Abstract
Bactericidal/permeability-increasing protein (BPI) has been known for some time to function in killing bacteria and in neutralizing the effects of bacterial endotoxin lipopolysaccharide. In the present study, BPI is found to be a novel endogenous inhibitor of angiogenesis. Within the sub-μM range, BPI shows a concentration-dependent inhibition of endothelial cell (EC) proliferation that is mediated by cell detachment and subsequent induction of apoptosis. As measured by flow cytometric analysis of the percentage of subdiploid cells, apoptosis induction was half-maximal at about 250 nmol/L BPI. Apoptosis was confirmed by quantification of cells with nuclear fragmentation. Apoptosis was found to be EC specific. In an in vitro collagen gel-based angiogenesis assay, BPI at 1.8 μmol/L inhibited tube formation by 81% after only 24 hours. Evidence for in vivo inhibition of angiogenesis was obtained, using the chorioallantoic membrane assay in which BPI was seen to be significantly effective at concentrations as low as 180 nmol/L. This newly discovered function of BPI might provide a possible therapeutic modality for the treatment of various pathologic disorders that depend on angiogenesis.
The 55-kd bactericidal protein produced by human neutrophils is most well known as the bactericidal/permeability-increasing factor (BPI),1,2 but it is also known as the cationic antibacterial protein of 57 kd (CAP57)3 and as the bactericidal protein of 55 kd molecular mass (BP55).4,5 BPI is most bactericidal against gram-negative bacteria1 and has been shown to neutralize both the pyrogenicity of bacterial lipopolysaccharide (LPS) in rabbits and the ability of LPS to initiate the coagulation pathway of theLimulus amoebocyte lysate or to cause tumor necrosis factor (TNF) release from human monocytes.6 BPI functions by binding to the lipid A moiety of LPS,7 whereby neutralizing the ability of this endotoxin to up-regulate the expression of complement receptors on neutrophils and to cause a systemic inflammatory response during bacterial infection. Lipid A consists of a phosphorylated diglucosamine moiety attached to 2 fatty acyl chains. Because BPI is cationic and is known to bind the polysulfated glycosaminoglycan heparin,8 it may function by effectively interacting with the anionic phosphate groups on LPS. Relatedly, many proteins isolated from blood are cationic and bind heparin. One of these proteins, platelet factor-4 (PF4), is a potent anticoagulant, perhaps the strongest binder of heparin, is antiangiogenic, and is also known to be bactericidal.9 10
Until now, aside from its antibacterial effects, no other activities for BPI have been reported. Given chemical similarities between BPI and PF4 and the fact that they share at least some biological activities, it was postulated that they may display other functional similarities. Probably the most clinically important activity of PF4 is its ability to inhibit angiogenesis.11,12 Angiogenesis, or the outgrowth of capillaries from existing vasculature, occurs during physiological processes, such as embryogenesis, the menstrual cycle, and wound healing, but it is also involved in numerous pathologic disorders requiring vascular outgrowth, such as cancer, arthritis, and atherosclerosis. A major interest in angiogenesis has developed in the field of oncology because it has been recognized that tumors, which are dependent on angiogenesis for outgrowth and metastasis, can be treated by attacking their blood supply.13 14
Here it is reported that BPI, like PF4, is indeed an effective inhibitor of angiogenesis. We found that BPI inhibits endothelial cell (EC) growth at low doses, leading to the inhibition of angiogenesis both in vitro and in vivo. The commonality between antiangiogenic and antibacterial proteins and the role of angiogenesis during inflammation are discussed. The fact that BPI is now found to be an endogenous inhibitor of angiogenesis makes it an important pharmacologic candidate for future antiangiogenic therapy in the clinic.
Materials and methods
Purification of BPI
Venous blood was obtained in the form of leukocyte concentrates from the Red Cross, St. Paul, Minnesota. Erythrocytes were lysed in a 2-step process, involving ammonium chloride and hypotonic shock. Cytoplasmic granules were obtained by centrifugation as described previously,4 except that polymorphonuclear leukocytes were lysed by nitrogen cavitation. BPI was then purified by column chromatography as previously described.5In the final step, the sample was applied to a 1 × 180 cm molecular sieving column of Toyopearl HW55S (TosoHaas, Philadelphia, PA) that had been equilibrated with 0.05 mol/L glycine buffer, pH 2.5, containing 0.5 mol/L NaCl. Protein concentration was determined according to Hartree. Purity was confirmed by visualization of an SDS polyacrylamide gel following electrophoresis of 1 μg purified BP55 protein and silver staining of the gel, also as previously described.15 BPI preparations were proven to be LPS free by demonstration of lack of TNFα secretion inducing capacity in macrophages,16 and by lack of E-selectin induction on ECs.
Cell culture
Human umbilical vein-derived endothelial cells (HUVECs) were isolated from the vein of human umbilical cords and routinely cultured in RPMI-1640 (Life Technologies, Breda, The Netherlands) supplemented with 20% heat inactivated human pooled serum (HS; provided by the University Hospital Maastricht), 2 mmol/L l-glutamine (Life Technologies, Breda, The Netherlands), 50 ng/mL streptomycin, and 50 U/mL penicillin (ICN Biomedicals) in a 0.2% gelatin-coated 75 cm2 tissue culture flask at 37°C at 5% CO2. HUVECs used for experiments were cultured without addition of extra growth factors and used for experiments between passage 2 and 4. Human microvascular endothelium was isolated from foreskins and cultured in the same medium.
Bovine microvascular ECs were kindly provided by Dr M. Furie (State University of New York, Stony Brook, NY) and were cultured on gelatin-coated flasks in MEM-α (Life Technologies, Breda, The Netherlands) supplemented with 15% fetal calf serum (FCS; Boehringer Ingelheim Biowhittaker, Verviers, Belgium), 50 U/mL penicillin, and 50 ng/mL streptomycin.
Proliferation assay
HUVECs were seeded in a 96-well culture plate coated with 1 mg/mL fibronectin (2 hours at 20°C) at a concentration of 3000 cells per well in a volume of 50 μL. The cells were allowed to adhere for 3 hours at 37°C at 5% CO2, and subsequently, 50 μL culture medium with 20 ng/mL basic fibroblast growth factor (bFGF), with or without BPI, was added. The cells were cultured for 72 hours. During the last 6 hours of the assay, the culture was pulsed with 0.5 μCi [methyl-3H]-thymidine (Amersham Life Science) per well. Activity was measured using liquid scintillation. Measurements were done in triplicate.
Detachment of cells was measured in HUVECs, cultured as described above, and with or without the control proteins bovine serum albumin (BSA) and PF4. Cultures were analyzed under an inverted microscope. In a microscopic field of 40 μm2, adhered and detached cells were counted to give an indication of the amount of dying cells in time. At day 3, detached cells were collected, adherent cells were trypsinized, and all cells were stained by mixing equal volumes of cell suspension and trypan blue solution (2 mg/mL; Serva, Germany). The percentage of dead cells was calculated after counting under an inverted microscope. Analysis of significance in differences was performed with the Mann-Whitney U test.
Apoptosis assay
HUVECs were seeded at a concentration of 3000 cells per well in a 1 mg/mL, fibronectin-coated, 96-well culture plate. The cells were cultured during 72 hours either in normal culture medium; in the presence of BPI, PF4, and BSA at different concentrations, or under serum deprivation. In some experiments, cells were cultured with or without 5 IU heparin (Leo Pharmaceutical Products, Weesp, The Netherlands). After 72 hours of culture, the cells were harvested with 0.125% trypsin. Part of the cells was used to make cytospin preparations on glass slides for determination of cell morphology after haematoxillin/eosin staining. The remaining cells were centrifuged at 1500 rpm for 5 minutes, washed once with PBS, and subsequently fixed in 70% ethanol at −20°C for 2 hours. The cells were pelleted at 1500 rpm and resuspended in DNA extraction buffer (90 volumes 0.05 mol/L Na2HPO4.2H2O, 10 volumes of 0.025 mol/L citric acid, and 1 volume of 10% Triton-X100 [in distilled water, pH7.4]) and incubated at 37°C for 20 minutes. After this incubation period, propidium iodide was added at a concentration of 20 μg/mL, and the DNA profile of the HUVECs was analyzed on the flow cytometer (FACS Calibur; Becton Dickinson). In some experiments, the caspase inhibitor z-VAD.FMK (Alexis Biochemicals) was used (100 μmol/L) to inhibit apoptosis.17 Statistical significance was determined using the Mann-Whitney U test.
In vitro angiogenesis
Sprouting and tube formation of ECs were studied with the use of cytodex-3 beads overgrown with ECs in a 3-dimensional gel.18 19 Bovine microvascular ECs (BCEs) were mixed with gelatin-coated cytodex-3 microcarrier beads (Sigma, The Netherlands) at a concentration of 25 cells per bead and cultured for 72 hours in a tissue culture plate in RPMI-1640, supplemented with 20% HS, 2 mmol/Ll-glutamine, 50 ng/mL streptomycin, and 50 U/mL penicillin. The beads were spun down and resuspended in a concentration of 25 beads per 100 μL, in 8 volumes of vitrogen-100 (Collagen, Fremont, CA), 1 volume 10 × concentrated α-MEM (Life Technologies, Breda, The Netherlands), 1 volume 11.76 mg/mL sodium bicarbonate, and 20 ng/mL bFGF. This mixture (100 μL) was suspended to each well of a 96-well culture plate, after which gelation was allowed to take place at 37°C. After gelation medium was applied on top of the gel containing 20 ng/mL bFGF with or without BPI at concentrations as indicated and with or without 5 IU/mL heparin. After 24 hours, photographs were made. For quantification, these images were analyzed using NIH image computer software. Statistical analysis was done using the Mann-Whitney U test.
Chorioallantoic membrane assay
Fertilized white leghorn chicken eggs were incubated for 3 days at 37°C and 60% humidity. On the third day, a hatch was made in the eggshell. The eggs were further incubated at 37°C and 60% humidity until day 7. On this day, a silicone ring was placed directly onto the chorioallantoic membrane (CAM) and was left to stabilize for 2 hours. Subsequently, the treatment of the CAMs started by daily addition of 65 μL of either vehicle (saline), 180 nmol/L or 540 nmol/L BPI dissolved in saline. On day 10, the CAMs were photographed. Quantification of vascularization was performed by enumeration of intersections with 3 concentric rings that were superimposed on the photographs.
Results
BPI inhibits EC growth
Potential angiogenic effects from BPI were first investigated in vitro with the use of an EC proliferation assay with cultured HUVECs. EC proliferation was measured using a [3H]-thymidine incorporation assay and by enumeration of living cells following 3 days of culture of HUVECs in the presence of various concentrations of BPI. Both bFGF-induced (20 ng/mL) (Figure 1) and spontaneous (not shown) EC proliferation were inhibited by BPI in a similar concentration-dependent fashion. BPI inhibited 80% proliferation at 1.8 μmol/L, whereas the half-maximal response was observed at about 250 nmol/L (Figure 1A). To discriminate between cytostatic and cytotoxic effects, proliferation was also investigated by quantifying the number of living cells, using the trypan blue dye-exclusion method. Similar results were found in both assays. In these experiments, BSA was used as a negative control, and the angiogenesis inhibitor PF411 was used as a positive control. The dye-exclusion method indicated that BPI is cytotoxic for ECs, whereas PF4 has been recognized as being cytostatic and not cytotoxic20 (Figure 1B). Figure 1C demonstrates that HUVECs are at least an order of magnitude more sensitive to BPI than to PF4, in the [3H]-thymidine incorporation assay.
BPI inhibits proliferation of HUVECs.
(A) Proliferation was measured after 72 hours of culture in the presence of 0.18, 0.54, or 1.8 μmol/L BPI by analysis of [3H]-thymidine incorporation. (B) Cell death induced by BPI, PF4, or BSA, or after starvation by culture in the presence of low serum concentrations (1%), as determined by trypan blue exclusion. (C) Comparison of proliferation inhibition by BPI (n = 4) and PF4 (n = 9), plotted as percentage of thymidine incorporation under control conditions. In panel A and B, the results of 3 independent experiments performed in triplicate are shown (*P < .05).
BPI inhibits proliferation of HUVECs.
(A) Proliferation was measured after 72 hours of culture in the presence of 0.18, 0.54, or 1.8 μmol/L BPI by analysis of [3H]-thymidine incorporation. (B) Cell death induced by BPI, PF4, or BSA, or after starvation by culture in the presence of low serum concentrations (1%), as determined by trypan blue exclusion. (C) Comparison of proliferation inhibition by BPI (n = 4) and PF4 (n = 9), plotted as percentage of thymidine incorporation under control conditions. In panel A and B, the results of 3 independent experiments performed in triplicate are shown (*P < .05).
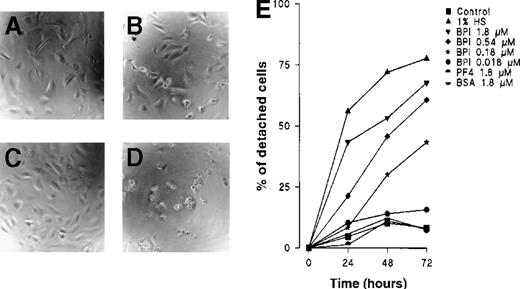
In assays that determine the fraction of detached cells over time, a time-dependent effect from BPI was observed. At a concentration of 1.8 μmol/L, BPI induced a half-maximal response within 24 hours and a maximal response after 48 hours (Figure 2). Neither PF4 nor BSA had any effect on EC attachment to the matrix. In related experiments, BPI functioned similarly in cultures of human microvascular endothelium and of BCE.
BPI induces HUVECs to detach from the matrix.
(A) Control HUVECs after 24 hours, (B) HUVECs cultured with 1.8 μmol/L BPI for 24 hours, (C) control HUVECs after 72 hours of culture, (D) HUVECs cultured with 1.8 μmol/L BPI for 72 hours. After 24, 48, and 72 hours, HUVECs were cultured under the indicated conditions and analyzed under an inverted microscope for the ratio of detached cells. The mean results of 3 independent experiments are shown. For clarity, error bars have been left out; variation was < 20% in all cases (E).
BPI induces HUVECs to detach from the matrix.
(A) Control HUVECs after 24 hours, (B) HUVECs cultured with 1.8 μmol/L BPI for 24 hours, (C) control HUVECs after 72 hours of culture, (D) HUVECs cultured with 1.8 μmol/L BPI for 72 hours. After 24, 48, and 72 hours, HUVECs were cultured under the indicated conditions and analyzed under an inverted microscope for the ratio of detached cells. The mean results of 3 independent experiments are shown. For clarity, error bars have been left out; variation was < 20% in all cases (E).
BPI induces apoptosis in ECs
As shown in Figure 2, BPI induced the detachment and shrinkage of HUVECs. To evaluate whether BPI-induced cell death occurring in EC cultures involves induction of apoptosis, flow cytometric analysis was performed to assess the DNA profile. For 3 days, HUVECs were cultured in the presence of different concentrations of BPI, BSA, and PF4, as well as under serum-deprived conditions. Following cell harvesting, staining with propidium iodide and DNA extraction, FACS analysis was used to analyze the percentage of cells with a subdiploid DNA content. Having fragmented (subdiploid) DNA is indicative of apoptosis. For ECs cultured in the presence of either BSA or PF4, the amount of cells in the subdiploid peak was comparable to that for HUVECs cultured under normal conditions. Serum deprivation basically starves cells and is known to trigger the apoptotic signal. In the presence of 1.8 μmol/L BPI, a 10-fold to 13-fold elevation in apoptosis was observed as compared with that in control cultures. BPI concentrations of 180 nmol/L, 540 nmol/L, and 1.8 μmol/L induced apoptosis in 15%, 27%, and 30% of the cells, respectively (Figure3A). These levels are comparable to those observed in serum-starved cultures. BPI-induced apoptosis was confirmed on the basis of cell morphology that was assessed in haematoxillin/eosin stained cytospin preparations (Table1). A third line of evidence that confirmed induction of apoptosis by BPI was the use of z-VAD.FMK in cultures of HUVECs with BPI or with serum deprivation. The addition of z-VAD.FMK at a concentration of 100 μmol/L significantly inhibited the apoptosis induction by BPI with 71% ± 2%, whereas the cell detachment was not blocked by adding z-VAD.FMK. These results indicate the induction of apoptosis as a result of cell detachment and not vice versa.
BPI induces apoptosis in ECs.
(A) HUVECs were cultured for 3 days in normal culture medium; in medium with 1% human serum; or in medium containing BPI, BSA, or PF4. After partial DNA extraction and propidium iodide staining, analysis of subdiploid ECs under the different conditions was performed (n = 3, *P < .025, **P < .014). (B) Apoptosis induction by BPI is EC specific (n = 3, *P < .025). Percentage of subdiploid cells was also determined in Ls174T human colon adenocarcinoma (n = 4) and in normal human foreskin–derived fibroblasts (n = 4).
BPI induces apoptosis in ECs.
(A) HUVECs were cultured for 3 days in normal culture medium; in medium with 1% human serum; or in medium containing BPI, BSA, or PF4. After partial DNA extraction and propidium iodide staining, analysis of subdiploid ECs under the different conditions was performed (n = 3, *P < .025, **P < .014). (B) Apoptosis induction by BPI is EC specific (n = 3, *P < .025). Percentage of subdiploid cells was also determined in Ls174T human colon adenocarcinoma (n = 4) and in normal human foreskin–derived fibroblasts (n = 4).
BPI induces nuclear fragmentation in HUVECs
| Condition . | Percentage of cells with fragmented nuclei . | |
|---|---|---|
| Experiment 1 . | Experiment 2 . | |
| Control | 5* | 3 |
| BPI (1.8 μmol/L) | 79 | 69 |
| PF4 (1.8 μmol/L) | 7 | 4 |
| BSA (1.8 μmol/L) | 4 | 5 |
| 1% HS | 99 | 71 |
| Condition . | Percentage of cells with fragmented nuclei . | |
|---|---|---|
| Experiment 1 . | Experiment 2 . | |
| Control | 5* | 3 |
| BPI (1.8 μmol/L) | 79 | 69 |
| PF4 (1.8 μmol/L) | 7 | 4 |
| BSA (1.8 μmol/L) | 4 | 5 |
| 1% HS | 99 | 71 |
HUVECs were cultured for 3 days under indicated conditions, collected, cytospinned, and stained with haematoxillin/eosin. Percentage of fragmented nuclei was microscopically scored. BPI, bactericidal/permeability-increasing protein; HUVEC, human umbilical vein-derived endothelial cell; PF4, platelet factor-4; BSA, bovine serum albumin; HS, human pooled serum.
The effects of BPI are EC specific as indicated by the analysis of apoptosis induction by BPI in non-ECs (the human adenocarcinoma cell-line Ls174T and freshly isolated human fibroblasts) as compared with HUVECs (Figure 3B).
Heparin blocks the apoptosis inducing activity of BPI
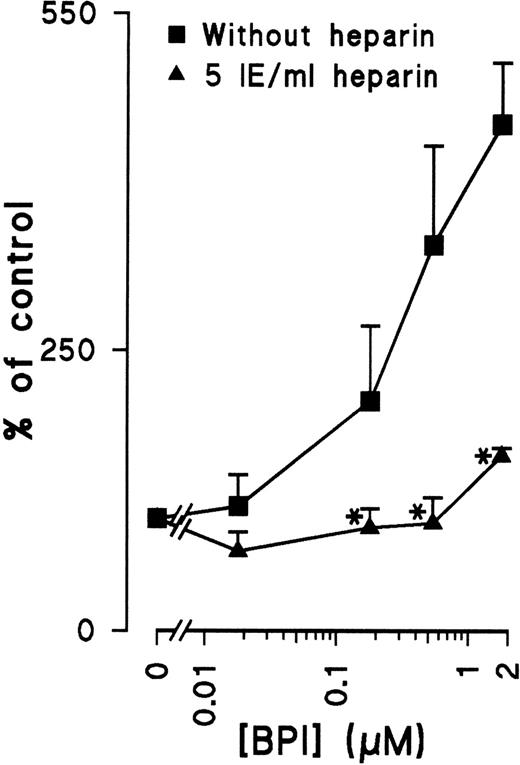
Many cytokines that are known to regulate angiogenesis have the capacity to bind heparin. Because BPI is also known to bind heparin,8 the dependence of heparin on the antiproliferative effect of BPI was investigated. Indeed, the addition of heparin to EC cultures inhibits the induction of apoptosis by BPI (Figure 4), suggesting that heparin does play a crucial role in the regulation of BPI-mediated inhibition of EC growth.
Heparin prevents BPI-induced apoptosis.
HUVECs were cultured with or without heparin and different concentrations of BPI for 72 hours. After this period, DNA fragmentation was analyzed with flow cytometry (results shown are mean of 3 independent experiments ± SEM). *Significant difference (P < .05) compared with cultures without heparin.
Heparin prevents BPI-induced apoptosis.
HUVECs were cultured with or without heparin and different concentrations of BPI for 72 hours. After this period, DNA fragmentation was analyzed with flow cytometry (results shown are mean of 3 independent experiments ± SEM). *Significant difference (P < .05) compared with cultures without heparin.
BPI inhibits in vitro angiogenesis
To further investigate the effects of BPI in subsequent steps of the angiogenic process, an in vitro angiogenesis assay was used in which gelatin-coated beads overgrown with ECs were embedded in a seminatural collagen matrix. Because ECs of human macro- or microvessel origin cannot, or can only marginally, be induced to form tubes,21 bovine capillary ECs were used in these studies. Under a stimulus of 20 ng/mL bFGF, ECs were induced to sprout and to form tubes into the matrix. BPI efficiently inhibited BCE tube formation in a concentration and time-dependent fashion. At 1.8 μmol/L, 81% inhibition was noted after only 24 hours. Half-maximal responses were observed at a concentration of 80 nmol/L. Heparin at a concentration of 5 IU/mL inhibits the BPI inhibition of in vitro angiogenesis by 56% ± 13%. PF4 also significantly inhibited angiogenesis by 77% at the same concentration (Figure5).
BPI inhibits in vitro angiogenesis in a collagen matrix.
BCEs were cultured on gelatin-coated Cytodex-3 beads in a collagen matrix. Sprouting was induced by addition of 20 ng/mL bFGF (panel A). Panel B shows sprouting of BCEs in the presence of 20 ng/mL bFGF and 1.8 μmol/L BPI. Quantification of results was performed by NIH-image software (n = 3, *P < .013).
BPI inhibits in vitro angiogenesis in a collagen matrix.
BCEs were cultured on gelatin-coated Cytodex-3 beads in a collagen matrix. Sprouting was induced by addition of 20 ng/mL bFGF (panel A). Panel B shows sprouting of BCEs in the presence of 20 ng/mL bFGF and 1.8 μmol/L BPI. Quantification of results was performed by NIH-image software (n = 3, *P < .013).
BPI inhibits angiogenesis in vivo
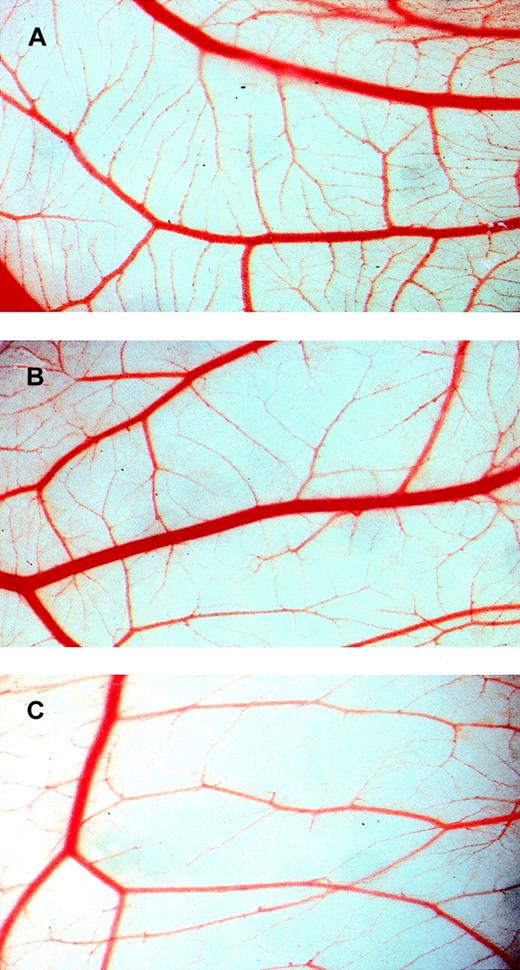
To study inhibition of angiogenesis in vivo, the chick embryo CAM assay was used. This assay measures developmental angiogenesis and is routinely used to obtain the first indication of angiogenic activity in vivo. CAMs were studied in intact eggs in which windows of 1 × 1.5 cm were made at day 3 of development. Treatment was initiated at day 7. In CAMs treated daily with BPI between days 7 and 10 of development, a profound inhibition of microvessel formation was observed, whereas larger, preexisting vessels were apparently unaffected. Figure 6 shows the development of CAM vasculature at day 10 after fertilization and treatment with 180 nmol/L (B), 540 nmol/L (C) BPI, or vehicle alone (A). Microvessel formation was inhibited by 42% at 180 nmol/L and 48% at 540 nmol/L. Treatment with vehicle alone resulted in the development of normal vasculature at day 10 similar to that observed in untreated CAMs.
BPI inhibits angiogenesis in the CAM assay.
CAMs were treated for 4 days with vehicle alone (A), 180 nmol/L BPI (B), or 540 nmol/L BPI (C).
BPI inhibits angiogenesis in the CAM assay.
CAMs were treated for 4 days with vehicle alone (A), 180 nmol/L BPI (B), or 540 nmol/L BPI (C).
Discussion
A novel biological activity for BPI has been reported in this study. BPI has joined the list of antiangiogenic agents that include PF4, angiostatin,22 and endostatin.23More and more angiogenesis inhibitors are continually being discovered, and only a few will ever be used in therapeutic applications. BPI, aside from providing a potential treatment against sepsis, might also be used in the treatment of cancer and/or other angiogenesis-related pathological disorders. In its capacity as an antiangiogenic agent, BPI works specifically on and is cytotoxic to ECs, whereas both PF4 and endostatin, for example, are cytostatic, arresting ECs in the cell cycle.11,20 The concentration at which BPI is half-maximally effective at inhibiting in vitro angiogenesis, 80 nmol/L, is considered to be quite low relative to that of many other inhibitors of angiogenesis,22,23 like PF411 that demonstrates a similar effectivity at a concentration about 10-fold higher in inhibiting proliferation; however, the antiangiogenic effects in the in vitro assay were quite similar.20
These studies have been performed using native, full-length BPI that has a molecular mass of 55 kd. The recombinant 23 kd N-terminal sequence from BPI has also been reported8 as being functional in neutralizing the effects from bacterial endotoxin. It is unknown if it also demonstrates antiangiogenic activity as does native BPI. A peptide derived from the B-sheet domain of that N-terminal sequence of BPI24 also has the ability to neutralize LPS, albeit 50-fold less effective; yet demonstrates no effect on EC proliferation (data not presented). It may be that both ends of BPI are required for antiangiogenic activity, although this requirement remains to be tested.
ECs cultured in the presence of native BPI detach from the matrix and undergo apoptosis. Induction of apoptosis can be a rapid event, for example, following signaling via death-domain containing receptors such as the Fas-Fas ligand interaction in leukocytes. Because of the fact that BPI-induced apoptosis in ECs was demonstrated to occur as a rather delayed phenomenon, it is suggested that the effect from BPI occurs indirectly, perhaps via interaction(s) with some adhesion receptor(s) to inhibit attachment of ECs to the extracellular matrix, thereby inducing apoptosis. The fact that the caspase inhibitor z-VAD.FMK does not prevent detachment of ECs favors the view that the apoptosis is a result of detachment, which may explain the delayed appearance of apoptotic morphology. Induction of apoptosis has also been demonstrated with another antiangiogenic agent, an anti–αvβ3-integrin antibody, which binds the EC surface αvβ3-integrin25 to directly trigger apoptosis.26 Future studies should elicit which cell surface molecule(s) functions as the receptor for BPI on the endothelium.
A specific question that arises from these data is “What, if any, is the relationship between the antibacterial and antiangiogenic effects of BPI?” The former function involves interactions of BPI with the outer membrane of bacteria, whereas the latter function involves interactions presumably with cell surface receptor(s) on ECs. Part of the explanation may be that, during bacterial infections and ongoing inflammatory responses, angiogenesis is heavily stimulated. Low levels of BPI have been found circulating in the blood of patients experiencing bacterially induced or sterile inflammation.27Inflammatory cytokines, such as TNFα, interferon, TGFβ, and interleukin-1, play an important role in this process. It is feasible that a feedback mechanism for inhibition of angiogenesis after turning down the inflammation may have evolved. These considerations are similar to those that have been discussed for PF4 in having a role in turning down angiogenesis after wound healing.
Comparing what is known about the molecular processes of BPI in these 2 biologically different functions may provide some clue to any possible relationship. Surprisingly, the LPS-neutralizing effect from BPI occurs at a half-maximal concentration of about 20 nmol/L,24 which is close to that reported here for its anti-angiogenic effect, 80 nmol/L. BPI has a strong affinity for negatively charged lipids in membranes, and it, therefore, binds to the negatively charged LPS in the outer membrane of gram-negative bacteria. Through its network of positive charges, BPI is thought to bind sugar phosphate groups in LPS, thereby neutralizing this bacterial endotoxin.2,28 This process might also be a regulatory pathway of BPI, through its binding to negatively charged receptors in the EC membrane. In support of this idea, BPI is known to bind the polyanionic molecule heparin8 via its cationic residues. BPI in fact contains 3 heparin-binding sequences.8 These amino acid sequences are similar in composition to many other angiogenic and angiostatic compounds.29
Initially, chemical similarities and heparin-binding abilities between BPI and vasoactive molecules like PF4, interleukin-8 (IL8),12,30 and other chemokines, urged us to investigate the effect of BPI on angiogenesis. Although these similarities with PF4 and IL8 suggest shared mechanisms and/or receptors, the effect of BPI on ECs is completely different. IL8 acts as an angiogenic factor, and PF4, while also being angiostatic, does not induce apoptosis. These points suggest that BPI acts via a completely different mechanism and receptor(s). Although the IL8 receptors are known, a receptor for PF4 has yet to be identified. Possibly the cell-associated form of heparin, heparan sulfate, and/or other interaction molecule containing some polyanionic carbohydrate moiety present in the extracellular matrix could be the receptor and be bound by BPI. The possibility thus arises that BPI detaches ECs by binding heparan sulfate on the extracellular matrix side of ECs, thus preventing them from migrating through the extracellular matrix and leading to the induction of apoptosis. The binding of heparin by BPI can also have another role. Heparin is known to stabilize angiogenic factors such as bFGF and vascular endothelial growth factor. Heparin binding by BPI might compete for this stimulatory pathway. In addition, bFGF is normally stored in the extracellular matrix where it is bound to heparan sulfate and can be released by heparin.31 BPI, therefore, could block that process. This proposal is supported by the in vitro observation that BPI, in the presence of heparin, loses its antiangiogenic activity.
In conclusion, BPI, produced in neutrophils, provides the human host with a natural defense against bacterial infection and also plays a role in the balance among angiogenic and angiostatic agents working together in the body. BPI, therefore, aside from providing a potential treatment against septic shock, might also be used in the treatment of cancer and/or other angiogenesis-related pathological disorders.
Reprints:Daisy W. J. van der Schaft, Tumor Angiogenesis Laboratory, Department of Internal Medicine, University Hospital Maastricht, P.O. Box 5800, 6202 AZ Maastricht, The Netherlands; e-mail:DvdSchaft@hotmail.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. BPI inhibits proliferation of HUVECs. / (A) Proliferation was measured after 72 hours of culture in the presence of 0.18, 0.54, or 1.8 μmol/L BPI by analysis of [3H]-thymidine incorporation. (B) Cell death induced by BPI, PF4, or BSA, or after starvation by culture in the presence of low serum concentrations (1%), as determined by trypan blue exclusion. (C) Comparison of proliferation inhibition by BPI (n = 4) and PF4 (n = 9), plotted as percentage of thymidine incorporation under control conditions. In panel A and B, the results of 3 independent experiments performed in triplicate are shown (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.176/4/m_bloo01332001x.jpeg?Expires=1769288187&Signature=fFRQuS5PR7givb8W-LEvU3tFG8tlej3Bb6RF4W5dZY3w071lH0wnRLy-QnPyG1ijlP108ks~~3Aa1k8TPT7VHx6JP02b7fwTF34i8cIh9cHc4YgzBD~-PZsg5vOQilYKOw97g6yGyWxlZ0RAHqGQtqIZeRmzPg9qahKJa2ZHOwvRRtbsUPQIcH51jU4AvoJ2WHqoHtFg6B519do0bUsFH8VSHwQOtPjme5xi1iE-zN9vP8lxRwhlE70DB8BDyyq-7DHTOUr-YpKIK0RImfNzhrFBphKYW5PMyqx8lQKycCj8Vje2kHHJbjaCmdzqt8WzN1hv0HZHmyoEpni3PElImg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal