values in 1 experiment representative of 3 similar experiments.
PWM enhances the susceptibility of myeloma cells to lysis mediated by IL-2–activated PBMCs in a dose-dependent manner.
MER cells were cultured with varying amounts of PWM for 3 days and then used as targets in standard 4-hour 51Cr release assays. The E:T ratio was 30:1. The data represent the mean ± SD of triplicate values in 1 experiment representative of 3 similar experiments.
PWM specifically enhances the susceptibility of myeloma cells to cytolysis by IL-2–activated effectors
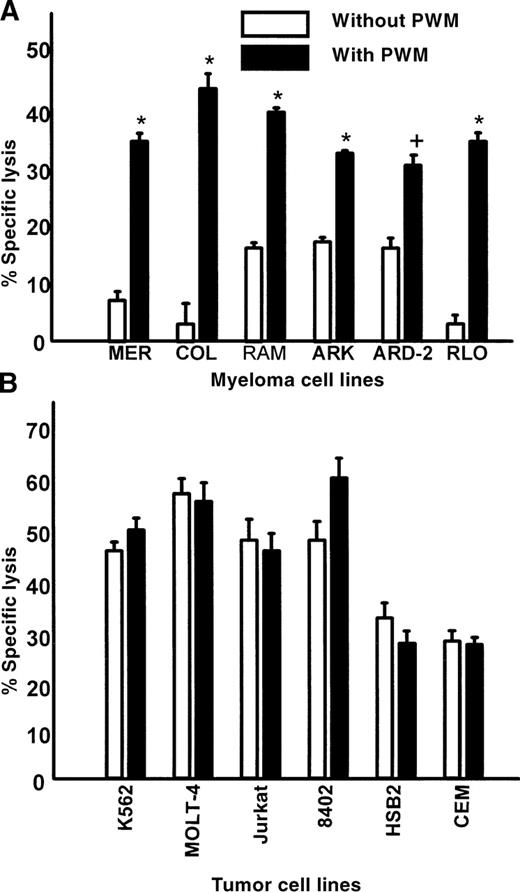
Six myeloma cell lines were treated with 1% PWM and then used as targets in standard 4-hour 51Cr release assays. Figure3A shows
Myeloma cell lines display enhanced susceptibility to lysis mediated by IL-2–activated PBMCs after PWM treatment; nonmyeloma cell lines are not significantly affected by PWM treatment.
(A) Myeloma cell lines MER, COL, RAM, ARK, ARD-2, and RLO were cultured with or without 1% PWM for 3 days and then used as targets in standard 4-hour 51Cr release assays. The E:T ratio was 30:1. The data represent mean values ± SEM of 3 individual experiments. (B) T-cell lines MOLT-4, Jurkat, 8402, HSB2, and CEM, as well as the erythroleukemia cell line K562, were used as targets in standard 4-hour51Cr release assays. Cells were treated with or without 1% PWM for 3 days. The E:T ratio was 30:1. The data represent mean values ± SEM of 3 individual experiments. Asterisk indicatesP < .0001; plus sign, P < .01.
Myeloma cell lines display enhanced susceptibility to lysis mediated by IL-2–activated PBMCs after PWM treatment; nonmyeloma cell lines are not significantly affected by PWM treatment.
(A) Myeloma cell lines MER, COL, RAM, ARK, ARD-2, and RLO were cultured with or without 1% PWM for 3 days and then used as targets in standard 4-hour 51Cr release assays. The E:T ratio was 30:1. The data represent mean values ± SEM of 3 individual experiments. (B) T-cell lines MOLT-4, Jurkat, 8402, HSB2, and CEM, as well as the erythroleukemia cell line K562, were used as targets in standard 4-hour51Cr release assays. Cells were treated with or without 1% PWM for 3 days. The E:T ratio was 30:1. The data represent mean values ± SEM of 3 individual experiments. Asterisk indicatesP < .0001; plus sign, P < .01.
that myeloma cell lines were significantly more sensitive to IL-2–activated effectors after treatment with PWM. There was a twofold to tenfold increase in susceptibility of myeloma cells to killing by IL-2–activated PBMCs after treatment with PWM. Similar results were also found for 6 of 7 fresh myeloma tumor cells and 1 primary chronic lymphocytic leukemia (CLL) sample treated with PWM (Table 1). To determine if the effect of PWM was exclusive to B-cell tumors, several cell lines of erythroid and T-cell origin were treated with PWM and used as targets. The susceptibility of these nonmyeloma cells to lysis remained unchanged after treatment with PWM (Figure 3B). These data suggest that the effects of PWM were relatively specific for myeloma and B-cell tumors.
PWM treatment increases the susceptibility of primary myeloma cells to cell-mediated cytolysis
| Target Cell . | Number of Effectors . | Percentage Lysis Without PWM . | Percentage Lysis With PWM . | Increase in CD9 . |
|---|---|---|---|---|
| MM1 | 4 | 1 ± 0.9 | 8 ± 3.4 | Yes |
| MM2 | 6 | 2 ± 2.7 | 11 ± 5.2 | Yes |
| MM3 | 5 | 1 ± 1.7 | 1 ± 1.4 | No |
| MM4 | 3 | 0 | 19 ± 3.0 | Yes |
| MM5 | 3 | 5 ± 3.5 | 40 ± 11.6 | Yes |
| MM6 | 2 | 3 ± 0.7 | 16 ± 0.7 | Yes |
| MM7 | 6 | 1 ± 2.0 | 27 ± 11.8 | Yes |
| CLL1 | 3 | 4 ± 2.8 | 22 ± 3.5 | Yes |
| Target Cell . | Number of Effectors . | Percentage Lysis Without PWM . | Percentage Lysis With PWM . | Increase in CD9 . |
|---|---|---|---|---|
| MM1 | 4 | 1 ± 0.9 | 8 ± 3.4 | Yes |
| MM2 | 6 | 2 ± 2.7 | 11 ± 5.2 | Yes |
| MM3 | 5 | 1 ± 1.7 | 1 ± 1.4 | No |
| MM4 | 3 | 0 | 19 ± 3.0 | Yes |
| MM5 | 3 | 5 ± 3.5 | 40 ± 11.6 | Yes |
| MM6 | 2 | 3 ± 0.7 | 16 ± 0.7 | Yes |
| MM7 | 6 | 1 ± 2.0 | 27 ± 11.8 | Yes |
| CLL1 | 3 | 4 ± 2.8 | 22 ± 3.5 | Yes |
Freshly isolated primary myeloma cells from 7 patients and tumor cells from 1 patient with CLL were cultured with or without 1% PWM for 3 days and then used as targets in 51Cr-release assays. Effector cells were IL-2–stimulated PBMCs from normal donors. E:T ratios were 30:1. The data represent the mean ± SD of the percentage of lysis values for the number of effectors tested with each tumor target. The ability of PWM to increase cell surface CD9 expression in the tumor cells by a minimum of 25% by fluorescence-activated cell sorter (FACS) analysis is indicated.
IL-2–stimulated NK and T cells are both effective at lysing myeloma tumor cells
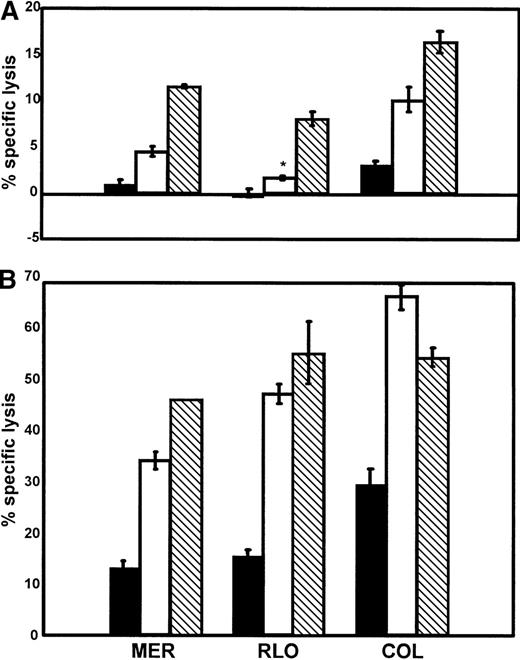
Purified NK and T cells were generated in order to determine which cell population was responsible for lysing the myeloma tumor cells. When either enriched NK or T cells were used as effectors in standard 4-hour 51Cr release assays, they were found to be significantly (P < .05) more robust in eliminating the myeloma tumor cell targets (Figure 4A) than IL-2–activated PBMCs. PWM-treated myeloma cells were also more sensitive to lysis by both enriched NK and T cells (Figure 4B). Uniformly, there was significantly (P < .05) greater cytolysis of PWM-treated versus untreated myeloma cells by all 3 effector-cell populations. These data indicate that IL-2–activated NK cells and T cells are both capable of killing PWM-treated myeloma tumor cells.
NK cells and T cells are significantly more effective at lysing PWM-treated myeloma tumor cell lines than untreated cells.
Untreated (A) or PWM-treated (B) myeloma tumor cell lines (MER, COL, RLO) were used as targets in 4-hour 51Cr release assays. Enriched NK (□) and T () cells were generated as described in “Materials and methods” and then used as effectors along with total PBMCs (▪). All effectors were cultured in the presence of IL-2 for at least 3 days prior to use. E:T ratios are 30:1. The data represent mean values ± SEM of 4 individual experiments. P< .05. *Indicates not significant.
NK cells and T cells are significantly more effective at lysing PWM-treated myeloma tumor cell lines than untreated cells.
Untreated (A) or PWM-treated (B) myeloma tumor cell lines (MER, COL, RLO) were used as targets in 4-hour 51Cr release assays. Enriched NK (□) and T () cells were generated as described in “Materials and methods” and then used as effectors along with total PBMCs (▪). All effectors were cultured in the presence of IL-2 for at least 3 days prior to use. E:T ratios are 30:1. The data represent mean values ± SEM of 4 individual experiments. P< .05. *Indicates not significant.
PWM treatment does not enhance binding of myeloma cells to effectors
Conjugate binding assays were performed to ascertain whether the enhanced susceptibility of PWM-treated cells to lysis mediated by IL-2–activated PBMCs was the result of greater binding of these targets to effectors. Myeloma target cells (MER, COL) were either untreated or treated with PWM. PBMCs from healthy individuals were used to generate IL-2–activated PBMCs; these cells were then labeled with a lipophilic fluorescent dye, PKH26, to allow identification of effectors. Targets and effectors were combined in a 1:1 ratio and allowed to bind for a period of 5 to 10 minutes at 37°C. Aliquots were then removed for analysis; the number of unbound and bound effectors was counted. A total of at least 1000 effectors were counted in 3 experiments. The percentage of bound cells was not significantly altered when tumor cells were treated with PWM (Table2). There were also no significant differences observed when targets or effectors were varied. Simultaneous standard 4-hour 51Cr release assays were conducted that showed that PKH26-labeled effectors were equally effective at lysing myeloma cell targets as their unlabeled counterparts (data not shown). These results suggest that the enhanced susceptibility of PWM-treated cells to lysis by IL-2–activated PBMCs is not due to increased binding of targets to effectors.
Conjugate binding assays
| Targets . | Effectors . | |
|---|---|---|
| Donor 1 . | Donor 2 . | |
| COL | 25 ± 2.1 | 27 ± 2.3 |
| COL + PWM | 21 ± 2.7 | 25 ± 5.5 |
| MER | 22 ± 2.4 | 22 ± 3.0 |
| MER + PWM | 20 ± 4.2 | 23 ± 4.0 |
| Targets . | Effectors . | |
|---|---|---|
| Donor 1 . | Donor 2 . | |
| COL | 25 ± 2.1 | 27 ± 2.3 |
| COL + PWM | 21 ± 2.7 | 25 ± 5.5 |
| MER | 22 ± 2.4 | 22 ± 3.0 |
| MER + PWM | 20 ± 4.2 | 23 ± 4.0 |
PWM treatment of myeloma cells did not result in increased binding to effectors. Myeloma cell lines COL and MER were used as targets. IL-2–activated PBMC effectors from 2 healthy donors were labeled with the fluorescent lipophilic dye PKH26. The E:T-cell ratio was 1:1. Effectors and targets were allowed to bind for 5 to 10 minutes at 37°C prior to counting.
These data represent the percentage binding ± SEM for 3 individual experiments with each donor. At least 1000 effectors were counted for each E:T combination. The differences were not statistically significant.
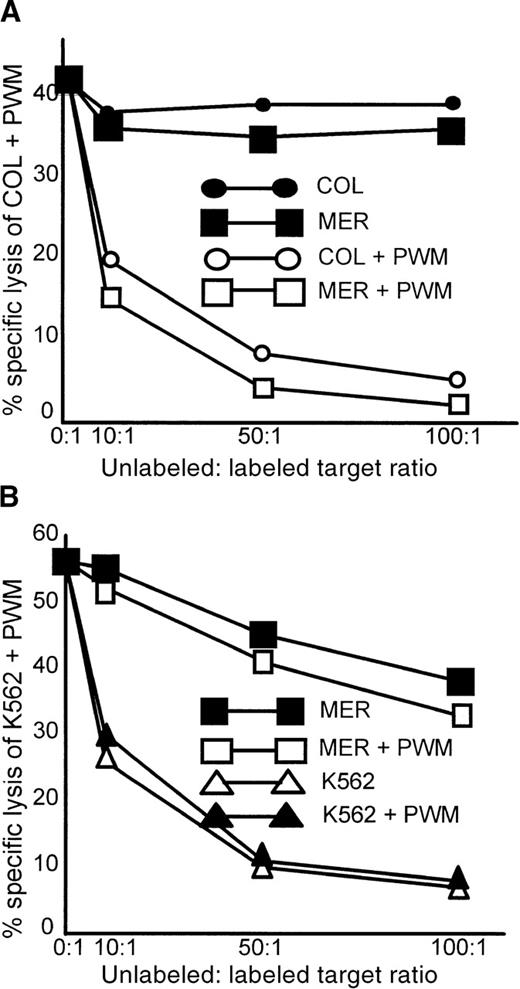
A common determinant appears to be responsible for enhanced susceptibility to lysis of these myeloma cell lines
Cold-target inhibition assays were performed with the use of the myeloma cell lines COL and MER. COL treated with PWM (COL + PWM) was the labeled target. Unlabeled competitors were COL, COL + PWM, MER, and MER + PWM (Figure5A). Only PWM-treated competitors were able to inhibit lysis of the labeled target. Especially notable was the fact that MER cells treated with PWM were equally capable of inhibiting the lysis of COL + PWM. Although MER cells were unrelated to COL cells, they were still able to completely inhibit lysis of the labeled target.
Only PWM-treated myeloma cell lines can inhibit the lysis of PWM-treated, labeled myeloma cells.
Lysis of the nonmyeloma cell line K562 + PWM cannot be inhibited by myeloma cell lines. Cold-target inhibition assays were performed as described in “Materials and methods.” (A) The labeled target was COL + PWM, and unlabeled competitors were MER, MER + PWM, COL, and COL + PWM. The data represent the mean of triplicate values of 1 of 3 similar experiments. (B) The labeled target was the erythroleukemia cell line K562 treated with PWM. Unlabeled competitors were K562, K562 + PWM, MER, and MER + PWM. The data represent the mean of triplicate values from 1 of 2 similar experiments.
Only PWM-treated myeloma cell lines can inhibit the lysis of PWM-treated, labeled myeloma cells.
Lysis of the nonmyeloma cell line K562 + PWM cannot be inhibited by myeloma cell lines. Cold-target inhibition assays were performed as described in “Materials and methods.” (A) The labeled target was COL + PWM, and unlabeled competitors were MER, MER + PWM, COL, and COL + PWM. The data represent the mean of triplicate values of 1 of 3 similar experiments. (B) The labeled target was the erythroleukemia cell line K562 treated with PWM. Unlabeled competitors were K562, K562 + PWM, MER, and MER + PWM. The data represent the mean of triplicate values from 1 of 2 similar experiments.
To determine whether nonmyeloma cells behaved in a similar manner, the NK-susceptible erythroleukemia cell line K562 was treated with PWM and used as a labeled target in a cold-target inhibition assay (Figure 5B). K562 and K562 + PWM, as well as the myeloma cell line MER and MER + PWM, were used as competitors. Lysis of the labeled target, K562 + PWM, was equally inhibited by both PWM-treated and untreated K562 cells. Neither untreated nor PWM-treated MER cells were able to significantly inhibit the lysis of K562 + PWM. This was true even at unlabeled-to-labeled target cell ratios of 100:1. PWM treatment had no effect on K562 cells and their susceptibility to lysis. This suggests that a common determinant is expressed by myeloma cells treated with PWM that is not found on untreated myeloma cells and not induced by PWM treatment of the nonmyeloma cell line K562.
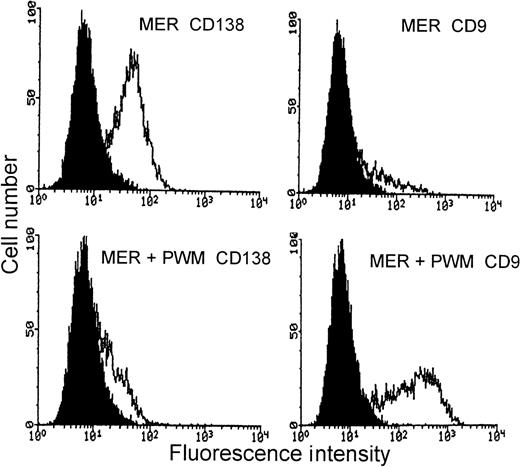
A phenotypic study of PWM-treated myeloma cells
To determine whether PWM induced phenotypic changes in myeloma cells that might be associated with their increased susceptibility to lysis by IL-2–activated effectors, flow cytometry studies were conducted with the use of a panel of 19 mAbs to various cell surface molecules (Table 3). These included markers expressed on B cells at some stage of their differentiation and adhesion molecules that are known to be involved in cell-to-cell interactions, such as CD11a (leukocyte function–associated antigen 1 [LFA-1]), CD54 (intercellular adhesion moledule 1 [ICAM-1]), CD49d (very late antigen 4 [VLA-4]), and CD80 (B7).33,34 Since MHC class I antigen expression is associated with sensitivity of cells to NK-mediated lysis,35 we looked at changes in both class I and class II MHC antigens in these studies. Associated with PWM treatment was an increase in CD9 expression and a decrease in CD138 (syndecan-1) expression. CD9 is a cell surface marker associated with the early stages of B-cell differentiation and is also found on the surface of eosinophils, basophils, platelets, megakaryocytes, and activated T and B cells.10,31,36,37 CD138, or syndecan-1, is expressed at high levels on myeloma cell lines and plasma cells, but is not expressed on Epstein-Barr virus–transformed B lymphoblastoid cells, myelomonocytic cells, or T-cell lines.38-40 Therefore, PWM treatment of myeloma cells resulted in a selective increase in the expression of CD9 and a decrease in the expression of CD138 (Figure6). Similar results with 4 other myeloma cell lines were also observed (data not shown).
Cell surface phenotype of MER cell line
| mAb . | Distribution (Function) . | MER . | MER + PWM . |
|---|---|---|---|
| Percentage of Positive Cells (RFI) . | |||
| CD3 | Mature T cells (associated with TCR) | 0 (1.07) | 0 (1.09) |
| CD5 | T cells, B-cell subset | 0 (0.97) | 0 (1.0) |
| CD9 | Platelets, pre-B cells, activated T cells | 21 (8.9) | 60 (34) |
| HLA-DR | (Class II MHC antigen) | 100 (6.4) | 100 (6.4) |
| CD10 | Lymphoid progenitors (common acute lymphoblastic leukemia antigen [CALLA]) | 0 (0.92) | 0 (1.0) |
| CD11a | Leukocytes (LFA-1, αL integrin chain) | 62 (9.1) | 75 (8.1) |
| CD11b | Granulocytes, monocytes, NK cells (mac-1, αM integrin chain) | 0 (1.0) | 0 (0.99) |
| CD18 | Leukocytes (β2 integrin chain) | 88 (14.3) | 94 (18.7) |
| CD19 | Pan B-cell | 94 (14.9) | 98 (28) |
| CD20 | Pan B-cell | 100 (6.3) | 100 (9.8) |
| HLA-A, B, and C | (Class I MHC antigen) | 100 (34.9) | 100 (16.5) |
| CD34 | Hematopoietic stem cells and progenitors | 0 (0.92) | 0 (1.0) |
| CD38 | Plasma cells, activated T cells | 95 (19.8) | 95 (18.3) |
| CD49d | Monocytes, B cells, T cells (VLA-4 α chain, α4 integrin chain) | 97 (23.2) | 99 (26.6) |
| CD54 | Activated cells (ICAM-1) | 90 (14.3) | 97 (18.7) |
| CD56 | NK cells and T cells, some myeloma plasma cells (isoform of N-CAM) | 0 (1.16) | 0 (0.94) |
| CD80 | Activated B cells and T cells, Burkitt's lymphomas (B:T cell interactions, B7) | 100 (11.4) | 100 (24.6) |
| CD138 | Plasma cells, syndecan-1, BB4 | 78 (5.3) | 28 (1.8) |
| Surface immunoglobulin | B cells | 100 (2.7) | 100 (2.2) |
| mAb . | Distribution (Function) . | MER . | MER + PWM . |
|---|---|---|---|
| Percentage of Positive Cells (RFI) . | |||
| CD3 | Mature T cells (associated with TCR) | 0 (1.07) | 0 (1.09) |
| CD5 | T cells, B-cell subset | 0 (0.97) | 0 (1.0) |
| CD9 | Platelets, pre-B cells, activated T cells | 21 (8.9) | 60 (34) |
| HLA-DR | (Class II MHC antigen) | 100 (6.4) | 100 (6.4) |
| CD10 | Lymphoid progenitors (common acute lymphoblastic leukemia antigen [CALLA]) | 0 (0.92) | 0 (1.0) |
| CD11a | Leukocytes (LFA-1, αL integrin chain) | 62 (9.1) | 75 (8.1) |
| CD11b | Granulocytes, monocytes, NK cells (mac-1, αM integrin chain) | 0 (1.0) | 0 (0.99) |
| CD18 | Leukocytes (β2 integrin chain) | 88 (14.3) | 94 (18.7) |
| CD19 | Pan B-cell | 94 (14.9) | 98 (28) |
| CD20 | Pan B-cell | 100 (6.3) | 100 (9.8) |
| HLA-A, B, and C | (Class I MHC antigen) | 100 (34.9) | 100 (16.5) |
| CD34 | Hematopoietic stem cells and progenitors | 0 (0.92) | 0 (1.0) |
| CD38 | Plasma cells, activated T cells | 95 (19.8) | 95 (18.3) |
| CD49d | Monocytes, B cells, T cells (VLA-4 α chain, α4 integrin chain) | 97 (23.2) | 99 (26.6) |
| CD54 | Activated cells (ICAM-1) | 90 (14.3) | 97 (18.7) |
| CD56 | NK cells and T cells, some myeloma plasma cells (isoform of N-CAM) | 0 (1.16) | 0 (0.94) |
| CD80 | Activated B cells and T cells, Burkitt's lymphomas (B:T cell interactions, B7) | 100 (11.4) | 100 (24.6) |
| CD138 | Plasma cells, syndecan-1, BB4 | 78 (5.3) | 28 (1.8) |
| Surface immunoglobulin | B cells | 100 (2.7) | 100 (2.2) |
RFI indicates relative fluorescence intensity. The percentage and level of expression of 19 cell surface molecules is shown for MER and MER + PWM. MER cells were labeled with mAbs specific for each cell surface molecule as described in “Materials and methods.” Labeled cells were analyzed by flow cytometry. The data show the percentage of positive cells and the RFI. RFI = 10(Experimental MFI/256 − Control MFI/256) where 256 indicates the number of gates used in the analysis and MFI indicates mean fluorescense intensity.
Expression of CD9 and CD138 cell surface markers on PWM-treated MER myeloma cells.
Top panels represent untreated MER cells. The lower panels represent MER cells treated with 1% PWM for 3 days. In the left panels, MER cells are labeled with the anti-CD138 mAb BB4. In the right panels, MER cells are labeled with the anti-CD9 mAb ALB6. The curve on the left in each panel is IgG1 control antibody, and the curve on the right in each panel represents the expression of the cell surface marker of interest.
Expression of CD9 and CD138 cell surface markers on PWM-treated MER myeloma cells.
Top panels represent untreated MER cells. The lower panels represent MER cells treated with 1% PWM for 3 days. In the left panels, MER cells are labeled with the anti-CD138 mAb BB4. In the right panels, MER cells are labeled with the anti-CD9 mAb ALB6. The curve on the left in each panel is IgG1 control antibody, and the curve on the right in each panel represents the expression of the cell surface marker of interest.
Primary myeloma cells from 51 patients were analyzed for CD9 expression. Tumor cells from 43% of patients (22/51) lacked cell surface CD9 expression. Primary tumor cells from 6 of these patients were incubated for 3 days with PWM (Table 1). PWM induced CD9 expression in 5 of the 6 tumor samples, with 25% to 68% of the cells becoming CD9-positive. The increase in CD9 expression in the 5 primary myeloma tumor cells directly correlates with their increase in susceptibility to cell-mediated cytolysis (Table 1).
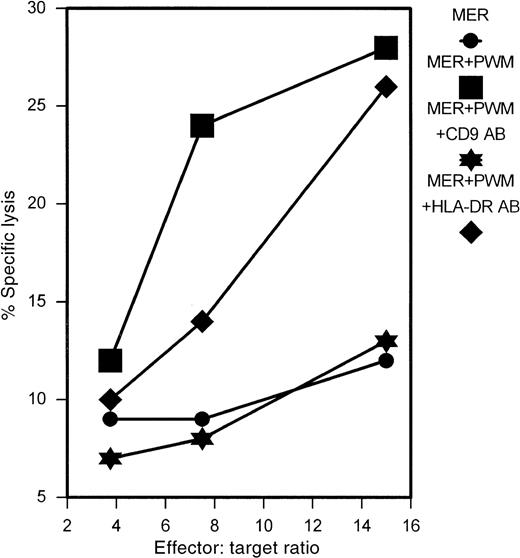
mAb and oligonucleotides directed against CD9 abrogate the effects of PWM
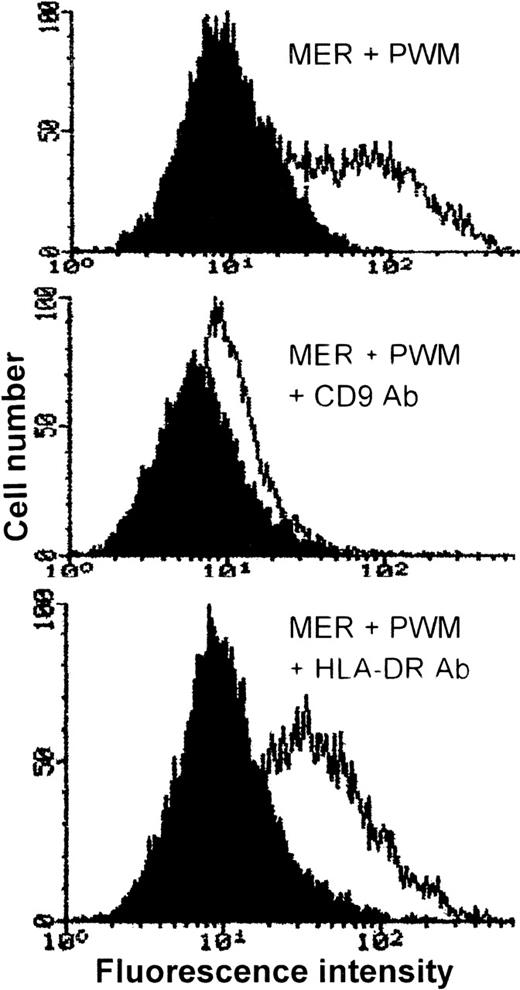
To determine whether the PWM-induced expression of CD9 was responsible for the enhanced sensitivity of myeloma cells to cytolysis, we attempted to block CD9 effects with antibody and antisense oligonucleotides. Using the mAb ALB6, directed against CD9, we were able to completely abrogate the effects of PWM on the myeloma cell line MER, as shown in Figure 7. Incubation of MER cells with an isotype-matched HLA-DR–specific antibody had no effect. To further confirm the specificity of the effect, cells were stained with CD9 after the 24-hour antibody treatment. As shown in Figure 8, there was a selective down-regulation of cell surface CD9 expression in MER cells treated with anti-CD9 antibody for 24 hours.
Treatment of MER myeloma cells with anti-CD9 mAb ALB6 abrogates the effects of PWM.
MER cells were incubated in the presence of ALB6 or anti–HLA-DR mAb (10 μg/mL) for 24 hours. PWM (1%) was then added, and the cells were allowed to incubate for an additional 2 hours prior to labeling with51Cr. Cells were then used as targets in 4-hour51Cr release assays after extensive washing. This experiment represents 1 of 3 similar experiments.
Treatment of MER myeloma cells with anti-CD9 mAb ALB6 abrogates the effects of PWM.
MER cells were incubated in the presence of ALB6 or anti–HLA-DR mAb (10 μg/mL) for 24 hours. PWM (1%) was then added, and the cells were allowed to incubate for an additional 2 hours prior to labeling with51Cr. Cells were then used as targets in 4-hour51Cr release assays after extensive washing. This experiment represents 1 of 3 similar experiments.
Incubation of MER myeloma cells with anti-CD9 mAb, ALB6, downmodulates the cell surface expression of CD9.
MER cells were incubated in the presence of ALB6 (middle panel), anti-HLA DR mAb (10 μg/mL) (lower panel), or no antibody (upper panel) for 24 hours. PWM (1%) was then added to each culture, and the cells were allowed to incubate for an additional 2 hours prior to staining. The curve on the left in each panel is IgG1 control antibody, and the curve on the right represents CD9 expression.
Incubation of MER myeloma cells with anti-CD9 mAb, ALB6, downmodulates the cell surface expression of CD9.
MER cells were incubated in the presence of ALB6 (middle panel), anti-HLA DR mAb (10 μg/mL) (lower panel), or no antibody (upper panel) for 24 hours. PWM (1%) was then added to each culture, and the cells were allowed to incubate for an additional 2 hours prior to staining. The curve on the left in each panel is IgG1 control antibody, and the curve on the right represents CD9 expression.
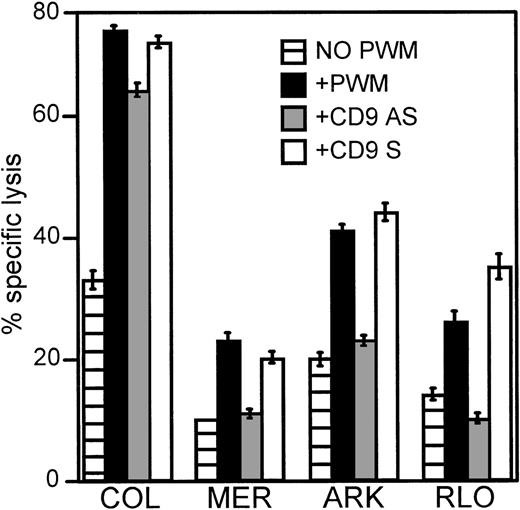
To further examine the role of CD9 in PWM-enhanced susceptibilty of myeloma cells to cytolysis, we treated myeloma cells with custom-made antisense oligonucleotides designed to bind an 18–base pair area located near the start site of the CD9 gene. In Figure9, myeloma cells were cultured in the presence of 150 μmol/L of antisense or sense oligonucleotides for a period of 2 hours at 37°C. PWM (1%) was then added, and the cells were incubated for an additional 2 hours. Cells were then washed and labeled with 51Cr. Untreated and PWM-treated cells were handled in a similar manner, but no oligonucleotides were introduced in their cultures. Using CD9 antisense oligonucleotides, we were able to completely abrogate the effects of PWM in 3 of 4 myeloma cell lines tested and reduce the effect in the other.
Antisense oligonucleotides against CD9 reduce or abrogate the enhanced susceptibility to lysis induced by PWM.
Myeloma cell lines COL, MER, ARK, and RLO were used as targets in 4-hour 51Cr release assays. These myeloma cells were first cultured in the presence of 150 μmol/L CD9 antisense or sense oligonucleotides for 2 hours followed by an additional 2 hours in the presence of 1% PWM prior to labeling with 51Cr. E:T ratios were 30:1. The data represents the mean values ± SEM of 3 similar experiments. P ≤ .01.
Antisense oligonucleotides against CD9 reduce or abrogate the enhanced susceptibility to lysis induced by PWM.
Myeloma cell lines COL, MER, ARK, and RLO were used as targets in 4-hour 51Cr release assays. These myeloma cells were first cultured in the presence of 150 μmol/L CD9 antisense or sense oligonucleotides for 2 hours followed by an additional 2 hours in the presence of 1% PWM prior to labeling with 51Cr. E:T ratios were 30:1. The data represents the mean values ± SEM of 3 similar experiments. P ≤ .01.
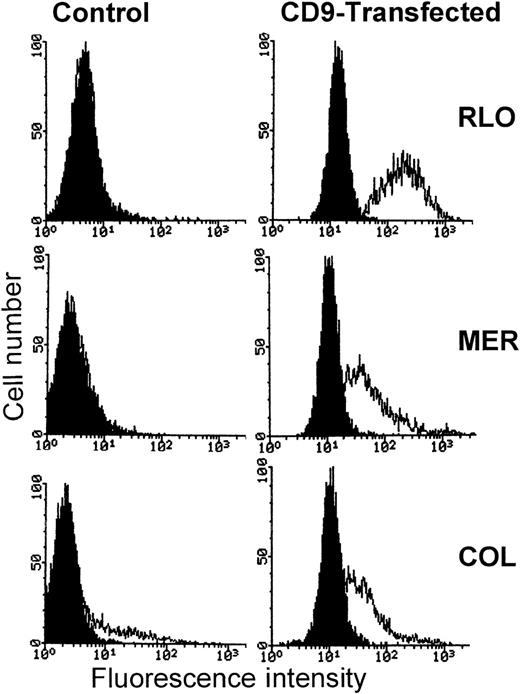
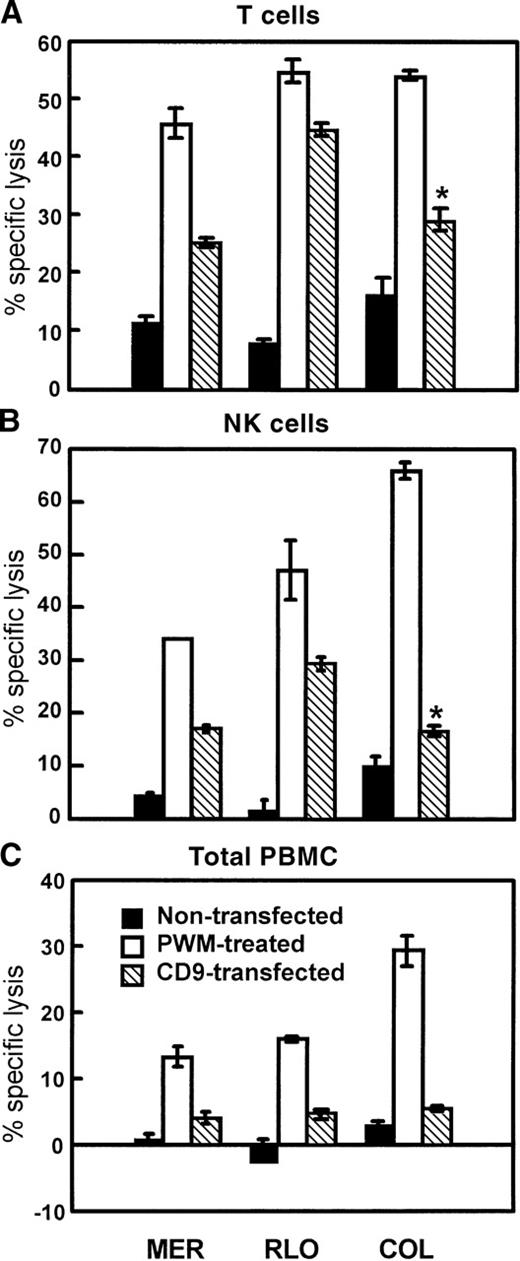
CD9-transfected myeloma cell lines are more sensitive to cell-mediated lysis than control transfected or nontransfected parental cells
Myeloma cell lines MER, RLO, and COL were stably transfected with pcDNA3 or a pcDNA3-CD9 construct by electroporation. Transfected cells were selected with the use of G418 at a concentration of 350 to 400 μg/mL. In Figure 10, fluorescence-activated cell sorter (FACS) analysis of CD9-transfected cells is shown. The expression of CD9 is greatly enhanced in the CD9-transfected cells compared with empty vector–transfected or nontransfected cell lines. PBMCs, purified T cells, and enriched NK cells were then used as effectors in 4-hour 51Cr release assays with CD9-transfected and control cells as targets. PWM-treated targets were used as positive controls. Figure11 demonstrates that CD9-transfected myeloma cell lines were significantly more sensitive to cell-mediated lysis than the nontransfected parental cell lines. Cells transfected with vector alone were equivalent in susceptibility and CD9 expression to the nontransfected parental cells (data not shown). The degree of enhanced susceptibility to cytolysis correlated with the degree of CD9 expression. As noted earlier, for the PWM-treated myeloma cell lines, CD9-transfected myeloma cells were also more susceptible to both T-cell– and NK-cell–mediated cytolysis.
Comparison of CD9 expression on the surface of CD9-transfected and parental myeloma cell lines.
Left panels represent parental cell lines. The right panels represent CD9-transfected cell lines. The curve on the left in each panel is IgG1 control antibody, and the curve on the right in each panel represents the expression of CD9 using antibody ALB6.
Comparison of CD9 expression on the surface of CD9-transfected and parental myeloma cell lines.
Left panels represent parental cell lines. The right panels represent CD9-transfected cell lines. The curve on the left in each panel is IgG1 control antibody, and the curve on the right in each panel represents the expression of CD9 using antibody ALB6.
The sensitivity of CD9-transfected myeloma cells to cell-mediated cytolysis is significantly greater than nontransfected parental cell lines.
Nontransfected, untreated, PWM-treated, and CD9-transfected cells were used as targets in 4-hour 51Cr release assays. IL-2–activated T cells (A), IL-2–activated NK cells (B), and IL-2–activated PBMCs (C) were used as effectors. E:T ratios were 30:1. The data represents the mean values ± SEM of 4 similar experiments.P ≤ .01. Asterisk indicatesP ≤ .05.
The sensitivity of CD9-transfected myeloma cells to cell-mediated cytolysis is significantly greater than nontransfected parental cell lines.
Nontransfected, untreated, PWM-treated, and CD9-transfected cells were used as targets in 4-hour 51Cr release assays. IL-2–activated T cells (A), IL-2–activated NK cells (B), and IL-2–activated PBMCs (C) were used as effectors. E:T ratios were 30:1. The data represents the mean values ± SEM of 4 similar experiments.P ≤ .01. Asterisk indicatesP ≤ .05.
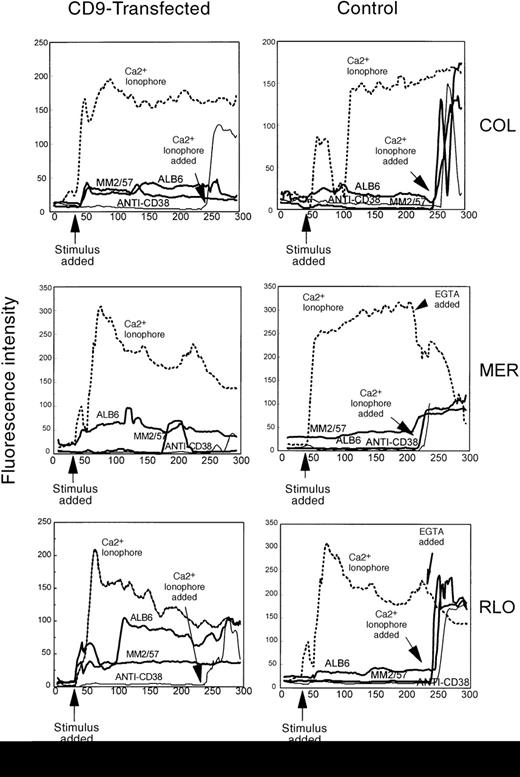
Enhanced susceptibility to cytolysis of CD9-transfected myeloma cells correlates with an increase in calcium influx
In Figure 12, control and CD9-transfected myeloma cells were loaded with the calcium-sensitive fluorescent dye fluo3/AM and placed in media containing Ca++. Upon addition of anti-CD9 antibody, intracellular calcium levels were much higher in the CD9-transfected myeloma cell lines. Both antibodies directed against CD9, ALB6 (IgG1) and MM2/57 (IgG2a), elicited a response. Anti-CD38 antibody was used as a negative control since CD38 is expressed equally on the surface of both transfected and nontransfected myeloma cells (data not shown). Anti-CD38 antibody did not induce a change in intracellular calcium levels in any of the cells tested. Myeloma cell lines with the highest cell surface levels of CD9 appeared to allow the highest levels of Ca++ to enter the cells, suggesting that CD9 may act as a Ca++ channel. When cells were placed in media lacking Ca++ or when EGTA was added prior to stimulation by the anti-CD9 antibodies ALB6 or MM2/57, no response was observed (data not shown). This suggests that the increase in intracellular Ca++ induced by anti-CD9 antibodies was due to the influx of Ca++ from outside the cells, and not due to the mobilization of intracellular stores of calcium.
Increased calcium flux in CD9-transfected myeloma cell lines.
Nontransfected and CD9-transfected myeloma cell lines COL, MER, and RLO were loaded with fluo-3/AM and placed in 8-chamber slides coated with poly-L-lysine. Both nontransfected and CD9-transfected cells were stimulated with anti-CD9 antibodies (ALB6 and MM2/57, 20 μg/mL), Ca++ ionophore A23187 (5 μmol/L), and anti-CD38 antibodies (25 μg/mL). Data acquisition and analysis were performed on a Meridian ACAS 570 image analyzer. The data represent 4 to 5 of at least 20 cells observed with similar results from a total of 5 individual experiments.
Increased calcium flux in CD9-transfected myeloma cell lines.
Nontransfected and CD9-transfected myeloma cell lines COL, MER, and RLO were loaded with fluo-3/AM and placed in 8-chamber slides coated with poly-L-lysine. Both nontransfected and CD9-transfected cells were stimulated with anti-CD9 antibodies (ALB6 and MM2/57, 20 μg/mL), Ca++ ionophore A23187 (5 μmol/L), and anti-CD38 antibodies (25 μg/mL). Data acquisition and analysis were performed on a Meridian ACAS 570 image analyzer. The data represent 4 to 5 of at least 20 cells observed with similar results from a total of 5 individual experiments.
Discussion
Previous reports have indicated that the stage of differentiation and activation of B cells influences their susceptibility to cell-mediated lysis.41 Therefore, in attempting to modulate the sensitivity of resistant myeloma cells to cell-mediated cytolysis, we treated myeloma cells with various proliferating, activating, and differentiating agents. PWM, one such agent, is known to induce the proliferation and differentiation of B cells.8 9 We have shown that the susceptibility of myeloma cells to IL-2–activated PBMCs can be significantly increased by treatment with PWM. The induction of sensitivity to lysis by PWM appears to be relatively selective for myeloma cells or B-cell tumors, as evidenced by the lack of enhanced susceptibility to lysis by a panel of nonmyeloma cell lines after treatment with PWM. Increased binding of targets to effectors does not appear to be the cause of enhanced sensitivity to killing mediated by IL-2–activated PBMCs. PWM-treated myeloma cells are more sensitive to lysis by IL-2–activated PBMCs, T cells, and NK cells.
The results of cold-target inhibition assays suggested that enhanced susceptibility to lysis of myeloma cells was due to a newly acquired cell surface determinant that was not induced in nonmyeloma cells treated in a similar manner. Previous studies have correlated decreases in MHC class I antigen with enhanced NK sensitivity.35Adhesion molecules such as CD54 (ICAM-1), CD11a (LFA-1), CD49d (VLA-4), and others also play a role in cytolysis mediated by T cells and NK cells.33 42 The expression of these adhesion molecules was found to remain unchanged after PWM treatment of myeloma cells. These findings, along with the results of conjugate binding assays, suggest that a postbinding event contributes to the enhanced cytolysis of PWM-treated myeloma cells.
From a panel of 19 cell surface molecules tested, the levels of 2 cell surface molecules were observed to change dramatically upon treatment with PWM. CD9 levels were found to increase and CD138 levels to decrease in cells treated with PWM. CD138, or syndecan-1, is expressed by mature plasma cells and myeloma cells.38-40 However, CD9 is a pre-B cell marker. Its expression is lost upon B-cell differentiation, but then reappears upon B-cell activation.37,43 CD9 is also constitutively expressed on megakaryocytes and platelets.10,31 36 Although PWM treatment induces myeloma cells to express less CD138, the levels of CD38 and surface immunoglobulin remained unchanged. Therefore, these results do not strictly support the hypothesis that a less mature phenotype correlates with greater susceptibility to lysis. There was neither a significant change in the percentage of positive cells nor a shift in fluorescence intensity for most of the other cell surface molecules examined.
PWM treatment induced a minor change in the levels of expression of MHC class I and CD80 (B7). The decrease in the intensity of MHC class I expression and increase in the intensity of CD80 expression are consistent with a more sensitive phenotype. These molecules may participate in the enhancement of susceptibility of myeloma cells to cell-mediated cytolysis. However, the most dramatic phenotypic change was in CD9 expression. The percentage of cells expressing CD9 increased threefold, and the cell surface intensity increased nearly fourfold. It was, therefore, of great interest to examine and identify the potential role of this molecule in target-effector interactions.
The function of CD9 is unknown; however, anti-CD9 antibodies have been shown to induce the aggregation and activation of platelets.12,14,15 Antibodies directed against CD9 have been shown to initiate the influx of Ca++ in platelets.17,19,20 Studies have also demonstrated that anti-CD9 antibodies are involved in increasing diacylglycerol formation, phosphoinositide hydrolysis, and protein phosphorylation.44,45 Since previous studies have implicated CD9 in signal transduction,15 44-46 it is interesting to speculate that a similar role for CD9 in enhancing the sensitivity of myeloma cells to killing by IL-2–activated PBMCs may exist. This possibility appears plausible, particularly in view of the lack of increased binding of PWM-treated myeloma cells to IL-2–activated effectors and the increased calcium flux elicited by anti-CD9 antibodies.
Studies using antibodies to downmodulate cell surface CD9 expression and antisense oligonucleotides directed against CD9 illustrate the need for the expression of CD9 in order for myeloma cells to be sensitive to IL-2–activated T cells, NK cells, and PBMCs. CD9-transfected myeloma cells were also found to be more susceptible to cytolysis than control transfected or nontransfected counterparts. This does not discount the possibility that CD9 is acting in association with other cell surface or intracellular molecules to achieve its effects. Many studies have shown association of CD9 with integrins and other molecules.10 46-48 CD9, which is a member of the transmembrane 4 superfamily, has a structure that resembles the structure of a transport molecule. As others have shown in platelets and as we have shown in CD9-transfected myeloma cells, CD9 appears to be involved in initiating calcium flux in these cells. This suggest that CD9 may be a Ca++ channel or may physically associate with Ca++ channels on the cell surface.
Since calcium plays an important role in a variety of cellular events, including cell-mediated cytotoxicity,49 50 we hypothesize that an increase in calcium flux in myeloma cells that express high levels of CD9 may induce greater sensitivity to cytolysis. As we have shown, CD9-transfected cells express higher levels of CD9 and are more susceptible to lysis by PBMCs, T cells, and NK cells. Anti-CD9 antibodies induce an influx of calcium when introduced into the media of CD9-transfected cells. One possibility is that CD9 may aid in the recruitment of calcium to the area of target-effector contact.
Whether the effects of CD9 are mediated through G proteins or through the activation of kinases or whether CD9 is itself a Ca++channel is still unresolved. It will be important to determine whether the increased Ca++ influx initiates an apoptotic cascade or whether the mere presence of higher levels of Ca++ is sufficient to allow effectors to lyse these tumor cells more efficiently. The latter seems to be more likely as the addition of CD9 antibody to transfected myeloma cells in the presence of media containing Ca++ was not observed to cause any cytotoxicity. Further studies are needed to ascertain the mechanism by which CD9 and the calcium flux it initiates are able to produce a more sensitive phenotype in myeloma cells. Understanding the details of these processes and the molecules involved will aid in the construction of more effective therapies for the treatment of neoplasias, such as multiple myeloma.
We have established a system for modulating the sensitivity of myeloma cells to killing mediated by IL-2–activated PBMCs. Such studies may enable investigators to design more efficient therapies that can circumvent the immunoresistance of neoplastic cells. Although PWM has limited utility as a therapeutic agent, analysis of drugs and biological modifiers for their ability to augment CD9 expression in myeloma cells may lead to new treatments. It also allows us to study cell surface molecules, such as CD9, to gain a better understanding of their function and their importance in cell-mediated cytolysis.
Acknowledgments
We thank Dr Claude Boucheix for donating mAb ALB6, which recognizes CD9, and for providing us with the CD9 expression vector, and Dr J. Wijdenes for donating the anti-CD138 mAb BB4. We also thank Jeff Woodliff for his expert flow cytometry analysis and Priscilla Gray for her technical assistance. Finally, we thank Dr Alberto Bianchi for the preliminary work performed and Dr Joshua Epstein for providing us with myeloma cell lines and advice throughout this project.
Supported in part by funding from the National Institutes of Health (grant CA62201), the Veterans Administration, and the University of Arkansas for Medical Sciences Graduate Student Research Fund.
Reprints:Jacki Kornbluth, Department of Pathology, St Louis University School of Medicine, 1402 South Grand Blvd, St Louis, MO 63104.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal