Abstract
Gender differences in vascular thromboses are well known, and there is evidence that platelets may be involved in these differences and that sex hormones affect platelet function. We characterized the expression of the estrogen receptor (ER ), estrogen receptor β (ER β), progesterone receptor (PR), and androgen receptor (AR) in the megakaryocyte lineage. Megakaryocytes generated ex vivo from normal human CD34+ stem cells contained RNA for ER β and AR, which increased with cell differentiation. Platelets and human erythroleukemia (HEL) cells also contained ER β and AR transcripts. No ER or PR messenger RNA or protein was detected in the megakaryocyte lineage. Immunofluorescence microscopy showed that ER β protein was present in glycoprotein (GP) IIb+ megakaryocytes and the HEL megakaryocytic cell line in a predominantly cytoplasmic location. AR showed a cytoplasmic and nuclear distribution in GPIIb+ and GPIIb− cells derived from CD34+ cells and in HEL cells. Western immunoblotting confirmed the presence of ER β and AR in platelets. Megakaryocyte and HEL AR expression was up-regulated by 1, 5, and 10 nmol/L testosterone, but down-regulated by 100 nmol/L testosterone. These findings indicate a regulated ability of megakaryocytes to respond to testosterone and suggest a potential mechanism through which sex hormones may mediate gender differences in platelet function and thrombotic diseases.
Thromboembolic complications of coronary and cerebral atherosclerotic diseases are the number 1 cause of death for both men and women in our society.1 Gender differences in the epidemiology of thromboembolic diseases have been described in a number of clinical settings. The incidence of cardiovascular disease is lower in premenopausal women than in men, but is increased for women after menopause,2 suggesting cardioprotective effects of estrogens. Observational studies led to the generally accepted belief that hormone replacement therapy (HRT) in postmenopausal women was beneficial, and one of the proposed mechanisms of the beneficial effects of HRT was the ability to lower low-density lipoprotein cholesterol and raise high-density lipoprotein cholesterol.3,4 However, recent data from the Heart and Estrogen/Progestin Replacement Study (HERS) indicated that HRT in postmenopausal women with known coronary artery disease produced no overall benefit, despite the expected improvement in serum cholesterol.5 These unexpected findings raise the possibility that HRT may have a biologic effect that counteracts its beneficial effect on lipids. Substantial evidence indicates that HRT predisposes to venous thrombosis,6,7 but little information exists regarding a possible prothrombotic effect of HRT on platelets in the coronary circulation, where high shear forces exert an especially important effect on the generation of platelet thrombi.8
There is both indirect and direct evidence that sex hormones affect human platelet biology. For example, several physiologic properties of platelets from women have been shown to vary with the phase of the menstrual cycle. We have found that platelets from women bound more fibrinogen during the luteal phase (defined as 14 or fewer days from the onset of the next menstrual cycle, or the second half of the menstrual cycle) of the menstrual cycle than during the follicular phase, suggesting a hormonal regulation of glycoprotein (GP) IIb-IIIa activation.9 The number of α2-adrenergic receptors has been shown to peak at the onset of menses and to drop to 74% to 79% of that value during the middle of the cycle.10 Tarantino et al11 observed that platelet adhesion to type I collagen showed a biphasic periodicity during the menstrual cycle. These studies suggest estrogen and/or progesterone regulate platelet activation and function. Estrogen- and androgen-responsive genes, such as nitric oxide synthase (an inhibitor of platelet aggregation), superoxide dismutase, gp130, and thromboxane A2, are present in megakaryocytes and/or platelets,12-15 but little is known about the existence or function of estrogen or androgen receptors in this cell lineage. In vitro human platelet aggregation induced by arachidonic acid is enhanced by androgens,16 and androgen therapy has improved platelet counts in patients with myelodysplasia and thrombocytopenia.17 Platelet production is also reported to respond to hormonal manipulation in mice, where castration decreases thrombocytopoiesis and testosterone restores platelet production.18 Thus, although there is a substantial body of evidence showing sex hormones affect megakaryocytes and/or platelet physiology, the mechanism of action is not clear.
To explore the possibility that gender differences in platelet function are due to sex-hormone–mediated genomic or nongenomic events, we investigated whether the megakaryocyte/platelet lineage contains receptors for sex hormones. We found that megakaryocytes and platelets express the estrogen receptor (ER) β and the androgen receptor (AR), and that AR expression is regulated by testosterone. Thus, genomic effects in megakaryocytes and/or signaling properties in platelets could contribute to the known gender differences in platelet function.
Methods and materials
Reagents
Apyrase was the generous gift of Dennis Perry (McMaster University, Hamilton, Ontario, Canada). Bovine serum albumin (BSA), prostaglandin E1 (PGE1), prostaglandin I2 (PGI2), normal goat serum, charcoal, and dextran were obtained from Sigma (St Louis, MO). RPMI 1640, Ham's F12K medium, Stem Pro culture media, 10 × HEPES buffer, and bovine insulin were from GibcoBRL (Gaithersburg, MD). Heat-inactivated fetal bovine serum (FBS) and penicillin/streptomycin solution were from Gemini Bio-Product (Calabasas, CA). Pegylated recombinant human megakaryocytic growth and differentiation factor (PEG-rhMGDF) was a gift of Amgen (Thousand Oaks, CA). Mouse and rabbit immunoglobin (Ig)–G were purchased from Pierce (Rockford, IL). Restriction enzymes were purchased from New England Biolabs (Beverly, MA). General laboratory reagents were obtained from either Sigma or Baker Scientific (Bridgeport, NJ).
Human subjects
Informed consent was obtained from all volunteers and patients. All studies were approved by the Johns Hopkins University School of Medicine Institutional Review Board and were conducted according to the principles of the Helsinki Declaration.
Enrichment of CD34+ cells from leukopheresis units or bone marrow
To obtain stem cells from leukopheresis units, low-density mononuclear cells from normal donors were first separated by centrifugation over HISTOPAQUE 1077 (Sigma) according to the manufacturer's suggested protocol. Mononuclear cells were washed twice in 1% BSA in phosphate-buffered saline (PBS) (pH = 7.4, 0.137 mol/L NaCl, 4.3 mmol/L Na2HPO4, 1.4 mmol/L KH2PO4, 2.7 mmol/L KCl) without Ca+2 and Mg+2. Cells were incubated with biotinylated anti-CD34+ antibody (gift from the Johns Hopkins Oncology Center, Baltimore, MD) for 30 minutes and washed, and then CD34+cells were isolated from the mononuclear cell fraction by passage over an avidin column (CellPro, Bothell, WA). In a second protocol, donor bone marrow for allogeneic transplantation was processed by our clinical Graft Engineering Laboratory (Johns Hopkins University, Baltimore, MD). Small mononuclear cells were separated from marrow by clinical elutriation centrifugation. This lymphoid-rich fraction was labeled with biotinylated anti-CD34 antibody and passed over a CEPRATE column (CellPro). The CD34-depleted fraction was washed, relabeled with biotinylated anti-CD34 antibody, washed again, and passed over an avidin column as previously described for leukopheresis products. Yields from the 2 preparation techniques were as follows: leukopheresis, 0.8 to 1.2 × 106 CD34+cells from 1 × 109 mononuclear cells; CD34-depleted bone marrow fraction, 3 to 7 × 106CD34+ cells from 1 × 109 mononuclear cells. CD34+ cells were resuspended in Stem Pro culture media without phenol red and without serum, cultured with 1 ng/mL IL-3 for 2 days, and immediately harvested (termed day-0 cells) or treated with 50 ng/mL PEG-rhMGDF for 7 days (termed day-7 cells). Approximately 25% of the day-7 cells were megakaryocytes as assessed by flow cytometry with the use of the megakaryocyte-specific marker GPIb or GPIIb.
Cell lines and cell culture
The human erythroleukemia (HEL) and Dami megakaryocytic cell lines were obtained from the American Type Culture Collections (ATCC; Rockville, MD). The Dami cells were obtained from the ATCC in 1989 and demonstrate greater GPIIIa (integrin β3) expression than do our HEL cells, despite their likelihood of being a subclone of HEL. PC3 and LNCap, both human prostate cancer cell lines, were gifts from Dr John Isaacs, and T47D, a human breast cancer cell line19 was a gift from Dr Saraswati Sukumar (both from Johns Hopkins University). These cell lines were used as controls for ER α, ER β, the progesterone receptor (PR), and AR. HEL and Dami cells were grown in RPMI 1640 containing 10% serum and 1% penicillin/streptomycin. T47D cells were grown in RPMI 1640 containing 10% serum, 1% penicillin/streptomycin, and 0.2 IU/mL insulin. PC3 cells were cultured in Ham's F12K media containing 10% FBS and 1% penicillin/streptomycin with 2 mmol/L L-glutamine, and 1.5 g/L sodium bicarbonate. Depending on the experiment, cells were cultured in FBS (GibcoBRL) or charcoal-stripped FBS. The FBS was stripped with the use of a standard protocol (Sigma) containing 0.25% charcoal (vol/vol) and 0.0025% dextran (vol/vol) in 10 mmol/L HEPES (pH 7.4).
Platelet preparation
Whole blood was obtained into acid-citrate-dextrose (0.1 mol/L trisodium citrate, 0.11 mol/L dextrose, and 71 mmol/L citric acid monohydrate) anticoagulant with the use of a 19-gauge needle and platelet-rich plasma (PRP), prepared as previously described.20 The top, middle, and bottom one third (by volume) of the PRP were designated PRP-upper, PRP-middle, and PRP-lower, respectively. Total red blood cells, white blood cells, and platelets were counted. Platelets were obtained by centrifugation of PRP at 800g for 20 minutes. Gel-filtered platelets were also prepared from PRP obtained as described previously. Then, 7.5 μmol/L PGI2 in 1:50 dilution and freshly prepared apyrase (20 μL/mL) were added, and the PRP was centrifuged at 800g for 20 minutes. The platelet pellet was resuspended in 1 mL buffer (138 mmol/L NaCl, 12 mmol/L NaHCO3, 10 mmol/L KCl, 5.5 mmol/L glucose, 0.36 mmol/L Na2HPO4, 0.35% BSA, and 10 mmol/L HEPES, pH 7.4) containing apyrase and PGI2. Platelets were purified over a Sepharose CL-2B (Pharmacia, NJ) column. The eluate possessed normal adenosine-diphosphate–inducible aggregation in the presence of fibrinogen.
Reverse transcription–polymerase chain reaction
Total RNA was prepared from different cell samples with the use of a single-step guanidine thiocyanate/phenol kit (RNA STAT-60; Tel-Test, Friendswood, TX). One microgram of total RNA was reverse-transcribed in a 20-μL reaction mixture containing 50 mmol/L KCl, 10 mmol/L Tris-HCl; pH 8.3, 4 mmol/L MgCl2, 1 mmol/L dNTPs (Pharmacia), 100 pmol random hexanucleotides (Pharmacia), 15 units of ribonuclease inhibitor (Gibco BRL), and 220 units M-MLV reverse transcriptase (Gibco BRL) at 37°C for 1 hour. Polymerase chain reaction (PCR) conditions varied slightly according to primers, but the ranges were as follows: 30 to 35 cycles of 1 minute at 94°C, 30 to 60 seconds at 52°C to 60°C, and 30 to 60 minutes at 72°C. Then, 1× PCR buffer (20 mmol/L Tris-HCl, pH 8.0, 50 mmol/L KCl) was used in the presence of 200 mmol/L deoxynucleotide-triphosphates (dNTPs), and 2.0 mmol/L MgCl2, 10 pmol of each primer per reaction, and 2.5 units of Gibco Taq polymerase. Then, 10 μL of the PCR reactions was separated on 2% agarose gels and visualized by ethidium bromide staining. All reverse transcription (RT) – PCR analyses were confirmed at least twice in separate experiments.
The PCR primers for the ER α were as follows: 5′-CAG GGG TGA AGT GGG GTC TGC TG-3′ (sense) (priming site in exon 4, nucleotides 1060 to 1083 as numbered by Green et al21); 5′-ATG CGG AAC CGA GAT GAT GTA GC-3′ (antisense) (priming site in exon 6, nucleotides 1520 to 1543). These primers yield a product of 247 base pairs (bp). The primers for the ER β were as follows: 5′-ATC TTT GAC ATG CTC CTG GC-3′ (sense) (priming site at nucleotides +985 to +1005 as numbered by Mosselman et al22); 5′-ACG CTT CAG CTT GTG ACC TC-3′ (antisense). These primers yield a product of 515 bp. An optimized PCR buffer was used for these primers (1×: 80 mmol/L Tris-HCl, pH 9.0, 20 mmol/L [NH4]2SO4). The primers for the PR were as follows: 5′-AAG GAG GGC CTG CCG CAG GTC TAC-3′ (sense) (priming site in exon 1; nucleotides +1591 to +1614 with respect to starting ATG codon as numbered by Misrahi et al23); 5′-GAA CGC CCA CTG GCT GTG GGA GAG-3′ (antisense) (priming site in exon 4). These primers yield a product of 247 bp. The primers for the AR were as follows: 5′-CAG ATG GCT GTC ATT CAG TAC TC-3′ (sense) (priming site at nucleotides +2559 to +2581 as numbered by Lubahn et al24); 5′-TGC TGA AGA GTA GCA GTG CTT TC-3′ (antisense). These primers yield a product of 247 bp. The primers for the human β actin were as follows: 5′-TAC CTC ATG AAG ATC CTC A-3′ (sense) (priming site at nucleotides 2227 to 2245 as numbered by Nakajima- Iijima et al25); 5′-TTC GTG GAT GCC ACA GGA C-3′ (antisense). These primers yield a product of 247 bp. There was no sequence similarity among these primers and they are specific for their corresponding complementary DNA (cDNA).
Immunostaining and fluorescence microscopy
Cells were washed once in PBS containing 0.05% sodium azide (Sigma), cytofuged onto glass slides, fixed in 4% paraformaldehyde for 10 minutes at 4°C, washed 3 times in PBS, and washed twice in PBS with 50 mmol/L NH4Cl. Cells were permeabilized with 0.05% saponin in PBS containing 10% normal goat serum for 30 minutes at 22°C. The first primary antibody incubation was performed in PBS containing 10% normal goat serum and 0.05% saponin for 1 hour at 37°C followed by washing in PBS containing 0.05% saponin 3 times for 5 minutes each at 22°C. Cells were then incubated with the first fluorochrome-conjugated secondary antibody for 30 minutes at 37°C followed by washing 3 times in PBS containing 0.05% saponin for 5 minutes each at 22°C. The nucleic acid staining dye Dapi (10 mg/mL) was diluted in the preparatory solution A of Slowfade Antifade kit (Molecular Probes, Eugene, OR) in 1:100 dilution. After an initial primary and secondary antibody staining, the procedure was repeated for the second primary and secondary antibody staining, and the slides were then mounted in the preparatory solution A containing Dapi. Each fluorochrome was analyzed individually by means of an inverted confocal laser scanning Fluorescence Microscope (LSM; Zeiss, Germany).
We used 3 primary antibodies in dual color immunofluorescence confocal microscopy: (1) SZ22, a mouse monoclonal antibody specific for human platelet GPIIb (integrin αIIb)26 (Immunotech, Westbrook, ME), was used at 20 μg/mL; (2) C-19, a rabbit polyclonal IgG specific for a peptide of the human AR (Santa Cruz Biotechnology, Santa Cruz, CA), was used at 4 μg/mL; and (3) purified rabbit antiserum to human ER β (Alexis Corporation, San Diego, CA) was used at 20 μg/mL. Secondary antibodies were a 1:125 dilution of fluorescein-isothiocyanate–conjugated goat antimouse (Jackson ImmunoResearch Laboratories, West Grove, PA) and a 1:100 dilution of Rhodamine Red X-conjugated goat antirabbit (Jackson ImmunoResearch Laboratories).
Western immunoblotting
Immunoblotting was performed as previously described.26Cells were lysed in 15 mmol/L Hepes, pH 7.0, 145 mmol/L NaCl, 0.1 mmol/L MgCl2, 10 mmol/L ethylene glycol-bis-N,N,N′,N′-tetraacetic acid (EGTA), 1% Triton X-100, 1 mmol/L NaVO4, 250 μg/mL 4-2-aminoethyl-benzene sulfonylfluoride, 15 μg/mL of protease inhibitors (chymostatin, antipain, and pepstatin), and 55 μg/mL of the protease inhibitor leupeptin. Protein concentration was determined by the Bradford technique. For AR analysis, the lysing detergent was 2% sodium dodecyl sulfate (SDS). Lysates were separated by 8% SDS–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Amersham, Buckinghamshire, England). A series of polyclonal antisera were used to probe for ER β: L-20 and N-19 (Santa Cruz Biotechnology, Inc), anti–ER β (Upstate Biotechnology, Lake Placid, NY), and Ab-1 (Oncogene Research Products, Cambridge, MA). A series of polyclonal antisera were used to probe for AR: PG-21 (Upstate Biotechnology), C19 and N-20 (Santa Cruz Biotechnology), and NCL-ARp (Vector Laboratories, Burlingame, CA). Numerous experiments indicated that the anti–ER β from Upstate Biotechnology, and PG21 yielded the best results. Blocking peptides from the amino termini of ER β and AR were purchased from Upstate Biotechnology, and Santa Cruz Biotechnology, respectively. The specificity of fragments detected by immunoblot was assessed by preincubating the primary antibody with the various peptides. For 10 μg/mL of antibody, 35 to 70 μmol/L peptide was used.
Radioimmunoassay
Total testosterone was measured with the use of the Coat-A-Count Kit (Diagnostic Products, Los Angeles, CA) according to the manufacturer's protocol. Testosterone levels in charcoal-stripped FBS were undetectable (lower limit of detection is 0.14 nmol/L) and in FBS was 3.5 nmol/L.
Results
Identification of RNA transcripts for sex hormone receptors
Specific nuclear and cytoplasmic receptors mediate the biologic effects of sex hormones. We surveyed the megakaryocyte/platelet lineage for the expression of ER α, ER β, PR, and AR. Using total RNA from various cell lines and oligonucleotide primers specific for each receptor cDNA, we probed for the respective transcripts by RT-PCR. T47D, a breast cancer cell line, served as positive control for all 4 receptors (Figure 1, lane 7) and demonstrated the ability of our primers to amplify the expected targets. ER β and AR transcripts were identified in day-0 CD34+ cells not treated with PEG-rhMGDF (lane 1) and appeared to increase after CD34+ cells were treated with PEG-rhMGDF for 7 days (lane 2). The ER β and AR RT-PCR products from the day-7 cells were cloned, and nucleotide sequencing confirmed them to be authentic ER β and AR transcripts (data not shown). Approximately 25% of the day-7 cells expressed the megakaryocyte-specific marker GPIIb, and many showed an increased cell size and a nucleus with several lobes, morphologic features typical of megakaryocytes (Figures 2 and3). ER α and PR messenger RNAs (mRNAs) were not observed in the day-0 or day-7 cells (Figure 1, lanes 1 and 2), or after longer exposure to PEG-rhMGDF (not shown). The absence of ER α and PR transcripts in CD34+ or CD34+-derived cells was not due to poor quality RNA, since β actin (Figure 1, bottom panel), ER β, and AR transcripts were readily amplified in all RNA preparations.
RT-PCR analysis of sex hormone receptors ER , ER β, PR, and AR in the megakaryocytic lineage.
Total RNA was reverse-transcribed and amplified by PCR with the use of primers specific for ER α, ER β, PR, AR, and β actin transcripts. All PCR primer pairs were separated by at least 1 intron to clearly identify products of cDNA amplification. The cells from which RNA was extracted were as follows: CD34+ cells prior to treatment with PEG-rhMGDF (D0 [day 0] CD34) (lane 1); CD34+ cells treated for 7 days with PEG-rhMGDF (D7 [day 7] CD34) (lane 2); gel-filtered platelets (lane 3); the PC3 prostate cancer cell line (lane 4); the Dami megakaryocytic cell line (lane 5); the HEL megakaryocytic cell line (lane 6); the T47D breast cancer cell line (lane 7). Lanes 8 and 9 contain products from PCRs using genomic DNA or no template, respectively. Lane 10 contains molecular weight marker (Φ × 174 DNA digested with HaeIII endonuclease). Sizes of PCR products are shown in “Materials and methods.”
RT-PCR analysis of sex hormone receptors ER , ER β, PR, and AR in the megakaryocytic lineage.
Total RNA was reverse-transcribed and amplified by PCR with the use of primers specific for ER α, ER β, PR, AR, and β actin transcripts. All PCR primer pairs were separated by at least 1 intron to clearly identify products of cDNA amplification. The cells from which RNA was extracted were as follows: CD34+ cells prior to treatment with PEG-rhMGDF (D0 [day 0] CD34) (lane 1); CD34+ cells treated for 7 days with PEG-rhMGDF (D7 [day 7] CD34) (lane 2); gel-filtered platelets (lane 3); the PC3 prostate cancer cell line (lane 4); the Dami megakaryocytic cell line (lane 5); the HEL megakaryocytic cell line (lane 6); the T47D breast cancer cell line (lane 7). Lanes 8 and 9 contain products from PCRs using genomic DNA or no template, respectively. Lane 10 contains molecular weight marker (Φ × 174 DNA digested with HaeIII endonuclease). Sizes of PCR products are shown in “Materials and methods.”
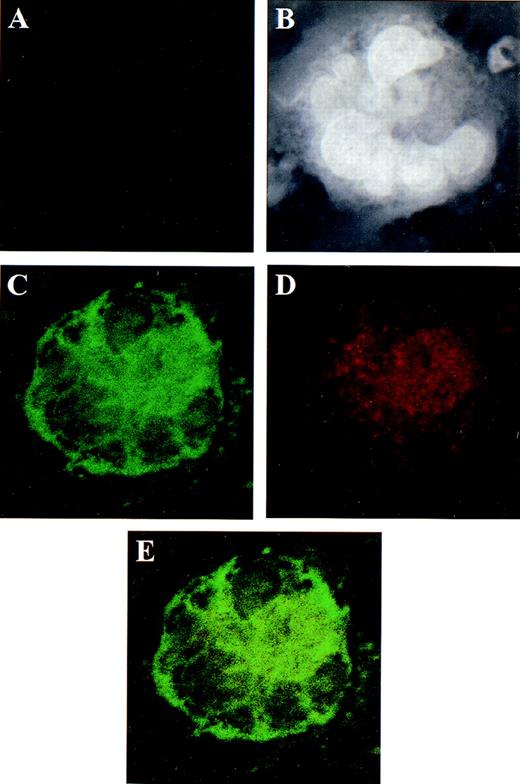
Localization of ER β in human megakaryocytes using dual color immunofluorescence microscopy.
CD34+ bone marrow cells, grown in serum-free and phenol-red–free medium, were treated for 7 days with PEG-rhMGDF and harvested for microscopy. (A) Negative control using mouse and rabbit IgGs as irrelevant primary antibodies. The same field is shown in panels B through E. (B) Dapi staining of nucleic acid. The polyploid nucleus is easily appreciated. (C) GPIIb detected by monoclonal antibody SZ22 (green). (D) ER β detected by specific rabbit polyclonal antisera (red). (E) The co-localization of GPIIb and ER β shown by summing the channels used in panels C and D. Although both GPIIb and ER β localize predominantly to the cytoplasm, the intensity of the GPIIb stain dominates in panel E to the extent that less yellow color is appreciated. Original magnification of panels B through E: ×1300.
Localization of ER β in human megakaryocytes using dual color immunofluorescence microscopy.
CD34+ bone marrow cells, grown in serum-free and phenol-red–free medium, were treated for 7 days with PEG-rhMGDF and harvested for microscopy. (A) Negative control using mouse and rabbit IgGs as irrelevant primary antibodies. The same field is shown in panels B through E. (B) Dapi staining of nucleic acid. The polyploid nucleus is easily appreciated. (C) GPIIb detected by monoclonal antibody SZ22 (green). (D) ER β detected by specific rabbit polyclonal antisera (red). (E) The co-localization of GPIIb and ER β shown by summing the channels used in panels C and D. Although both GPIIb and ER β localize predominantly to the cytoplasm, the intensity of the GPIIb stain dominates in panel E to the extent that less yellow color is appreciated. Original magnification of panels B through E: ×1300.
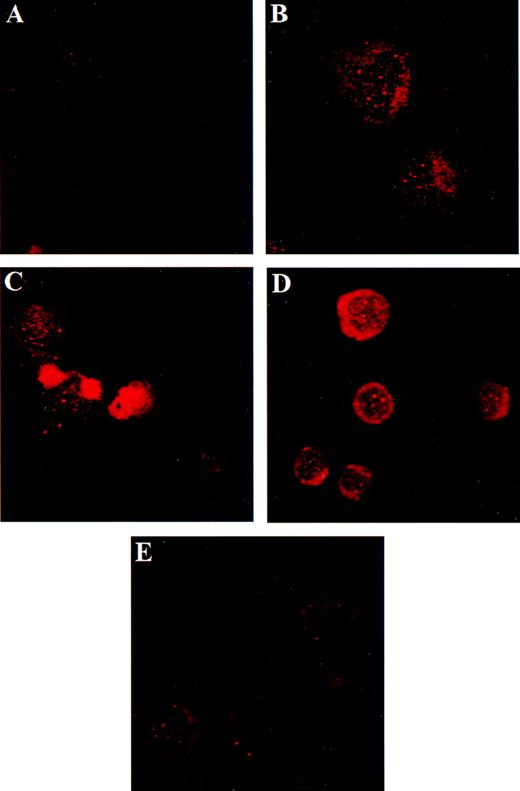
Localization of AR in human megakaryocytes using dual color immunofluorescence microscopy.
Similar to Figure 2 except that antisera specific for AR were used. (A) Negative control using mouse and rabbit IgGs as irrelevant primary antibodies. The same field of cells is shown in panels B through E. (B) Dapi staining of nucleic acid. (C) AR detected by specific rabbit polyclonal antisera C-19 (red). (D) GPIIb (green). (E) The co-localization of GPIIb and AR shown by summing the channels used in panels C and D. Panels F and G contain the same cell to emphasize the polyploid features of the megakaryocyte. (F) Dapi staining of nucleic acid. (G) Co-localization of GPIIb and the AR. Original magnification for panels B through E: ×1000. Original magnification for panels F and G: ×600.
Localization of AR in human megakaryocytes using dual color immunofluorescence microscopy.
Similar to Figure 2 except that antisera specific for AR were used. (A) Negative control using mouse and rabbit IgGs as irrelevant primary antibodies. The same field of cells is shown in panels B through E. (B) Dapi staining of nucleic acid. (C) AR detected by specific rabbit polyclonal antisera C-19 (red). (D) GPIIb (green). (E) The co-localization of GPIIb and AR shown by summing the channels used in panels C and D. Panels F and G contain the same cell to emphasize the polyploid features of the megakaryocyte. (F) Dapi staining of nucleic acid. (G) Co-localization of GPIIb and the AR. Original magnification for panels B through E: ×1000. Original magnification for panels F and G: ×600.
We studied mRNA obtained from platelets prepared by density centrifugation and by gel filtration. The former was contaminated by leukocytes, while the latter had no leukocytes (Table1). Like the cells of bone-marrow origin, gel-filtered platelets also contained ER β and AR, but not ER α or PR transcripts (Figure 1, lane 3). The upper two thirds of platelet-rich plasma corroborated these results, but the lower third reproducibly did not show ER β or AR transcripts (data not shown). Whether this discrepancy was due to substantial leukocyte contamination and a relative lack of platelet mRNA in the lower third of the PRP or to a true difference in platelets of different density is not clear. The megakaryocytic cell lines Dami and HEL also contained transcripts for ER β and AR, but not ER α or PR (Figure 1, lanes 5 and 6). The PC3 prostate cancer cell line is known to lack the AR,27but there are no prior reports as to which ER it contains (Figure 1, lane 4). These data support the presence of ER β and AR transcripts, but not ER α or PR transcripts, in the megakaryocytic lineage.
Cell counts on different platelet preparations*
| Preparation . | Platelet count per mm3 . | White blood cell count per mm3 . | Red blood cell count per mm3 . | Volume used to make total RNA . |
|---|---|---|---|---|
| PRP-upper | 500 000 | 550 | 0 | 6 mL |
| PRP-middle | 398 000 | 330 | 0 | 3 mL |
| PRP-lower | 17 500 | 18 800 | 2 950 000 | ∼3 mL |
| Gel filtered | 235 500 | 0 | 0 | ∼3 mL |
| Preparation . | Platelet count per mm3 . | White blood cell count per mm3 . | Red blood cell count per mm3 . | Volume used to make total RNA . |
|---|---|---|---|---|
| PRP-upper | 500 000 | 550 | 0 | 6 mL |
| PRP-middle | 398 000 | 330 | 0 | 3 mL |
| PRP-lower | 17 500 | 18 800 | 2 950 000 | ∼3 mL |
| Gel filtered | 235 500 | 0 | 0 | ∼3 mL |
This procedure was repeated on several occasions to confirm the absence of red blood cells in the upper and middle platelet-rich plasma (PRP) fractions.
Localization and distribution of the estrogen receptor β and androgen receptor in the cultured CD34+ cells
Because the day-7 CD34+-derived cells contained a heterogeneous population of cells (ie, both megakaryocytes and nonmegakaryocytic hematopoietic cells), we could not be certain of the cell type that gave rise to the transcripts observed in Figure 1. We used dual color immunofluorescence confocal microscopy with antibodies specific for ER β, AR, and the megakaryocyte-specific marker GPIIb to assess protein expression in cells of CD34+ origin that had been treated with PEG-rhMGDF for 7 days. ER β was present in GPIIb+ cells in a predominantly cytoplasmic location (Figures 2D and 2E). Similarly, intense staining for AR was seen in GPIIb+ day-7 cells (Figures 3C, 3E, and 3G). The identity of the GPIIb-negative, AR-positive cells is unknown, but such cells were also observed in the day-0 CD34+ cells (not shown), suggesting AR expression occurs early in hematopoiesis. AR showed primarily a nuclear distribution under these conditions. In both Figures 2 and 3, the same microscopic field is shown in each panel to demonstrate co-localization of the megakaryocyte-specific marker and the ER β and AR. Specificity of staining is demonstrated by the lack of fluorescence with the use of control mouse and rabbit IgG (Figures2A and 3A). We also observed ER β and AR in GPIIb-expressing HEL cells, a transformed cell line with megakaryocytic properties (data not shown). Thus, the expression of ER β and AR protein correlated with the RNA data.
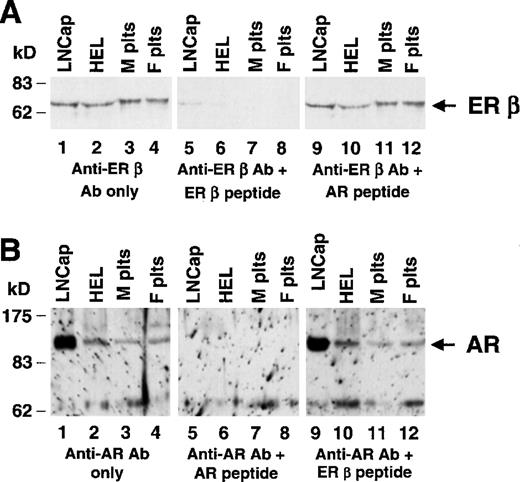
Expression of the estrogen receptor β and androgen receptor in human platelets
Members of the family of steroid receptors have been shown to possess nongenomic activity,28,29 and we sought to determine whether the corresponding protein was present in platelets that are anucleate. Immunofluorescence microscopy identified the AR in normal human platelets from men and women, but we were not able to observe ER β in platelets of either sex by this technique (not shown). The anti–ER β antisera used in the immunofluorescence analyses do not work on Western blotting (according to the manufacturer), and perhaps they were less efficient in detecting their target under the conditions of our platelet immunofluorescence experiments. However, Western blot analysis showed both ER β and AR protein in human platelets (Figure 4). Specificity for both platelet ER β and AR was demonstrated by the ability of the appropriate peptide to block antibody binding (Figure 4, lanes 5-8), while an irrelevant peptide did not block antibody binding (Figure 4, lanes 9-12). LNCap and HEL cells contained the expected approximately 65-kd ER β and approximately 110-kd AR proteins. Figure 4A suggests that ER β in platelets may be slightly larger than in HEL and LNCap cells. A second preparation of anti–ER β antisera also suggested platelet ER β was larger in platelets (not shown). To some extent, these studies were limited by reagents (see “Discussion”), but perhaps platelet ER β has alternately spliced mRNAs or posttranslation modifications. In data not shown, a different anti–AR antibody bound to the same 110-kd band on Western blot, providing further evidence that this polypeptide in platelet and HEL cells is the authentic AR. We suspect the signal at approximately 62 kd may represent a proteolytic fragment of AR, which has been repeatedly observed with AR from prostatic cells,30-32 since we observed almost exclusively the approximately 62-kd fragment until lysing cells in an SDS buffer.
Identification of the AR and ER β in normal human platelets.
Lysates from human cell lines or normal human platelets were analyzed by immunoblotting with polyclonal antisera specific for ER β (panel A) and for the AR (panel B). Total lysates were made from the prostate carcinoma cell line LNCap (lane 1), HEL (lane 2), normal male platelets (lane 3), and normal female platelets (lane 4). For both panels A and B, the same molarity of peptide was used in lanes 5-8 as in lanes 9-12, and the filter shown in lanes 5-8 was stripped and reprobed and shown to contain ER β and AR, respectively (not shown). (A) In all lanes, 40 μg of protein lysates were loaded. Filters were probed with a 1.5 μg/mL of anti-ER β (Upstate Biotechnology, Inc) without (lanes 1-4) or with (lanes 5-12) the indicated competing peptide. (B) Twenty micrograms of LNCap and HEL cell lysates and 40 μg of platelet lysates were electrophoresed. Filters were probed with 0.15 μg/mL PG-21 (Upstate Biotechnology, Inc) without (lanes 1-4) or with (lanes 5-12) the indicated competing peptide, as described in “Materials and methods.”
Identification of the AR and ER β in normal human platelets.
Lysates from human cell lines or normal human platelets were analyzed by immunoblotting with polyclonal antisera specific for ER β (panel A) and for the AR (panel B). Total lysates were made from the prostate carcinoma cell line LNCap (lane 1), HEL (lane 2), normal male platelets (lane 3), and normal female platelets (lane 4). For both panels A and B, the same molarity of peptide was used in lanes 5-8 as in lanes 9-12, and the filter shown in lanes 5-8 was stripped and reprobed and shown to contain ER β and AR, respectively (not shown). (A) In all lanes, 40 μg of protein lysates were loaded. Filters were probed with a 1.5 μg/mL of anti-ER β (Upstate Biotechnology, Inc) without (lanes 1-4) or with (lanes 5-12) the indicated competing peptide. (B) Twenty micrograms of LNCap and HEL cell lysates and 40 μg of platelet lysates were electrophoresed. Filters were probed with 0.15 μg/mL PG-21 (Upstate Biotechnology, Inc) without (lanes 1-4) or with (lanes 5-12) the indicated competing peptide, as described in “Materials and methods.”
Ex vivo effect of sex hormones
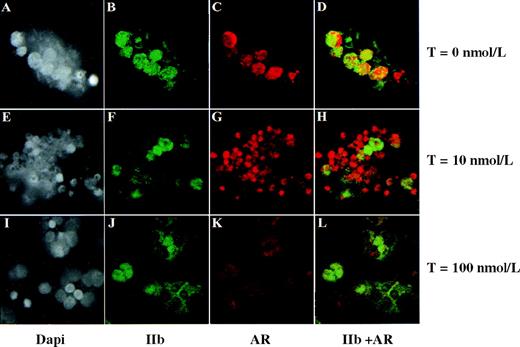
Sex hormones can autoregulate their corresponding receptors,33,34 often in a complex fashion. In these next studies, we wanted to determine whether sex hormones affected receptor expression in HEL cells. Using estradiol, we observed no changes in ER β expression (data not shown). However, as shown in Figure5, HEL-cell AR expression varied according to the testosterone exposure, with an increase in AR at 1, 5, and 10 nmol/L testosterone (compare Figure 5B-5D with Figure 5A, the no-testosterone control). However, AR expression was reduced when cells were treated with 100 nmol/L testosterone (Figure5E). The lack of AR induction in FBS containing 100 nmol/L testosterone (Figure 5E) was a consistent finding, suggesting that regulation of AR expression is not a linear function of testosterone concentration. A similar dose-response was seen when cells were cultured in charcoal-stripped FBS (data not shown). The reason for the punctate appearance of the AR seen in HEL cell nuclei is unknown, but in light of the numerous alternately spliced forms of the AR mRNA that have been reported, perhaps this represents the “speckle” or “coiled body” nuclear structures known to be rich in splicing factors.35,36 We further pursued these hormonal effects on AR expression using ex vivo–generated megakaryocytes. As with HEL cells, we observed prominent AR expression in megakaryocytes treated with 10 nmol/L testosterone (Figure 6G) and a reduction in AR expression with 100 nmol/L testosterone (Figure 6K compared with Figures 6C and 6G). The difference in AR expression between HEL cells and megakaryocytes in response to no testosterone (Figure 5A versus Figure 6C) is most likely due to the persistent inhibitory effects of ethanol on AR expression37 at the 48-hour time point (Figure 5) versus loss of ethanol via evaporation by 13 days (Figure 6).
Testosterone modulates AR expression in HEL cells.
HEL cells were grown for 48 hours in 10% FBS, with or without testosterone, and stained for AR as described above. Cells were incubated with 0 nmol/L (panel A), 1 nmol/L (panel B), 5 nmol/L (panel C), 10 nmol/L (panel D), and 100 nmol/L (panel E) testosterone. Testosterone requires ethanol to go into suspension, and all experiments had ethanol at a final concentration of 0.001%. The reduction in AR in cells treated with ethanol alone (panel A) is expected.37 Original range of magnifications: ×800 to ×2400.
Testosterone modulates AR expression in HEL cells.
HEL cells were grown for 48 hours in 10% FBS, with or without testosterone, and stained for AR as described above. Cells were incubated with 0 nmol/L (panel A), 1 nmol/L (panel B), 5 nmol/L (panel C), 10 nmol/L (panel D), and 100 nmol/L (panel E) testosterone. Testosterone requires ethanol to go into suspension, and all experiments had ethanol at a final concentration of 0.001%. The reduction in AR in cells treated with ethanol alone (panel A) is expected.37 Original range of magnifications: ×800 to ×2400.
Testosterone modulates AR expression in megakaryocytes.
CD34+ cells, grown in serum-free and phenol-red–free medium, were cultured in PEG-rhMGDF and 0 nmol/L, 10 nmol/L, or 100 nmol/L testosterone (T) as indicated for 13 days. Each experiment had a final concentration of 0.01% ethanol. Each row represents the same field of cells. Only approximately 10% of the total population of CD34-derived cells were negative for AR, suggesting that AR expression is widespread among CD34-derived cells. Staining with Dapi, GPIIb, and AR were as described in Materials aand methods. Original range of magnification for panels A through J: ×1200 to ×1400.
Testosterone modulates AR expression in megakaryocytes.
CD34+ cells, grown in serum-free and phenol-red–free medium, were cultured in PEG-rhMGDF and 0 nmol/L, 10 nmol/L, or 100 nmol/L testosterone (T) as indicated for 13 days. Each experiment had a final concentration of 0.01% ethanol. Each row represents the same field of cells. Only approximately 10% of the total population of CD34-derived cells were negative for AR, suggesting that AR expression is widespread among CD34-derived cells. Staining with Dapi, GPIIb, and AR were as described in Materials aand methods. Original range of magnification for panels A through J: ×1200 to ×1400.
Discussion
Although sex hormones were first reported to affect platelet function more than 25 years ago, little mechanistic data exist and no previous information has been available on either ER β or AR in the megakaryocyte lineage. The major findings in this study are the following: (1) ER β and AR can be identified in normal human megakaryocytes and platelets; (2) both ER β and AR transcripts are up-regulated during megakaryocyte differentiation; and (3) megakaryocytic AR expression is regulated by hormonal manipulation. Although the downstream effects of ER β and AR in these cells are unknown, potential genomic effects in megakaryocytes and/or signaling properties in platelets may contribute to the known gender differences in platelet function and vascular disease.
A survey of the megakaryocyte/platelet lineage for receptors for estrogen, progesterone, and testosterone was performed, and transcripts for ER β and AR were identified. No transcripts were detected for ER α or PR in the megakaryocytic lineage, although we cannot exclude the possibility of an alternately spliced ER α or PR mRNA not detected by our PCR primers. However, for ER α at least, no platelet protein was detected by Western immunoblotting (data not shown). Prior to the identification of ER β, Tarantino et al11 used monoclonal antibody H222 to show the presence of an ER in the S01 megakaryocytic cell line and in megakaryocytes, although no other markers of the megakaryocyte lineage were used. This antibody was raised against an ER purified from the MCF-7 breast cancer cell line38 that is now known to contain both ER α and ER β.39 Perhaps there is some cross-reactivity of H222 with ER β and the signal detected in megakaryocytes by Tarantino et al11 was ER β.
CD34+ stem cells are pluripotent and give rise to a heterogeneous population, even when induced to differentiate with PEG-rhMGDF. Immunofluorescence staining of this population demonstrated both ER β and AR proteins in GPIIb-positive megakaryocytes (Figures 2 and 3). Several isoforms of both ER β and AR have been described in other tissues,40-42 and our immunoblotting studies raised the possibility of a platelet ER β of greater-than-expected size (Figure 4 and data not shown). However, for unclear reasons, these size differences were not consistently observed with all 4 ER β antibodies used, prohibiting firm conclusions about a possible difference in platelet ER β size.
Steroid receptors bind steroid response elements within and adjacent to target genes, although the mechanism by which steroid receptors regulate transcriptional activity is not well understood.43,44 ER β may affect transcription differently than ER α, and presumably it is ER β that mediates the known estrogenic effects in megakaryocyte genes. Megakaryocytic AR most likely mediates the androgenic regulation of platelet thromboxane A2 receptor expression,15 and a similar mechanism could regulate other androgen-responsive genes involved in platelet function. Androgen therapy has been used for decades in the treatment of bone-marrow failure states, such as aplastic anemia45and paroxysmal nocturnal hemoglobinuria.46 Our findings support a hypothesis that in certain hormonal milieus, sex hormones may affect hematopoiesis. Along these lines, an AR has been identified in erythroid precursors47 that may contribute to the higher hemoglobin concentrations in men than in women.
An increasing number of nongenomic effects of sex hormones have been reported,28,29,48,49 including effects in platelets.50 The cytoplasmic distribution of megakaryocyte and platelet ER β and AR raises the possibility that some hormonal effects on the megakaryocyte lineage may be nongenomic. In vitro testosterone appears to enhance platelet aggregation,16,51 and our own studies suggest that estradiol inhibits aggregation of platelets from men (unpublished observations). Since these nongenomic effects of sex hormones may not always be mediated through the corresponding hormone receptor,52 further studies are needed to assess the role of platelet ER β and AR in these immediate, nontranscriptional effects.
The effects of hormones on a given tissue is complex, in part because receptor levels vary among tissues and even in the same tissue at different developmental or pathologic states.53Tissue-specific effects are manifest further by the ability of testosterone to both up-regulate54 and down-regulate33,34 AR mRNA. Using a range of testosterone concentrations, we found that low concentrations of testosterone (1 to 10 nmol/L) up-regulate AR expression, while 100 nmol/L down-regulates AR expression (Figure 5). Similar findings were seen with megakaryocytes (Figure 6). Such nonlinear dose-response to testosterone concentration has also been seen in LNCap cells, in which a low concentration of testosterone has been shown to increase AR protein (by decreasing AR turnover rate without changing mRNA levels), while higher concentrations cause AR down-regulation.55,56 Posttranslational destabilization of AR mRNA appears to be the predominant mechanism resulting in down-regulation of AR mRNA by androgen in some cell types,57 and perhaps this is the effect of higher testosterone concentrations in the megakaryocyte lineage. A thorough characterization of this complex aspect of megakaryocyte biology was not the intention of these studies. Rather, our goal was to assess whether the megakaryocytic AR was functional, as assessed by some phenotypic change in response to its ligand, and our data support the transcriptional functionality of the AR in megakaryocytes.
Cardiovascular disease is a complex, multifactorial process in which the gender of an individual and the individual's unique platelet physiology affect the propensity to develop or resist thrombosis. The interaction of these 2 traits may be linked to the expression of sex hormones and their corresponding receptors. The identification of ER β and AR in the megakaryocyte lineage may enable new strategies for investigating the prothrombotic effects of estrogens and androgens suggested previously by HERS and other studies. It is important to note that we found comparable levels of ER β and AR in the platelets of both men and women, suggesting that receptor levels per se could not account for any gender difference in platelet function. However, our data do not exclude the possibility that variations over time in either the ligands (estradiol or testosterone) or in the expression of the receptors themselves could alter events of downstream ER β and AR engagement and, hence, megakaryocyte and/or platelet physiology.
Supported in part by grants HL58564 and HL03454 from the National Institutes of Health.
Reprints:Paul F. Bray, Smith Tower 1295, Baylor College of Medicine, 6550 Fannin, Houston, TX 77030; e-mail: pbray@bcm.tmc.edu
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. RT-PCR analysis of sex hormone receptors ER , ER β, PR, and AR in the megakaryocytic lineage. / Total RNA was reverse-transcribed and amplified by PCR with the use of primers specific for ER α, ER β, PR, AR, and β actin transcripts. All PCR primer pairs were separated by at least 1 intron to clearly identify products of cDNA amplification. The cells from which RNA was extracted were as follows: CD34+ cells prior to treatment with PEG-rhMGDF (D0 [day 0] CD34) (lane 1); CD34+ cells treated for 7 days with PEG-rhMGDF (D7 [day 7] CD34) (lane 2); gel-filtered platelets (lane 3); the PC3 prostate cancer cell line (lane 4); the Dami megakaryocytic cell line (lane 5); the HEL megakaryocytic cell line (lane 6); the T47D breast cancer cell line (lane 7). Lanes 8 and 9 contain products from PCRs using genomic DNA or no template, respectively. Lane 10 contains molecular weight marker (Φ × 174 DNA digested with HaeIII endonuclease). Sizes of PCR products are shown in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2289/5/m_bloo00703001w.jpeg?Expires=1770395914&Signature=tciYDUdjAiUaUDxamHz-vIcEtnS0Ddvj~L~lKb6q~0ugMklgwKGhhjKYVi4UJ1vnMf70InOhNB3hJwcp0Bdm4cJEFnhvFBfEoxgB68qdxDF01KQmBVJiiXE4UNQ90T52unlVvu20s6ktgsTrD7ivlBFE-iEr1DQSEj2L5ta-3saGWUBSKuNSo51-QDd75Z7hqPHX1ljZVJAQHwg1Eej70l25cR8ybi1opoWFPjxWEp5wo31juB4JX9yBIZfTTaLfwcXlPtokv5XoIuteSQ~flX43K6ytm4vxJmo3EOqNWmjvoy2JDeymP6r4~wgDxFxfQoGo5QJwKc8wGqCCvhEHfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal