Abstract
We describe thrombogenic tissue factor (TF) on leukocyte-derived microparticles and their incorporation into spontaneous human thrombi. Polymorphonuclear leukocytes and monocytes transfer TF+particles to platelets, thereby making them capable of triggering and propagating thrombosis. This phenomenon calls into question the original dogma that vessel wall injury and exposure of TF within the vasculature to blood is sufficient for the occurrence of arterial thrombosis. The transfer of TF+ leukocyte-derived particles is dependent on the interaction of CD15 and TF with platelets. Both the inhibition of TF transfer to platelets by antagonizing the interaction CD15 with P-selectin and the direct interaction of TF itself suggest a novel therapeutic approach to prevent thrombosis.
Vessel wall injury and exposure of tissue factor (TF) within the vascular wall to blood are considered necessary for the initiation of arterial thrombosis.1-3 This paradigm has been broadened by the observation that TF is also present in circulating blood.4-6 We have previously demonstrated the presence of TF in thrombi and have shown that ex vivo thrombus and fibrin formations were reduced by the addition of a TF inhibitor to blood. These results indicate that circulating TF is potentially active,4 which implies the existence of a thrombogenic pool of circulating TF. In our study, TF in thrombi appeared mostly on membranous structures that were proximate to platelets. In fact, polymorphonuclear (PMN) leukocytes from both circulating blood and rabbit liver tested positive for TF by staining.4,7 In addition, leukocytes coincubated with platelets generate more procoagulant activity than either cell type alone.8,9 Taken together, these data suggest that leukocytes are a potential source of TF microparticles that adhere to platelets within a thrombus. Leukocytes and platelets are known to interact via CD15 (a leukocyte membrane-bound carbohydrate known as sialyl Lewisx) with CD62P (P-selectin), which is an α granule–derived activation-dependent adhesion molecule found on platelets.10-13
This study examines whether (1) a cell line that was derived from monocytes (THP-1 cells) can transfer TF+ fragments to platelets during thrombus formation and (2) the above-mentioned adhesion molecules are involved in the formation of platelet-TF hybrids. Furthermore, inhibitory monoclonal antibodies (mAbs) to the identified membrane proteins were used to inhibit the transfer of preexisting TF to platelets. Our data suggest that monocytes and possibly PMN leukocytes are the source of circulating TF which is transferred to platelets, thereby making F platelets capable of triggering and propagating thrombosis. This transfer process is mediated by the interaction of CD15 with platelets and by TF itself. The inhibition of TF transfer to platelets suggests a novel therapeutic approach to prevent thrombosis.
Materials and methods
Reagents
We used the following in this study: human recombinant factor VIIa (FVIIa) (gift from Novo-Nordisk, Gentofte, Denmark); Spectrozyme Xa (American Diagnostica, Greenwich, CT); monomeric bovine type I collagen (Vitrogen; Collagen Corp, Palo Alto, CA); Tyrode's solution, sterile bovine serum albumin (BSA), citrate-dextrose solution (ACD), tumor necrosis factor α (TNF-α), and prostaglandin E1 (Sigma Chemical Co, St Louis, MO); Roswell Park Memorial Institute medium (RPMI 1640) with L-glutamine, Hanks' balanced salt solution (HBSS), phosphate-buffered saline (PBS), and a penicillin-streptomycin combination (Gibco Life Technologies, Braunschwedg, Germany); THP-1 cells (American Type Culture Collection, Bethesda, MD); and DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). Purified factor X was prepared from human plasma.14 Immunopurified rabbit antihuman TF polyclonal antibody (pAb-TF) and digoxygenin-labeled FVIIa (Dig-VIIa) were prepared as reported.15 Anti-TF mAb (hTF1) has been described.16
Preparation of platelets and THP-1 cells
Platelet-rich plasma was prepared from citrated blood of healthy donors (who had given informed consent) by centrifugation (Sorvall RT 6000B Refrigerated Centrifuge; DuPont, Wilmington, Delaware) at 250g for 10 minutes. The plasma was mixed with modified Tyrode's solution I (Tyrode's solution, ACD 1:10 vol/vol, 0.35% BSA [pH 6.5], and 20 μmol/L prostaglandin E1). Isolated platelets were washed 2 times in modified Tyrode's solution II (Tyrode's solution, 0.35% BSA [pH 6.5], and 20μmol/L prostaglandin E1) followed by a final washing in modified Tyrode's solution III (Tyrode's solution, 0.35% BSA, 2 mmol/L magnesium, and 100 U/mL penicillin/streptomycin). The platelet preparations were used for TF transfer experiments after 30 minutes at rest. THP-1 cells from a human monocytic cell line were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), L-glutamine, and 2% penicillin/streptomycin. Before induction of TF, THP-1 cells were thoroughly washed in HBSS and then incubated with 1 μg/mL TNF-α (cell concentration, 20 000 to 30 000 cells per μL) overnight.
Perfusion experiments on collagen- and platelet-coated glass slides
Microscope slides (Superfrost/Plus, Fisher Scientific, Springfield, NJ) were incubated with 10 μg/mL collagen overnight at 4 °C followed by washing 3 times in PBS and blocking in 1% BSA/PBS for 30 minutes. Perfusion systems for the slides were previously reported.17 Two approaches for the assessment of TF transfer to platelets were established: (1) Collagen-coated glass slides were mounted in a parallel plate flow chamber.17 The induced THP-1 cells were mixed with platelets and perfused at a shear rate of 500s−1 over collagen-coated slides. (2) A platelet carpet was first deposited on the collagen-coated slide by incubating isolated platelets in the presence of 1 mmol/L magnesium chloride and 2.5 mmol/L calcium chloride for 2 hours at 37°C. The platelets deposited on collagen were then perfused with TF-expressing THP-1 cells. All perfusions were performed for 10 minutes at room temperature.
Immunocytochemistry
Immediately after perfusion, the slides were removed from the chamber, gently rinsed in PBS, placed in freshly prepared 4% phosphate-buffered paraformaldehyde (pH 7.4), and fixed for 1 hour at room temperature. The slides were washed in PBS, blocked with 1.2% peroxide in methanol and goat serum, and incubated with 1 μg/mL anti-TF pAb (pAb-TF, anti-sTF). Bound primary antibody was detected using a biotin streptavidin-amplified detection system (BioGenex Laboratories, San Ramon, CA) developed with 3,3-diaminobenzidine tetra-hydrochloride. After counter staining with hematoxylin, the slides were examined microscopically. Controls for immunostaining consisted of a nonspecific antibody as the primary antibody.
Immunoelectron microscopy
Preembedding with double-immunogold labeling.
Human blood was perfused over collagen-coated slides to obtain thrombi as described.4 The thrombi were then fixed in 4% paraformaldehyde. Nonspecific binding was reduced by washing the thrombi in 1.5% BSA and 0.5% ovalbumin in PBS (pH 7.4) twice for 5 minutes each washing. Incubation with 10 μg/mL rabbit anti-hTF pAb was performed in 0.1% BSA/PBS for 1 hour. Simultaneously, we used 10 μg/mL anti-CD15 antibody (mouse monoclonal antineutrophil/monocyte carbohydrate epitope, immunoglobulin M [IgM])(Dako, Hialeah, FL). The samples were then washed 3 times in 0.1% BSA/PBS for 5 minutes each washing. The samples were incubated with secondary goat antirabbit IgG coupled to 5-nm colloidal gold particles (Biocell Laboratories, Rancho Dominguez, CA) or goat antimouse IgM coupled to 10-nm colloidal gold particles. The samples were then diluted 1:40 in 0.1% BSA/PBS and 0.05% Tween 20 (pH 7.4) for 1 hour.
Alternatively, primary and secondary antibodies were applied in a single step and lead to identical results. After 3 washes in PBS for 5 minutes each, the samples were fixed in 2% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.4) for 1 hour, briefly washed in 0.1 mol/L Na cacodylate buffer, and postfixed in 2% osmium tetroxide. The samples were then dehydrated in a series of aqueous ethanol solutions, exposed to propylene oxide, and embedded in Epon 812 according to Luft.18 Ultrathin sections were cut on a Reichert Ultracut S and stained with 5% aqueous uranyl acetate followed by lead citrate.19 Electron micrographs were acquired with a JEOL 1210 at 100 kV. The controls, which were treated with mouse preimmune serum or nonspecific monoclonal IgG, revealed no significant stain, and a negligible background of fewer than 10 gold grains was noted in cells greater than 10 μm2.
On-section immunogold labeling with ultrathin cryosections.
Human venous thrombi were obtained from a 38-year-old patient. Samples were fixed overnight with 4% formaldehyde in 0.1 mol/L PBS (pH 7.4). After storage in PBS for 6 days, samples were dissected into small fragments not more than 1 μL and postfixed in 3% formaldehyde with 0.1% glutaraldehyde in PBS for 2 hours. Sample preparation was done as described by Tokuyasu20 with the following modifications: neutralization of free aldehydes was achieved by rinsing in PBS and incubating in 50 mmol/L ammonium chloride in PBS for 1 hour. The samples were immersed overnight in cryoprotection buffer consisting of 1.7 mol/L sucrose and 15% polyvinyl-pyrrolidone (10 000 MW) in 10 mmol/L PBS (pH 7.4) at 4°C and frozen in liquid nitrogen.
Ultrathin (9-nm) cryosections were prepared at −100°C with a Reichert Ultracut S + FCS using a diamond knife (Diatome) in the presence of an antistatic line (Diatome). Cryosections were collected in 2.3 mol/L aqueous sucrose onto formvar/carbon–coated 200-mesh nickel grids. The sections were thawed over PBS prior to immunolabeling, which was performed at room temperature. The sections were floated on 0.05 mol/L glycine in PBS for 15 minutes to inactivate free aldehyde groups and then on 2.5% BSA/PBS and 2.5% ovalbumin for 15 minutes to block unspecific binding sites. Sections were incubated with 10 μg/mL hTF1 in 2% BSA/PBS for 1 hour. After 6 washes in BSA/PBS, the sections were incubated with secondary goat antimouse IgG (Amersham, Arlington Heights, IL), conjugated to 10-nm gold at 1:20 dilution in 2% BSA with 0.1% Tween 20 in PBS for 1 hour, and washed in BSA/PBS. For a control, we used sections treated with normal mouse serum, which resulted in a negligible background of not more than 10 gold grains in cells larger than 10 μm.2The sections were postfixed in 2% glutaraldehyde in PBS for 5 minutes, washed, and stained with 0.4% uranyl acetate in 1.8% methyl cellulose (25 cP) for 5 minutes.
THP-1 cell–derived cell fragments and measurement of TF activity
Conditioned medium from TNF-α–induced THP-1 cells was used as a source of microparticles. THP-1 cells were removed by centrifugation at 2000g for 10 minutes, and the THP-1 cell pellet was washed in HBSS and pelleted again. Cell particles and fragments present in the supernatant were concentrated by centrifugation at 16 000g for 15 minutes. The absence of THP-1 cells in the particle suspension was confirmed by light microscopy. The protein concentration of the particles was determined according to the manufacturer's instructions using a microplate protocol (DC Protein Assay, Bio-Rad Laboratories). Absorbance was read at 650 nm, and sample protein concentrations were calculated from a standard curve using BSA. We assayed 40 μL concentrated particles for TF activity using HEPES (4-[2-Hydroxyethyl]-1-piperazineethanesulfonic acid) buffer with 1 nmol/L FVIIa, 150 nmol/L factor X, and 5 mmol/L calcium chloride. At intervals, samples were transferred to a microtiter plate in which each well contained 100 μL EDTA (ethylenediamine tetraacetic acid) buffer (50 mmol/L Bicine [pH 8.5], 20 mmol/L EDTA, and 1 mg/mL BSA), which terminates production of factor Xa. Chromogenic substrate Spectrozyme Xa (final concentration 0.5 mmol/L) was added to each well, and the increase in OD was monitored at 405 nm for 10 minutes by using a kinetic enzyme-linked immunoabsorbent assay (ELISA) plate reader at 35°C (Tmax, Molecular Devices, Sunnyvale, CA). Activity was quantified by reference to purified human rTF of known concentration.
Flow cytometry
Particles from TNF-α–induced THP-1 cells were freshly prepared and resuspended in PBS for flow cytometric analysis of surface membrane proteins. Particles were blocked in normal goat serum and incubated with an mAb to 20 μg/mL CD15 (clone AHN1.1, Ancell), 10 μg/mL TF (hTF-1), and 10 μg/mL CD18 (clone IB4, Ancell, Bayport, MN), respectively, for 2 hours at room temperature. Mouse IgG1 served as a control. After 2 washings in 1% BSA/PBS, samples were incubated with a secondary rabbit antimouse antibody conjugated to phycoerythrin (PE) (Molecular Probes, Eugene, OR) for 1 hour at room temperature. Flow cytometry was performed immediately thereafter, and 10 000 events were acquired for each measurement.
To minimize the amount of antibodies needed to inhibit the TF transfer, the parallel flow chamber was replaced by a cone and plate viscometer that was suitable for small sample volumes (250 μL instead of 4 mL).21 Isolated platelets were activated on collagen-coated glass plates within plastic wells; TF-containing particles were added, and the mixture was subjected to 500s−1 wall shear rates in the viscometer21for 30 minutes. To examine whether blocking antibodies to the identified adhesion proteins on the microparticle surface inhibit the attachment of TF to platelets, TF-containing microparticles were preincubated with either a blocking antibody to 200 μg/mL CD15 (anti-LewisX, clone AHN1.1, Ancell) or to 200 μg/mL TF (hTF-1) for 15 minutes at room temperature before adding the microparticles to the platelets. After exposing the platelet-particle mixture to shear stress, platelet-particle hybrids were fixed in 1% paraformaldehyde for 15 minutes, washed in PBS, incubated with 0.02% Triton X-100 in PBS for 5 minutes at 37°C, and washed twice with PBS.
Platelet-particle hybrids were further blocked with 1% BSA/PSA and normal goat serum, then stained with 1 μg/mL rabbit anti-TF pAb for 2 hours at room temperature or overnight at 4°C. After rinsing twice with PBS, samples were incubated with a secondary goat antirabbit antibody conjugated to PE. To double stain for TF and a specific platelet antigen on platelet-particle hybrids, the sample was simultaneously incubated with 1 μg/mL mouse anti–platelet glycoprotein IIb/IIIa complex (anti-GPIIb/IIIa) mAb (anti-CD41a, Ancell) directly conjugated to fluorescein isothiocyanate (FITC). Rabbit IgG, followed by the secondary antibody described above, and mouse IgG-FITC were used as a negative staining control. The incubation was performed at 4°C for 2 hours. Flow cytometry was performed immediately thereafter.
Attachment of the TF-containing particles to platelets was inhibited by hTF-1 (see “Results”). To ensure that the mAb did not inhibit detection of TF by the pAb, preliminary experiments binding pAb with and without the addition of hTF-1 were performed. The presence of hTF-1 had no significant effect on the binding.
Confocal laser scanning microscopy
Platelet-TF hybrids were examined using a Leica TCS-SP (ultraviolet [UV]) confocal laser scanning microscope (Leica, Heidelberg, Germany) equipped with a 4-channel spectrophotometer scan head and 4 lasers (argon-UV [Ar-UV], Ar, krypton, and helium-neon [HeNe]). For these studies, the platelet-TF hybrids were illuminated simultaneously with the λ 488- and 568-nm laser lines. The pinhole size was adjusted such that resultant “optical sections” were approximately 0.25- to 0.3-μm thick. To ensure that the signal did not “spill over” from one channel into another, the acousto-optical tunable filter was adjusted, and the spectrophotometer windows in each channel were set so that signals from one channel were not detected in the other channel.
Results
TF+ microparticles are transferred from leukocytes to platelets
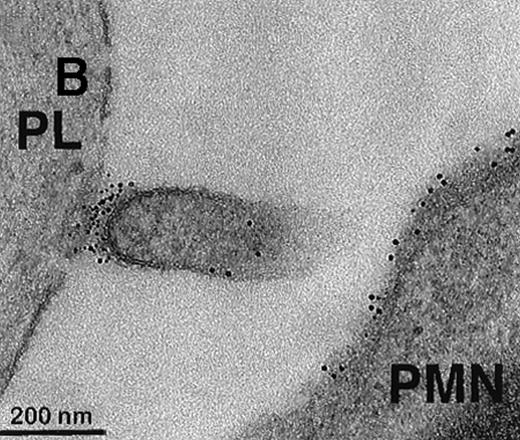
To further visualize the structure of TF on platelets, immunoelectronmicroscopy was performed on thrombi generated ex vivo by perfusing human blood directly from a donor's arm over collagen-coated slides. TF+ pseudopodia extending from PMN leukocytes were found to contact platelets within the thrombus (Figure1). Adhesion molecules present on the leukocyte surface, which may possibly be involved in the adhesion to platelets during thrombus formation, were also identified. A colocalization of CD15 and TF on pseudopodia and small membrane particles was demonstrated by double immunolabeling (Figure 1). To confirm the presence of TF+ particles within thrombi, a human venous thrombus obtained from a patient was examined by immunoelectronmicroscopy. TF+ particles were present within the thrombus and also intermingled with fibrin strands (Figure2).
Collagen-coated glass slides were perfused with native human blood, and the deposited thrombi were double immunostained for TF and CD15.
(A) Electronmicroscopy showing platelets and PMN leukocytes within a thrombus. Note the PMN leukocyte–derived membrane attached to the platelet surface depicted in the boxed field. (B) Closeup examination of the boxed field: the structure double stains for CD15 (10-nm gold grains) and TF (5-nm gold grains). (C) TF on small particle-like structures in proximity to PMN leukocytes.
Collagen-coated glass slides were perfused with native human blood, and the deposited thrombi were double immunostained for TF and CD15.
(A) Electronmicroscopy showing platelets and PMN leukocytes within a thrombus. Note the PMN leukocyte–derived membrane attached to the platelet surface depicted in the boxed field. (B) Closeup examination of the boxed field: the structure double stains for CD15 (10-nm gold grains) and TF (5-nm gold grains). (C) TF on small particle-like structures in proximity to PMN leukocytes.
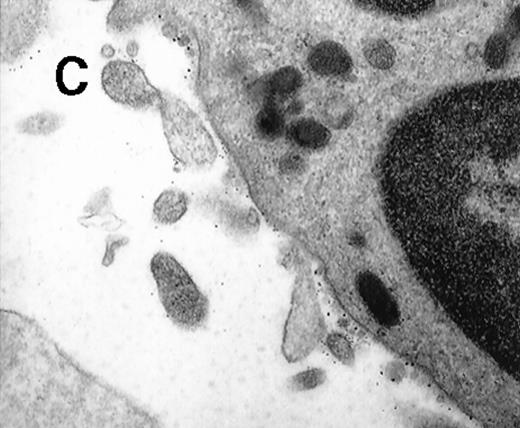
Ultrathin cryosections through a human venous thrombus.
TF within the thrombus was detected by on-section labeling using mAb anti-TF (hTF1) followed by goat antimouse IgG conjugated to 10-nm colloidal gold. (A) Electronmicroscopy shows TF+ cell fragments adjacent to the fibrin strands. (B) Closeup of the region in the boxed field: a clearly discernible bilayered cell membrane and organelle substructure are indicative of the cellular origin of the TF+ structures.
Ultrathin cryosections through a human venous thrombus.
TF within the thrombus was detected by on-section labeling using mAb anti-TF (hTF1) followed by goat antimouse IgG conjugated to 10-nm colloidal gold. (A) Electronmicroscopy shows TF+ cell fragments adjacent to the fibrin strands. (B) Closeup of the region in the boxed field: a clearly discernible bilayered cell membrane and organelle substructure are indicative of the cellular origin of the TF+ structures.
THP-1 cells as a model to study the transfer of TF+ cell fragments to platelets
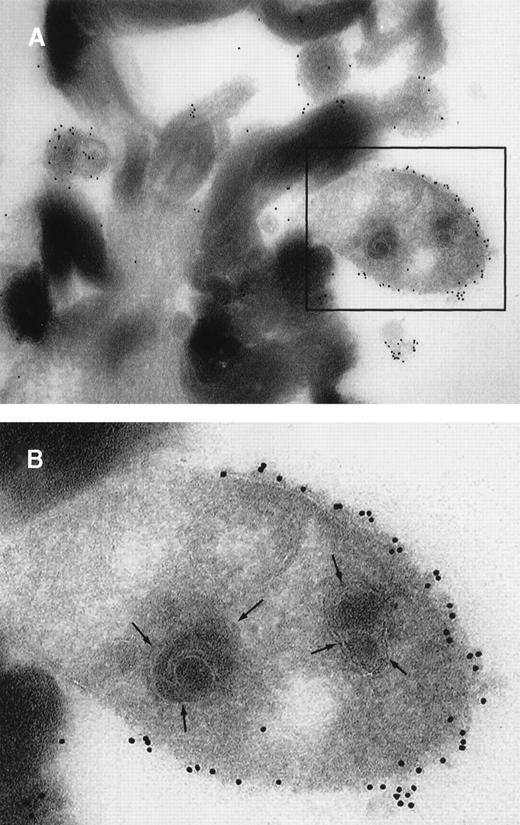
We then established a perfusion model to study whether leukocytic cells can transfer TF+ particles to platelets. Platelets isolated from human blood were deposited on collagen-coated slides that were then mounted in a parallel plate flow chamber. THP-1 cells were treated with TNF-α to induce the production of TF microparticles22 23; the suspension was then perfused at 500s−1 over the previously formed platelet carpet. In this model system, platelets, which usually do not contain TF (Figure3A), became TF+ after being exposed to TF+ THP-1 cells and THP-1 cell–derived cell fragments (Figure 3B).
Results of immunocytochemistry performed on platelets.
(A) The platelets were isolated from circulating blood and deposited on collagen-coated slides. Immunocytochemistry was performed to detect TF. When stained, the platelets did not test positive for TF. Hemotoxylin was used for counter staining. (B) THP-1 cells induced to produce TF and also TF+ microparticles by treatment with TNF-α were perfused over a platelet carpet on a collagen-coated glass slide that was mounted in a parallel plate flow chamber.14The induced THP-1 cells were perfused over the platelets at 500s−1. Note that platelets turned a brown color, indicating TF+, after exposure to TF containing THP-1 cells (original magnification × 200).
Results of immunocytochemistry performed on platelets.
(A) The platelets were isolated from circulating blood and deposited on collagen-coated slides. Immunocytochemistry was performed to detect TF. When stained, the platelets did not test positive for TF. Hemotoxylin was used for counter staining. (B) THP-1 cells induced to produce TF and also TF+ microparticles by treatment with TNF-α were perfused over a platelet carpet on a collagen-coated glass slide that was mounted in a parallel plate flow chamber.14The induced THP-1 cells were perfused over the platelets at 500s−1. Note that platelets turned a brown color, indicating TF+, after exposure to TF containing THP-1 cells (original magnification × 200).
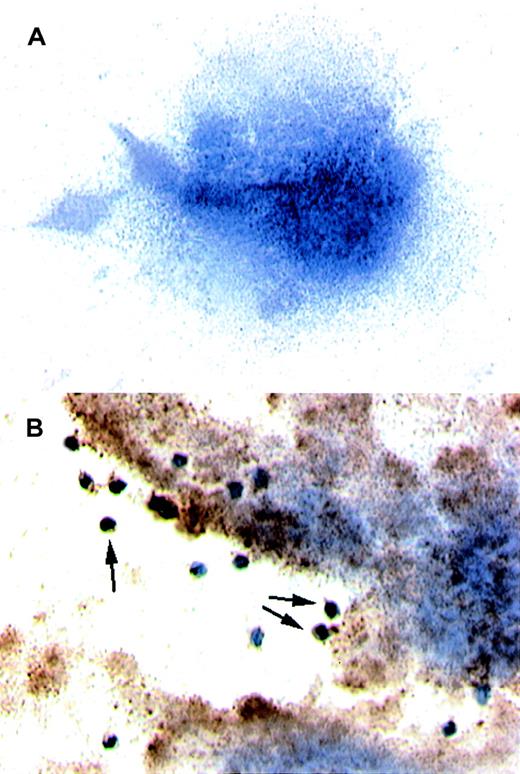
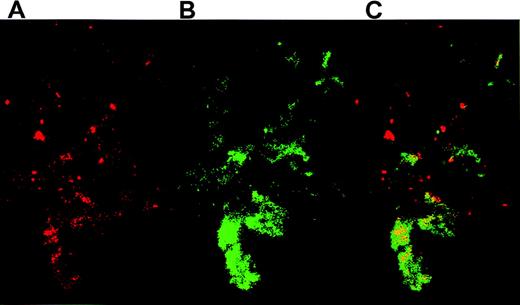
Confocal microscopy demonstrated the colocalization of TF+cell fragments and platelets. After coincubating activated platelets with THP-1–derived TF particles overnight, platelet-particle hybrids were then stained for TF and CD41. TF fragments on platelets were visualized by anti-TF pAbs that were detected with a secondary antirabbit antibody conjugated to PE (Figure4A), whereas the platelets were recognized by FITC-conjugated anti-GPIIb (CD41) antibodies (Figure 4B). Confocal microscopy revealed a high degree of colocalization between TF and platelets (Figure 4C). Identical results were obtained using an anti-TF mAb (not shown).
Platelets activated on collagen were incubated overnight with THP-1 cell–derived TF particles.
The platelet-particle hybrids were then double stained: TF particles on platelets were visualized by anti-TF pAb followed by staining with a secondary goat antirabbit antibody conjugated to PE. The platelets were simultaneously stained by an FITC-conjugated mAb directed against GPIIb. (A) Confocal microscopy reveals TF particles stained red. (B) GPIIb on platelets were stained green. (C) An overlay of panels A and B shows the colocalization of TF and platelets.
Platelets activated on collagen were incubated overnight with THP-1 cell–derived TF particles.
The platelet-particle hybrids were then double stained: TF particles on platelets were visualized by anti-TF pAb followed by staining with a secondary goat antirabbit antibody conjugated to PE. The platelets were simultaneously stained by an FITC-conjugated mAb directed against GPIIb. (A) Confocal microscopy reveals TF particles stained red. (B) GPIIb on platelets were stained green. (C) An overlay of panels A and B shows the colocalization of TF and platelets.
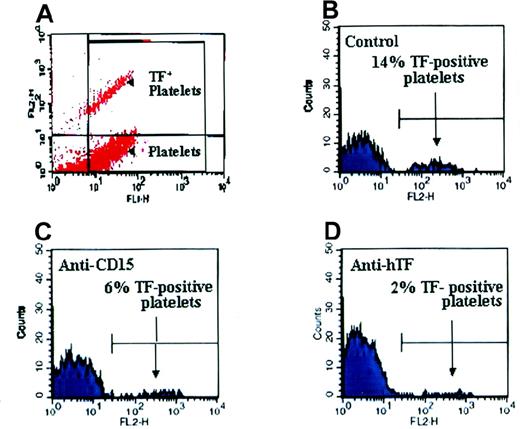
The TF cell fragments derived from stimulated THP-1 cells were further characterized by qualitative and quantitative flow cytometric analysis. Conditioned medium from TNF-α–induced THP-1 cells was used as a source of particles. CD15 and TF were abundantly present on the microparticle surface (detectable in approximately 60% and 45% of the particles, respectively; Figure5A and 5B), whereas CD18 was only detectable in approximately 14% of the particles (Figure 5C). In addition, measurement of the Xa generation by a chromogenic assay showed that THP-1 cell–derived fragments produced 370 pμ Xa per min in suspension, which reflects the high procoagulant activity of leukocyte-derived TF particles.
Characterization of THP-1 cell–derived microparticles by flow cytometry.
Using mAbs, we detected CD15, TF, and CD18 and acquired 10 000 events for each measurement. Plots show the PE staining on particles (immunofluorescence 2 [LFL-2]). Staining showed that (A) CD15+ was present in 60% of the particles tested, (B) TF was present in 45%, and (C) CD18+ was present in 14%.
Characterization of THP-1 cell–derived microparticles by flow cytometry.
Using mAbs, we detected CD15, TF, and CD18 and acquired 10 000 events for each measurement. Plots show the PE staining on particles (immunofluorescence 2 [LFL-2]). Staining showed that (A) CD15+ was present in 60% of the particles tested, (B) TF was present in 45%, and (C) CD18+ was present in 14%.
The role of CD15 and TF on the transfer of TF+cell fragments to platelets
At the platelet-PMN leukocyte contact site (Figure 1), an intense cluster of TF and CD15 labeling was evident. This suggests a possible involvement of these molecules in the transfer of TF+ particles from leukocytes to platelets. For these experiments we employed a microcone and microplate viscometer using small sample volumes of 250 μL. Anti-CD15 and anti-hTF (hTF-1) mAbs reduced the formation of platelet-TF hybrids (Figure6).
Inhibiting the transfer of TF cell fragments to platelets.
Platelets were activated on collagen, and (A) platelet-particle hybrids were double stained. TF was visualized by rabbit pAbs against hTF followed by goat antirabbit antibodies conjugated to PE, whereas platelets were stained with an FITC-conjugated mAb directed against GPIIb. Plots in panels B, C, and D show the GPIIb+ particles (platelets). (B) TF was found on 14% of platelets. (C) A blocking anti-CD15 antibody inhibited the attachment of TF-containing particles to platelets. (D) The anti-TF mAb (hTF-1) also reduced TF adherence to platelets.
Inhibiting the transfer of TF cell fragments to platelets.
Platelets were activated on collagen, and (A) platelet-particle hybrids were double stained. TF was visualized by rabbit pAbs against hTF followed by goat antirabbit antibodies conjugated to PE, whereas platelets were stained with an FITC-conjugated mAb directed against GPIIb. Plots in panels B, C, and D show the GPIIb+ particles (platelets). (B) TF was found on 14% of platelets. (C) A blocking anti-CD15 antibody inhibited the attachment of TF-containing particles to platelets. (D) The anti-TF mAb (hTF-1) also reduced TF adherence to platelets.
Discussion
The presence of thrombogenic TF activity in circulating blood has recently been demonstrated by us,4 but the source of platelet-associated TF and the mechanism of its transfer have not yet been identified. In this study we present data indicating that monocytes and possibly PMN leukoctyes were involved in the transfer of TF+ particles to platelets. Studies on THP-1 cells revealed that the adherence of TF+ particles to platelets was mediated by CD15. The role of P-selectin in thrombogenesis was previously shown in a baboon model in which antibodies to P-selectin inhibited fibrin deposition in a polyester fiber arteriovenous shunt,24 although the formation of TF-platelet hybrids was not considered. Moreover, it has been shown that P-selectin knockout mice show a hemostatic defect.25 The addition of anti-CD15 mAbs or oligosaccharides containing sialyl Lewisx inhibits CD15 binding to P-selectin on platelets. This action has been shown to prevent both platelet-leukocyte adherence10,26,27 and the recurrence of arterial thrombosis in animal models.27 The data we present in this paper provide an explanation for these observations, namely that the CD15 and P-selectin interactions mediate the formation of highly procoagulant platelet aggregates containing TF particles from leukocytic cells.
Platelets became TF+ after exposure to TF+particles (Figure 3). Our findings substantiate previous ex vivo observations suggesting that leukocytes are one possible source of TF+ structures in thrombi.4 Monocytes have been documented to express TF. Although TF+ neutrophils have repeatedly been observed by us and others, we do not know whether these cells synthesize TF. Further studies are needed to evaluate whether PMN leukocytes acquire TF from other cells and then deposit it onto platelet thrombi or whether the leukocytes themselves are able to express TF.
Notably, it has been suggested that circulating microparticles are a thrombogenic species.28,29 Previously it was shown that TF modulates the migration of TF-containing monocytes through an endothelial cell monolayer. Antibodies to TF and TF fragments were inhibitory, thus implicating TF as an adhesive molecule and suggesting the presence of a TF receptor on endothelial cells.30 A role for TF in cell adhesion and migration mediated by interaction with intracellular actin-binding protein 280 was also recently reported.31 Ultrastructural data on the location of TF expressed by various cell lines demonstrated that TF was at the cell surface and appeared in a spotty pattern at the base and apex of budding processes and in close proximity to actin-rich regions.32 Immobilized ligands for TF accelerated the adhesion and spreading of TF-expressing cells, which indicates that cellular TF may be involved in cell adhesion.32 Taken together, these observations implicate TF-leukocyte interactions in TF-dependent coagulation and thrombotic events and support the concept of TF as an adhesive molecule.
Suspensions of TF+ particles exhibited considerable TF activity, which points to the potential thrombogenicity of leukocyte-derived TF-bearing particles. It should be emphasized that under normal conditions, most cell surface TF is encrypted, which means that the TF binds to FVIIa and anti-TF, but it is not capable of initiating coagulation. Encrypted TF allows circulating TF+leukocytes to be present in the circulation without generalized coagulation ensuing.33-36 The transfer of TF+microparticles to platelets during thrombogenesis clearly favors the propagation of thrombosis. The activation of coagulation factors IX and X occurs very close to platelet surfaces, thereby favoring the formation of factors IXa:VIIIa and Xa:Va complexes on their highly procoagulant surfaces. In our experiments, platelets seem to acquire TF after thrombus formation has been initiated and therefore perpetuate the process of thrombus growth. Whether platelet-associated TF may be able to participate in the initiation of thrombus formation remains to be clarified.
Our findings demonstrate that TF+ microparticles are present on platelets within thrombus-forming platelet-TF hybrids. Thus, the data presented here show monocytes and possibly PMN leukocytes as sources of TF+ membrane particles that are transferred to platelets, thereby enabling them to trigger and propagate thrombosis. The TF transfer process is mediated by the interaction of CD15 and TF with platelets, and the inhibition of TF transfer may be a novel therapeutic approach to prevent thrombosis.
Acknowledgments
We thank Professor J. T. Fallon, Department of Pathology, Mount Sinai School of Medicine (MSSM), New York, NY, for his excellent assistance, and Veronia Gulle for her extensive technical support. We also thank David Varon, Sheba Medical Center, Tel-Hashomer, Israel, for the use of his viscometer, and Scott Henderson, MSSM-CLSM core facility, for help with confocal laser scanning microscopy.
Supported in part by grants HL 29019 and HL 54469 from the National Heart Lung and Blood Institute, National Institutes of Health (NIH), Bethesda, MD; shared instrumentation grant 1 S10 RR0 9145-01 from NIH; and instrumentation grant DBI-9724504 from the National Science Foundation (Arlington, VA) Major Research.
Reprints:Yale Nemerson, Division of Thrombosis Research, Department of Medicine, Box 1269, 1 Gustave L. Levy Pl, New York, NY 10029; e-mail: yale.nemerson@mssm.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 5. Characterization of THP-1 cell–derived microparticles by flow cytometry. / Using mAbs, we detected CD15, TF, and CD18 and acquired 10 000 events for each measurement. Plots show the PE staining on particles (immunofluorescence 2 [LFL-2]). Staining showed that (A) CD15+ was present in 60% of the particles tested, (B) TF was present in 45%, and (C) CD18+ was present in 14%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.170/4/m_bloo01342005y.jpeg?Expires=1769113876&Signature=FJcaRhApf4yRc0mAxZViDJLyj3XwOJy5FiaNx5GVOwoXlu5g8XAA~5G8y49QhdQt8EutRvAqWLmdZ68jxySa~iZOPDy-YM1gG0ULIcL6y8~Z50zacTxgl1x5DB4QT8kS3hSdow4t4GW5EUKhLbq4dzKpPrbhcggXJEMz8FgXyWtkHC32wZyIgfxTaI3ZRUsYMzFKYqyegcxNLCowFDuJDsNxSTwKWQiDydxdhBUdGSx40DWc6pcMqm~KbTQssoeFnDinBNHA-TgSqdwYAOhp66kvyCosVc5Nv4seoNZ5QrHiSez2qh2qyOtS7Qszx6NPgoKCb9dFhZOlv4c22f1c5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal