Abstract
Peripheral blood stem cells (PBSC) obtained from granulocyte-colony stimulating factor (G-CSF)-mobilized donors are increasingly used for allogeneic transplantation. Despite a 10-fold higher dose of transplanted T cells, acute graft-versus-host disease (GVHD) does not develop in higher proportion in recipients of PBSC than in recipients of marrow. T cells from G-CSF-treated experimental animals preferentially produce IL-4 and IL-10, cytokines characteristic of Th2 responses, which are associated with diminished GVHD-inducing ability. We hypothesized that G-CSF-mobilized PBSC contain antigen-presenting cells, which prime T-lymphocytes to produce Th2 cytokines. Two distinct lineages of dendritic cells (DC) have been described in humans, DC1 and DC2, according to their ability to induce naive T-cell differentiation to Th1 and Th2 effector cells, respectively. We have used multicolor microfluorometry to enumerate DC1 and DC2 in the peripheral blood of normal donors. G-CSF treatment with 10 to 16 μg/kg per day for 5 days increased peripheral blood DC2 counts from a median of 4.9 × 106/L to 24.8 × 106/L (P = .0009), whereas DC1 counts did not change. Purified DC1, from either untreated or G-CSF treated donors, induced the proliferation of allogeneic naive T cells, but fresh DC2 were poor stimulators. Tumor necrosis factor- (TNF-)-activated DC1 induced allogeneic naive T cells to produce IFN-γ, which is typical of Th1 responses, whereas TNF--activated DC2 induced allogeneic naive T cells to produce IL-4 and IL-10, which are typical of Th2 responses. PBSC transplants contained higher doses of DC2 than marrow transplants (median, 2.4 × 106/kg versus 0.5 × 106/kg) (P = .006), whereas the dose of DC1 was comparable. Thus, it is conceivable that transplantation of G-CSF-stimulated PBSC does not result in overwhelming acute GVHD because the graft contains predominantly Th2-inducing DC. Adoptive transfer of purified DC2 may be exploited to induce immune deviation after transplantation of hematopoietic stem cells or organ allografts.
Granulocyte-colony stimulating factor (G-CSF) has been used in the clinic for its ability to mobilize hematopoietic stem cells from the bone marrow into the bloodstream of patients or normal donors.1,2 Administration of G-CSF followed by leukapheresis allows the harvest of a sufficient number of stem cells to engraft autologous or allogeneic recipients.3-7 Clinical data and results in experimental animals indicate that allografts from G-CSF-treated donors have peculiar immunologic features. In humans, G-CSF-mobilized peripheral blood stem cell (PBSC) grafts do not cause a higher incidence of acute graft-versus-host disease (GVHD) than marrow grafts, despite at least a 10-fold higher T-cell dose,1 and they achieve better engraftment across human leukocyte antigen (HLA) barriers.8,9 PBSC grafts, however, may be associated with an increased incidence of chronic GVHD.10 11
T cells from blood or spleen cells of G-CSF-treated mice show decreased ability to induce GVHD in allogeneic recipients, presumably because of their polarization toward T helper 2 (Th2) cells, which produce the cytokines interleukin-4 (IL-4) and IL-10.12,13 In nature, Th2 cells are involved in allergic responses dominated by the B-cell production of IgE and the recruitment of eosinophils and basophils.14 In contrast, Th1 cells produce interferon (IFN)-γ and promote the generation of cytotoxic T lymphocytes and mononuclear phagocytes, which protect against viruses and other intracellular microbes.14 Thus, their functional heterogeneity is likely to account for a distinct role of Th1 and Th2 cells in transplantation responses. T-cell polarization to Th1 or Th2 depends on the type of antigen-presenting cell that initiates the immune response in humans and rodents.15-17
Dendritic cells (DC) are the only antigen-presenting cells that can prime naive T cells to a new antigen.18 Two distinct lineages of DC have been described in humans. Myeloid DC (or DC1) express myeloid antigens CD11c, CD13, and CD33, originate from myeloid bone marrow precursors, and require the presence of GM-CSF for their survival.19-23 In human peripheral blood, DC1 are identified as negative for lymphoid and myeloid cell-specific markers (lin−) and HLA-DR+/CD11c+.29-31 DC1 produce high levels of IL-12 when stimulated with tumor necrosis factor (TNF)-α or CD40L24,25 and drive T-cell differentiation into Th1. Lymphoid DC (or DC2) have been recently described in human peripheral blood and lymphoid tissues as HLA-DR+/lin−/CD11c−/CD4+/IL-3Rα+plasmacytoid cells.26-31 DC2 lack myeloid markers,26,27,30,31 have high levels of pre-T-cell receptor α chain expression,32 and depend on IL-3, and not granulocyte-macrophage colony-stimulating factor (GM-CSF), for their survival and differentiation.15,27,30 They have been designated DC2 because, after appropriate activation, they can induce T-cell differentiation into Th2 cells.15
In the current study, we demonstrate that G-CSF treatment selectively increases the number of DC2 in the peripheral blood, so that PBSC used for allogeneic transplantation contain more DC2 than marrow but a similar number of DC1.
Materials and methods
Cell samples
Blood, leukapheresis, and bone marrow samples were obtained from normal or G-CSF-treated donors after they gave written informed consent to protocols approved by the Institutional Review Board at Fred Hutchinson Cancer Research Center. PBSC donors were treated with human recombinant G-CSF (Neupogen; Amgen, Inc, Thousand Oaks, CA) 10 to 16 μg/kg per day for 5 days subcutaneously. Leukapheresis was performed on day 5 using a continuous flow blood cell separator (Cobe Laboratories, Lakewood, CO). In selected experiments, buffy coat cells from normal blood donors were provided by the Puget Sound Blood Center (Seattle, WA).
Monoclonal antibodies
Phycoerythrin (PE)- and biotin-conjugated anti-IL-3Rα (CD123), PE-conjugated anti-CD45RA, and fluorescein isothiocyanate (FITC)- and PE-conjugated isotype control murine IgG2b monoclonal antibodies (mAbs) were purchased from PharMingen (San Diego, CA). All other mAbs were purchased from Becton Dickinson (San Jose, CA).
Flow microfluorometry
Cells were stained without further separation to minimize selective loss. Erythrocytes were lysed after staining, using FACS Lysing Solution (Becton Dickinson) according to the manufacturer's instructions. Dendritic cells were identified as positive for anti-HLA-DR peridin chlorophyll protein (PerCP)-conjugated and negative for a mixture of FITC-conjugated mAbs specific for lineage markers on T cells (CD3), B cells (CD19, CD20, surface IgM), natural killer cells (CD16, CD56), monocytes (CD14), neutrophils (CD16), and progenitor cells (CD34). Anti-CD11c PE or IL-3Rα PE were used for identification of DC1 and DC2 subpopulations, respectively. Selected samples were stained with CD4 PE or CD11c PE, and with CD11c APC or IL-3Rα biotin plus streptavidin allophycocyanin-conjugated. Three-color analysis was performed using a FACScan (Becton Dickinson), and 4 color-analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson). The number of total white blood cells (WBC) in the samples was determined using a NE8000 Sysmex (TOA, Kobe, Japan) automated cell counter. The absolute number of DC1 and DC2 was calculated from the WBC count multiplied by the proportion of each subpopulation among the WBC, as determined by flow cytometric analysis.
DC separation for functional assays
PBMC from normal or G-CSF treated donors were isolated by Ficoll–Hypaque density gradient centrifugation at 800g for 25 minutes, then washed twice in phosphate-buffered saline. PBMC were then depleted of CD14+ monocytes and CD19+ B cells by labeling with anti-CD14 and anti-CD19 magnetic microbeads (Miltenyi-Biotec, Bergisch Gladbach, Germany) and depleting the labeled cells on a high-gradient magnetic separation column (VarioMACS; Miltenyi-Biotec). Recovered cells were labeled with anti-HLA-DR magnetic microbeads (Miltenyi-Biotec), then positively selected on a magnetic separation column (MidiMACS; Miltenyi-Biotec). The immunomagnetic purification was performed according to the manufacturer's instructions. The entire procedure lasted 4 to 6 hours, during which cells were kept at 4°C. Aliquots of enriched cells were checked for purity and were consistently more than 96% HLA-DR+. Cells were then stained with FITC lineage markers, CD11c PE, and IL-3Rα biotin plus streptavidin TC, then sorted on a FACS Vantage (Becton Dickinson). Aliquots of sorted cells were reanalyzed for their purity, which was consistently greater than 95% for DC1 and greater than 98% for DC2. Cross-contamination of each DC subset with cells belonging to the other subset was consistently below the detection limit of the assay.
T-cell separation
CD4+CD45RA+ naive T cells were purified by using the CD4 MACS Multisort Kit (Miltenyi-Biotec) according to the manufacturer's instructions. Briefly, Ficoll-separated mononuclear cells were first labeled with anti-CD4 magnetic microbeads and positively selected on a magnetic separation column. Then the microbeads were enzymatically removed from the antibody by using MACS release reagent (Miltenyi-Biotec). CD4+ cells were then labeled with anti-CD45RA microbeads (Miltenyi-Biotec) and positively selected on a magnetic separation column. Aliquots of sorted naive T cells were restained with anti-CD4 FITC and anti-D45RA PE, and their purity was consistently greater than 98%. In some experiments, purified CD4+/CD45RA+ cells were frozen in aliquots of 107/mL in RPMI-1640 medium containing 20% fetal calf serum (FCS) and 10% dimethyl sulfoxide for use at a later date. Viability after thawing was consistently greater than 90%.
In vitro activation of DC1 and DC2
Purified DC1 and DC2 were cultured for 2 to 6 days in flat-bottomed 96-well plates at 10 to 50 × 103 cells/ 200 μL in complete RPMI–HEPES containing 10% heat-inactivated FCS (Gibco BRL, Grand Island, NY). The following cytokines were added: 10 ng/mL (150 IU/mL) human recombinant GM-CSF (Genzyme, Cambridge, MA), 50 ng/mL human recombinant IL-3 (R&D Systems, Minneapolis, MN), and 10 ng/mL (1100 IU/mL) human recombinant TNF-α (R&D Systems).
T-cell proliferation assays
Fresh or in vitro-activated DC1 and DC2 were cocultured with autologous or allogeneic naive CD4+CD45RA+cells to test for stimulatory activity. Stimulator cells were suspended in complete medium containing 15% FCS and irradiated at 1500 cGy, and serial dilutions were prepared beginning at 5 to 10 × 103 cells/well. Responding T cells were plated at 5 × 104 cells/well. All cocultures were performed in round-bottomed 96-well plates. Cultures were maintained in a humidified atmosphere at 37°C and 5% CO2. Cultures were pulsed with 1 μCi/well 3H-thymidine for 8 to 18 hours before harvest on day 6 to measure proliferation.
Cytokine production assay
Naive CD4+CD45RA+ T cells were cocultured with allogeneic DC1 and DC2, harvested after 6 days, and replated at 5 × 104 cells/well in round-bottomed 96-well plates in the presence of PMA (25 ng/mL) and ionomycin (1 μg/mL). After 48 hours supernatants were harvested and frozen until analysis. Cytokines were analyzed by enzyme-linked immunosorbent assay (ELISA). ELISA kits for human IFN-γ, IL-4, and IL-10 were purchased from Endogen (Boston, MA). The lower limits of detection were 2.6 pg/mL for IFN-γ, 2.8 pg/mL for IL-10, and 3 pg/mL for IL-4. Specific activity of rH IFN-γ and IL-4 used in the assay were, respectively, 6.8 × 103 and 2.9 × 104 IU/μg. Specific activity was not available for IL-10.
Statistical analysis
Proliferation and cytokine data are summarized with means ± 1 SD. Statistical comparisons were performed using t tests for independent samples.
Results
Phenotype of dendritic cells in the peripheral blood of normal and G-CSF–treated donors
DC were identified as HLA-DR+ cells and negative for granulocytes, monocytes, natural kill cells, T cells, B cells, and CD34 cell lineage markers (Figure1A), and they constituted on average 0.5% PBMC from normal donors. They showed light scatter properties intermediate between lymphocytes and monocytes (Figures 1B,1C). They were larger and more granular than resting lymphocytes but smaller and less granular than monocytes. DC were analyzed for the expression of the adhesion molecule CD11c, typical of DC1 lineage, and of the IL-3 receptor (IL-3R)α chain typically bright in the DC2 lineage (Figure 1D). The intensity of CD4 expression was higher on DC2 (Figure 1E) than on DC1. DC from G-CSF-treated donors showed the same pattern of expression of CD11c, IL-3Rα, and CD4 as in normal donors (Figures 1D, 1E) and the same light scatter properties (Figure 1C).
Phenotype of blood DC before and after G-CSF treatment.
Peripheral blood samples were collected from the same donor before (left) and after (right) G-CSF treatment. After lysis of erythrocytes, cells were labeled with anti-HLA-DR PerCP, anti-IL-3Rα or anti-CD4 PE, anti-CD11c APC, and a mixture of FITC-conjugated mAbs specific for lineage markers CD3, CD14, CD16, CD19, CD20, CD34, CD56, and IgM expressed on lymphocytes, monocytes, granulocytes and progenitor cells (lineage FITC). Cells were then analyzed by 4-color flow cytometry. (A) Dendritic cells were characterized by positive HLA-DR and negative lineage markers. (B) Scatter profile of granulocytes (Gr), monocytes (Mo), and lymphocytes (Ly). (C) Scatter profile of DC, as gated in A. (D) DC were analyzed for the expression of IL-3Rα and CD11c or (E) CD4 and CD11c.
Phenotype of blood DC before and after G-CSF treatment.
Peripheral blood samples were collected from the same donor before (left) and after (right) G-CSF treatment. After lysis of erythrocytes, cells were labeled with anti-HLA-DR PerCP, anti-IL-3Rα or anti-CD4 PE, anti-CD11c APC, and a mixture of FITC-conjugated mAbs specific for lineage markers CD3, CD14, CD16, CD19, CD20, CD34, CD56, and IgM expressed on lymphocytes, monocytes, granulocytes and progenitor cells (lineage FITC). Cells were then analyzed by 4-color flow cytometry. (A) Dendritic cells were characterized by positive HLA-DR and negative lineage markers. (B) Scatter profile of granulocytes (Gr), monocytes (Mo), and lymphocytes (Ly). (C) Scatter profile of DC, as gated in A. (D) DC were analyzed for the expression of IL-3Rα and CD11c or (E) CD4 and CD11c.
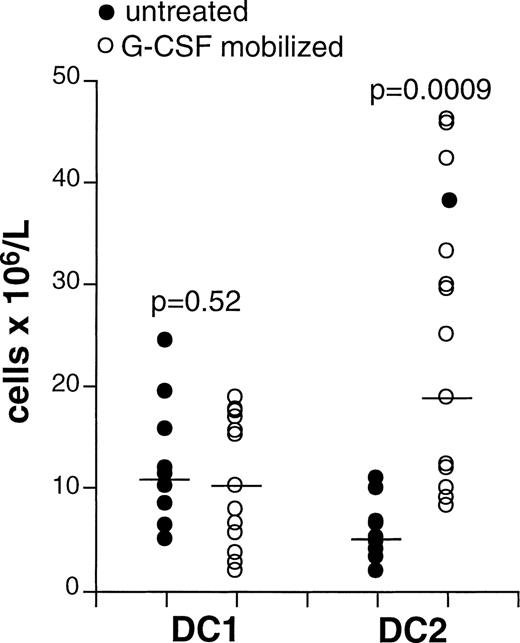
G-CSF treatment mobilizes DC2 but not DC1
G-CSF treatment induces mobilization of leukocytes into the bloodstream. In our series, the median WBC count was 5500 × 106/L in normal donors and 31,200 × 106/L in G-CSF-treated donors. The increase in the number of leukocytes in the blood of G-CSF-treated patients was mainly caused by the mobilization of granulocytes and monocytes (data not shown). The median DC1 count was not different in normal or G-CSF-treated donors (Figure 2 ). In contrast, the median DC2 count was 4.9 × 106/L (range, 1.9-10.8 × 106/L) in the blood of normal donors and 5-fold higher at 24.8 × 106/L (range 7.9-45.5 × 106/L) in the blood of G-CSF treated donors (P = .0009) (Figure 2). Therefore, the ratio between DC2 and DC1 was higher in G-CSF-treated donors as a consequence of the selective increase of DC2.
G-CSF treatment mobilizes DC2 but not DC1.
Peripheral blood samples were collected from normal (n = 9) and G-CSF-treated (n = 13) donors. DC1 and DC2 phenotype was characterized as shown in Figure 1, and absolute counts were determined as described in “Materials and methods.” Samples from 3 donors were collected before and after G-CSF treatment, whereas other samples were collected from independent groups of donors. Each symbol represents a single sample. Horizontal lines in each series represent median values. P values were determined using ttests for independent samples.
G-CSF treatment mobilizes DC2 but not DC1.
Peripheral blood samples were collected from normal (n = 9) and G-CSF-treated (n = 13) donors. DC1 and DC2 phenotype was characterized as shown in Figure 1, and absolute counts were determined as described in “Materials and methods.” Samples from 3 donors were collected before and after G-CSF treatment, whereas other samples were collected from independent groups of donors. Each symbol represents a single sample. Horizontal lines in each series represent median values. P values were determined using ttests for independent samples.
Proliferative response of naive allogeneic T cells to fresh DC1 but not DC2
DC1 and DC2 were purified as described in “Materials and methods” (Figure 3) and then tested for their ability to induce the proliferation of allogeneic naive T cells because this is a specific function of DC. DC1 obtained from the same donor before and after G-CSF treatment were both powerful stimulators of naive allogeneic but not autologous T cells (Figures 4A to 4C). In contrast, DC2 induced weak, if any, proliferation of naive allogeneic T cells. Thus, in normal and in G-CSF-treated donors, fresh DC1 but not DC2 can induce allogeneic naive T cells to proliferate in vitro.
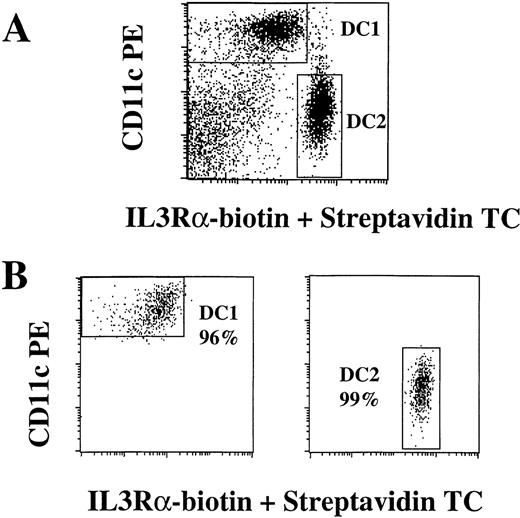
Purification of DC1 and DC2.
Mononuclear cells were enriched for DC by the depletion of CD14+ monocytes and CD19+ B cells, followed by the positive selection of HLA-DR-expressing cells using immunomagnetic beads, as described in “Materials and methods.” HLA-DR+ cells were labeled with anti-CD11c PE, anti-IL-3Rα biotin plus streptavidin TC, and FITC-conjugated antibodies to lineage markers CD3, CD14, CD16, CD19, CD20, CD34, CD56, and IgM. CD11c+, FITC− DC1 (left) and IL-3Rαbright, FITC− DC2 (right) were sorted on a FACSVantage using the gates shown in (A). DC1 and DC2 were reanalyzed after separation for purity assessment (B). Results are representative of more than 10 experiments.
Purification of DC1 and DC2.
Mononuclear cells were enriched for DC by the depletion of CD14+ monocytes and CD19+ B cells, followed by the positive selection of HLA-DR-expressing cells using immunomagnetic beads, as described in “Materials and methods.” HLA-DR+ cells were labeled with anti-CD11c PE, anti-IL-3Rα biotin plus streptavidin TC, and FITC-conjugated antibodies to lineage markers CD3, CD14, CD16, CD19, CD20, CD34, CD56, and IgM. CD11c+, FITC− DC1 (left) and IL-3Rαbright, FITC− DC2 (right) were sorted on a FACSVantage using the gates shown in (A). DC1 and DC2 were reanalyzed after separation for purity assessment (B). Results are representative of more than 10 experiments.
Proliferative responses of naive allogeneic T cells to DC1 and DC2.
DC1 and DC2 from normal (A, B) or G-CSF-treated (C, D) donors were purified as shown in Figure 3. Purified DC were used as stimulators either fresh (A, C) or after 48 hours pre-activation in vitro by GM-CSF, IL-3, and TNF-α (B, D). Unfractionated PBMC were used as control stimulators. CD4+/CD45RA+ naive T cells were purified by immunomagnetic beads, as described in “Materials and methods.” Autologous or allogeneic, purified CD4+/CD45RA+ T cells were used as responders in a 6-day culture assay. T-cell proliferation was measured by 3H]TdR incorporation. Proliferation of autologous T cells was always less than 500 cpm when cocultured with fresh DC (n = 2) and less than 2000 cpm when cocultured with activated DC (n = 3).
Proliferative responses of naive allogeneic T cells to DC1 and DC2.
DC1 and DC2 from normal (A, B) or G-CSF-treated (C, D) donors were purified as shown in Figure 3. Purified DC were used as stimulators either fresh (A, C) or after 48 hours pre-activation in vitro by GM-CSF, IL-3, and TNF-α (B, D). Unfractionated PBMC were used as control stimulators. CD4+/CD45RA+ naive T cells were purified by immunomagnetic beads, as described in “Materials and methods.” Autologous or allogeneic, purified CD4+/CD45RA+ T cells were used as responders in a 6-day culture assay. T-cell proliferation was measured by 3H]TdR incorporation. Proliferation of autologous T cells was always less than 500 cpm when cocultured with fresh DC (n = 2) and less than 2000 cpm when cocultured with activated DC (n = 3).
Proliferative response of naive allogeneic T cells to activated DC2
The difference in stimulatory ability between fresh DC1 and DC2 could result from their level of expression of costimulatory molecules or state of cell activation. Reports from several laboratories have indicated that costimulatory molecules CD40, CD80, and CD86 are expressed at low levels on resting DC1 and at even lower levels on resting DC2 isolated from human blood.29-31 We tested whether DC2 activation with cytokines would induce allo-stimulatory activity. DC2 from G-CSF-treated donors did not express CD40, CD80, or CD86 (Figure5, upper panel). Purified DC1 and DC2 were activated in vitro for 48 hours with GM-CSF, IL-3, and TNF-α. GM-CSF and IL-3 were chosen because they are essential for the survival of DC115,26,30 and DC2,27,28,30 respectively, and TNF-α was used to activate the expression of CD86 and antigen-presenting function.18 21 After activation, DC2 from G-CSF-treated donors up-regulated the expression of CD40, CD80, and CD86 (Figure 5, lower panel). When used to stimulate naive allogeneic CD4+CD45RA+ T cells, activated DC1 and DC2 were both able to stimulate T-cell proliferation, whether they were obtained from untreated or G-CSF-treated donors (Figures 4B to 4D).
Surface phenotype of fresh or activated DC2 from G-CSF-treated donors.
DC2 from G-CSF-treated donors were purified as shown in Figure 3. DC2 were tested for the expression of CD40, CD80, and CD86 either fresh (upper panels) or after 48 hours pre-activation in vitro by GM-CSF, IL-3, and TNF-α (lower panels). IL-3Rα (CD123) expression confirms that the DC2 population was not contaminated with other cell types that might have expanded during in vitro culture. Fluorescence signal of cells stained with the specific antibody is shown in solid black, whereas the background of cells stained with an isotype-matched antibody of irrelevant specificity is shown in white with a black contour.
Surface phenotype of fresh or activated DC2 from G-CSF-treated donors.
DC2 from G-CSF-treated donors were purified as shown in Figure 3. DC2 were tested for the expression of CD40, CD80, and CD86 either fresh (upper panels) or after 48 hours pre-activation in vitro by GM-CSF, IL-3, and TNF-α (lower panels). IL-3Rα (CD123) expression confirms that the DC2 population was not contaminated with other cell types that might have expanded during in vitro culture. Fluorescence signal of cells stained with the specific antibody is shown in solid black, whereas the background of cells stained with an isotype-matched antibody of irrelevant specificity is shown in white with a black contour.
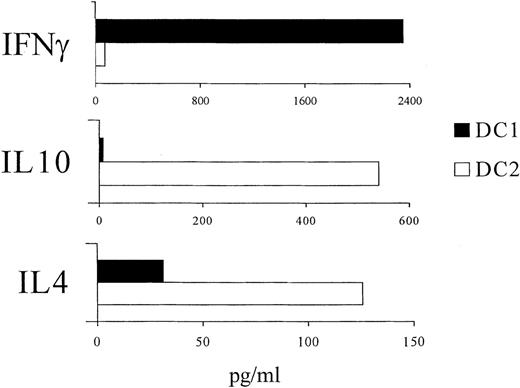
Th2 polarization of naive allogeneic T cells stimulated by activated DC2
To assess Th1 versus Th2 polarization, CD4+CD45RA+ naive T cells were cultured with activated DC1 or DC2 from G-CSF-treated allogeneic donors and restimulated with PMA plus ionomycin to release cytokines in the supernatant. T cells primed by DC1 produced predominantly IFN-γ whereas T cells primed by DC2 produced predominantly IL-10 and IL-4 (Figure 6). T cells primed by DC from normal donors showed the same pattern of cytokine production, depending on the DC subset (not shown). Thus, in G-CSF-treated and normal donors, activated DC1 induced naive allogeneic T cells to differentiate into IFN-γ-producing effector Th1 cells, whereas activated DC2 induced naive allogeneic T cells to differentiate into IL-4 and IL-10-producing effector Th2 cells.
Cytokine secreted by CD4+/CD45RA+ T cells after stimulation with activated, allogeneic DC1 or DC2.
Allogeneic DC1 or DC2 were purified from G-CSF-treated donors, as shown in Figure 3, and activated for 48 hours in vitro with GM-CSF, IL-3, and TNF-α. CD4+CD45RA+ naive T cells were purified by immunomagnetic beads, as described in “Materials and Methods.” Purified CD4+/CD45RA+ T cells were stimulated by pre-activated DC1 or DC2 for 6 days. Then cells were restimulated with PMA plus ionomycin. Supernatants were harvested after 48 hours and frozen until analyzed by ELISA for the presence of IFN-γ, IL-10, and IL-4. Results are representative of 3 identical experiments showing similar results.
Cytokine secreted by CD4+/CD45RA+ T cells after stimulation with activated, allogeneic DC1 or DC2.
Allogeneic DC1 or DC2 were purified from G-CSF-treated donors, as shown in Figure 3, and activated for 48 hours in vitro with GM-CSF, IL-3, and TNF-α. CD4+CD45RA+ naive T cells were purified by immunomagnetic beads, as described in “Materials and Methods.” Purified CD4+/CD45RA+ T cells were stimulated by pre-activated DC1 or DC2 for 6 days. Then cells were restimulated with PMA plus ionomycin. Supernatants were harvested after 48 hours and frozen until analyzed by ELISA for the presence of IFN-γ, IL-10, and IL-4. Results are representative of 3 identical experiments showing similar results.
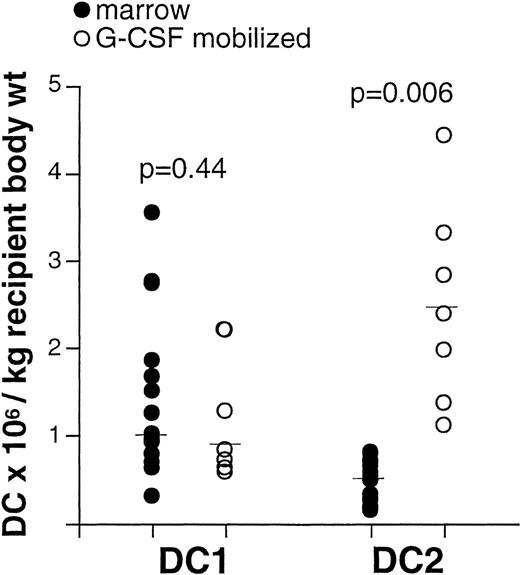
Recipients of unmodified blood stem cell products from G-CSF–treated donors receive more DC2 than recipients of unmodified marrow products
DC were identified in normal marrow by their lineage−, HLA-DR+, CD4+, and CD11c+ (DC1) or IL-3Rα+ (DC2) phenotype (not shown). We determined the count of DC1 and DC2 in a series of samples from either marrow or blood stem cell products from G-CSF-treated donors, collected for the purpose of allogeneic transplantation. The dose of total nucleated cells received by recipients of blood stem cells (median, 743.8 × 106/kg body weight) (n = 7) was higher than for recipients of marrow transplants (median, 256.1 × 106/kg body weight) (n = 15). The DC1 dose did not differ between recipients of marrow and blood stem cells (Figure 7). In contrast, the dose of DC2 received by recipients of blood stem cells from G-CSF-treated donors (median, 2.4 × 106/kg body weight, n = 7) was higher than the dose received by recipients of marrow transplants (median, 0.5 × 106/kg body weight, n = 15) (P = .006; Figure 7). Thus, in this study, recipients of G-CSF-mobilized blood stem cells received more Th2-inducing antigen-presenting cells than recipients of marrow transplants.
Recipients of unmodified stem cell products from G-CSF-treated donors receive more DC2 than recipients of unmodified marrow.
Sample aliquots were obtained from either marrow (n = 15) or peripheral blood stem cells (n = 7) intended for allogeneic transplantation. After lysis of erythrocytes, cells were labeled with FITC-conjugated antibodies to lineage markers CD3, CD14, CD16, CD19, CD20, CD34, CD56, IgM, anti-HLA-DR PerCP, and either anti-- CD11c or anti-IL-3Rα PE for the identification of DC1 and DC2, respectively. Absolute DC counts were determined as described in “Materials and methods.” Each symbol represents a single sample. Median values are represented by horizontal lines in each series. P values were determined using t tests for independent samples.
Recipients of unmodified stem cell products from G-CSF-treated donors receive more DC2 than recipients of unmodified marrow.
Sample aliquots were obtained from either marrow (n = 15) or peripheral blood stem cells (n = 7) intended for allogeneic transplantation. After lysis of erythrocytes, cells were labeled with FITC-conjugated antibodies to lineage markers CD3, CD14, CD16, CD19, CD20, CD34, CD56, IgM, anti-HLA-DR PerCP, and either anti-- CD11c or anti-IL-3Rα PE for the identification of DC1 and DC2, respectively. Absolute DC counts were determined as described in “Materials and methods.” Each symbol represents a single sample. Median values are represented by horizontal lines in each series. P values were determined using t tests for independent samples.
Discussion
Antigen-specific T-cell responses are characterized by distinct profiles of secreted cytokines. Here we have confirmed recent findings by Rissoan et al15 demonstrating that polarization of the T-cell response into Th1 or Th2 depends on the type of antigen-presenting cell: in humans, CD11c+ DC1 induce Th1 responses, whereas IL-3Rα+ DC2 induce Th2 responses. We have used direct staining and multiple color microfluorometry to enumerate DC subsets in the peripheral blood and bone marrow of healthy persons. We have found that G-CSF treatment of normal stem cell donors selectively increases the number of DC2, but not DC1, in the blood and that PBSC used for allogeneic transplantation contain more DC2 than marrow but a similar number of DC1.
G-CSF may increase the number of DC2 in the circulation by either stimulating their production in the marrow, increasing survival, inducing mobilization, or decreasing migration out of the vascular space and recruitment into lymphoid organs. It is unknown whether G-CSF has a direct effect on maturation of DC2 from their CD34+/IL-3Rα+ precursors. It is established, however, that G-CSF can mobilize several types of leukocytes, including CD34+ progenitors, from the marrow into the peripheral blood, possibly by down-regulating expression of the α4β1 integrin.33 Therefore, it is conceivable that G-CSF also facilitates the physiological migration of DC2 from the marrow, through the blood, into peripheral lymphoid organs, where DC2 present antigenic peptides to T cells.16
DC2 isolated from the blood of normal or G-CSF-treated donors are unable to activate proliferation of naive allogeneic T cells until after ex vivo activation by CD40L or TNF-α.27,28 In contrast, DC1 freshly isolated from human blood can activate naive allogeneic T cells.24,30,31 Expression of costimulatory molecules CD40, CD80, and CD86 is relatively low or absent in circulating DC1 and DC2, so that there must be other differences in the state of differentiation or activation of DC1 and DC2 that account for the difference in their allo-stimulatory activity. It is possible that blood DC1 and DC2 differ in their T-cell activation potential because of their distinct roles in the immune response. DC1 capture microbial and environmental antigens in the periphery and migrate into the lymph nodes, where they activate T-cell immunity. Conversely, DC2 migrate to the lymph nodes during fetal life, before exposure to exogenous proteins. It has been proposed that DC2 might be responsible for maintaining peripheral T-cell tolerance to self-antigens.15 Therefore, inducing proliferation of antigen-specific T cells might be obligatory for DC1 but not DC2. During inflammatory or immune responses initiated by macrophages and DC1, however, the secretion of TNF-α and the expression of CD40L induce full activation of DC2. Under these circumstances, DC2 initiate the proliferation of antigen-specific T cells, leading to the expansion of Th2 clones and the secretion of IL-4 and IL-10. These cytokines produce a negative feedback on Th1 differentiation and terminate the immune and inflammatory responses.
Our findings that G-CSF induces mobilization of DC2 in humans are intriguing in view of the reports that the administration of G-CSF induces Th2 polarization of splenocytes in mice.12,13 It is conceivable that G-CSF might increase the availability of DC2 in peripheral lymphoid organs, leading to the presentation of self-peptides and Th2 polarization in the donor. In several experimental models, donor Th2 cells have a decreased potential to induce acute GVHD.12,13,34-37 It is also possible that donor DC2 contribute to the pathogenesis of GVHD after transfer to the recipient through the indirect presentation of host antigen to donor T cells and the induction of Th2 responses. Interestingly, umbilical cord blood, another source of allogeneic stem cells for transplantation associated with relatively low incidence of acute GVHD, contains DC2 but not DC1.38 Direct presentation of host alloantigens by resident host DC, however, is likely to exert a dominant effect on the activation of donor T cells, resulting in both acute and chronic GVHD.39
The role of DC on engraftment remains to be defined. Donor DC engraft and are functional in recipients of allogeneic transplants, and their persistence has been associated with prolonged organ transplant survival.40-44 There are no reports yet on the role of donor DC in the engraftment of hematopoietic stem cells, but one would expect that variation in the DC1-DC2 ratio in the graft could have important consequences on sensitization of the host and on engraftment outcome. Donor DC1 would activate allogeneic host T cells to produce IFN-γ and generate cytotoxic responses against the graft, favoring rejection. Donor DC2, instead, would induce host T cells to produce IL-4 and IL-10, which suppress Th1 and cytotoxic responses,14 favoring engraftment. Anti-donor Th2 responses are associated with prolonged survival of organ grafts in experimental models.45-47 Thus, DC2 hold a potential for use in cell therapy for the induction of tolerance to hematopoietic cells and solid organ transplants. The role of G-CSF in mobilizing DC2 could be exploited to collect DC2 for adoptive therapy.
Acknowledgments
We thank Dr Michael Loken for his expert assistance in the design of the 4-color flow microfluorometric assays and Jennifer Brackensick for typing the manuscript.
Supported by National Institutes of Health grants AI33484, AI37678, and CA18029.
Reprints not available from the author.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 4. Proliferative responses of naive allogeneic T cells to DC1 and DC2. / DC1 and DC2 from normal (A, B) or G-CSF-treated (C, D) donors were purified as shown in Figure 3. Purified DC were used as stimulators either fresh (A, C) or after 48 hours pre-activation in vitro by GM-CSF, IL-3, and TNF-α (B, D). Unfractionated PBMC were used as control stimulators. CD4+/CD45RA+ naive T cells were purified by immunomagnetic beads, as described in “Materials and methods.” Autologous or allogeneic, purified CD4+/CD45RA+ T cells were used as responders in a 6-day culture assay. T-cell proliferation was measured by 3H]TdR incorporation. Proliferation of autologous T cells was always less than 500 cpm when cocultured with fresh DC (n = 2) and less than 2000 cpm when cocultured with activated DC (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2484/4/m_bloo00801004x.jpeg?Expires=1767732251&Signature=pv2YKVuWEh5lU-GltRYe5dFfokxB8V9tVJuVYseP41JOSRiWah3notVvkdLQKaw1AabIzdHLfAkGCnQmT7kRoWq9rXv8GbJPWLbORx3w06UkEf6RK4S4mgcVr2purNXMWsswexlG7j1XPCnpqm0bRUFu9TaEVYchkJfJgvXljOI4Sdr39kRW~B7eKoQ45RLC6JOBYZVB1uvVpxPjH0iVf5L-Mb3Cwov6Qanb7Z~oVhyg3UlWSlq3ZiEQi~Yozu~jgxUGrh8f0Hi-bGlA-U2szYvYTqu4DyWHIEKZayYoxL1RI48oS5Uu8EKZxLlpdztxj3rb13kdkm6J-76SN23O7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal