Abstract
Endotoxin tolerance (ET) has been described as a temporary alteration in the lipopolysaccharide (LPS) response of monocytic cells after an initial LPS exposure with respect to the production of soluble immunomodulators. Apart from the LPS response, monocytic cells play an important role in initiation of the specific immune response as antigen-presenting cells. This study investigated the capacity of human blood monocytes to induce T-cell stimulation in ET. First, the expression of monocyte surface molecules, important for T-cell interaction, was analyzed by flow cytometry. In vitro priming of peripheral blood mononuclear cells with LPS clearly down-regulates major histocompatibility complex class II molecules and the costimulatory molecule CD86. Both changes were dependent on the endogenous interleukin (IL)-10 and less so on the transforming growth factor-β. In contrast, other accessory molecules on monocytes were only marginally down-regulated (CD58), were not significantly changed during ET (CD40), or even remained up-regulated after initial LPS priming (CD54, CD80). Second, an impact of these phenotypic alterations on the accessory function of monocytes was observed. This was manifested as diminished T-cell proliferation and interferon (IFN)-γ release in response to the presence of different recall antigens. Neutralizing IL-10 during LPS priming prevented the diminished T-cell IFN-γ production but had little effect on T-cell proliferation. These data confirm that ET is an appropriate model of the monocyte functional state in immunoparalysis, which is frequently observed in patients after septic shock, trauma, or major surgery.
Endotoxin tolerance (ET) was first described as the temporary insensitivity of a host to a repeated lipopolysaccharide (LPS) challenge with respect to systemic inflammation parameters. This phenomenon is associated with functional alterations to the monocytic cells1 and can be imitated in vitro. After pretreatment with a first activating LPS dose, a second LPS stimulation of monocytic cells causes a reduced production of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-12, IL-6, as well as nitric oxide and IL-10 when compared with non-LPS prestimulated controls.2-6
However, we and others have shown that this monocytic state does not represent a complete deactivation but rather a specific reorientation in the reaction of the cells to LPS. In response to a second LPS stimulation the production of IL-1 receptor antagonist (IL-1RA) was not altered,5 and TNF receptor II gene expression was actually increased.7
We recently demonstrated that the reduced monocyte TNF-α production capacity observed during ET is mediated by IL-10 and transforming growth factor (TGF)-β produced during the primary LPS stimulation. In fact, neutralization of endogenous IL-10 and TGF-β during LPS priming prevented the loss of LPS-induced TNF-α production in peripheral blood mononuclear cells (PBMCs).5 Furthermore, exposure to IL-10 in combination with TGF-β could mimic ET in terms of the reduced production of TNF-α and IL-10.5,8In contrast, neutralization of endogenous IL-10 and TGF-β failed to prevent reduced monocytic IL-12 production capacity, indicating other factors are involved in the initiation of ET.6
In contrast to previous studies, which defined the functional state of monocytic cells during ET only in terms of the altered capacity to produce soluble immunomodulators, we intended to characterize LPS-tolerized monocytes in terms of their antigen-presenting capacity. Here we show the differentially altered expression of human monocyte molecules important for T-cell stimulation after an initial in vitro exposure of total PBMCs to LPS. We also determined the capacity of these primed monocytes to stimulate various T-cell responses. Furthermore, we tested the role of endogenous mediators produced during the initial LPS exposure in the alterations expressed by the monocytes.
Materials and methods
Preparation and culture of PBMCs
Human PBMCs from various healthy donors were isolated from citrated venous blood by density gradient centrifugation using Ficoll-Paque (Pharmacia, Freiburg, Germany). Cells were usually cultured at a concentration of 106 cells/mL in RPMI 1640 medium tested for very low (< 0.01 EU/mL) endotoxin content (Biochrom KG, Berlin, Germany) supplemented with 10% (v/v) fetal bovine serum and 2 mmol/L glutamine (both from Biochrom KG). For inhibiting monocyte adherence, culture vessels were coated with use of 100 μL/cm2 of 1% (w/v) poly(2-hydroxyethylmethacrylate) (ICN Biomedicals, Eschwege, Germany) as described elsewhere.9
If not otherwise indicated, PBMCs were primed with 2 ng/mL LPS fromEscherichia coli 0127 B8 (Sigma-Aldrich Chemie, Deisenhofen, Germany) or were cultured without stimulation (control group) for the first 24 hours. For neutralization experiments either 5 μg/mL anti-IL-10 monoclonal antibody (MoAb) (clone CB/RS/1),10anti-TGF-β MoAb (clone 1D11.16; Genzyme, Munich, Germany),5 or control murine immunoglobulin (Ig)G1 MoAb (clone 11 711.11; R&D Systems, Wiesbaden-Nordenstadt, Germany), were present during priming period.
For the following experiments cells were extensively washed and recultured under secondary culture conditions:
1. For flow cytometric analysis at 36, 48, 72, and 120 hours, reculturing was performed without any stimulation on coated culture vessels in medium as described previously.
2. For detection of monocyte TNF-α production capacity, PBMCs were cultured for another 12 hours without stimulation and subsequently exposed to LPS (100 ng/mL) for 6 hours, afterward culture supernatant was recovered for TNF-α measurement.
3. To assay T-cell stimulation, PBMCs were recultured as described below.
Flow cytometric analysis
To quantify the expression of monocyte surface molecules, PBMCs from primary culture (12 and 24 hours) and secondary culture (36, 48, 72, 96, and 120 hours) were stained with the following antibodies: R-phycoerythrin (PE)-labeled antihuman leukocyte antigen (HLA)-DR MoAb (clone L243; Becton Dickinson, Heidelberg, Germany), fluorescein isothiocyanate (FITC)-labeled anti-CD40 MoAb (clone 5C3; Pharmingen, Hamburg, Germany), PE-labeled anti-CD86 MoAb (clone 2331; Pharmingen), FITC-labeled anti-CD80 MoAb (clone BB1; Pharmingen), PE-labeled anti-CD58 MoAb (clone AICD58; Coulter Immunotech, Hamburg, Germany), FITC-labeled anti-CD54 MoAb (clone 84H10; Coulter Immunotech), or the respective isotype controls: FITC-labeled mouse IgG1 MoAb (clone 679.1Mc7; Coulter Immunotech), PE-labeled mouse IgG2a MoAb (clone X39; Becton Dickinson). To discriminate the monocyte population, additional staining with R-phycoerythrincyanin (PE-Cy5)-labeled anti-CD14 MoAb (clone RMO52; Coulter Immunotech) was performed.
To assess the major histocompatibility complex (MHC) class II expression patterns on monocytes at 36 hours, the following antibodies were used in addition to anti-HLA-DR MoAb and the respective isotope control: anti-HLA-DP MoAb (clone B7/21; Becton Dickinson), control murine IgG1 MoAb (clone 11711.11; R&D Systems) and PE-labeled goat antimouse IgG (H, L) F(ab′)2 (Jackson ImmunoResearch Laboratories, West Groove, PA), FITC-labeled anti-HLA-DQ MoAb (clone SK10; Becton Dickinson) and FITC-labeled mouse IgG1 MoAb (clone X40; Becton Dickinson).
The flow cytometric analyses were performed by means of a FACScan instrument with LYSYS II software (Becton Dickinson); 30,000 to 40,000 PBMCs per measurement were analyzed, and monocytes were gated based on their CD14 expression and light-scatter properties.
T-cell stimulation assay
To determine the capacity of monocytes to induce antigen-dependent T-cell stimulation, PBMCs were recultured in triplicate assays at 106 cells/mL in RPMI 1640 medium (see above) supplemented with 10% (v/v) human AB serum (Sigma-Aldrich Chemie) and 2 mmol/L glutamine (Biochrom KG) in the presence of either 2.5 μg/mL candidin (Allergopharma, Reinbek, Germany), 0.08 IU/mL tetanus toxoid (Tetasorbat SSW; SmithKline Beecham Pharma, Munich, Germany), 5 purified protein derivative (PPD)-S/mL tuberculin (Tuberkulin GT 100 Behring; Chiron Behring & Co, Marburg, Germany), or medium. To assay T-cell DNA synthesis, cells were labeled after 3 days or (in kinetic assays) after the indicated times for a further 15 hours with 1 μCi/mL [5′-3H]thymidine (Amersham Buchler & Co KG, Braunschweig, Germany) in the continued presence of antigen. After harvesting cells, the incorporated radioactivity was measured using a beta counter (Inotech; Dunn Labortechnik, Asbach, Germany). For detection of T-cell interferon (IFN)-γ production, supernatant was recovered after 4 days of antigen stimulation. Of note, at the onset of each T-cell proliferation assay the ratio of CD4+and CD14+ cells was verified to be similar in all groups (data not shown).
Cytokine quantification assays
Detection of TNF-α and IFN-γ concentrations in PBMC culture supernatant was realized by the commercially available enzyme-linked immunosorbent assay (ELISA) kits from Medgenix (Ratingen, Germany).
Statistical analysis
Statistical analyses were performed either by Wilcoxon matched-pairs signed-ranks test (Figure 1, Tables1 and 2), Friedman 2-way ANOVA (Figure 2), or Mann-Whitney U test (Figure 1) using SPSS software (SPSS, Chicago, IL).
LPS differentially alters surface expression of HLA-DR and accessory molecules in monocytes in a time-dependent manner.
PBMCs were cultured for the first 24 hours with 2 ng/mL or without LPS in the presence of either control murine IgG1, anti-IL-10, or anti-TGF-β MoAb and recultured afterward without further stimulation. Surface molecule expression as assessed by flow cytometry is presented as the ratio of the mean fluorescence intensities (MFI) between cells treated with or without LPS for each of the 3 antibodies. Mean data (± SEM) from 5 independent experiments are shown. Statistical analyses were performed for LPS/IgG1-treated groups by Wilcoxon matched-pairs signed-rank test (*P < .05 versus cultures at 0 hours) or for LPS/neutralizing MoAb-treated groups by Mann- Whitney U test (#P < .05, ##P < .01 for LPS/anti-IL-10 MoAb versus LPS/IgG1 MoAb-treated group at the same time point; +P < .05 for LPS/anti-TGF-β MoAb- versus LPS/IgG1 MoAb-treated group at the same time point), respectively.
LPS differentially alters surface expression of HLA-DR and accessory molecules in monocytes in a time-dependent manner.
PBMCs were cultured for the first 24 hours with 2 ng/mL or without LPS in the presence of either control murine IgG1, anti-IL-10, or anti-TGF-β MoAb and recultured afterward without further stimulation. Surface molecule expression as assessed by flow cytometry is presented as the ratio of the mean fluorescence intensities (MFI) between cells treated with or without LPS for each of the 3 antibodies. Mean data (± SEM) from 5 independent experiments are shown. Statistical analyses were performed for LPS/IgG1-treated groups by Wilcoxon matched-pairs signed-rank test (*P < .05 versus cultures at 0 hours) or for LPS/neutralizing MoAb-treated groups by Mann- Whitney U test (#P < .05, ##P < .01 for LPS/anti-IL-10 MoAb versus LPS/IgG1 MoAb-treated group at the same time point; +P < .05 for LPS/anti-TGF-β MoAb- versus LPS/IgG1 MoAb-treated group at the same time point), respectively.
Expression of different MHC class II species on monocytes is down-regulated during ET; a comparison with monocyte TNF- production capacity
| . | Control . | LPS primed . |
|---|---|---|
| HLA-DR [MFI] | 2427.4 ± 514.85 | 267.8 ± 86.21* |
| HLA-DQ [MFI] | 529.1 ± 164.15 | 66.6 ± 42.24* |
| HLA-DP [MFI] | 1731.5 ± 620.01 | 136.1 ± 49.67* |
| TNFα [pg/mL] | 5113.8 ± 2510.00 | 50.8 ± 16.57* |
| . | Control . | LPS primed . |
|---|---|---|
| HLA-DR [MFI] | 2427.4 ± 514.85 | 267.8 ± 86.21* |
| HLA-DQ [MFI] | 529.1 ± 164.15 | 66.6 ± 42.24* |
| HLA-DP [MFI] | 1731.5 ± 620.01 | 136.1 ± 49.67* |
| TNFα [pg/mL] | 5113.8 ± 2510.00 | 50.8 ± 16.57* |
PBMCs were cultured with (2 ng/mL) or without LPS for 24 hours and subsequently washed. Monocyte HLA-DR, HLA-DP, and HLA-DQ expression was analyzed by flow cytometry at 36 hours. In parallel, cells were restimulated at 36 hours with 100 ng/mL LPS and 6 hours later TNF-α concentrations in culture supernatant were assessed by ELISA. Mean (±SEM) data from 5 independent experiments are presented. Statistical analysis was performed by Wilcoxon matched-pairs signed-rank test (*P < .05 versus untreated cultures).
MFI indicates mean fluorescence intensity; TNF, tumor necrosis factor.
ET depresses T-cell proliferation and IFN-γ production in response to various protein recall antigens
| . | . | . | Control . | LPS primed . |
|---|---|---|---|---|
| A | 3H incorporation | Can | 29.5 ± 11.30 | 9.0 ± 4.15* |
| [specific cpm × 103/culture] | TT | 34.3 ± 9.55 | 5.2 ± 2.41* | |
| Tbc | 33.5 ± 9.73 | 3.5 ± 1.50* | ||
| B | IFN-γ [U/mL] | Can | 17.2 ± 10.80 | 3.3 ± 1.80* |
| TT | 9.5 ± 4.00 | 1.6 ± 0.20* | ||
| Tbc | 21.1 ± 5.70 | 2.2 ± 1.00* |
| . | . | . | Control . | LPS primed . |
|---|---|---|---|---|
| A | 3H incorporation | Can | 29.5 ± 11.30 | 9.0 ± 4.15* |
| [specific cpm × 103/culture] | TT | 34.3 ± 9.55 | 5.2 ± 2.41* | |
| Tbc | 33.5 ± 9.73 | 3.5 ± 1.50* | ||
| B | IFN-γ [U/mL] | Can | 17.2 ± 10.80 | 3.3 ± 1.80* |
| TT | 9.5 ± 4.00 | 1.6 ± 0.20* | ||
| Tbc | 21.1 ± 5.70 | 2.2 ± 1.00* |
PBMCs were cultured in the presence or absence of 2 ng/mL LPS. After 24 hours, cells were washed and recultured in the presence of either 2.5 μg/mL candidin (Can), 0.08 IU/mL tetanus toxoid (TT), 5 PPD-S/mL tuberculin (Tbc), or without stimulation. (A) Cellular [5′-3H]thymidine incorporation after 3 days of secondary culture is given as specific cpm (Figure 3). (B) IFN-γ concentrations in culture supernatant were assessed by ELISA after 4 days of secondary culture. Mean (±SEM) data from 6 independent experiments are given. Statistical analysis was performed by Wilcoxon matched-pairs signed-rank test (*P < .05 versus untreated cultures).
IFN indicates interferon; Can, candidin; TT, tetanus toxoid; Tbc, tuberculin.
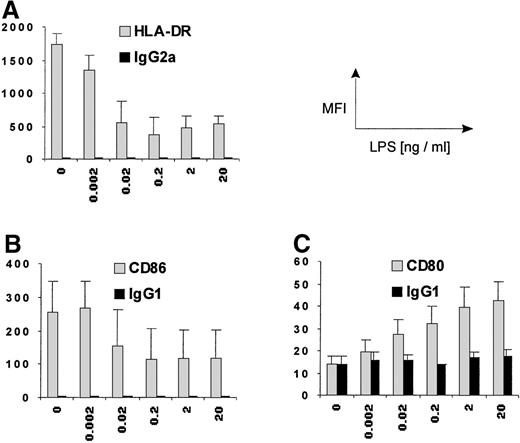
LPS priming alters surface expression of HLA-DR and costimulatory molecules in monocytes in a dose-dependent fashion.
PBMCs were cultured with different concentrations of LPS for 24 hours, and monocyte HLA-DR (A), CD86 (B), and CD80 (C) expression was assessed by flow cytometry. Mean (± SEM) data from 3 independent experiments are shown. Dark columns indicate the respective isotypic controls. For the expression of all 3 surface molecules, significant dose dependency was demonstrated by Friedman test (P < .05). Note the different scales for the 3 markers.
LPS priming alters surface expression of HLA-DR and costimulatory molecules in monocytes in a dose-dependent fashion.
PBMCs were cultured with different concentrations of LPS for 24 hours, and monocyte HLA-DR (A), CD86 (B), and CD80 (C) expression was assessed by flow cytometry. Mean (± SEM) data from 3 independent experiments are shown. Dark columns indicate the respective isotypic controls. For the expression of all 3 surface molecules, significant dose dependency was demonstrated by Friedman test (P < .05). Note the different scales for the 3 markers.
Results and discussion
Isolated human PBMCs, primed with a low-dose (2 ng/mL) of LPS during 24 hours and washed afterward, were LPS-tolerant for some days as assessed by the clearly reduced LPS-induced TNF-α production when compared with PBMCs with no LPS priming (Table 1). We investigated whether monocytes, rendered tolerant this way, demonstrated an altered expression of surface molecules important for T-cell activation.
The prerequisite for any antigen-specific activation of T cells is recognition of MHC-bound antigenic peptides expressed on antigen-presenting cells (APCs) by the T-cell receptor complex.11 In addition, costimulatory interactions, which complement signals transduced from the T-cell receptor complex, as well as adhesive interactions, which enable intimate intercellular contacts, are necessary for effective T-cell stimulation. One major costimulatory signal of resting T cells is provided through engagement of T-cell CD28 by B7 molecules (CD86, CD80). This interaction promotes T-cell IL-2 production and proliferation, and its absence inhibits T-cell responses and induces T-cell anergy or death.12,13 T-cell receptor signaling leads to the induction of CD154, the ligand of the accessory molecule CD40 on APCs. Expression of CD154 is further enhanced by CD28 engagement.14 The CD40/CD154 interaction seems to be especially important for T-cell activation if the APCs are B cells.15 Adhesion molecules like CD58 (leukocyte function-associated antigen-3) and CD54 (intercellular adhesion molecule-1) have been implicated not only in intercellular adhesion, but also in direct costimulation of T cells.16CD54-mediated costimulation was more effective in CD8+ than in CD4+ cells.17
In this study, therefore, we analyzed the expression of the surface molecules MHC class II, CD86, CD80, CD40, CD58, and CD54 on LPS-primed human monocytes using flow cytometry. We recently demonstrated the participation of IL-10 and TGF-β in the initiation of ET.5 Therefore, we simultaneously tested the influence of endogenous IL-10 and TGF-β during LPS priming on the expression of the monocyte surface molecules listed above using neutralizing MoAbs. Figure 1 shows the time course of monocyte expression patterns before (0 hour), during (12 and 24 hours), and after (36-120 hours) LPS priming in the presence of either control MoAb, anti-IL-10 MoAb, or anti-TGF-β MoAb. Expression patterns of LPS-primed monocytes are presented relative to the expression patterns of monocytes with no LPS priming at the same time point.
After an initial up-regulation within the first 12 hours, the expression of the MHC class II molecule HLA-DR had already decreased by the end of the LPS priming period and was strongly depressed during the following 4 days tested. The CD86 expression was moderately down-regulated after 12 hours of LPS exposure and further decreased until 48 hours. It had not recovered by the 4th or 5th day of culture. CD58 expression on monocytes was only moderately down-regulated after LPS priming. After an increase during initial LPS priming, monocyte CD40 expression corresponded to untreated controls; however, CD54 and CD80 expression remained up-regulated moderately and strongly, respectively. Similar LPS-induced alterations of surface expression were observed in purified monocytes (data not shown). The relative up-regulation of CD80 expression reflects the presence of only a small number of surface molecules because freshly isolated monocytes are weakly if at all positive for CD80 (Figure 2). Taking the down-regulation of CD86 into account, the totality of T-cell CD28 ligands was decreased in ET.
The effects were already evident with a few picograms per milliliter of LPS, that is, at concentrations below those found in plasma of patients with septic shock.18 Figure 2 shows the alterations of monocyte HLA-DR, CD86, and CD80 expression in a LPS dose-dependent manner.
Because we observed the strongest down-regulating effect of LPS priming on HLA-DR expression, we examined whether the expression of other monocyte MHC class II molecules was also diminished during ET. PBMCs were rendered tolerant by exposing them for the first 24 hours to 2 ng/mL LPS. As shown in Table 1, the down-regulation of HLA-DR at 36 hours was accompanied by a reduction of HLA-DP and HLA-DQ expression. No down-regulation of the expression of MHC class II molecules on B cells was observed in our study (data not shown).
The down-regulation of CD86 and CD58 expression could be prevented completely if endogenous IL-10 was neutralized during LPS priming (Figure 1). Anti-IL-10 MoAb increased and prolonged initial HLA-DR up-regulation but failed to fully prevent its down-regulation at later time points. The expression of CD40, CD80, and CD54 was further increased after IL-10 neutralization. The use of anti-TGF-β MoAb increased levels of all surface molecules tested during the LPS priming period but had marginal effect afterward. The combination of anti-TGF-β MoAb and anti-IL-10 MoAb did not amplify the effect of IL-10 neutralization alone, when tested with respect to HLA-DR and CD86 expression (data not shown).
IL-10 is produced by activated monocytes at high levels and has been described to act in a negatively autoregulatory fashion to limit the activation-induced proinflammatory state.19 The extent to which IL-10 counteracts the initial LPS-induced increase in expression of the analyzed monocyte surface molecules (Figure 1) reflects the differential sensitivity of these molecules toward IL-10 signaling in resting monocytic cells. In fact, IL-10 strongly down-regulates monocyte HLA-DR and CD86 expression but has only minor inhibitory effect on CD58, CD40, and CD54 expression (references 20-23 and our unpublished data). In contrast, IL-10 has been shown to moderately up-regulate monocyte CD80 expression.21 Thus, the differentially altered expression of monocyte surface molecules participating in T-cell interaction during ET reflects, at least partly, the autoregulatory action of endogenous IL-10.
We also investigated whether these changes in monocyte expression had an effect on their capacity to present antigen to autologous T cells in an MHC class II-dependent manner. If this were the case, reduction of monocytic MHC class II and CD86 expression should limit monocyte accessory function in ET. After LPS priming, PBMCs were recultured in the presence or absence of the recall antigen candidin for 1, 2, 3, and 4 days. Induction of T-cell proliferation was determined by measuring cellular incorporation of 3H-thymidine, which is a direct indicator of DNA synthesis. Figure 3 shows reduced antigen-specific DNA synthesis in LPS-primed PBMC cultures compared to unprimed controls, which was observed after 3 days.
The capacity of monocytes to stimulate T-cell DNA synthesis in response to the protein recall antigen candidin is diminished during ET; study of different kinetics.
PBMCs were cultured in the presence or absence of 2 ng/mL LPS. Cells were washed and recultured at 24 hours with (2.5 μg/mL) or without candidin. After the time periods indicated, cells were pulsed with [5′-3H]thymidine for a further 15 hours. Results of the cellular radioactive labeling are presented as specific cpm (the difference between cpm of culture in the presence of antigen and cpm of culture in the absence of antigen). Representative data from 1 of 3 independent experiments in triplicate assays (mean ± SEM) are given.
The capacity of monocytes to stimulate T-cell DNA synthesis in response to the protein recall antigen candidin is diminished during ET; study of different kinetics.
PBMCs were cultured in the presence or absence of 2 ng/mL LPS. Cells were washed and recultured at 24 hours with (2.5 μg/mL) or without candidin. After the time periods indicated, cells were pulsed with [5′-3H]thymidine for a further 15 hours. Results of the cellular radioactive labeling are presented as specific cpm (the difference between cpm of culture in the presence of antigen and cpm of culture in the absence of antigen). Representative data from 1 of 3 independent experiments in triplicate assays (mean ± SEM) are given.
To further verify the diminished antigen-presenting activity of monocytes during ET, we tested different T-cell responses after challenge with the recall antigens candidin, tetanus toxoid, and tuberculin. The proliferation (Table 2A) and IFN-γ secretion (Table2B) of T cells were strongly diminished in ET following challenge with each of the 3 antigens for 3 or 4 days, respectively. Similarly, LPS-primed purified monocytes showed reduced capacity to stimulate T cells in the presence of these antigens. In contrast, LPS priming of T cells did not impair their antigenic response when cocultured with nonprimed monocytes (data not shown).
Finally, we investigated whether IL-10 neutralization during LPS priming could prevent the suppression of T-cell responses. PBMCs were primed with LPS or not in the presence of either anti-IL-10 MoAb or a control MoAb. After extensive washing, stimulation with candidin, tetanus toxoid, or tuberculin was performed. Figure4 presents the individual antigen-specific3H-thymidine incorporation and IFN-γ production in cultures of 2 different donors after 3 or 4 days of antigen stimulation, respectively. Depending on the individual, the T-cell reactivity toward the different antigens varied considerably. Neutralization of endogenous IL-10 only marginally reduced LPS-induced suppression of antigen-specific T-cell DNA synthesis (Figure 4A). In contrast, neutralization of endogenous IL-10 completely repressed down-regulation of antigen-induced IFN-γ production (Figure 4B). No IFN-γ was detectable in any of the groups in the absence of antigen exposition.
Neutralization of endogenous IL-10 during LPS priming completely ameliorates the ET-associated suppression of T-cell IFN-γ production but not the suppression of T-cell proliferation in response to different protein recall antigens.
PBMCs from 2 donors were cultured for the first 24 hours with (2 ng/mL) or without LPS in the presence of either a control murine IgG1 or anti-IL-10 MoAb and recultured with either 2.5 μg/mL candidin (Can), 0.08 IU/mL tetanus toxoid (TT), 5 PPD-S/mL tuberculin (Tbc), or without stimulation. (A) Cellular [5′-3H]thymidine incorporation after 3 days of antigen stimulation is given from experiments in triplicate assays (mean ± SEM). (B) IFN-γ production after 4 days of antigen stimulation was assessed by ELISA.
Neutralization of endogenous IL-10 during LPS priming completely ameliorates the ET-associated suppression of T-cell IFN-γ production but not the suppression of T-cell proliferation in response to different protein recall antigens.
PBMCs from 2 donors were cultured for the first 24 hours with (2 ng/mL) or without LPS in the presence of either a control murine IgG1 or anti-IL-10 MoAb and recultured with either 2.5 μg/mL candidin (Can), 0.08 IU/mL tetanus toxoid (TT), 5 PPD-S/mL tuberculin (Tbc), or without stimulation. (A) Cellular [5′-3H]thymidine incorporation after 3 days of antigen stimulation is given from experiments in triplicate assays (mean ± SEM). (B) IFN-γ production after 4 days of antigen stimulation was assessed by ELISA.
In summary, these data demonstrate for the first time that ET of monocytic cells not only diminishes their proinflammatory action but also their ability to elicit antigen-specific T-cell responses. IL-10, which is involved in this phenomenon, is known to suppress T-cell stimulation by altering monocyte accessory function.20,21The reduction of monocyte MHC class II expression and T-cell stimulation capacity after LPS priming were even stronger than after IL-10 (10 ng/mL) priming (manuscript in preparation). These results are consistent with ET being associated with incomplete repression of MHC class II down-regulation in monocytes and decreased antigen-specific T-cell responses, if endogenous IL-10 is neutralized during LPS priming (Figures 1 and 4). Our data indicate that IL-10-independent factors are involved in mediating the impaired accessory function of LPS-primed monocytes, as is also known for IL-12 suppression in ET.6TGF-β does not appear to be one of these factors.
Neutralization of IL-10 during LPS priming of PBMCs was sufficient to completely prevent decreased T-cell IFN-γ production but not to normalize T-cell DNA synthesis. T cells can effect distinct biologic responses as a function of the level of T-cell receptor occupancy, as has been previously described.24 Consistent with our data, the induction of T-cell proliferation requires a greater number of MHC molecule-peptide complexes to interact with the T cell than is required for the release of T-cell IFN-γ.
LPS is well known as an activator of monocytic cells. It induces the expression of monokines and cytotoxic agents25,26 and up-regulates the accessory function of monocytic cells.27The latter is consistent with the initially increased expression of MHC class II, and several accessory molecules, by LPS seen in our study (Figure 1). After the LPS-induced proinflammatory state, however, the ET state follows where monocytic cells do not adequately express cytokines when reexposed to LPS. Here we demonstrate that ET is also associated with impaired monocyte accessory function.
Massive LPS-induced release of proinflammatory cytokines in vivo has been associated with induction of acute septic shock.28,29We have observed that subsequent to septic shock, as well as to major surgery or polytrauma, there often follows a clinical state called immunoparalysis.30-32 Although immunoparalysis can be induced by multiple mechanisms, it is accompanied by common functional abnormalities of the blood monocytes: (1) a drastically decreased expression of surface MHC class II and CD86 molecules, which is associated with a diminished antigen presentation capacity, and (2) a reduced ex vivo LPS-induced production of TNF-α, IL-10, and reactive oxygen species, whereas the capacity to produce IL-1RA was not altered (references 5, 33, and 34 and our unpublished data). The diminished HLA-DR expression on peripheral blood monocytes is regarded as a diagnostic indicator of immunoparalysis, whose persistence correlates with a high risk for persistent infections and fatal outcome.34-36 IL-10, systemically released during septic events and injury-induced neuroendocrine stress, seems to be essential in the pathogenesis of immunoparalysis.32 37 Therefore, the monocyte functional state in patients with immunoparalysis is similar to that observed in experimental ET. Modern medicine is now able to potentially control acute hyperinflammatory situations, but there is no established therapy to deal with the high risk associated with immunoparalysis. We propose that the monocyte functional state during in vitro ET is an appropriate model of the monocyte alterations observed during immunoparalysis and may help in the development of novel therapeutic strategies.
Acknowledgments
The authors especially thank Christa Liebenthal for technical help and Dr Nigel E. A. Crompton for accurately proofreading the manuscript.
Supported in part by the Deutsche Forschungsgemeinschaft (SFB 421, TP-B2 Volk).
Reprints:Robert Sabat, Institut für Medizinische Immunologie, Charité, Schumannstr. 20/21, D-10098 Berlin; e-mail:robert.sabat@charite.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 3. The capacity of monocytes to stimulate T-cell DNA synthesis in response to the protein recall antigen candidin is diminished during ET; study of different kinetics. / PBMCs were cultured in the presence or absence of 2 ng/mL LPS. Cells were washed and recultured at 24 hours with (2.5 μg/mL) or without candidin. After the time periods indicated, cells were pulsed with [5′-3H]thymidine for a further 15 hours. Results of the cellular radioactive labeling are presented as specific cpm (the difference between cpm of culture in the presence of antigen and cpm of culture in the absence of antigen). Representative data from 1 of 3 independent experiments in triplicate assays (mean ± SEM) are given.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.218/4/m_bloo01304003x.jpeg?Expires=1769091283&Signature=LEOdtYL34s6Xe-izEjNgm7Lbb7-eIyMC454kePRuFF84cQkI9ruAbUBBndu0R~JYx3J6x6fp~5yq9w20cJy5XcaIebwrDc1cW~Ip74lPP~se3XMuMGpsz89DaP~E0GCfd6RPZQzylkmXdbOS5r45AzbMn2UzCoQ1xzpbbRjKRXUQPRMN0ZABdZlHU6vJDGTVNeL8iwwe1b73djfy-K31U3hrutxxogl7CUkxOBGlfv3pORb4kLqW90XYpQiG8S4VAiEdAz2c3JZR6vVE5QJSi1WWF68VXxf-5EPy38lpG7ydHyz~5FFhbY7Lye-bEkerGDpth29Kot3jNDKXJxGM3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Neutralization of endogenous IL-10 during LPS priming completely ameliorates the ET-associated suppression of T-cell IFN-γ production but not the suppression of T-cell proliferation in response to different protein recall antigens. / PBMCs from 2 donors were cultured for the first 24 hours with (2 ng/mL) or without LPS in the presence of either a control murine IgG1 or anti-IL-10 MoAb and recultured with either 2.5 μg/mL candidin (Can), 0.08 IU/mL tetanus toxoid (TT), 5 PPD-S/mL tuberculin (Tbc), or without stimulation. (A) Cellular [5′-3H]thymidine incorporation after 3 days of antigen stimulation is given from experiments in triplicate assays (mean ± SEM). (B) IFN-γ production after 4 days of antigen stimulation was assessed by ELISA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.218/4/m_bloo01304004x.jpeg?Expires=1769091283&Signature=Wo5A502xwVtagM7FuM4hu7DQ~r2EraZf6ynXgev1~Yu~bMh8MOfDhzbIk0hyvMKu0IqcznoUoEOGe9AeZbZBAlyiUDiS8HgOfAsh9ZUQEPTh22dZ0ePlkjgeYDOSb1SvQZXWQnqt4GNGd70AE~CXtho3eNHWGtt16ZZ0cJM3R-AjeuwIzX-Pjlx9dRj-qjhVUieeoAs7SyQk2wyMr27BhFSif~2XwPEJKik0S7f1ealZsqn1RxHCXX2rUUGhJearUiE4JnU842ed~6W6Sf0vFJbIcfExbL4~W7xyYsW-4fKshicpWeOrWW-3xVWGmfmQCJdk6DTnbFa-6n9FSX9wgw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal