Abstract
Immune heparin-induced thrombocytopenia (HIT) is associated with antibodies directed against a complex of platelet factor 4 (PF4) and heparin. We were able to affinity purify anti-PF4–heparin IgG (HIT IgG) from the plasma of 2 patients with HIT. Under conditions that were more physiological and sensitive than those in previous studies, we observed that this HIT IgG caused platelet aggregation on the addition of heparin. Platelets activated with HIT IgG increased their release and surface expression of PF4. We quantitated, for the first time, the binding of affinity-purified HIT iodine 125–IgG to platelets as they activated in a plasma milieu. Binding of the HIT IgG was dependent on heparin and required some degree of platelet activation. Blocking the platelet FcγRII with the monoclonal antibody IV.3 did not prevent HIT IgG binding to activated platelets. We concluded that anti-PF4–heparin IgG is the component in these HIT plasmas that induces platelet aggregation. The Fab region of HIT IgG binds to PF4–heparin on the surface of activated platelets. We propose that only then does the Fc portion of the bound IgG further activate the same or adjacent platelets through the Fc receptor. Our data support a dynamic model of platelet activation in which released PF4 enhances further antibody binding and more release.
Immune heparin-induced thrombocytopenia (HIT) is a potentially serious complication of heparin therapy.1,2Patient plasma usually contains antibodies that aggregate platelets in the presence of heparin.3,4 In addition, antibodies directed against complexes of platelet factor 4 (PF4) and heparin are associated with this disease.5-7 However, HIT plasma that aggregates platelets does not always contain anti-PF4–heparin antibodies and vice versa.6,8 9 Understanding how HIT antibodies interact with platelets and whether the anti-PF4–heparin antibodies bind platelets and cause aggregation is important for understanding the pathophysiology of HIT. Unfortunately, conflicting models have been proposed to describe the interaction of HIT antibodies with platelets, and none of the previous studies directly investigated the binding of affinity-purified anti-PF4–heparin IgG to platelets.
Visentin et al10 report that, with flow cytometry, IgG from some patients with HIT bound to resting, washed platelets. Blocking the platelet Fc receptor (FcγRII) with the monoclonal antibody IV.3 abolished antibody binding, which implied that only the Fc portion of HIT IgG interacts with platelets. Visentin et al10 and others2,11 12 contend that HIT IgG forms an immune complex with circulating PF4–heparin and that the complex binds to the platelet FcγRII through the Fc portion of the IgG and causes platelet activation.
This model is disputed by Horne and Alkins,13 who investigated the binding of iodinated total IgG from patients with HIT to washed platelets. Antibody binding was reliably observed only with thrombin-activated platelets and was dependent on the addition of approximately equimolar concentrations of PF4 and heparin. In contrast to the findings of Visentin et al,10 they observed that Fc receptors were not important for the binding of HIT antibodies. Specifically, HIT antibodies bound to platelets in the presence of IV.3. Horne and Alkins13 and others5 14-16support a model in which HIT IgG binds by its Fab region to PF4–heparin complexes already on the platelet surface.
These studies by Visentin et al10 and Horne and Alkins13 are valuable, but both investigated the binding of HIT antibodies to platelets under static conditions in which the washed platelets were either fully activated or held quiescent by prostaglandin E1. There was no opportunity for secreted platelet proteins to modulate antibody binding and no information describing how binding changed during activation. We have studied the binding of HIT IgG to platelets under more physiological conditions using affinity-purified anti-PF4–heparin IgG from HIT plasma. This IgG provides much greater sensitivity and is a more clearly defined antibody than the total IgG or plasma used by others.10 13Importantly, platelet aggregation was induced by the affinity-purified HIT IgG, and antibody binding was studied in a plasma milieu with platelets exposed to shear forces. Our data also help to resolve the role played by the platelet FcγRII in HIT, which previously had only been investigated in artificial experiments.
Materials and methods
Purification of HIT IgG
Plasma was collected with informed consent from healthy individuals and from 2 patients with HIT (patient 1 and patient 2). The affinity purification of HIT anti-PF4–heparin IgG (HIT IgG) and a clinical summary of these patients has been previously described.17 Briefly, patients 1 and 2 were 72- and 67-year-old women. Thrombocytopenia developed in each of them after approximately 1 week of heparin therapy, and findings on14C-serotonin release and platelet aggregation assays were positive. Other causes of thrombocytopenia were clinically excluded.
HIT plasma was passed through a heparin-PF4–agarose column, and bound antibodies were eluted with 0.13 to 1.5 mol/L NaCl gradient. IgG was further purified on a protein G Sepharose column. Normal IgG, which did not bind PF4–heparin, was purified from the pooled plasma of 3 healthy persons by affinity for protein G Sepharose. The molecular weights of the HIT and normal IgG were characterized under native conditions by size exclusion high-performance liquid chromatography using a Zorbax G-450 column (Hewlett Packard, Palo Alto, CA) and an aqueous solvent of 0.2 mol/L NaCl, 0.1 mol/L phosphate, pH 7.0, running at 1 mL/minute. The IgG was predialyzed against the running buffer and was detected at 210 nm. The IgG eluted as a single peak with an average molecular weight of 157 kd when compared with gel filtration molecular weight standards (Bio-Rad Laboratories, Hercules, CA). No larger IgG aggregates were present. IgG was iodinated with Na 125I using iodogen (Pierce, Rockford, IL).
IgG binding during platelet aggregation
The methods for platelet preparation and platelet aggregometry are based on those previously described.4Platelet-rich plasma (PRP) was prepared by centrifuging citrated blood at 170g for 15 minutes. Plasma was obtained by centrifuging the remaining blood at 1600g for 15 minutes. The concentration of platelets was determined with an automated blood cell counter (Sysmex NE-8000, Kobe, Japan). Platelet aggregometry was performed in silicone-coated glass tubes at 37°C by monitoring the increase in optical transmittance of the platelet suspension with a ChronoLog (Chrono-Log, Havertown, PA) aggregometer. Aggregation was recorded once a second on a personal computer with an 8-bit analog-to-digital converter (Pocket Sampler; Jaycar Electronics, Sydney, Australia).
The aggregation reaction involved stirring PRP (300 × 106 platelets/mL) with HIT IgG (12 μg/mL = 80 nmol/L), 125I-IgG tracer (≈0.07 μg/mL, ≈13 kBq/mL), in 650 μL plasma. Aggregation was induced by the addition of heparin (0.5 U/mL) or collagen (4μg/mL). The aggregation reaction was sampled by removing, in duplicate, 80 μL platelets with a wide-mouth siliconized pipette tip. These samples were layered onto a 800 μL cushion of 17% sucrose18 in phosphate-buffered saline (PBS) within a microfuge tube then centrifuged at 11,000g for 2 minutes. The supernatant (free counts) was carefully removed from the top down, and the pellet (bound counts) were briefly rinsed with 200 μL PBS without resuspension. The bottom of the tube, containing the pellet, was cut off, and the radioactivity level was determined with a γ-counter. The amount of bound IgG was determined by multiplying the proportion of total counts bound by the amount of IgG added per platelet.
PF4 expression and release during platelet activation
In a siliconized glass aggregometer tube, 650 μL citrated PRP (300 × 106 platelets/mL) was stirred at 37°C with heparin plus HIT IgG, collagen plus heparin, collagen alone, or heparin alone. Heparin was used at 0.5 U/mL, collagen was used at 4 μg/mL, and affinity-purified anti-PF4–heparin IgG from HIT patient 2 was used at 12 μg/mL. Aggregation was only observed when platelets were activated with HIT IgG, collagen plus heparin, or collagen alone. Activation was arrested after 8 to 9 minutes by adding 65 μL ETP and chilling on ice (ETP consists of 107 mmol/L EDTA, 12 mmol/L theophylline, and 2.8 μmol/L prostaglandin E1). Platelets were pelleted by centrifugation (3500g, 2 minutes) in the siliconized tube. The plasma supernatant was passed though a 0.2 μm filter to remove residual platelets19 and was stored at −20°C before the PF4 concentration was measured.
To measure PF4 expression, the platelet pellets were resuspended in 220 μL of a solution containing 2% bovine serum albumin (BSA), 10% ETP, 50 μg/mL IV.3, and PBS. Ninety-microliter platelets were incubated on ice in microfuge tubes for 10 minutes with an equal volume of iodinated sheep anti-PF4 IgG (affinity-purified polyclonal; Cedarlane Laboratories, Hornby, Ontario, Canada) or sheep total IgG (purified from sheep serum by affinity for protein G Sepharose). Free125I-IgG was removed from the platelets by direct centrifugation (9400g, 2 minutes) and 2 washes with PBS. The bottom of the microfuge tube, containing the pellet, was cut off, and the radioactivity level was determined with a γ-counter. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis indicated that neither sheep IgG preparation contained PF4.
PF4 assay
A competition radioimmunoassay was developed to measure the PF4 concentration in plasma derived from activated and inactivated platelets (see above). Microtiter wells that could be individually separated (MaxiSorp–BreakApart; Nunc, Roskilde, Denmark) were coated with affinity-purified sheep anti-PF4 IgG and blocked with BSA. Dilutions of test plasma and purified PF47 standards (0-2 μg/mL) were prepared in a solution containing 1% BSA, 0.38% trisodium citrate, 10% ETP, and PBS–Tween. When the test plasma contained 0.5 U/mL heparin, the same heparin concentration was maintained throughout dilution of the test and standard PF4 solutions.
Standard or unknown PF4 solution (110 μL) was equilibrated for 30 minutes with an equal volume of 125I-PF4 solution (≈0.2 μg/mL, 4 KBq/mL) containing polybrene (50 μg/mL). One hundred microliters of PF4 mixture was incubated, in duplicate, for 15 minutes in the wells coated with anti-PF4 IgG. Wells were washed 3 times with PBS–Tween and then broken apart, and radioactivity was measured in a γ-counter. The PF4 concentration was determined by comparing counts with the appropriate standard curve, depending on the presence or absence of 0.5 U/mL heparin. The standard curve with heparin was shifted by 0.25 μg/mL to the right of the curve without heparin. The most sensitive region of the PF4 standard curve was 0.05 to 1 μg/mL PF4, and samples were diluted to fall within this range. Polybrene was required in this assay to prevent heparin from abolishing the binding between PF4 and antibody.
Results
HIT IgG binding during platelet aggregation
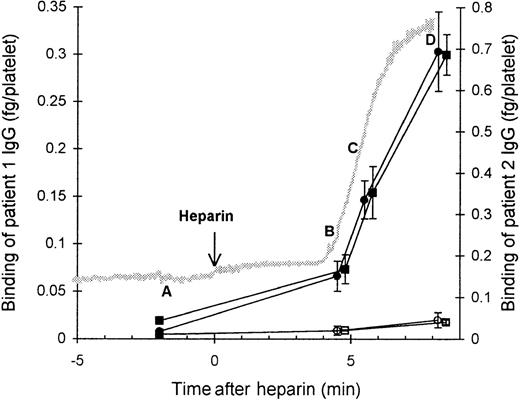
We investigated the binding of affinity-purified HIT125I-IgG to platelets at various time points (A, B, C, and D) during platelet aggregation induced by the antibody and heparin (Figure 1). Before heparin was added to the suspension of PRP and HIT IgG (time point A), the platelets remained nonaggregated, and negligible binding of HIT IgG was detected. The addition of heparin triggered platelet aggregation. The beginning of aggregation (time point B) was associated with low but detectable binding of HIT IgG. At time point C, when platelets were approximately 50% aggregated, there was approximately half-maximal HIT IgG binding. The greatest IgG binding was observed once platelets were fully aggregated (time point D). The nonspecific binding of normal125I-IgG remained low throughout, indicating minimal entrapment of IgG during aggregation. These data suggest that some degree of platelet activation is required for HIT IgG binding to platelets.
Binding of affinity-purified anti-PF4–heparin HIT IgG to platelets during heparin-induced platelet aggregation.
Platelet-rich plasma (300 × 106 platelets/mL) was mixed with HIT IgG (12 μg/mL) and 125I-IgG tracer in a platelet aggregometer at 37°C for 12 minutes before heparin (0.5 U/mL) was added. The reaction was sampled 2 minutes before heparin (time point A), either at the start (time point B) or in the middle (time point C) of aggregation, and when platelets were fully aggregated (time point D). Samples were centrifuged through a 17% sucrose cushion to separate platelets from unbound IgG. Specific binding of patient 1 (▪) and patient 2 (•) HIT IgG was measured by using HIT125I-IgG tracer, whereas nonspecific binding (open symbols) was determined with normal 125I-IgG instead. The gray trace shows a typical aggregation profile that progresses from unaggregated to fully aggregated on an arbitrary scale. IgG bound is the mean ± SE of 3 to 8 experiments.
Binding of affinity-purified anti-PF4–heparin HIT IgG to platelets during heparin-induced platelet aggregation.
Platelet-rich plasma (300 × 106 platelets/mL) was mixed with HIT IgG (12 μg/mL) and 125I-IgG tracer in a platelet aggregometer at 37°C for 12 minutes before heparin (0.5 U/mL) was added. The reaction was sampled 2 minutes before heparin (time point A), either at the start (time point B) or in the middle (time point C) of aggregation, and when platelets were fully aggregated (time point D). Samples were centrifuged through a 17% sucrose cushion to separate platelets from unbound IgG. Specific binding of patient 1 (▪) and patient 2 (•) HIT IgG was measured by using HIT125I-IgG tracer, whereas nonspecific binding (open symbols) was determined with normal 125I-IgG instead. The gray trace shows a typical aggregation profile that progresses from unaggregated to fully aggregated on an arbitrary scale. IgG bound is the mean ± SE of 3 to 8 experiments.
Role of platelet activation and platelet FcγRII
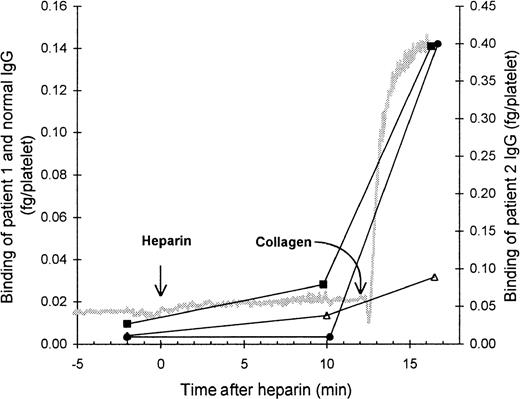
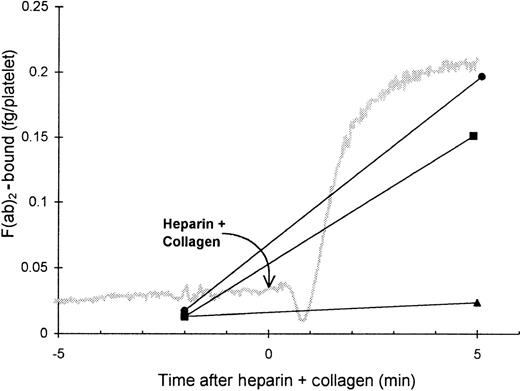
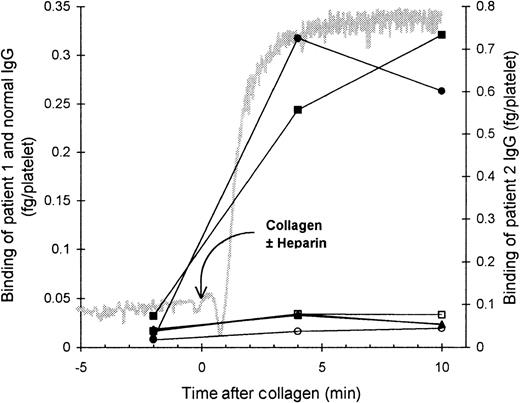
The role of the FcγRII in the binding of HIT IgG to platelets was investigated by blocking the FcγRII with the monoclonal antibody IV.3. As expected, the presence of IV.3 prevented HIT IgG and heparin from inducing platelet aggregation, and no binding of HIT125I-IgG to these platelets was detected. However, after collagen was added to activate the platelets, the HIT125I-IgG did bind (Figure 2). Thus, blocking the FcγRII did not prevent binding of HIT IgG if the platelets could be activated by another pathway. This indicates that IgG binding to activated platelets must have occurred by the Fab region of HIT IgG, probably binding to PF4–heparin immobilized on the platelet surface. The same conclusion was reached when HIT anti-PF4–heparin F(ab′)2 bound to platelets aggregated with collagen plus heparin (Figure3). The action of IV.3 appears to be that it precludes HIT IgG from activating platelets. This prevents the release of PF4 from platelet α-granules and minimizes the expression of PF4–heparin on the platelet surface so there is little binding of HIT IgG.
Effect of blocking platelet Fc receptor on the binding of HIT IgG during platelet aggregation.
Platelet-rich plasma (300 × 106 platelets/mL) was mixed with the monoclonal antibody IV.3 (anti-FcγRII, 50 μg/mL), HIT IgG (12 μg/mL), and 125I-IgG tracer in a platelet aggregometer for 12 minutes before heparin (0.5 U/mL) was added. Aggregation was induced by the addition of collagen (4 μg/mL) 12 minutes after heparin was added. The reaction was sampled 2 minutes before heparin, 10 minutes after heparin (when full aggregation would have occurred without IV.3), and when the collagen-induced aggregation was complete. Samples were centrifuged through a 17% sucrose cushion to separate platelets from unbound IgG. Specific binding of patient 1 (▪) and patient 2 (•) was quantitated by the inclusion of the same HIT 125I-IgG tracer, whereas nonspecific binding (▵) was measured by the binding of normal 125I-IgG. The gray trace shows a typical aggregation profile. IgG bound is the mean of 2 experiments.
Effect of blocking platelet Fc receptor on the binding of HIT IgG during platelet aggregation.
Platelet-rich plasma (300 × 106 platelets/mL) was mixed with the monoclonal antibody IV.3 (anti-FcγRII, 50 μg/mL), HIT IgG (12 μg/mL), and 125I-IgG tracer in a platelet aggregometer for 12 minutes before heparin (0.5 U/mL) was added. Aggregation was induced by the addition of collagen (4 μg/mL) 12 minutes after heparin was added. The reaction was sampled 2 minutes before heparin, 10 minutes after heparin (when full aggregation would have occurred without IV.3), and when the collagen-induced aggregation was complete. Samples were centrifuged through a 17% sucrose cushion to separate platelets from unbound IgG. Specific binding of patient 1 (▪) and patient 2 (•) was quantitated by the inclusion of the same HIT 125I-IgG tracer, whereas nonspecific binding (▵) was measured by the binding of normal 125I-IgG. The gray trace shows a typical aggregation profile. IgG bound is the mean of 2 experiments.
Binding of HIT F(ab′)2 to platelets.
Platelet-rich plasma (300 × 106 platelets/mL) was mixed with HIT or normal F(ab′)2 (8 μg/mL) and125I-F(ab′)2 tracer in a platelet aggregometer for 12 minutes before heparin (0.5 U/mL) plus collagen (4 μg/mL) was added to induce aggregation. Platelets were sampled 2 minutes before heparin plus collagen and 5 minutes after heparin plus collagen and were centrifuged through a 17% sucrose cushion to separate platelets from unbound F(ab′)2. Symbols indicate the specific binding of patient 1 (▪) and patient 2 (•) F(ab′)2 and nonspecific binding of Normal F(ab′)2 (▴). The gray trace shows a typical aggregation profile. F(ab′)2 bound is the mean of 2 experiments.
Binding of HIT F(ab′)2 to platelets.
Platelet-rich plasma (300 × 106 platelets/mL) was mixed with HIT or normal F(ab′)2 (8 μg/mL) and125I-F(ab′)2 tracer in a platelet aggregometer for 12 minutes before heparin (0.5 U/mL) plus collagen (4 μg/mL) was added to induce aggregation. Platelets were sampled 2 minutes before heparin plus collagen and 5 minutes after heparin plus collagen and were centrifuged through a 17% sucrose cushion to separate platelets from unbound F(ab′)2. Symbols indicate the specific binding of patient 1 (▪) and patient 2 (•) F(ab′)2 and nonspecific binding of Normal F(ab′)2 (▴). The gray trace shows a typical aggregation profile. F(ab′)2 bound is the mean of 2 experiments.
Specificity of affinity-purified HIT IgG
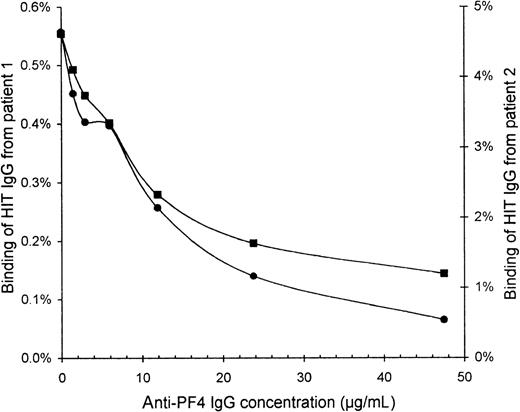
To confirm that the HIT IgG indeed recognized PF4, we blocked the binding of HIT IgG to activated platelets by preincubating the platelets with affinity-purified sheep anti-PF4 IgG. The binding of HIT125I-IgG tracer (without unlabeled HIT IgG) was increasingly inhibited with higher concentrations of anti-PF4 (Figure4). At 48 μg/mL anti-PF4, the binding of HIT IgG from patients 1 and 2 was reduced to one quarter and one eighth, respectively, of the binding without anti-PF4.
Inhibition of HIT IgG binding to platelets by anti-PF4.
Platelets that were activated by collagen plus heparin were resuspended in PBS containing 2% BSA, 6 mg/mL human IgG, 10% ETP, and 50 μg/mL IV.3. Platelets (60 × 106) were blocked for 30 minutes with various concentrations of affinity-purified sheep anti-PF4 IgG before HIT 125I-IgG tracer was added for 10 minutes. All incubations were performed at 0°C to minimize further platelet activation. After centrifugation and 2 washes with PBS, free125I-IgG was removed. Binding of HIT IgG from patient 1 (▪) and patient 2 (•) is expressed as a percentage of the total radioactivity added because the IgG concentration of the125I-IgG tracer alone cannot be accurately measured. Background binding of normal 125I-IgG was only 0.05%.
Inhibition of HIT IgG binding to platelets by anti-PF4.
Platelets that were activated by collagen plus heparin were resuspended in PBS containing 2% BSA, 6 mg/mL human IgG, 10% ETP, and 50 μg/mL IV.3. Platelets (60 × 106) were blocked for 30 minutes with various concentrations of affinity-purified sheep anti-PF4 IgG before HIT 125I-IgG tracer was added for 10 minutes. All incubations were performed at 0°C to minimize further platelet activation. After centrifugation and 2 washes with PBS, free125I-IgG was removed. Binding of HIT IgG from patient 1 (▪) and patient 2 (•) is expressed as a percentage of the total radioactivity added because the IgG concentration of the125I-IgG tracer alone cannot be accurately measured. Background binding of normal 125I-IgG was only 0.05%.
Release of PF4 from platelets
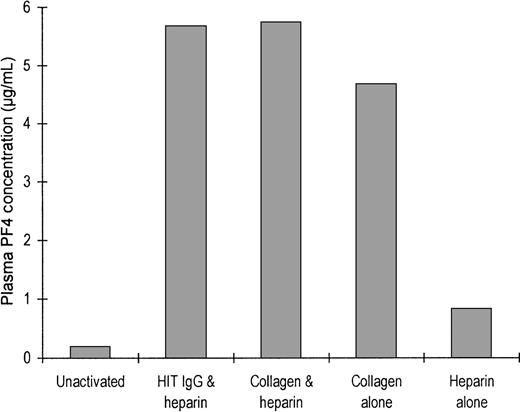
To show that PF4 is released during platelet activation, we quantitated the PF4 released from platelets during incubation of PRP with various agonists (Figure 5). The plasma from unstimulated platelets, anticoagulated with citrate, contained 0.19 μg/mL PF4. When platelets were aggregated by the addition of either HIT IgG plus heparin, collagen alone, or collagen plus heparin, the plasma contained approximately 5 μg/mL PF4. Treatment of platelets with heparin alone released a lesser amount of PF4, and the resultant plasma contained 0.82 μg/mL PF4. The released PF4 may be absorbed onto the platelet surface, resulting in elevated surface expression of the protein. PF4 release by HIT IgG without heparin was not investigated because IgG alone does not trigger platelet activation.
Release of PF4 after platelet activation.
Either 650 μL citrated platelet-rich plasma (300 × 106 platelets/mL) was not activated or it was activated with heparin and HIT IgG, collagen and heparin together, collagen alone, or heparin alone after being stirred in an aggregometer. Activation was arrested when ETP was added and the plasma was chilled on ice. Platelets were removed by centrifugation (for use in the step described in Figure 6), and the plasma PF4 concentration was measured by competition radioimmunoassay. Bars show the mean values of 3 experiments.
Release of PF4 after platelet activation.
Either 650 μL citrated platelet-rich plasma (300 × 106 platelets/mL) was not activated or it was activated with heparin and HIT IgG, collagen and heparin together, collagen alone, or heparin alone after being stirred in an aggregometer. Activation was arrested when ETP was added and the plasma was chilled on ice. Platelets were removed by centrifugation (for use in the step described in Figure 6), and the plasma PF4 concentration was measured by competition radioimmunoassay. Bars show the mean values of 3 experiments.
Increased expression of PF4 on the platelet surface after activation
The relative surface expression of PF4 on activated and nonactivated platelets was measured by the binding of sheep anti-PF4125I-IgG (Figure 6). Unstimulated citrated platelets displayed little specific binding of anti-PF4 antibodies. Aggregation of platelets with either HIT IgG plus heparin, collagen alone, or collagen plus heparin resulted in a marked increase in PF4 expression. Platelets treated with heparin alone had only a modest increase in surface PF4 compared with unstimulated platelets.
Expression of PF4 on the platelet surface after activation.
Citrated PRP either was not activated or it was activated with heparin and HIT IgG, collagen and heparin together, collagen alone, or heparin alone. Activation was arrested after ETP was added and the plasma was chilled on ice. Plasma was removed by centrifugation (for use in the step described in Figure 5). Surface expression of PF4 was determined by the binding of sheep anti-PF4 125I-IgG (dark bars). Open bars represent the nonspecific binding of nonimmune sheep IgG. Graph illustrates the percentage anti-PF4 125I-IgG bound to platelets as a proportion of the total radioactivity added and shows the mean of 2 experiments.
Expression of PF4 on the platelet surface after activation.
Citrated PRP either was not activated or it was activated with heparin and HIT IgG, collagen and heparin together, collagen alone, or heparin alone. Activation was arrested after ETP was added and the plasma was chilled on ice. Plasma was removed by centrifugation (for use in the step described in Figure 5). Surface expression of PF4 was determined by the binding of sheep anti-PF4 125I-IgG (dark bars). Open bars represent the nonspecific binding of nonimmune sheep IgG. Graph illustrates the percentage anti-PF4 125I-IgG bound to platelets as a proportion of the total radioactivity added and shows the mean of 2 experiments.
These data support the notion that the surfaces of activated platelets are covered in PF4 that binds heparin and HIT IgG. The bound IgG activates other platelets through FcγRII, which initiates a chain reaction that leads to a rapid increase in platelet aggregation and HIT IgG binding.
Role of heparin
Figure 7 demonstrates that heparin is required to facilitate the binding of HIT IgG to aggregated platelets. Platelets aggregated with collagen alone did not bind HIT IgG. Binding only occurred when heparin was included with the collagen. This supports the concept that PF4 must form a complex with heparin before it is recognized by HIT IgG.
Heparin dependence in the binding of HIT IgG to collagen-aggregated platelets.
Platelet-rich plasma (300 × 106 platelets/mL) was mixed with HIT or normal IgG (12 μg/mL) and 125I-IgG tracer in a platelet aggregometer for 12 minutes before collagen (4 μg/mL) ± heparin (0.5 U/mL) was added. Platelets were sampled 2 minutes before collagen, 4 minutes after collagen (when platelets were consistently fully aggregated), and 10 minutes after collagen by centrifugation through 17% sucrose. Solid symbols indicate the specific binding of patient 1 (▪) and patient 2 (•) IgG and nonspecific binding of normal IgG (▴) in the presence of heparin. Corresponding open symbols represent binding without heparin. Gray trace shows a typical aggregation profile. Values are the means of 2 experiments.
Heparin dependence in the binding of HIT IgG to collagen-aggregated platelets.
Platelet-rich plasma (300 × 106 platelets/mL) was mixed with HIT or normal IgG (12 μg/mL) and 125I-IgG tracer in a platelet aggregometer for 12 minutes before collagen (4 μg/mL) ± heparin (0.5 U/mL) was added. Platelets were sampled 2 minutes before collagen, 4 minutes after collagen (when platelets were consistently fully aggregated), and 10 minutes after collagen by centrifugation through 17% sucrose. Solid symbols indicate the specific binding of patient 1 (▪) and patient 2 (•) IgG and nonspecific binding of normal IgG (▴) in the presence of heparin. Corresponding open symbols represent binding without heparin. Gray trace shows a typical aggregation profile. Values are the means of 2 experiments.
The amount of IgG bound to platelets was double (Figure 7) the amount when FcγRII was blocked by IV.3 (Figure 2). This probably reflects differences in the degree of platelet activation between the 2 figures and not a component of Fc binding. In Figure 7 platelets were activated by both collagen and HIT IgG and so probably expressed more PF4–heparin antigen than did Figure 2, in which activation by HIT IgG was prevented by IV.3. Support for this view comes from our observation that platelets activated by HIT IgG plus heparin bound more anti-PF4 IgG than those activated by collagen plus heparin (Figure 6).
Discussion
In this study we demonstrated that HIT IgG, affinity purified on heparin-PF4–agarose, induces heparin-dependent platelet aggregation (Figure 1). This finding extends that of Greinacher et al,20 who showed that HIT antibodies, isolated by affinity for endothelial cells, could bind PF4–heparin on enzyme-linkedimmunosorbent assay and activate platelets. However, our data contribute the strongest evidence that the anti-PF4–heparin antibody is the heparin-dependent platelet-aggregating factor, known to be present in the plasma of patients with HIT, and they imply that the anti-PF4–heparin antibody may cause in vivo platelet activation and, consequently, thromboembolism. However, this mechanism may not necessarily reflect the pathophysiology in all patients with HIT.
We provide, for the first time, fundamental data on the binding of affinity-purified HIT anti-PF4–heparin IgG to platelets during platelet aggregation. We used concentrations of platelets,21 heparin,22,23 and specific anti-PF4–heparin IgG17 that were achievable in vivo. Significantly, no exogenous PF4 was added, and PF4 released from the platelets was available to modulate further antibody binding to the platelets. Our findings are particularly significant because the platelet aggregation was induced by the HIT IgG itself rather than by thrombin. The use of affinity-purified antibody provided high sensitivity.
Figure 1 indicates that before the addition of heparin, there was little or no binding of HIT IgG to platelets. We only detected that HIT IgG bound to platelets when heparin was added to the platelets and aggregation began. The HIT IgG binding then increased sharply until platelets were fully aggregated. There is clearly a close correlation between the degree of aggregation and the amount of HIT IgG bound. This confirms that platelet aggregation by HIT IgG is a dynamic process by which activation promotes further antibody binding. We speculate that a very low level of HIT IgG binding to the platelets occurred before aggregation.
We confirmed that PF4 is strongly implicated in forming the platelet antigen recognized by HIT IgG because the binding of HIT IgG to activated platelets was inhibited by the preincubation of platelets with sheep anti-PF4 IgG (Figure 4). Furthermore, the activation of platelets by HIT IgG plus heparin, or by collagen, triggered a substantial increased in the expression of PF4 on the platelet membrane (Figure 6). Of note, incubating platelets with heparin alone was sufficient to elevate modestly the surface expression of PF4.
Many reports demonstrate that HIT antibodies optimally bind to PF4–heparin in a narrow range of PF4:heparin ratios. In our hands, this ratio corresponded to at least 20 μg PF4/U heparin, regardless of whether the PF4–heparin was immobilized on enzyme-linked immunosorbent assay wells or was free in solution. We have demonstrated that the ratio of PF4:heparin is perhaps not as important in a more physiological and sensitive system. We added 0.5 U/mL heparin to platelet-rich plasma, but the PF4 was derived solely from that already present in the plasma or the platelets, or both. The plasma PF4 concentration increased from ≈0.19 μg/mL in citrated platelet-rich plasma to ≈5.6 μg/mL when platelets were fully aggregated by HIT IgG (Figure 5). Consequently, heparin was always in excess, but the increase in PF4 concentration would have moved the PF4:heparin ratio toward the optimum. It is likely that this contributed to the increased binding of HIT IgG during platelet aggregation.
Previously, we and others5,10,17 20 observed that HIT IgG binds weakly to PF4 alone, immobilized into microtiter wells in the absence of heparin. Therefore, we investigated the importance of heparin in the binding of HIT IgG to platelet-surface PF4. We observed that binding of anti-PF4–heparin IgG to collagen-aggregated platelets only occurred in the presence of heparin (Figure 7), despite the fact that platelets aggregated by collagen alone expressed significant amounts of PF4 on their surfaces (Figure 6). We concluded that PF4 on the platelet surface may not be modified in the same way as PF4 adsorbed into microtiter wells. Instead heparin is required to facilitate HIT antibody binding, perhaps by inducing a conformational change within PF4.
Aster24 indicates that understanding the orientation of IgG on the platelet surface is important. If the Fab region of IgG can bind platelets, then anti-PF4–heparin antibodies of IgA or IgM classes may opsonize platelets for destruction in vivo, and this may explain why HIT can develop in patients with only IgA or IgM anti-PF4–heparin antibodies.25 Figure 2 indicates that, as expected,26 27 IV.3 prevented HIT IgG from aggregating platelets in the presence of heparin, and these nonaggregated platelets did not bind HIT 125I-IgG. Platelet aggregation was then induced by the addition of collagen, which acts through a mechanism independent of FcγRII. Once aggregated, the platelets bound HIT IgG despite the presence of IV.3. Similarly, F(ab′)2fragments of HIT IgG also bound to platelets aggregated by collagen and heparin (Figure 3). This implies that the binding of HIT IgG to platelets occurs when the Fab portion of IgG recognizes PF4–heparin already on the platelet surface. Consequently, HIT IgG binding is dependent on platelet activation—needed to increase PF4 expression on the platelet surface—and not on the availability of FcγRII. The effect of IV.3 is simply to prevent HIT IgG from activating platelets through the Fc receptor.
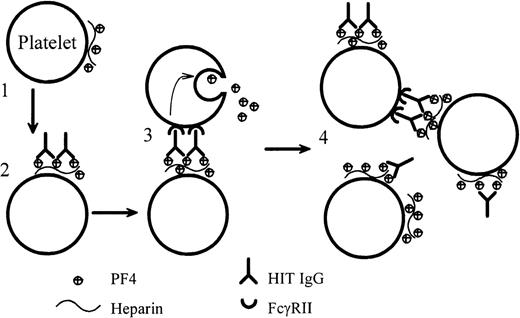
We present definitive evidence that anti-PF4–heparin IgG from patients with HIT can cause heparin-dependent platelet aggregation. For the first time, we also report the dynamic binding of HIT IgG to platelets as the IgG (plus heparin) induces platelet aggregation under conditions that are as close as possible to those found in vivo. Our data support a mechanism of platelet activation by HIT IgG that is summarized in Figure 8. Initially, on the addition of heparin, tiny amounts of PF4–heparin complexes form on the platelet surfaced. The Fab portion of HIT IgG binds to this antigen, and the Fc region of the bound HIT IgG cross-links FcγRII on the same or adjacent platelets. This triggers platelet activation and degranulation. The PF4 that is released binds more heparin and forms more antigen on platelets. Thus, positive feedback accelerates platelet activation until all the platelets are fully aggregated.
Dynamic model of platelet activation by HIT IgG.
(1) Complexes of PF4–heparin form on the surfaces of weakly activated platelets. (2) HIT IgG binds to the platelet-bound PF4–heparin through the Fab region. (3) The Fc portion of the bound IgG cross-links FcγRII on the same (not illustrated) or adjacent platelets and produces strong platelet activation. (4) PF4, released from the activated platelets, forms more complexes with heparin on platelets and promotes antibody binding. In this model, blocking FcγRII with IV.3 would still permit the Fab part of IgG to bind platelets, but IV.3 would inhibit platelet activation.
Dynamic model of platelet activation by HIT IgG.
(1) Complexes of PF4–heparin form on the surfaces of weakly activated platelets. (2) HIT IgG binds to the platelet-bound PF4–heparin through the Fab region. (3) The Fc portion of the bound IgG cross-links FcγRII on the same (not illustrated) or adjacent platelets and produces strong platelet activation. (4) PF4, released from the activated platelets, forms more complexes with heparin on platelets and promotes antibody binding. In this model, blocking FcγRII with IV.3 would still permit the Fab part of IgG to bind platelets, but IV.3 would inhibit platelet activation.
These data on the dynamic binding of HIT IgG to platelets in the presence of heparin provide further insights into the pathophysiology of heparin-induced thrombocytopenia and thrombosis.
Acknowledgments
We wish to thank Irene Bate and Johanne Lonie (Gradipore Ltd.) for performing size exclusion high-performance liquid chromatography on the IgG.
Supported by a grant from the National Health and Medical Research Council (Australia) and an NSW Research and Development Infrastructure grant. P.N. is an Australian Postgraduate Award Scholar.
Reprints:Beng H. Chong, Department of Haematology, Prince of Wales Hospital, Randwick, New South Wales, 2031, Australia; e-mail:b.h.chong@unsw.edu.au.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal