Abstract
Hemophilia B is caused by the absence of functional coagulation factor IX (F.IX) and represents an important model for treatment of genetic diseases by gene therapy. Recent studies have shown that intramuscular injection of an adeno-associated viral (AAV) vector into mice and hemophilia B dogs results in vector dose–dependent, long-term expression of biologically active F.IX at therapeutic levels. In this study, we demonstrate that levels of expression of approximately 300 ng/mL (6% of normal human F.IX levels) can be reached by intramuscular injection of mice using a 2- to 4-fold lower vector dose (1 × 1011 vector genomes/mouse, injected into 4 intramuscular sites) than previously described. This was accomplished through the use of an improved expression cassette that uses the cytomegalovirus (CMV) immediate early enhancer/promoter in combination with a 1.2-kilobase portion of human skeletal actin promoter. These results correlated with enhanced levels of F.IX transcript and secreted F.IX protein in transduced murine C2C12 myotubes. Systemic F.IX expression from constructs containing the CMV enhancer/promoter alone was 120 to 200 ng/mL in mice injected with 1 × 1011vector genomes. Muscle-specific promoters performed poorly for F.IX transgene expression in vitro and in vivo. However, the incorporation of a sequence from the -skeletal actin promoter containing at least 1 muscle-specific enhancer and 1 enhancer-like element further improved muscle-derived expression of F.IX from a CMV enhancer/promoter-driven expression cassette over previously published results. These findings will allow the design of a clinical protocol for therapeutic levels of F.IX expression with lower vector doses, thus enhancing efficacy and safety of the protocol.
The severe bleeding disorder hemophilia B is caused by an absence of functional coagulation factor IX (F.IX). The X-linked disease affects 1 in 30 000 males in the United States. Current treatment for hemophilia is based on intravenous infusion of plasma-derived or recombinant clotting factor concentrates. Treatment can be episode-based (in response to a bleeding episode) or prophylactic (2-3 infusions per week). The latter is expensive and not without complications and is therefore not widely adopted in the United States. However, 30 years of experience with prophylactic treatment as pioneered in Sweden has shown that a continuous supply of clotting factor levels above 1% of normal (greater than 50 ng F.IX/mL plasma) results in prevention of most joint damage that constitutes the major morbidity of the disease. Furthermore, more life-threatening bleeding episodes, such as intracranial or retroperitoneal bleeds, are also prevented if a continuous level of factor can be maintained.1 2 These observations, as well as the availability of animal models, have contributed to the development of hemophilia into an important model for treatment of genetic diseases by gene therapy. The goal of a gene-based treatment is to achieve long-term expression of F.IX in the circulation of a severe hemophiliac in order to maintain a continuous supply of F.IX.
Progress toward gene therapy has been substantial over the past 2 years due to successful studies in a large animal model of hemophilia B using liver or muscle as a target organ for F.IX gene transfer with adeno-associated viral (AAV) vectors.3,4 In a recently developed approach, we have taken advantage of the observation that AAV vectors efficiently transfer genes to muscle fibers in vivo.5-7 AAV vectors are single-stranded, replication-deficient DNA viruses that can be produced in a helper virus-free system.8 Recombinant AAV contains an expression cassette flanked by viral inverted terminal repeats and is devoid of any sequences encoding viral gene products. The viral packaging limit is approximately 5 kilobases (kb). Gene transfer to nondividing cells such as muscle fibers is stable and often not associated with cellular immune responses against transduced fibers (as commonly observed with, for example, adenoviral vectors). Although F.IX is normally made in the liver, muscle cells are capable of synthesizing biologically active F.IX.9,10 In previous studies, we achieved sustained expression of therapeutic levels of F.IX in the circulation of experimental animals by intramuscular administration of AAV vectors for expression of human F.IX in immunodeficient mice or canine F.IX in hemophilia B dogs (up to 7% of normal human levels in mice and up to 1.4% in dogs).3 11 While these levels of expression are likely to have a therapeutic effect in a patient with severe hemophilia B, further improvements in expression are desirable. On a transcriptional level, this could be achieved by an improved expression cassette resulting in increased expression per delivered vector particle. Equally important, the ability to direct expression of a set amount of F.IX using a lower total dose of vector is an important therapeutic goal, because biodistribution to sites outside muscle (eg, to brain or gonads, an undesirable outcome) is a direct function of dose (in vector genomes) administered. Thus, the use of an improved vector to achieve higher levels of F.IX expression with lower total doses of vector will result in an added measure of safety for this gene therapy approach. In addition, optimization of gene expression on a transcriptional level will benefit not only a protocol for F.IX, but other gene therapy strategies that might use muscle-derived expression of the transgene product (eg, growth hormone, dystrophin, leptin, erythropoietin, insulin-like growth factor-1) as well.
In our initial studies in mice and dogs, we have used the cytomegalovirus (CMV) immediate early (IE) enhancer/promoter to drive sustained high levels of F.IX expression in muscle (for at least 2 years postvector administration). In this study, we demonstrate that expression of muscle-derived human F.IX can be enhanced by a combination of human α-skeletal actin and CMV enhancer/promoter sequences driving transgene expression.
Materials and methods
Construction of plasmids encoding AAV vectors
Cassettes for expression of human F.IX were constructed by standard cloning techniques in Escherichia coli.12 All cassettes were inserted between two 145-base pair (bp) AAV-2 inverted terminal repeats contained in plasmid DNA. Vector AAV-CMV-hF.IX-3 contained the previously published expression cassette based on the CMV IE enhancer/promoter, human F.IX (hF.IX) coding region (including a 1.4-kb portion of intron I of the human F.IX gene), and SV40 polyadenylation sequences.11,13 AAV-CMV-hF.IX-3/bGH was constructed by replacing the SV40 polyA sequences in AAV-CMV-hF.IX-3 with the bovine growth hormone (bGH) polyadenylation signal excised from plasmid pRc/RSV (Invitrogen, Carlsbad, CA). AAV-CMV-hF.IX-8 was created by further deleting intron I sequences in AAV-CMV-hF.IX-3 to a 0.3-kb sequence (by removal of a 1.1-kb ScaI fragment contained in the 1.4-kb portion of intron I, deletion of the SV40 polyA sequences, and addition of the entire 3′-untranslated [UT]/polyadenylation signal of the human F.IX gene [up to bp position 33 071].14,15 Vector AAV-CMV-hF.IX-2 was identical to the published canine F.IX vector,3 except that the canine F.IX complementary DNA (cDNA) was substituted by a 2.5-kb portion of human F.IX cDNA (up to the EcoRI site at position bp 2501 of the human F.IX cDNA16). This vector contains the CMV enhancer/promoter, a 0.4-kb chimeric CMV/β-globin intron upstream of the F.IX cDNA, and a human growth hormone polyA signal. AAV-HSA-hF.IX-1 contains a 2.2-kb region of the human α-skeletal actin (HSA) gene, which includes the promoter, first noncoding exon, and a portion of the first intron.17 This sequence was kindly provided by Dr Hardeman, The Children's Medical Research Institute, Wentworthville, New South Wales, Australia. HSA sequences in this vector replaced the CMV enhancer/promoter in AAV-CMV-hF.IX-3. Intron I was further truncated to a 0.3-kb sequence as described above in order not to exceed the packaging limit of AAV. AAV-HSA-hF.IX-3 is based on AAV-CMV-hF.IX-2. In this vector, CMV enhancer/promoter sequences and the chimeric intron were replaced with the 2.2-kb HSA sequence. Subsequently, a truncated version of intron I (253 bp) of the HSA gene was introduced between the transcription start site of the HSA promoter and the F.IX cDNA (HSA intron I is located 5′ to the translational start site in the HSA gene18). All of the 3′-UT of the F.IX cDNA was deleted to accommodate for the additional sequences. AAV-HSA/CMV-hF.IX-2 was created by adding a 1.2-kb portion of the HSA promoter region (bp position −1281 to −8417) 5′ to the CMV enhancer/promoter in AAV-CMV-hF.IX-2. Again, the entire 3′-UT of the F.IX cDNA was deleted in this vector. AAV-cTnT-hF.IX contains a 588-bp region from the chicken cardiac troponin T (cTnT) promoter (−550 to +38), which replaces the CMV enhancer/promoter in AAV-CMV-hF.IX-2. This sequence was generously provided by Dr Charles Ordahl, University of California, San Francisco, and has been demonstrated to direct expression of reporter genes in primary muscle cells.19 20
Production of AAV vectors
AAV vectors were produced in an adenovirus-free system by cotransfection of human embryonic kidney (HEK)-293 cells with the vector plasmid and 2 additional plasmids carrying adenoviral helper functions E2a, E4, and VA and AAV rep/cap functions.21 Vector was purified by 2 rounds of cesium chloride density gradient centrifugation and viral titers determined by quantitative dot blot hybridization. Titers are therefore given as vector genomes/mL. Vector was stored at −80°C in 1 × phosphate-buffered saline containing an osmotic stabilizer.
Transduction of myotubes in vitro
Murine C2C12 myoblasts were grown in DMEM medium containing 10% fetal bovine serum and antibiotics. Cells were plated in 6-well plates, allowed to reach confluence, and then differentiated to myotubes by exchanging media to DMEM containing 2% horse serum (differentiation media). Cells were incubated for 5 to 6 days in differentiation medium prior to transduction with AAV vector. Immediately prior to addition of vector, fresh differentiation media was added to the cells, and vector was added at multiplicities of infection (MOIs) ranging from 104 to 105 vector genomes/cell. The media was replaced on the following day. All transduction experiments were carried out in duplicate. MOIs were calculated for number of myoblasts used per well for formation of myotubes (2 × 106myoblasts per confluent 6-well). Supernatants (24-hour) were collected 7 to12 days posttransduction, and myotubes were subsequently harvested for RNA isolation. Concentrations of human F.IX in supernatants were measured by enzyme-linked immunosorbent assay (ELISA) as described.22 Human myotubes derived from primary human myoblasts were a gift from Dr Heiman-Petterson, Hahneman University, Philadelphia, PA. These cells were maintained and transduced as described for differentiated C2C12 myotubes, except that vector was added in OptiMEM medium (Gibco/BRL, Gaithersburg, MD), and the differentiation medium contained 10% horse serum.5Transduction experiments in other cell types (HEK-293 cells and human hepatoma [HepG2] cells) were carried out in 24-well plates as described.13 These cells were transduced at 50% confluence in DMEM/2% fetal bovine serum medium. Supernatants (24-hour) were harvested 96 hours posttransduction.
Analysis of F.IX mRNA in transduced myotubes
Total RNA was isolated from transduced myotubes using the RNAzol kit (Tel Test, Friendswood, TX). Reverse transcriptase–polymerase chain reaction (RT-PCR) was carried out on 1 μg of total RNA using an RT-PCR kit purchased from Applied Biosystems (Foster City, CA) and using primers specific for messenger RNAs (mRNAs) from AAV-hF.IX vectors. PCR products were separated by agarose gel electrophoresis. Duplicate Northern blots were performed by separation of 10 μg of total RNA using agarose gel electrophoresis under denaturing conditions. Following transfer to nylon membranes, 1 blot was hybridized with a radioactively labeled probe specific for the coding region of the human F.IX cDNA and the other to a β-actin probe. Phosphoimage analysis of the blots was performed using a Storm 860 phosphoimager (Molecular Devices, Sunnyvale, CA) and ImageQuaNT software (Molecular Devices). The β-actin signal was used to normalize the amount of RNA loaded in each lane, and the relative amounts of human F.IX transcript observed in the various RNA samples was calculated.
Animal experiments
Immunodeficient Rag-1 mice (4-6 weeks old, 20-25 g) were injected intramuscularly into 4 sites of the hind limbs with 1 × 1011 vector genomes of recombinant AAV (n = 4 for each vector) as described previously.11 Rag-1 mice lack recombinase activating gene 1 and are therefore devoid of mature B and T cells and circulating immunoglobulins.23 Quadriceps and tibialis anterior muscles were exposed by a 1-cm incision through the skin while the animals were under general anesthesia from an intraperitoneal injection of ketamine/xylazine. Vector was diluted in 1 × phosphate-buffered saline and slowly injected with a Hamilton syringe (50 μL of vector suspension per quadriceps and 25 μL per tibialis anterior). Incisions were closed with suture. Mice were bled retro-orbitally on a biweekly schedule for 10 to 12 weeks, and F.IX levels in plasma samples were determined by ELISA specific for human F.IX as described.22
Results
A series of AAV vectors for expression of the human F.IX cDNA was constructed and produced in high titer (greater than or equal to 1012 vector genomes/mL) in HEK-293 cells using an adenovirus-free production system that is based on the triple-transfection method (see “Materials and methods”). These vectors are derived from constructs that were successfully used for expression of therapeutic levels of human or canine F.IX in mice and hemophilic dogs.3,11 All expression cassettes were designed not to exceed the packaging limit of AAV (approximately 5 kb). While all vectors contain the human F.IX cDNA, they differ in enhancer/promoter, intron, and polyadenylation sequences (Table1). When tested for expression of muscle cell–derived F.IX in murine C2C12 myotubes, all constructs showed detectable expression and correct splicing when analyzed by RT-PCR (data not shown) but differed substantially with regard to transcript levels and secretion of F.IX into culture media (see below). For in vivo experiments, immunodeficient Rag-1 mice were chosen because intramuscular administration of vector results in antibody formation against the nonspecies-specific transgene product (human F.IX) in immunocompetent mice.11 We have previously shown that F.IX expression in Rag-1 mice is vector dose–dependent, and we therefore chose an intermediate dose of 1 × 1011 vector genomes/mouse for intramuscular administration of recombinant AAV vectors (based on experiments with 3 × 109 to 4 × 1011 AAV-CMV-hF.IX-3 vector genomes/mouse24).
Components of AAV vectors for expression of muscle-derived human F.IX (hF.IX)
| Vector . | Enhancer/Promoter . | Intron . | Human F.IX 3′-UT . | PolyA Signal . |
|---|---|---|---|---|
| AAV-CMV-hF.IX-3 | CMV IE | Portion of hF.IX intron I (1.4 kb) | 0.3 kb | SV40 late |
| AAV-CMV-hF.IX-3/bGH | CMV IE | Portion of hF.IX intron I (1.4 kb) | 0.3 kb | Bovine growth hormone (bGH) |
| AAV-HSA-hF.IX-1 | Human α-skeletal actin (HSA) | Portion of hF.IX intron I (0.3 kb) | 0.3 kb | SV40 late |
| AAV-CMV-hF.IX-8 | CMV IE | Portion of hF.IX intron I (0.3 kb) | Entire 1.6 kb | Human F.IX |
| AAV-CMV-hF.IX-2 | CMV IE | Chimeric CMV/β-globin mini-intron | 1.1 kb | Human growth hormone (hGH) |
| AAV-HSA/CMV-hF.IX-2 | HSA/CMV IE | Chimeric CMV/β-globin mini-intron | None | hGH |
| AAV-HSA-hF.IX-3 | HSA | HSA intron I | None | hGH |
| AAV-cTnT-hF.IX | Cardiac troponin I | Chimeric CMV/β-globin mini-intron | 1.1-kb | hGH |
| Vector . | Enhancer/Promoter . | Intron . | Human F.IX 3′-UT . | PolyA Signal . |
|---|---|---|---|---|
| AAV-CMV-hF.IX-3 | CMV IE | Portion of hF.IX intron I (1.4 kb) | 0.3 kb | SV40 late |
| AAV-CMV-hF.IX-3/bGH | CMV IE | Portion of hF.IX intron I (1.4 kb) | 0.3 kb | Bovine growth hormone (bGH) |
| AAV-HSA-hF.IX-1 | Human α-skeletal actin (HSA) | Portion of hF.IX intron I (0.3 kb) | 0.3 kb | SV40 late |
| AAV-CMV-hF.IX-8 | CMV IE | Portion of hF.IX intron I (0.3 kb) | Entire 1.6 kb | Human F.IX |
| AAV-CMV-hF.IX-2 | CMV IE | Chimeric CMV/β-globin mini-intron | 1.1 kb | Human growth hormone (hGH) |
| AAV-HSA/CMV-hF.IX-2 | HSA/CMV IE | Chimeric CMV/β-globin mini-intron | None | hGH |
| AAV-HSA-hF.IX-3 | HSA | HSA intron I | None | hGH |
| AAV-cTnT-hF.IX | Cardiac troponin I | Chimeric CMV/β-globin mini-intron | 1.1-kb | hGH |
Vector genomes were designed to approximate the size of wild-type AAV (approximately 4.5 kb) in order not to exceed the packaging limit for generation of recombinant AAV. All vectors include the entire coding region of the human F.IX cDNA plus the components listed above.
Expression from the CMV promoter is sufficient to achieve therapeutic levels
In our published experiments in mice and dogs, we have used 2 similar expression cassettes. Both use the CMV IE enhancer/promoter but differ in intron and polyadenylation sequences and in the amount of 3′-UT region of the F.IX cDNA. Hence, we decided to compare these vectors (AAV-CMV-hF.IX-2 and -3 as outlined in Table 1) for expression of human F.IX in vitro using murine C2C12 myotubes and in vivo using Rag-1 mice. Based on other investigators' observations on transgene expression, we additionally constructed vectors AAV-CMV-hF.IX-3-bGH and AAV-CMV-hF.IX-8. The first vector contains the bovine growth hormone polyadenylation signal, which has been shown to improve expression in the context of a plasmid DNA vector when substituted for the SV40 polyA (which is used in AAV-CMV-hF.IX-3).25 The second vector contains the entire human F.IX 3′-UT region and polyadenylation signal of the human F.IX message, which was found to increase expression of human F.IX in mice after hepatic gene transfer with an adenoviral vector.26
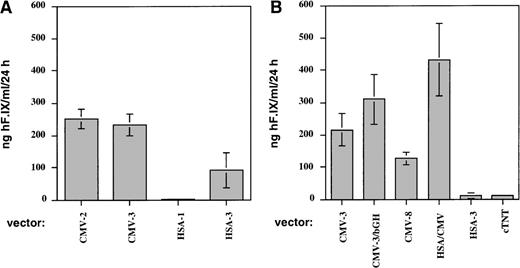
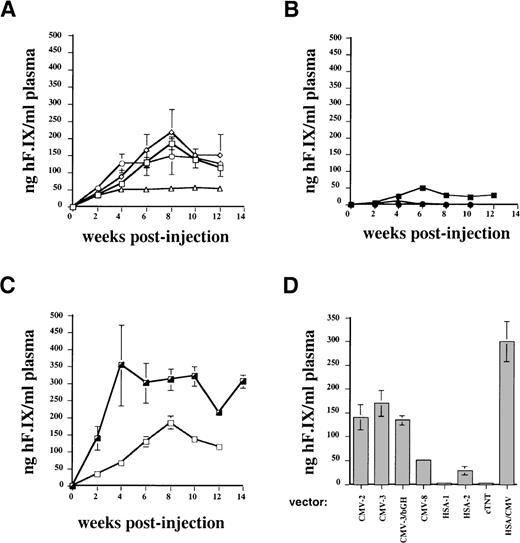
Figures 1 and 2A show the results of a comparison of these vectors in vitro and in vivo. Two experiments were performed by transduction of C2C12 cells. The combined results of these experiments (Figure 1) demonstrated that vectors using the SV40 or a growth hormone polyadenylation signal (AAV-CMV-hF.IX-2, -3, and -3-bGH) gave nearly identical levels of F.IX expression in myotubes and in plasma samples of Rag-1 mice. Levels of systemic expression in mice (n = 4 for each vector construct) were, on average, 120 to 200 ng/mL and therefore in the therapeutic range of expression (2.4%-4% of normal human F.IX levels; Figure 2A). Expression from the construct containing the human F.IX polyA sequence (CMV-8) was 2- to 4-fold lower, both in vitro and in vivo (Figure 1 and Figure 2A).
Expression of human F.IX in conditioned media of C2C12 murine myotubes after transduction with AAV vectors at an MOI = 105 vector genomes/cell.
All measurements of human F.IX concentrations were performed by ELISA. Transduction experiments were performed in duplicate. (A) Cells were transduced with either AAV-CMV-hF.IX-2 (CMV-2), AAV-CMV-hF.IX-3 (CMV-3), AAV-HSA-hF.IX-1 (HSA-1), or AAV-HSA-hF.IX-3 (HSA-3). F.IX values (ng/mL/24 hours) are average of 2 independent measurements performed on 24-hour supernatants at day 8 posttransduction. Vertical bars indicate standard deviation. (B) Cells were transduced with either AAV-CMV-hF.IX-3 (CMV-3), AAV-CMV-hF.IX-3-bGH (CMV-3-bGH), AAV-CMV-hF.IX-8 (CMV-8), AAV-HSA-hF.IX-3 (HSA-3), AAV-HSA-CMV-hF.IX-2 (HSA/CMV), or AAV-cTnT-hF.IX (cTnT). F.IX values (ng/mL/24 hours) are average of a total of 4 measurements (2 independent measurements per experiment) on 24-hour supernatants at day 8 posttransduction from 2 independent transduction experiments.
Expression of human F.IX in conditioned media of C2C12 murine myotubes after transduction with AAV vectors at an MOI = 105 vector genomes/cell.
All measurements of human F.IX concentrations were performed by ELISA. Transduction experiments were performed in duplicate. (A) Cells were transduced with either AAV-CMV-hF.IX-2 (CMV-2), AAV-CMV-hF.IX-3 (CMV-3), AAV-HSA-hF.IX-1 (HSA-1), or AAV-HSA-hF.IX-3 (HSA-3). F.IX values (ng/mL/24 hours) are average of 2 independent measurements performed on 24-hour supernatants at day 8 posttransduction. Vertical bars indicate standard deviation. (B) Cells were transduced with either AAV-CMV-hF.IX-3 (CMV-3), AAV-CMV-hF.IX-3-bGH (CMV-3-bGH), AAV-CMV-hF.IX-8 (CMV-8), AAV-HSA-hF.IX-3 (HSA-3), AAV-HSA-CMV-hF.IX-2 (HSA/CMV), or AAV-cTnT-hF.IX (cTnT). F.IX values (ng/mL/24 hours) are average of a total of 4 measurements (2 independent measurements per experiment) on 24-hour supernatants at day 8 posttransduction from 2 independent transduction experiments.
Expression of human F.IX as a function of time in plasma of Rag-1 mice following intramuscular injection of AAV vector at day 0.
(A) Systemic expression of human F.IX from CMV IE enhancer/promoter following intramuscular injection of AAV vectors into Rag-1 mice (n = 4 for each vector, 1 × 1011 vector genomes/mouse). Data points are mean expression levels at each time point, and vertical lines indicate standard deviation. AAV-CMV-hF.IX-2: open squares; AAV-CMV-hF.IX-3: open diamonds; AAV-CMV-hF.IX-3/bGH: open circles; AAV-CMV-hF.IX-8: open triangles. (B) Systemic expression of human F.IX from muscle-specific promoters (HSA or troponin) following intramuscular injection of AAV vector into Rag-1 mice (n = 4 for each vector, 1 × 1011 vector genomes/mouse). AAV-HSA-hF.IX-1: closed circle; AAV-HSA-hF.IX-3: closed square; AAV-cTnT-hF.IX: closed triangle. (C) Effect of HSA enhancer on systemic expression of human F.IX following intramuscular injection of AAV vector into Rag-1 mice (n = 4 for each vector, 1 × 1011 vector genomes/mouse). Note that vector AAV-HSA/CMV-hF.IX-2 (HSA/CMV-2, half-closed square) was constructed by the addition of a 1.2-kb 5′-flanking sequence of the HSA promoter 5′ to the CMV IE enhancer/promoter in vector AAV-CMV-hF.IX-2 (CMV-2, open square). The 3′-UT region of the human F.IX cDNA was deleted in the vector to accommodate the additional sequence. Animals injected with AAV-CMV-hF.IX-2 were identical to those shown in graph A. (D) Summary of systemic human F.IX expression in Rag-1 mice from AAV vectors. Columns represent average values of mean human F.IX expression levels for each vector for time points 6 to 12 weeks postinjection, ie, the plateau of expression (average of data points shown in graphs A-C). Vertical bars are standard deviation from the mean.
Expression of human F.IX as a function of time in plasma of Rag-1 mice following intramuscular injection of AAV vector at day 0.
(A) Systemic expression of human F.IX from CMV IE enhancer/promoter following intramuscular injection of AAV vectors into Rag-1 mice (n = 4 for each vector, 1 × 1011 vector genomes/mouse). Data points are mean expression levels at each time point, and vertical lines indicate standard deviation. AAV-CMV-hF.IX-2: open squares; AAV-CMV-hF.IX-3: open diamonds; AAV-CMV-hF.IX-3/bGH: open circles; AAV-CMV-hF.IX-8: open triangles. (B) Systemic expression of human F.IX from muscle-specific promoters (HSA or troponin) following intramuscular injection of AAV vector into Rag-1 mice (n = 4 for each vector, 1 × 1011 vector genomes/mouse). AAV-HSA-hF.IX-1: closed circle; AAV-HSA-hF.IX-3: closed square; AAV-cTnT-hF.IX: closed triangle. (C) Effect of HSA enhancer on systemic expression of human F.IX following intramuscular injection of AAV vector into Rag-1 mice (n = 4 for each vector, 1 × 1011 vector genomes/mouse). Note that vector AAV-HSA/CMV-hF.IX-2 (HSA/CMV-2, half-closed square) was constructed by the addition of a 1.2-kb 5′-flanking sequence of the HSA promoter 5′ to the CMV IE enhancer/promoter in vector AAV-CMV-hF.IX-2 (CMV-2, open square). The 3′-UT region of the human F.IX cDNA was deleted in the vector to accommodate the additional sequence. Animals injected with AAV-CMV-hF.IX-2 were identical to those shown in graph A. (D) Summary of systemic human F.IX expression in Rag-1 mice from AAV vectors. Columns represent average values of mean human F.IX expression levels for each vector for time points 6 to 12 weeks postinjection, ie, the plateau of expression (average of data points shown in graphs A-C). Vertical bars are standard deviation from the mean.
Muscle-specific promoters fail to produce therapeutic levels of expression
The use of muscle-specific cellular promoters, including that of the human α-skeletal actin gene (sequence up to position −200017) and the cardiac troponin I gene (cTnT, also expressed in skeletal muscle) gave low to undetectable levels of human F.IX in conditioned media of myotubes (Figure 1) and in plasma samples of Rag-1 mice following intramuscular injection (1 × 1011 vector genomes/mouse, n = 4; Figure2B). These disappointing F.IX levels correlated with low transcript levels by Northern blot analysis of RNA isolated from transduced C2C12 myotubes when compared with expression from the CMV enhancer/promoter (Figure 3).
A Northern blot demonstrating expression of recombinant human factor IX cDNA in C2/C12 mouse myoblasts.
Mouse myoblasts were plated in 6-well tissue culture plates at a density of 2.5 × 105 cells per well. The myoblasts were grown for 2 days until they had reached confluence and then stimulated to differentiate into myotubes. After 6 days of differentiation, the myotubes were transduced with AAV particles of the different hF.IX constructs (MOI = 5 × 104). Five days later, media and cellular RNA were harvested and analyzed by ELISA and Northern blot, respectively. Ten micrograms of RNA was examined for hF.IX (A) or b-actin (B) expression by Northern analysis and hybridization with the corresponding probes. The RNA samples loaded in each lane were lane 1, AAV-CMV-hF.IX3 (2.0 kb); lane 2, AAV-HSA-CMV-hF.IX2 (1.9 kb); lane 3, AAV-HSA-hF.IX3 (1.8 kb); lane 4, AAV-cTNT-hF.IX (3.0 kb); and lane 5, C2/C12 RNA only. The expected size for each expressed RNA is indicated in parentheses. (C) The levels of hF.IX transcript were normalized to the level of b-actin transcript, and the ratios are reported relative to the amount of hF.IX transcript expressed from the AAV-CMV-hF.IX-3 vector. The relative amount of hF.IX protein as assayed by ELISA are also reported.
A Northern blot demonstrating expression of recombinant human factor IX cDNA in C2/C12 mouse myoblasts.
Mouse myoblasts were plated in 6-well tissue culture plates at a density of 2.5 × 105 cells per well. The myoblasts were grown for 2 days until they had reached confluence and then stimulated to differentiate into myotubes. After 6 days of differentiation, the myotubes were transduced with AAV particles of the different hF.IX constructs (MOI = 5 × 104). Five days later, media and cellular RNA were harvested and analyzed by ELISA and Northern blot, respectively. Ten micrograms of RNA was examined for hF.IX (A) or b-actin (B) expression by Northern analysis and hybridization with the corresponding probes. The RNA samples loaded in each lane were lane 1, AAV-CMV-hF.IX3 (2.0 kb); lane 2, AAV-HSA-CMV-hF.IX2 (1.9 kb); lane 3, AAV-HSA-hF.IX3 (1.8 kb); lane 4, AAV-cTNT-hF.IX (3.0 kb); and lane 5, C2/C12 RNA only. The expected size for each expressed RNA is indicated in parentheses. (C) The levels of hF.IX transcript were normalized to the level of b-actin transcript, and the ratios are reported relative to the amount of hF.IX transcript expressed from the AAV-CMV-hF.IX-3 vector. The relative amount of hF.IX protein as assayed by ELISA are also reported.
Improved expression of muscle-derived F.IX by addition of skeletal actin regulatory sequences to the CMV enhancer/promoter
A 1.2-kb portion of the HSA promoter region (bp position −1281 to −8417) was added 5′ to the CMV enhancer/promoter in construct AAV-CMV-hF.IX-2 (Table 1). All of the 3′-UT of the F.IX cDNA was deleted to accommodate the additional HSA sequences. The resulting vector AAV-HSA/CMV-hF.IX-2 gave an approximately 2- to 3-fold increase in levels of secreted F.IX in culture media of C2C12 myotubes (Figures 1 and 3) compared with expression obtained from CMV sequences alone. In agreement with these data, a 2-fold increase in expression of systemic F.IX levels over the CMV constructs was measured in Rag-1 mice following intramuscular injection of the vector (1 × 1011 vector genomes/mouse, n = 4; Figure 2C), resulting in circulation of approximately 300 ng of human F.IX/mL of plasma, or 6% of normal human F.IX levels. Northern blot analysis showed 5-fold higher F.IX transcript levels in myotubes after transduction with AAV-HSA/CMV-hF.IX-2 in comparison with our previously published vector, AAV-CMV-hF.IX-3 (Figure 3). When vectors AAV-CMV-hF.IX-2 and AAV-HSA/CMV-hF.IX-2 were tested for transduction of HepG2 and HEK-293 cells, almost identical levels of F.IX expression were observed (Figure 4), indicating that expression from the CMV promoter in nonmuscle cells is not altered by inclusion of the muscle-specific HSA element. However, results in myotubes and mouse muscle demonstrate improved expression from this vector in muscle cells. Similar to expression in C2C12 murine myotubes, expression in human myotubes was enhanced 1.7-fold by addition of the 1.2-kb HSA sequence (Figure 4). Experiments in Rag-1 mice were repeated with different vector preparations of AAV-CMV-hF.IX-2, AAV-CMV-hF.IX-3, and AAV-HSA/CMV-hF.IX-2. AAV-HSA/CMV-hF.IX-2 gave reproducibly approximately 2-fold higher levels of systemic human F.IX expression at a dose of 1 × 1011vector genomes/mouse (data not shown).
Expression of human F.IX (ng/106 cells/24 hours) in conditioned media after in vitro transduction of cultured human cells (24-hour supernatants) with AAV vector.
MOIs were 104 vector genomes/cell. Results are shown for vectors AAV-CMV-hF.IX-2 (CMV) and AAV-HSA/CMV-hF.IX-2 (HSA/CMV). Cell types were HEK-293 cells, HepG2 cells, and human myotubes derived from primary myoblasts. Cells were transduced in triplicate. Values for myotubes were average for 24-hour supernatants at days 11 and 18 posttransduction; values for HEK-293 and HepG2 cells were average for 24-hour supernatants at day 4 posttransduction. Vertical lines are standard deviation.
Expression of human F.IX (ng/106 cells/24 hours) in conditioned media after in vitro transduction of cultured human cells (24-hour supernatants) with AAV vector.
MOIs were 104 vector genomes/cell. Results are shown for vectors AAV-CMV-hF.IX-2 (CMV) and AAV-HSA/CMV-hF.IX-2 (HSA/CMV). Cell types were HEK-293 cells, HepG2 cells, and human myotubes derived from primary myoblasts. Cells were transduced in triplicate. Values for myotubes were average for 24-hour supernatants at days 11 and 18 posttransduction; values for HEK-293 and HepG2 cells were average for 24-hour supernatants at day 4 posttransduction. Vertical lines are standard deviation.
Discussion
Previously published studies have established the feasibility of muscle-directed gene transfer for treatment of hemophilia B. Vector dose–dependent sustained expression of therapeutic F.IX levels was demonstrated in a large animal model using intramuscular injection of an AAV vector.3 Injection of up to 8.5 × 1012 vector genomes of recombinant AAV per kilogram did not result in detectable toxicity in the hemophilia B dogs used in that study. However, levels of systemic F.IX expression per delivered vector particle were clearly less than published for liver-directed approaches.13,27 Therefore, improvement of expression levels remains an important issue. In this investigation, we have shown that addition of a muscle-specific element of the skeletal actin promoter region to our CMV-driven expression cassette improved systemic muscle-derived F.IX expression substantially. Systemic levels of 6% of the normal human F.IX concentration were achieved with a 2- to 4-fold lower vector dose per animal than described previously with a vector containing the CMV enhancer/promoter alone.11 24These results further underline the feasibility of muscle as a target organ for systemic delivery of F.IX or other secreted proteins and give more insight into the use of the CMV enhancer/promoter sequence for transgene expression. Moreover, the potential use of this expression cassette in a clinical trial might additionally improve safety of the protocol because therapeutic levels of expression could be reached with a lower vector dose per kilogram.
Previous results suggested an approximately linear scale-up from the Rag-1 mouse model to hemophilia B dogs on a per-weight basis as a requirement to achieve therapeutic levels of expression in a large animal model.3 Both vectors used in these studies for expression of human and canine F.IX used the CMV enhancer/promoter, but they differed in polyadenylation and intron sequences. To confirm our interpretation of scale-up results, it was necessary to compare both vectors side by side in vitro and in vivo in Rag-1 mice. As shown in Figures 1A and 2A, vectors AAV-CMV-hF.IX-2 and -3 indeed expressed comparable F.IX levels. Minor differences in expression (less than or equal to 30%) are within the margin of error of the viral titer. Furthermore, replacement of the SV40 polyadenylation signal with a growth hormone polyA signal had no apparent effect on expression, indicating that mRNA processing is not likely the limiting factor for expression from our initially published human F.IX cassette (in vector AAV-CMV-hF.IX-3).11 13 The addition of human F.IX 3′-UT/polyA sequences slightly reduced rather than enhanced expression. It may be that these sequences contain an element that improves expression in hepatocytes where F.IX is normally synthesized and is not recognized in other cell types such as muscle fibers.
Substitution of CMV sequences with muscle-specific promoters was not advantageous to expression of F.IX. Muscle-specific skeletal actin and cardiac troponin promoters performed poorly in transgene expression when compared with the strong viral promoter element. This result may not be surprising given the experience of others that compared other muscle-specific promoters such as those from muscle creatine kinase (mck) and myosin genes with CMV or SV40 promoters for muscle-derived expression of transgenes in the context of adenoviral and plasmid vectors.28 29 However, a comparison between our and the cited studies is not straightforward because sequences of AAV, adenoviral, or plasmid vectors might influence transgene expression in different ways.
Interestingly, our results obtained in the in vitro test system (C2C12 myotubes) were predictive of results in vivo. However, this observation might not reflect a general pattern for other enhancer/promoter combinations. For example, in work with transgenic mice, the 1-kb region between the mck enhancer and the mck basal promoter has been implicated in enhancing muscle-specific expression synergistically with the 206-bp mck enhancer.30 This effect on expression was missed when the 1-kb region was evaluated in vitro using murine skeletal myocytes. Thus, in vivo testing of expression cassettes remains an important component for optimization studies in gene therapy.
The CMV enhancer/promoter has been successfully used for sustained muscle-derived expression of several transgenes from AAV vectors.3,5,7,11,31,32 Expression from this promoter is clearly influenced by target tissue and vector sequences. For example, efficient shutdown of expression in liver and lung has been reported for a variety of vectors, including AAV.13,33 In muscle, CMV promoter shutdown has also been observed in the context of a retroviral vector, but it could be prevented by addition of a muscle-specific enhancer element, the mck enhancer.34 In another study, addition of multiple copies of the mck enhancer to a constitutive cellular β-actin and other promoters synergistically improved expression in the context of retroviral vectors used to transduce murine muscle cells in vitro.35 Therefore, we surmised that transcriptional activity of the CMV promoter in muscle might be improved by addition of a strong, muscle-specific element.
The HSA promoter region up to position −2000 bp is well characterized for muscle-specific gene expression.17,18,36The region of bp position −153 to +239 is described as necessary and sufficient for muscle-specificity of the HSA promoter.17 The promoter contains at least 4 CArG boxes.18,37 The CArG box with the consensus sequence CC(A/T)6GG, also referred to as serum response element, binds to serum response factor and is found in several muscle-specific elements. A classical orientation- and position-independent enhancer is located in the region −153 to −87 of the human α-skeletal actin gene. This enhancer synergistically increases muscle-specific expression with sequences located −1300 to −628.17 The latter element acts in an orientation- and position-dependent fashion. Sequences upstream of the enhancer include additional CArG boxes, and sequences 3′ to −87 include transcription enhancer factor-1 (TEF-1) and Sp1 binding sites and a TATA box.17 The binding site for TEF-1 is also known as M-CAT element and is found, for example, in the promoters of the cTnT and the α-skeletal actin gene.38 39
The HSA sequences in vector AAV-HSA/CMV-hF.IX-2 (bp position −1281 to −84 of the HSA promoter) contain at least 4 CArG boxes, a putative Sp1 binding site, and a binding site for bifunctional factor YY1.17 39 This portion of the HSA gene also contains the synergistic enhancer elements discussed above (bp positions −153 to −87 and −1300 to −628 of the HSA promoter, respectively). The construct containing the HSA/CMV promoter (AAV-HSA/CMV-hF.IX-2) showed enhanced expression both in vitro and in vivo in muscle cells, compared with AAV-CMV-hF.IX-2. A comparison of these 2 constructs reveals 2 differences: the presence of the HSA enhancer element and the absence of the F.IX 3′-UT region in AAV-HSA/CMV-hF.IX-2. Theoretically, the difference in expression can result from either or both of 2 reasons. First, the addition of skeletal actin enhancer sequences enhances transcriptional activity of the CMV promoter in muscle cells or, secondly, human F.IX 3′-UT sequences downregulate transcript levels in a muscle cell-specific manner. The first interpretation of the data, in our opinion, is far more likely to be true, while the second possibility remains a formal one. These data would suggest that muscle-specific upregulation of the strong CMV enhancer/promoter element by HSA enhancer sequences results in promoter activity in muscle fibers that is far superior to expression from muscle-specific promoters and higher than expression from the CMV enhancer/promoter by itself. This synergistic effect on gene expression achieved by a combination of a strong viral enhancer/promoter element with strong enhancer and enhancer-like elements specific for the target tissue of gene transfer will be useful in future design of expression cassettes.
Acknowledgments
The authors thank Dr Heiman-Patterson for human myotubes, Dr Hardeman for the HSA DNA, Dr Ordahl for the troponin DNA, and J. H. Liu and N. Chung for technical assistance.
Supported by National Institutes of Health grants R01 HL53668 and P50 HL54500 to K.A.H, grant F32 HL09397 to J.N.H., a K. Dormandy Trust grant to P.A.F., and Avigen, Inc, a company in which K.A.H., L.B.C., and C.S. hold equity.
Reprints:Katherine A. High, The Children's Hospital of Philadelphia, Abramson Research Center, Room 310, 34th St and Civic Center Blvd, Philadelphia, PA 19104; e-mail: high@emailchop.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal