Hrk is a newly described proapoptotic member of the Bcl-2 family that is mainly expressed in hematopoietic tissues and cultured neurons. In this study we have examined the expression and activity of Hrk in hematopoietic progenitors. To address these issues, we used 3 growth factor-dependent murine hematopoietic cell lines, HCD-57, FDCP-Mix, and FL5.12. The expression of Hrk was undetectable in cells cultured with growth factors, but it was rapidly up-regulated on growth factor withdrawal. In contrast, the expression of Bcl-xL decreased and that of proapoptotic Bax, Bad, and Bak was unchanged or down-regulated after removal of growth factors. This pattern of expression correlated with the induction of apoptosis. Hrk was also up-regulated in human cell lines and in bone marrow-derived CD34+ cells cultured in the absence of growth factors. In addition, the levels of Hrk were up-regulated after treatment with the chemotherapeutic drug etoposide. Expression of prosurvival Bcl-xL or Bcl-2 proteins blocked the induction of Hrk. Hrk was induced in FDCP-Mix cells treated with ionomicin in the presence of IL-3, suggesting that cytosolic calcium may regulate the expression of this proapoptotic protein. Furthermore, ectopic expression of Hrk induced cell death of hematopoietic progenitors in the presence of IL-3. Thus, Hrk is specifically and rapidly induced in hematopoietic progenitors after growth factor deprivation or treatment with chemotherapeutic drugs, and this may be sufficient to induce apoptosis in these cells.
There is increasing evidence that most cells in multicellular organisms require constant stimulation by extracellular signals to survive. Committed hematopoietic progenitor cells require defined growth factors for survival, differentiation, and proliferation.1 Withdrawal of these growth factors leads to apoptosis, and this cell death mechanism has been proposed to play a critical role in the control of cell numbers in both hematopoietic precursors and their fully differentiated cell populations.2-4 Apoptosis is implemented by a death machinery that is evolutionarily conserved and activated in the dying cell. In mammals, the executory arm of apoptosis involves a family of death proteases, called caspases, that are activated in a proteolytic cascade to execute the cell death program.5 The activation of upstream caspases represents a critical checkpoint in the decision to survive or to die.6,7 Upstream caspases are controlled by several molecules, including proteins of the Bcl-2 family. Members of this family possess at least 1 of 4 conserved motifs known as Bcl-2 homology domains (BH1 to BH4), and can exert both prosurvival and proapoptotic activity.8
In hematopoietic progenitor cells, several mechanisms have been proposed to explain how growth factors promote survival through Bcl-2 family members. Several hematopoietins, including interleukin-3 (IL-3) and erythropoietin, have been shown to maintain the expression of prosurvival Bcl-2 family members, including Bcl-2, Bcl-xL, A1, and Mcl-1, at the transcriptional level.9,10 By contrast, RNA levels of proapoptotic Bcl-2 members, including Bax, Bad, and Bak, appear to be regulated independently of IL-3 in hematopoietic progenitor cells. Additionally, IL-3 and perhaps other growth factors can promote survival through phosphorylation and inactivation of proapoptotic BAD via activation of the Akt kinase.11
Some proapoptotic Bcl-2 family members, including Bik/Nbk, Blk, Bad, and Bid, contain only the BH3 motif that acts as a dimerization domain for prosurvival proteins, including Bcl-2 and Bcl-xL.12-15 The proapoptotic activity of these BH3-only proteins is mediated by the BH3 region, as deletion or mutation of this region abrogates their binding to Bcl-2 and Bcl-xL and their killing activity.16,17 Thus, these BH3-only Bcl-2 family proteins have been suggested to represent the physiologic antagonists of prosurvival Bcl-2 family members.8 Little is known about the regulation of the newly described BH3 subfamily of proapoptotic Bcl-2 family members. These BH3 proteins are evolutionarily conserved, in that they are structurally related to EGL-1, a nematode BH3-containing protein that interacts with and inhibits CED-9.18 Human Hrk and its mouse homologue (DP5) are members of this BH3 subfamily of proapoptotic proteins. Human Hrk was isolated as a Bcl-xL and Bcl-2-interacting protein that was found to be preferentially expressed in spleen and bone marrow.19 Mouse Hrk was identified during a screening for genes up-regulated after nerve growth factor withdrawal in primary sympathetic neurons.20 We report here that the expression of Hrk, but not that of related proapoptotic Bcl-2 family members, including Bad, Bax, and Bak, is rapidly induced by growth factor deprivation at the messenger RNA (mRNA) and protein levels in hematopoietic progenitors. This up-regulation of Hrk was inhibited by prosurvival Bcl-2 and Bcl-xL proteins. We also report that enforced expression of Hrk in the presence of growth factor induces cell death in hematopoietic progenitor cells.
Materials and methods
Cell culture
The erythropoietin-dependent HCD-57 cell line and its derivative HCD-57-Bcl-xL were maintained in Iscove's modified Dulbecco's medium (IMDM) (GIBCO-BRL, Grand Island, NY), supplemented with 0.1 U/mL of recombinant erythropoietin (Boehringer Mannheim, Indianapolis, IN) as previously described.9 FL5.12, FL5.12-Bcl-2,21 FDCP-Mix, and UT7 cell lines were grown in RPMI 1640 medium (Seromed Biochrom KG, Berlin, Germany), supplemented with 10% fetal calf serum (FCS) (Flow Laboratories, Irvine, CA), and 10% of Wehi 3B culture supernatant as an IL-3 source for the murine cells, or 5 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sigma, St Louis, MO) for UT7 cells. Mo7e cell line was cultured in IMDM supplemented with 20% FCS and 5 ng/mL IL-3 (Sigma). When indicated, cell lines were treated with 10 μg/mL etoposide or 1 μmol/L ionomycin (both from Sigma) for different time intervals and then analyzed for expression of Hrk. Bone marrow was obtained from healthy donors after appropriate informed consent. The CD34 positive bone marrow population was selected using an immunomagnetic system as previously described22 and then cultured in IMDM containing 20% FCS and recombinant stem cell factor, IL-3, and IL-6 (Immunex, Seattle, WA) at a final concentration of 100 ng/mL.
Transfection and cell death analysis
FL5.12 and FDCP-Mix cells (5 × 106) were transfected by electroporation (200 V, 950 μF) with a construct containing the human Hrk cDNA in the sense or inverted orientation, cloned into the unique EcoRI site of the pIRES-EGFP vector (Clontech Lab, Palo Alto, CA) or with a control pIRES-Neo plasmid. After 24 hours of transfection, cells were analyzed for expression of the green fluoresce protein and the uptake of propidium iodide by flow cytometry using a FACScan analyzer (Becton Dickinson, San Jose, CA). For propidium iodide uptake, cells were incubated in a solution containing 0.1% sodium citrate and 0.1 mg/mL propidium iodide, for 10 minutes and then analyzed.
In some experiments, 5 × 106 FL5.12 cells were transfected with 1 μg of pcDNA3-Hrk19 or control pcDNA3 plasmid. After 48 hours, cells were seeded into 96-well plates at 2000 cells per well in the presence of 1 mg/mL G418 and the number of wells containing viable cells were scored on day 12 after transfection.
Antibodies against Hrk and Western blot analysis
To examine the expression of Hrk, we synthesized a 35-amino acid peptide corresponding to residues that encompass the BH3 region of human Hrk.19 A rabbit was immunized and, after antigen boosting, the immune serum that recognized a protein of the expected molecular mass for human and mouse Hrk was used for Western blot analysis. The expression of Bcl-xL, Bax, Bad, Bak, and Hrk proteins was determined by Western blotting as previously described.9 Blots were incubated with rabbit antibodies against Bcl-x (Transduction Lab, Lexington, KY), Bax (Santa Cruz Biotechnology, Santa Cruz, CA), Bak (Upstate Biotechnology, Lake Placid, NY), and Hrk, or mouse anti-Bad antibodies (Transduction Lab), and then incubated with goat antirabbit or goat antimouse antibodies conjugated to alkaline phosphatase (Tropix, Bedford, MA). Bound antibody was detected by a chemiluminescence system (Tropix).
Messenger RNA expression analysis
Total RNA was prepared using TRIZOL reagent (GIBCO-BRL). To assess mRNA expression, a semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) method was used as previously described.9 The generated cDNA was amplified by using primers for murine Bcl-x, β-actin,9 and Hrk (5′TAGGCGACGAGCTGCA3′ and 5′CTCCAAGGACACAGGGTT3′). The amplification profile was as follows: 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. After 25 to 30 amplification cycles, the expected PCR products were size fractionated onto a 2% agarose gel and stained with ethidium bromide.
Assays for apoptotic cells
For DNA fragmentation analysis, cells (1 to 3 × 106) were washed with phosphate-buffered saline and pelleted by centrifugation. Genomic DNA was isolated from cell pellets as described previously.23 DNA samples were electrophoresed on a 2% agarose gel and stained with 0.1% ethidium bromide. The early apoptotic cells were detected with annexin V, labeled with fluorescein isothiocyanate (PharMingen, San Diego, CA) by flow cytometry.
Results
Hrk is up-regulated in hematopoietic progenitor cell lines cultured in the absence of specific growth factors
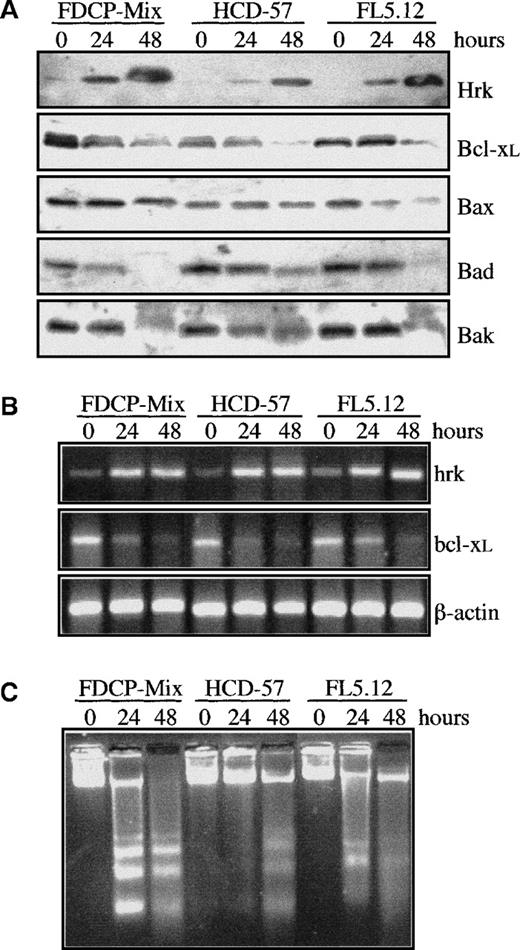
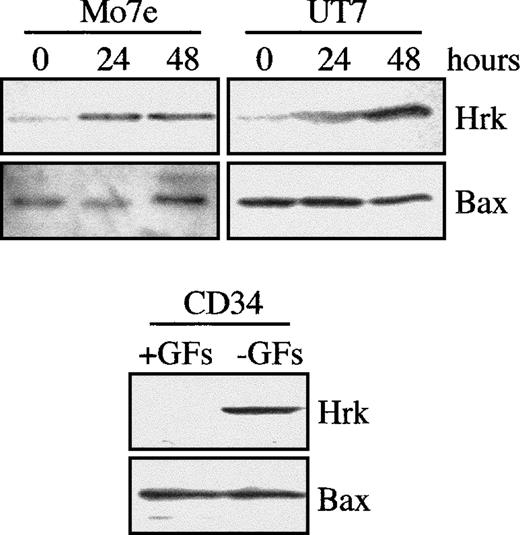
To examine the regulation of Hrk, we used 3 hematopoietic progenitor cell lines that required defined growth factors for survival and proliferation. FDCP-Mix and FL5.12 are myeloid and lymphoid cell lines, respectively, that are IL-3–dependent, whereas HCD-57 is an erythroid cell line that requires erythropoietin for proliferation and survival. When these 3 hematopoietic cell lines were cultured in the absence of growth factor, the protein levels of Hrk were clearly up-regulated by 24 hours and greatly increased by 48 hours after growth factor withdrawal (Figure 1A). In contrast, expression of Bcl-xL gradually decreased within 48 hours of culture in the absence of growth factor. In addition, the expression of proapoptotic members of the Bcl-2 family, Bax, Bak, and Bad, either remained unchanged or diminished, but none were up-regulated within 48 hours after growth factor withdrawal. To verify whether the increase in Hrk protein expression correlated with the level of mRNA, we determined the levels of Hrk by semiquantitative RT-PCR analysis (Figure 1B). In agreement with the protein results, Hrk was up-regulated and Bcl-xL mRNA levels were down-regulated within 48 hours of growth factor deprivation (Figure 1B). This expression pattern was accompanied by a loss of cell viability caused by the activation of an apoptotic process. The genomic DNA isolated from FDCP-Mix, HCD-57, and FL5.12 cells cultured in the absence of growth factor was degraded into oligonucleosomal fragments that are characteristic of apoptosis (Figure1C). Similar results have been obtained also with human hematopoietic progenitors. Induction of the Hrk protein but not of Bax was detected in human megakaryoblastic UT-7 and Mo7e cell lines cultured in the absence of GM-CSF and IL-3, respectively, for 24 and 48 hours, and in bone marrow-derived CD34+ cell population cultured without IL-3, IL-6, and stem cell factor for 36 hours (Figure2).
Analysis of Bcl-2 family members and apoptosis in murine hematopoietic progenitor cell lines after growth factor withdrawal.
(A) Western blot analysis of Hrk, Bcl-xL, Bax, Bad, and Bak in cells cultured in the presence (0 hours) or absence of IL-3 (FDCP-Mix, FL5.12) or erythropoietin (HCD-57) for 24 and 48 hours. (B) Semiquantitative RT-PCR analysis of Hrk and Bcl-xL mRNA at 0, 24, and 48 hours after growth factor deprivation. PCR products were electrophoresed onto a 2% agarose gel. β-actin mRNA was used as an amplification control. (C) DNA fragmentation analysis in cells cultured with or without growth factor. Cells were incubated for the indicated time points and genomic DNA fragmentation was monitored by electrophoresis onto a 2% agarose gel and staining with ethidium bromide.
Analysis of Bcl-2 family members and apoptosis in murine hematopoietic progenitor cell lines after growth factor withdrawal.
(A) Western blot analysis of Hrk, Bcl-xL, Bax, Bad, and Bak in cells cultured in the presence (0 hours) or absence of IL-3 (FDCP-Mix, FL5.12) or erythropoietin (HCD-57) for 24 and 48 hours. (B) Semiquantitative RT-PCR analysis of Hrk and Bcl-xL mRNA at 0, 24, and 48 hours after growth factor deprivation. PCR products were electrophoresed onto a 2% agarose gel. β-actin mRNA was used as an amplification control. (C) DNA fragmentation analysis in cells cultured with or without growth factor. Cells were incubated for the indicated time points and genomic DNA fragmentation was monitored by electrophoresis onto a 2% agarose gel and staining with ethidium bromide.
Expression of Hrk in human cell lines and CD34+ progenitor cells.
Two megakaryoblastic cell lines were cultured in the absence of IL-3 (Mo7e) or GM-CSF (UT7). Bone marrow-derived CD34+ cells were cultured with (+GFs) and without (−GFs) growth factors (IL-3, IL-6, and stem cell factor) for 36 hours. At the indicated time intervals, cells were analyzed for the expression of Hrk and Bax by Western blot.
Expression of Hrk in human cell lines and CD34+ progenitor cells.
Two megakaryoblastic cell lines were cultured in the absence of IL-3 (Mo7e) or GM-CSF (UT7). Bone marrow-derived CD34+ cells were cultured with (+GFs) and without (−GFs) growth factors (IL-3, IL-6, and stem cell factor) for 36 hours. At the indicated time intervals, cells were analyzed for the expression of Hrk and Bax by Western blot.
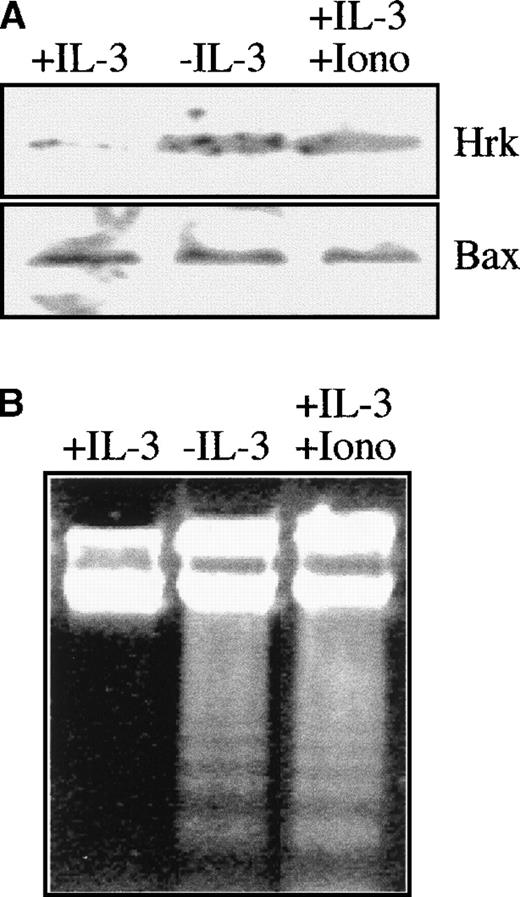
Previous data showed that the treatment of rat cortical neurons with inhibitors of calcium channels blocked the expression of Hrk mRNA and prevented apoptosis induced by amyloid-β protein.24 To analyze whether calcium flux could also affect the expression of Hrk in hematopoietic progenitors, we incubated FDCP-Mix cells with 1 μmol/L ionomycin in the presence of IL-3. As shown in Figure3A, the protein levels of Hrk but not of Bax increased by 24 hours after addition of ionomycin, and the expression was similar to that of cells cultured in the absence of IL-3. Furthermore, this up-regulation of Hrk correlated with the activation of an apoptotic process as assessed by genomic DNA fragmentation analysis (Figure 3B).
Analysis of Hrk and apoptosis in FDCP-Mix cells treated with ionomycin.
Cells were cultured for 24 hours with ionomycin in the presence of IL-3 and then analyzed. As controls, cells cultured in the presence or absence of IL-3 were also analyzed. (A) Expression of Hrk and Bax by Western blot. (B) DNA fragmentation analysis by electrophoresis onto a 2% agarose gel and staining with ethidium bromide.
Analysis of Hrk and apoptosis in FDCP-Mix cells treated with ionomycin.
Cells were cultured for 24 hours with ionomycin in the presence of IL-3 and then analyzed. As controls, cells cultured in the presence or absence of IL-3 were also analyzed. (A) Expression of Hrk and Bax by Western blot. (B) DNA fragmentation analysis by electrophoresis onto a 2% agarose gel and staining with ethidium bromide.
Induction of Hrk is rapid and unaffected by pretreatment with cycloheximide
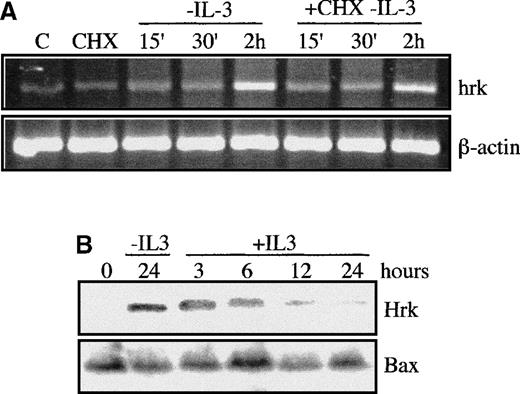
We focused next on the expression of Hrk during the first 2 hours after IL-3 withdrawal, to study whether the induction of Hrk was an early response to an apoptotic stimulus. The levels of Hrk mRNA in FDCP-Mix cells began to increase within 15 minutes and continued to accumulate by 2 hours after IL-3 deprivation (Figure4A). The kinetics of Hrk induction resembled that of immediate early genes such as c-myc25 or oncostatin M.26 Indeed, as expected for an immediate early gene, when we culture growth factor-starved cells with cycloheximide at a dose (20 μg/mL) that inhibited more than 90% of protein synthesis (data not shown), the expression of Hrk mRNA increased at a similar rate to that found in cycloheximide-free cultures within 2 hours after IL-3 withdrawal (Figure 4). In addition, the protein levels of Hrk reached after 24 hours of growth factor deprivation were clearly reduced by 6 hours after re-addition of IL-3 and returned to baseline by 12 to 24 hours (Figure 4B). By contrast, the steady-state protein levels of Bax were not modified during the same interval (Figure 4B). This result was consistent with the half-life of the Hrk protein, which was estimated to be around 6 hours as detected by pulse-chase labeling analysis (data not shown).
Expression of Hrk in FDCP-Mix cells treated with a protein synthesis inhibitor.
(A) IL-3-deprived cells were cultured with or without cycloheximide (CHX), and at the indicated time intervals, the mRNA levels of Hrk were analyzed by semiquantitative RT-PCR. As controls, cells maintained with IL-3 in the absence (first lane) or in the presence (second lane) of CHX were also analyzed and showed no induction of Hrk. PCR products were electrophoresed onto a 2% agarose gel. β-actin mRNA was used as an amplification control. (B) Cells were deprived of IL-3 for 24 hours and then stimulated for different time intervals with IL-3. The expression of Hrk and Bax proteins was determined by Western blotting with use of specific antibodies.
Expression of Hrk in FDCP-Mix cells treated with a protein synthesis inhibitor.
(A) IL-3-deprived cells were cultured with or without cycloheximide (CHX), and at the indicated time intervals, the mRNA levels of Hrk were analyzed by semiquantitative RT-PCR. As controls, cells maintained with IL-3 in the absence (first lane) or in the presence (second lane) of CHX were also analyzed and showed no induction of Hrk. PCR products were electrophoresed onto a 2% agarose gel. β-actin mRNA was used as an amplification control. (B) Cells were deprived of IL-3 for 24 hours and then stimulated for different time intervals with IL-3. The expression of Hrk and Bax proteins was determined by Western blotting with use of specific antibodies.
Expression of Hrk is induced in hematopoietic progenitor cells after treatment with etoposide
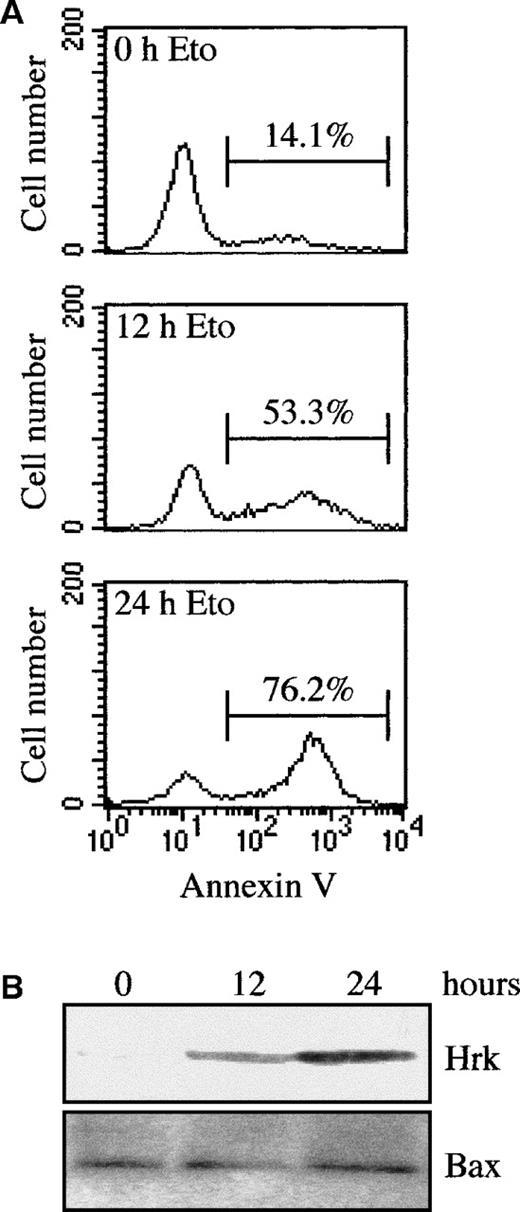
To determine whether the induction of Hrk was restricted to signals associated with growth factor withdrawal or, alternatively, it might be a response to an apoptotic signal, we analyzed the expression of Hrk in FDCP-Mix cells treated with the topoisomerase II inhibitor etoposide, an inducer of apoptosis.27 As shown in Figure5, when cells were cultured with etoposide in the presence of IL-3, a time-dependent increase in the number of apoptotic cells was observed, as assessed by flow cytometry analysis with annexin V (Figure 5A) and DNA fragmentation analysis (data not shown). By 12 hours of treatment, 53.3% of cells were annexin V positive, and by 24 hours after etoposide, most of the cells (76.2%) were apoptotic. Within 24 hours of treatment with etoposide, a clear correlation was observed between the increase in the number of apoptotic cells and the up-regulated expression of Hrk as assessed by Western blot analysis (Figure 5B). By contrast, etoposide treatment did not affect the expression of Bax. A similar pattern of expression was observed in FL5.12 and HCD-57 cells treated with etoposide (data not shown), indicating that in addition to growth factor withdrawal, the expression of Hrk can be up-regulated by chemotherapeutic drugs.
Analysis of Hrk and apoptosis in etoposide-treated FDCP-Mix cells.
(A) Cells were treated for different times with etoposide in the presence of IL-3 and then analyzed by flow cytometry with fluorescein isothiocyanate-labeled annexin V. Numbers above the selected regions indicate the percentage of apoptotic cells. (B) Western blot analysis of Hrk and Bax expression in FDCP-Mix cells treated with etoposide for 12 and 24 hours in the presence of IL-3.
Analysis of Hrk and apoptosis in etoposide-treated FDCP-Mix cells.
(A) Cells were treated for different times with etoposide in the presence of IL-3 and then analyzed by flow cytometry with fluorescein isothiocyanate-labeled annexin V. Numbers above the selected regions indicate the percentage of apoptotic cells. (B) Western blot analysis of Hrk and Bax expression in FDCP-Mix cells treated with etoposide for 12 and 24 hours in the presence of IL-3.
Bcl-2 and Bcl-xL prevent the induction of Hrk
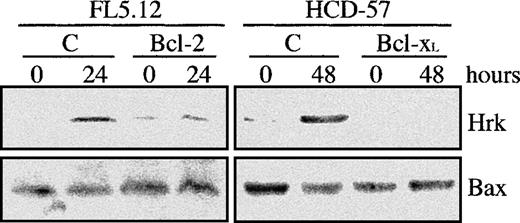
Overexpression of Bcl-2 and Bcl-xL has been shown to inhibit or delay the apoptotic cell death in a number of cellular systems,23,28-30 so that we studied whether ectopic expression of Bcl-xL or Bcl-2 could impede the up-regulation of Hrk in hematopoietic progenitor cell lines. HCD-57 cells transfected with Bcl-xL,9 and FL5.12 expressing constitutive levels of Bcl-221 were cultured in the absence of erythropoietin and IL-3, respectively, and the levels of Hrk were analyzed by Western blot. Figure 6shows a representative experiment. Both parental FL5.12, and HCD-57 displayed an increase in the expression of Hrk after growth factor withdrawal, in agreement with results shown in Figure 1. Furthermore, by 24 hours of culture without IL-3 and 48 hours in the absence of erythropoietin, most of the cells were apoptotic, as assessed by flow cytometry with annexin V and DNA fragmentation analysis (data not shown). Significantly, FL5.12-Bcl-2 cells and HCD-57-Bcl-xLdid not show any change in the expression of Hrk at the protein (Figure6) and mRNA levels (data not shown) after 24 or 48 hours of culture in the absence of IL-3 or erythropoietin, respectively. Moreover, these cells did not show any evidence of apoptosis within the same period after growth factor withdrawal (data not shown). These data indicate that Bcl-2 and Bcl-xL can repress the induction of Hrk and suggest that up-regulation of Hrk is triggered by an intracellular apoptotic signal that is inhibited by these prosurvival Bcl-2 family members.
Analysis of the Hrk expression in cells transfected with Bcl-2 or Bcl-xL.
FL5.12 cells transfected with human Bcl-2 cDNA and HCD-57 cells transfected with human Bcl-xL cDNA were cultured in the absence of IL-3 and Epo, respectively, for the indicated times and then analyzed for the expression of Hrk and Bax proteins by Western blot. As control, FL5.12 and HCD-57 cells subjected to the same culture conditions were also analyzed (lane C).
Analysis of the Hrk expression in cells transfected with Bcl-2 or Bcl-xL.
FL5.12 cells transfected with human Bcl-2 cDNA and HCD-57 cells transfected with human Bcl-xL cDNA were cultured in the absence of IL-3 and Epo, respectively, for the indicated times and then analyzed for the expression of Hrk and Bax proteins by Western blot. As control, FL5.12 and HCD-57 cells subjected to the same culture conditions were also analyzed (lane C).
Enforced expression of Hrk induces cell death in the presence of growth factor
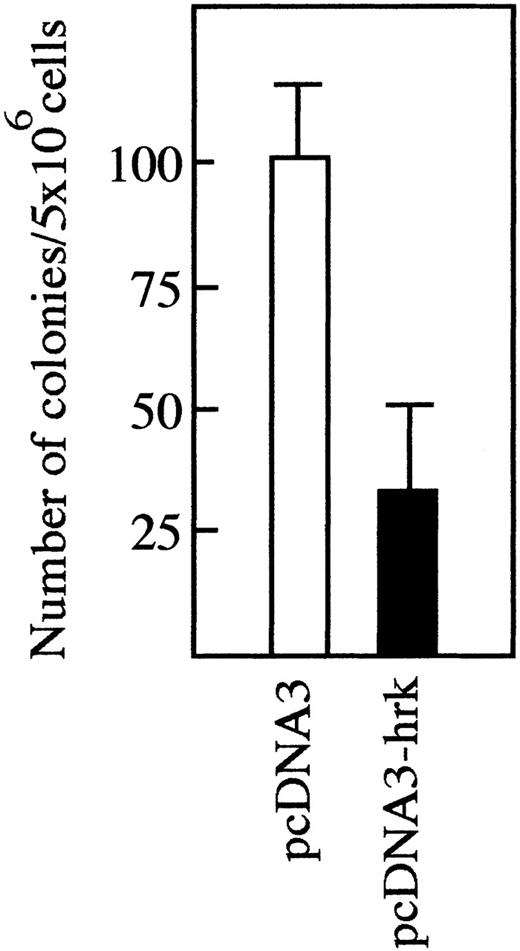
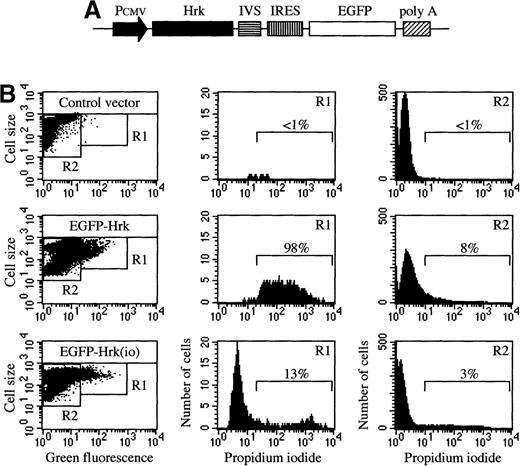
We transfected a plasmid producing human Hrk into IL-3-dependent FL5.12 cells but analysis of multiple clones selected after culture in G418 showed no detectable Hrk expression (data not shown). To test whether Hrk inhibits the expansion of these cells into colonies, we compared colony formation in FL5.12 cells transfected with a plasmid expressing Hrk or with a control plasmid. Transfection of the control plasmid into FL5.12 cells generated 100 ± 13 colonies in the presence of G418, whereas only 33 ± 18 were obtained by transfection of the Hrk plasmid (Figure 7), indicating that expression of Hrk impedes the formation of colonies in the presence of IL-3. To determine whether Hrk blocked the expansion of these cells by inducing cell death, we constructed a plasmid to coexpress Hrk and enhanced green fluorescence protein (EGFP). This construct contains an internal ribosomal entry site, allowing translation of Hrk and EGFP from a single bicistronic mRNA (Figure8A), which permits identifying Hrk-expressing cells on the basis of EGFP expression after transient transfection. When we examined FL5.12 cells 24 hours after transfection with Hrk in the sense orientation, 98% of the EGFP-positive cells were dead as determined by their incorporation of propidium iodide (Figure8B). In contrast, the great majority (more than 85%) of the cells transfected with Hrk in the inverted orientation that coexpressed EGFP remained alive as they excluded propidiun iodide (Figure 8B). Similar results were obtained with FDCPMix cells (data not shown). These results indicate that expression of Hrk causes the death of hematopoietic progenitor cells in the presence of growth factor.
Expansion of FL5.12 cells after transfection with Hrk.
Cells were transfected with pcDNA3 or pcDNA3-Hrk expression plasmids and then cultured into 96-well plates at 2000 cells per well in the presence of G418 for 12 days. Data are presented as the number of colonies (wells containing viable cells) per 5 × 106 transfected cells (mean of triplicate cultures ± SD).
Expansion of FL5.12 cells after transfection with Hrk.
Cells were transfected with pcDNA3 or pcDNA3-Hrk expression plasmids and then cultured into 96-well plates at 2000 cells per well in the presence of G418 for 12 days. Data are presented as the number of colonies (wells containing viable cells) per 5 × 106 transfected cells (mean of triplicate cultures ± SD).
Cell death analysis of FL5.12 cells transiently transfected with pIRES-EGFP-Hrk.
(A) Schematic structure of the bicistronic vector containing the internal ribosomal entry site (IRES) and the Hrk and EGFP cDNAs. (B) Flow cytometry analysis of cells after 24 hours of transfection with pIRES-EGFP containing Hrk in the sense or inverted orientation (io). Quadrants in the dot plots were set according to the green fluorescence of cells transfected with a negative control vector (pIRES-Neo). Histograms represent the percentage of cells stained with propidium iodide in the green fluorescence positive population (R1) and the green fluorescence negative population (R2). All histograms and dot plots are from a representative experiment (n = 3).
Cell death analysis of FL5.12 cells transiently transfected with pIRES-EGFP-Hrk.
(A) Schematic structure of the bicistronic vector containing the internal ribosomal entry site (IRES) and the Hrk and EGFP cDNAs. (B) Flow cytometry analysis of cells after 24 hours of transfection with pIRES-EGFP containing Hrk in the sense or inverted orientation (io). Quadrants in the dot plots were set according to the green fluorescence of cells transfected with a negative control vector (pIRES-Neo). Histograms represent the percentage of cells stained with propidium iodide in the green fluorescence positive population (R1) and the green fluorescence negative population (R2). All histograms and dot plots are from a representative experiment (n = 3).
Discussion
A number of cytokines and hematopoietic growth factors have been shown to promote viability of subpopulations of primitive progenitor cells, suggesting that the regulation of apoptosis plays a key role in hematopoiesis.1 Apoptosis is tightly regulated by members of the caspase and Bcl-2 families. Genetic and biochemical analyses have demonstrated that the activation of caspases is influenced by the Bcl-2 family members, which either suppress or induce apoptosis.8 In this scenario, a reasonable expectation would be that the activity and/or expression of Bcl-2 family members is regulated by signaling pathways that suppress or induce apoptosis.
Here we showed that Hrk, a newly described proapoptotic member of the Bcl-2 family, is specifically and rapidly induced in hematopoietic progenitor cell lines and bone marrow-derived CD34+ cells after growth factor deprivation, which is accompanied by activation of an apoptotic process. Consistent with this is the observation that the mRNA of mouse Hrk (DP5) was induced in sympathetic neurons cultured in the absence of nerve growth factor (NGF).20 However, the expression of Hrk in hematopoietic progenitor cell lines can be induced not only by growth factor withdrawal but also by treatment with etoposide, a chemotherapeutic drug that induces apoptosis. On the basis of these data, we hypothesize that the binding of growth factors (ie, IL-3, erythropoietin, GM-CSF) to their cognate receptors in hematopoietic progenitors activates a repressor pathway that silences the expression of proapoptotic Hrk. Alternatively, this repressor pathway may not be triggered by the growth factor but may be active during the normal development of hematopoietic progenitor cells. On growth factor withdrawal or treatment with certain chemotherapeutic drugs, the repressor mechanism may be released (ie, by posttranslational modification or binding to a specific factor), allowing the expression of Hrk. A similar mechanism may operate in neurons that up-regulate mouse Hrk and undergo apoptosis after NGF withdrawal or treatment with amyloid-β protein.20,24 The induction of mouse Hrk in cultured neurons treated with amyloid-β protein is blocked by inhibitors of voltage-dependent calcium channels and calcium release from the endoplasmic reticulum, suggesting that calcium fluxes could be involved in the regulation of Hrk expression.24 An increase in cytosolic calcium has been implicated in signal transduction pathways that mediate apoptosis.31,32 We have shown here that the expression of Hrk is induced in FDCP-Mix cells after incubation with ionomycin, a calcium ionophore that releases calcium from a variety of intracellular stores, including the endoplasmic reticulum and mitochondria.33 Thus, Hrk might be regulated in these cells by an intracellular calcium-sensitive pathway that is activated on growth factor withdrawal or treatment with chemotherapeutic drugs. Furthermore, it has been described that Bcl-2 either directly or indirectly regulates the flux of calcium across the endoplasmic reticulum membrane, thereby abrogating calcium signaling of apoptosis.34 Consistent with the model of calcium-regulated expression of Hrk, we have shown that overexpression of Bcl-2 or Bcl-xL inhibits the induction of Hrk and prevents apoptosis after growth factor withdrawal.
Deletion of Bcl-x in mice leads to apoptosis of hematopoietic progenitors and embryonic lethality.35 Thus, it appears that Bcl-xL is an important mediator of hematopoietic cell survival in vivo. Hematopoietic progenitor cells down-regulate the expression of Bcl-xL on growth factor deprivation, in addition to up-regulating Hrk. Furthermore, we demonstrate that enforced expression of Hrk into hematopoietic progenitor cell lines is sufficient to induce cell death in the presence of IL-3 within 24 hours. Consistent with this is the observation that transfection of Hrk into embryonic kidney 293T cells resulted in a loss of cell viability at 36 hours posttransfection.19 This suggests that induction of Hrk, which is normally silenced or expressed at low levels, might be a mechanism that participates in the activation of a cell death program linked to growth factor withdrawal or treatment with chemotherapeutic drugs, in hematopoietic progenitors. Because enforced coexpression of Hrk and the antiapoptotic protein Bcl-2 or Bcl-xL inhibits the death promoting activity of Hrk,19 it appears that the balance between the steady-state levels of Bcl-xL or Bcl-2 and those of Hrk likely contribute to either suppress or induce apoptosis. Future studies will need to address the molecular mechanisms that regulate the expression of Hrk and, more importantly, the role for Hrk in the regulation of normal hematopoiesis. To this end, mice deficient in Hrk will be very useful to study whether this proapoptotic protein is needed for the homeostasis of the hematopoietic compartment.
Supported by Comision Interministerial de Ciencia y Tecnologia Grant No. SAF-96/0274 to J.L.F-L., and grant P01 CA75136 from the National Institutes of Health to G.N. C.S. is a recipient of a predoctoral fellowship from the “Fundacion Marques de Valdecilla”; A.B. is a recipient of a NATO postdoctoral fellowship.
Reprints:Jose Luis Fernandez-Luna, Servicio de Inmunologia, Hospital Universitario Marques de Valdecilla, INSALUD, 39008 Santander, Spain; e-mail: inmflj@humv.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal