Abstract
The activity of hematopoietic stem cells in the developing liver of a C57BL/6 mouse embryo was quantified by a competitive repopulation assay. Different doses of fetal liver cells at days 11 to 18 of gestation were transplanted into irradiated mice together with 2 × 105 adult bone marrow cells. A long-term repopulation in myeloid-, B-cell, and T-cell lineage by fetal liver cells was evaluated at 20 weeks after transplantation. At day 12 of gestation multilineage repopulating activity was first detected in the liver as 50 repopulating units (RU) per liver. The number of RU per liver increased 10-fold and 33-fold by day 14 and day 16 of gestation, and decreased thereafter, suggesting a single wave of stem cell development in the fetal liver. A limiting dilution analysis revealed that the frequency of competitive repopulating units (CRU) in fetal liver cells at day 12 of gestation was similar to that at day 16 of gestation. Because of an increase of total fetal liver cell number, the absolute number of CRU per liver from days 12 to 16 of gestation increased 38-fold. Hence, the mean activity of stem cells (MAS) that is given by RU per CRU remained constant from days 12 to 16 of gestation. From these data we conclude that hematopoietic stem cells expand in the fetal liver maintaining their level of repopulating potential.
Recent studies on the development of hematopoietic system have revealed the initiation sites of hematopoiesis and proposed a migration of stem cells during development of the mouse embryo.1-5 Cells with long-term marrow repopulating activity have been detected in the yolk sac (YS) and the aorta-gonad-mesonephros (AGM) region at days 9 to 10 of gestation for the first time in an embryonic life of mice.6 7Hematopoietic stem cells are believed to migrate into the liver around day 11, and subsequently into the bone marrow and spleen, whereas the fetal liver remains as a main organ of definitive hematopoiesis during the embryonic period.
Myeloid and B-cell precursors sequentially increase in the fetal liver from mid-gestation to birth.1,8-10 However, the number of T-cell precursor cells peaks at day 13 of gestation.11 It is generally believed that hematopoietic stem cells also expand in the fetal liver, but the extent of their expansion and the kinetics of stem cell development remain poorly understood. It has been shown that fetal liver hematopoietic stem cells have a greater proliferative capacity than do adult bone marrow stem cells.12-14 It has also been reported that the frequency of hematopoietic stem cells in day 14-fetal liver cells is comparable to that in adult bone marrow cells.15 Fetal liver hematopoietic stem cells have been characterized in comparison with adult bone marrow stem cells. However, fetal liver stem cells at different time points of development have never been compared. Herein, we measured a long-term multilineage repopulating ability of the hematopoietic stem cells, which is referred to as stem cell activity, in the fetal liver at successive stages of gestation using 2 previously defined units.
We measured the numbers of repopulating units (RU)16 and competitive repopulating units (CRU)17 in fetal liver cells, based on a competitive repopulation assay,12 because they are complementary. The activity of repopulating cells is given by RU, whereas the number of these cells is given by CRU. Given both numbers of RU and CRU, a mean activity of stem cell (MAS = RU/CRU) was introduced to compare the repopulating ability of individual CRU on average between different cells examined. We demonstrate the total RU per liver increased until day 16 of gestation and decreased thereafter. The total CRU per liver also increased from day 12 to 16 of gestation, along with an increase of fetal liver cell number. Because MAS was similar at days 12 and 16 of gestation, we conclude that there is an expansion of hematopoietic stem cells in the fetal liver.
Materials and methods
Mice
C57BL/6 mice (B6-Ly5.2) and their congenic strain (B6-Ly5.1) were maintained in our animal facility. B6-F1 embryos were obtained from mating pairs of B6-Ly5.1 males and B6-Ly5.2 females. The day of a vaginal plug observed was designated as day 0 of gestation. Eight- to 10-week-old female mice were used as recipients in transplantation experiments.
Cells
Fetal liver was isolated from embryos at days 11 to 18 of gestation under a dissecting microscope. Cell suspension was prepared in Hank's balanced salt solution (HBSS, Life Technologies, Rockville, MD) containing 2% fetal calf serum (FCS) by repeated flushing through needles of 18 to 27 gauge. The cells were passed through a nylon mesh with pore size of 70 μm (Falcon 2350, Becton Dickinson Labware, Franklin Lakes, NJ). Bone marrow competitor cells of 8- to 10-week-old B6-Ly5.1 mice were suspended in HBSS containing 2% FCS with an 18-gauge needle and passed through a nylon mesh. Viability of cells was verified with the trypan blue dye exclusion.
Competitive repopulation assay
We performed population-type and limiting dilution-type assays, both based on a competitive repopulation assay12 to which the Ly5 system was adapted. A population-type assay was used to compare repopulating activity between fetal liver and bone marrow cells.18 A limiting dilution analysis was used to estimate a frequency of competitive repopulating units (CRU) in fetal liver cells as described.17 In both assays, 3 different numbers of fetal liver cells (B6-F1) were mixed with 2 × 105 bone marrow cells (B6-Ly5.1). A cell mixture in 200 μL of HBSS was injected into a group of B6-Ly5.2 mice irradiated at a single dose of 9.5 Gy. Five or more recipients per group were used for a population-type assay. Ten or more recipients per group were used for a limiting dilution-type assay.
Analysis of the recipients
At 4 and 20 weeks after transplantation, peripheral blood cells of the recipients were obtained by retro-orbital bleeding and stained with fluorescence isothiocyanate (FITC)–conjugated anti-Ly5.1 (A20) and biotinylated anti-Ly5.2 (104), followed by addition of streptavidin-allophycocyanin (SA-APC). The cells were simultaneously stained with PE-conjugated anti-B220 (RA3-6B2) or a mixture of phycoerythrin (PE)-conjugated anti-Mac-1 (M1/70) and Gr-1 (RB6-8C5), or a mixture of PE-conjugated anti-CD4 (GK1.4) and CD8 (53-6.7) antibodies. All antibodies and reagents were purchased from PharMingen, San Diego, CA. Multicolor analysis and cell sorting were performed on a dual laser FACS Vantage (Becton Dickinson, San Jose, CA).
In a population-type assay, contribution of fetal liver-derived cells against that of bone marrow competitor-derived cells was expressed by the test donor-derived cells/competitor-derived cells (T/C) ratio. The T/C ratio was defined as percentage of test donor-derived cells (Ly5.1/Ly5.2-double positive F1 cells) divided by percentage of competitor-derived cells (Ly5.1 cells) on FACS analysis of the peripheral blood as demonstrated in Figure1. Because Ly5.2 positive cells were gated out from the calculation, T/C ratio calculated was not influence by the residual host cells. Repopulating units (RU) was calculated using Harrison's method16 as follows:
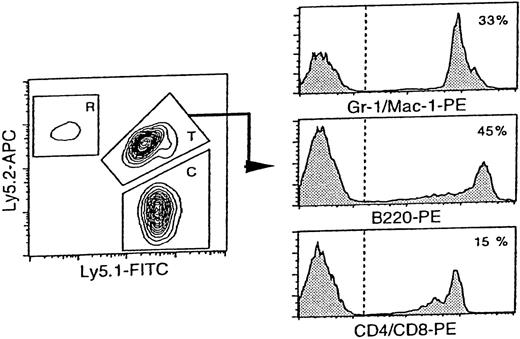
Reconstitution analysis of the mice transplanted with day 12-fetal liver cells at 20 weeks after transplantation.
The representative FACS profile of peripheral blood analysis is shown. The reconstitution in tri-lineage: myeloid, B cell, and T cell was a criterion for engraftment. T: test donor cells (fetal liver cells derived from F1 mouse), C: competitor cells (bone marrow cells from B6-Ly5.1), and R: residual host cells from B6-Ly5.2. T/C ratio: a ratio of (% test donor-derived cells)/ (% competitor-derived cells). In this case, T = 45.0%, C = 44.0%, R = 1.0%, and T/C ratio = 1.02.
Reconstitution analysis of the mice transplanted with day 12-fetal liver cells at 20 weeks after transplantation.
The representative FACS profile of peripheral blood analysis is shown. The reconstitution in tri-lineage: myeloid, B cell, and T cell was a criterion for engraftment. T: test donor cells (fetal liver cells derived from F1 mouse), C: competitor cells (bone marrow cells from B6-Ly5.1), and R: residual host cells from B6-Ly5.2. T/C ratio: a ratio of (% test donor-derived cells)/ (% competitor-derived cells). In this case, T = 45.0%, C = 44.0%, R = 1.0%, and T/C ratio = 1.02.
RU equals the percentage of donor cells times the number of competitor cells times 10−5 divided by 100 minus percentage of donor cells. By definition each RU represents the repopulating activity of 1 × 105 bone marrow cells.16 In this study, the number of bone marrow competitor cells was fixed as 2 × 105 cells. T/C ratio defined above was applied to Harrison's formula as follows: RU = T/C ratio × 2.
Limiting dilution analysis was performed as described.17 A recipient mouse was regarded as positive when the test donor-derived cells consisted of more than 1% of test donor and competitor-derived cells in the peripheral blood. The frequency of repopulating cells (CRU) was estimated on the basis of Poisson statistics as described.19-21 Reconstitution in myeloid-, T-cell, and B-cell lineage was verified by detecting the cells positive for Mac-1/Gr-1, B220 and CD4/CD8 within the gate of F1-test donor cells in both population and limiting dilution -type assays (see Figure 1). To compare the repopulating potentials between different sources of cells, we defined the mean activity of stem cells (MAS) as follows: MAS = RU/CRU.
Results
Measure of test donor-derived cells/competitor-derived cells ratio
Fetal liver cells were obtained from embryos of B6 mice from day 11 to day 18 of gestation, and subjected to a population-type competitive repopulation assay.18 At day 11 of gestation, the total liver cells that contained 2 × 105 cells per embryo on average were transplanted into lethally irradiated mice together with 2 × 105 adult bone marrow cells. At days 12 to 18 of gestation, 3 different numbers of fetal liver cells were transplanted. At 4 and 20 weeks after transplantation, peripheral blood cells were analyzed to evaluate contributions of fetal liver cells (test donor) and bone marrow cells (competitor) to host peripheral blood chimerism. Only myeloid lineage was taken into account for hematopoietic reconstitution at 4 weeks after transplantation, whereas the reconstitution in all myeloid, B-cell, and T-cell lineage was a criterion for reconstitution at 20 weeks after transplantation. As demonstrated in Figure 1, the cells derived from the fetal liver expressed both the Ly5.1 and Ly5.2 antigens (F1-type cells). Bone marrow cells used as competitor cells expressed only the Ly5.1 antigen (Ly5.1 cells). Therefore, in B6-Ly5.2 recipients, fetal liver-derived F1-type cells were distinguished from competitor-derived Ly5.1 cells and residual host-derived Ly5.2 cells.
We obtained the T/C ratio (% test donor-derived cells/% competitor-derived cells) to calculate RU.16 Under the irradiation condition used, remaining host cells could be detected in almost all recipient mice even 20 weeks after transplantation at a frequency of 6.5% ± 4.4% (n = 12). Use of Ly5.1, Ly5.2, and F1 cells has allowed more accurate estimation of stem cell activity by removing the influence of remaining host cells from the analysis. The T/C ratios for fetal liver cells at different days of gestation were listed in Table 1. In every reconstituted mouse, fetal liver-derived cells were detected in all myeloid-, B-, and T-cell lineages at 20 weeks after transplantation (data not shown).
Test donor-derived cells/competitor-derived cells (T/C) ratio at 4 and 20 weeks after transplantation
| Day of Gestation . | No. of Cells Tested . | T/C Ratio (mean ± SD) . | |
|---|---|---|---|
| 4 wk . | 20 wk . | ||
| 11 | 2.0 × 105 | nd | nd |
| 12 | 5.0 × 105 | 1.98 ± 1.48 (n = 9) | 7.18 ± 7.39 (n = 9) |
| 10.0 × 105 | 2.37 ± 1.49 (n = 8) | 11.53 ± 11.10 (n = 8) | |
| 15.0 × 105 | 4.79 ± 2.60 (n = 9) | 17.60 ± 7.08 (n = 9) | |
| 13 | 0.8 × 105 | 0.35 ± 0.20 (n = 5) | 0.86 ± 0.59 (n = 5) |
| 1.0 × 105 | 0.32 ± 0.25 (n = 5) | 1.36 ± 1.16 (n = 5) | |
| 2.0 × 105 | 0.77 ± 0.38 (n = 5) | 2.48 ± 1.51 (n = 5) | |
| 14 | 1.0 × 105 | 0.30 ± 0.18 (n = 5) | 1.44 ± 1.22 (n = 5) |
| 1.5 × 105 | 0.50 ± 0.10 (n = 5) | 1.60 ± 1.78 (n = 5) | |
| 2.0 × 105 | 0.64 ± 0.10 (n = 8) | 2.32 ± 1.05 (n = 8) | |
| 15 | 1.0 × 105 | 0.31 ± 0.30 (n = 9) | 1.20 ± 1.15 (n = 9) |
| 2.0 × 105 | 0.78 ± 0.42 (n = 7) | 2.95 ± 2.31 (n = 7) | |
| 3.0 × 105 | 1.10 ± 0.67 (n = 9) | 3.35 ± 1.95 (n = 9) | |
| 16 | 1.0 × 105 | 0.31 ± 0.19 (n = 5) | 1.40 ± 1.11 (n = 5) |
| 2.5 × 105 | 1.10 ± 0.75 (n = 5) | 3.29 ± 1.39 (n = 5) | |
| 5.0 × 105 | 1.53 ± 0.54 (n = 5) | 6.72 ± 2.78 (n = 5) | |
| 17 | 1.0 × 105 | 0.33 ± 0.16 (n = 8) | 1.15 ± 0.96 (n = 8) |
| 2.0 × 105 | 0.66 ± 0.47 (n = 6) | 2.13 ± 1.65 (n = 6) | |
| 3.0 × 105 | 1.63 ± 1.62 (n = 8) | 4.39 ± 2.94 (n = 8) | |
| 18 | 1.0 × 105 | 0.48 ± 0.28 (n = 5) | 1.31 ± 0.78 (n = 5) |
| 2.5 × 105 | 1.32 ± 0.65 (n = 5) | 2.39 ± 0.90 (n = 5) | |
| 5.0 × 105 | 2.63 ± 0.74 (n = 5) | 5.95 ± 1.93 (n = 5) | |
| Day of Gestation . | No. of Cells Tested . | T/C Ratio (mean ± SD) . | |
|---|---|---|---|
| 4 wk . | 20 wk . | ||
| 11 | 2.0 × 105 | nd | nd |
| 12 | 5.0 × 105 | 1.98 ± 1.48 (n = 9) | 7.18 ± 7.39 (n = 9) |
| 10.0 × 105 | 2.37 ± 1.49 (n = 8) | 11.53 ± 11.10 (n = 8) | |
| 15.0 × 105 | 4.79 ± 2.60 (n = 9) | 17.60 ± 7.08 (n = 9) | |
| 13 | 0.8 × 105 | 0.35 ± 0.20 (n = 5) | 0.86 ± 0.59 (n = 5) |
| 1.0 × 105 | 0.32 ± 0.25 (n = 5) | 1.36 ± 1.16 (n = 5) | |
| 2.0 × 105 | 0.77 ± 0.38 (n = 5) | 2.48 ± 1.51 (n = 5) | |
| 14 | 1.0 × 105 | 0.30 ± 0.18 (n = 5) | 1.44 ± 1.22 (n = 5) |
| 1.5 × 105 | 0.50 ± 0.10 (n = 5) | 1.60 ± 1.78 (n = 5) | |
| 2.0 × 105 | 0.64 ± 0.10 (n = 8) | 2.32 ± 1.05 (n = 8) | |
| 15 | 1.0 × 105 | 0.31 ± 0.30 (n = 9) | 1.20 ± 1.15 (n = 9) |
| 2.0 × 105 | 0.78 ± 0.42 (n = 7) | 2.95 ± 2.31 (n = 7) | |
| 3.0 × 105 | 1.10 ± 0.67 (n = 9) | 3.35 ± 1.95 (n = 9) | |
| 16 | 1.0 × 105 | 0.31 ± 0.19 (n = 5) | 1.40 ± 1.11 (n = 5) |
| 2.5 × 105 | 1.10 ± 0.75 (n = 5) | 3.29 ± 1.39 (n = 5) | |
| 5.0 × 105 | 1.53 ± 0.54 (n = 5) | 6.72 ± 2.78 (n = 5) | |
| 17 | 1.0 × 105 | 0.33 ± 0.16 (n = 8) | 1.15 ± 0.96 (n = 8) |
| 2.0 × 105 | 0.66 ± 0.47 (n = 6) | 2.13 ± 1.65 (n = 6) | |
| 3.0 × 105 | 1.63 ± 1.62 (n = 8) | 4.39 ± 2.94 (n = 8) | |
| 18 | 1.0 × 105 | 0.48 ± 0.28 (n = 5) | 1.31 ± 0.78 (n = 5) |
| 2.5 × 105 | 1.32 ± 0.65 (n = 5) | 2.39 ± 0.90 (n = 5) | |
| 5.0 × 105 | 2.63 ± 0.74 (n = 5) | 5.95 ± 1.93 (n = 5) | |
Competitive repopulation was performed with 3 different numbers of fetal liver cells for each day of gestation. A whole liver was transplanted for day 11 of gestation. At 4 and 20 weeks after transplantation, peripheral blood cells were analyzed to evaluate engraftment. T/C ratio, % test donor-derived cells divided by % competitor-derived cells on FACS analysis (see Figure 1). nd, not determined because of undetectable level of engraftment. The numbers of recipients analyzed are shown in parentheses.
In 2 independent experiments, fetal liver cells of gestation day 11 were transplanted into a total of 20 mice. Whole fetal liver cells from an embryo were transplanted into a lethally irradiated adult recipient. However, no mice showed a detectable level of reconstitution by test donor cells for an observation period of 38 weeks. Transplantation of gestation day 10-liver cells also resulted in no reconstitution (data not shown). These data indicated that fetal liver, before day 12 of gestation, did not contain hematopoietic stem cells capable of competing against 2 × 105 adult bone marrow cells, at least in an adult hematopoietic environment.
Developmental wave of hematopoietic stem cell activity in the fetal liver
RU has been used to indicate the repopulating potential in a cell population.16 On the basis of the data shown in Table 1, RU was calculated by multiplying the mean T/C ratio by a factor of 2. After calculating RU per 1 × 105 cells for each dose, the mean ± SD of the data for each gestation day was obtained and shown in Table 2. The total number of RU per liver was obtained by multiplying the number of RU per 1 × 105 by the total number of liver cells divided by 105. Data in Table 2 are graphically demonstrated in Figure 2.
RU at 4 and 20 weeks after transplantation
| Day of Gestation . | No of Cells/Liver . | RU/105 . | RU/Liver . | ||
|---|---|---|---|---|---|
| 4 wk . | 20 wk . | 4 wk . | 20 wk . | ||
| 11 | 2 × 105 | nd | nd | nd | nd |
| 12 | 2 × 106 | 0.64 ± 0.16 | 2.51 ± 0.32 | 13 ± 3 | 50 ± 6 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 13 | 5 × 106 | 0.76 ± 0.12 | 2.45 ± 0.29 | 38 ± 5 | 123 ± 15 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 14 | 2.0 × 107 | 0.64 ± 0.03 | 2.44 ± 0.40 | 128 ± 6 | 488 ± 78 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 15 | 4.5 × 107 | 0.71 ± 0.09 | 2.53 ± 0.38 | 320 ± 41 | 1136 ± 169 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 16 | 6.0 × 107 | 0.70 ± 0.16 | 2.71 ± 0.09 | 420 ± 90 | 1626 ± 54 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 17 | 5.5 × 107 | 0.80 ± 0.24 | 2.45 ± 0.42 | 440 ± 132 | 1348 ± 231 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 18 | 4.5 × 107 | 1.02 ± 0.05 | 2.30 ± 0.36 | 459 ± 23 | 1035 ± 162 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| Day of Gestation . | No of Cells/Liver . | RU/105 . | RU/Liver . | ||
|---|---|---|---|---|---|
| 4 wk . | 20 wk . | 4 wk . | 20 wk . | ||
| 11 | 2 × 105 | nd | nd | nd | nd |
| 12 | 2 × 106 | 0.64 ± 0.16 | 2.51 ± 0.32 | 13 ± 3 | 50 ± 6 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 13 | 5 × 106 | 0.76 ± 0.12 | 2.45 ± 0.29 | 38 ± 5 | 123 ± 15 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 14 | 2.0 × 107 | 0.64 ± 0.03 | 2.44 ± 0.40 | 128 ± 6 | 488 ± 78 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 15 | 4.5 × 107 | 0.71 ± 0.09 | 2.53 ± 0.38 | 320 ± 41 | 1136 ± 169 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 16 | 6.0 × 107 | 0.70 ± 0.16 | 2.71 ± 0.09 | 420 ± 90 | 1626 ± 54 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 17 | 5.5 × 107 | 0.80 ± 0.24 | 2.45 ± 0.42 | 440 ± 132 | 1348 ± 231 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| 18 | 4.5 × 107 | 1.02 ± 0.05 | 2.30 ± 0.36 | 459 ± 23 | 1035 ± 162 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
Repopulation units (RU) were calculated based on the mean T/C ratios listed in Table 1. The number of cells per liver is shown as the mean of 7 or 8 fetuses obtained for each day of gestation, except day 11 of gestation. The mean number of day 11 fetal liver cells was given by 31 fetuses. RU/105 is the number of RU per 1 × 105 cells tested. RU/liver shows the total number of RU per liver.
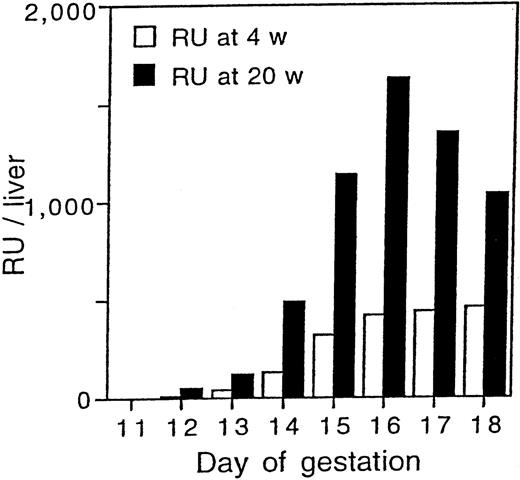
Development of stem cell activity in the fetal liver.
Graphical presentation of the data in Table 2. The mean of total RU/liver at 4 and 20 weeks after transplantation are shown.
Development of stem cell activity in the fetal liver.
Graphical presentation of the data in Table 2. The mean of total RU/liver at 4 and 20 weeks after transplantation are shown.
Repopulating activity in the liver was first detected at day 12 of gestation. The RU for 4 and 20 weeks after transplantation were 13 and 50 per liver, respectively. The total RU per liver at 4 weeks after transplantation gradually increased with day of gestation and reached a plateau by day 16. In contrast, RU obtained 20 weeks after transplantation showed a drastic increase with a peak at day 16 of gestation and decreased thereafter suggesting a single wave of stem cell development. The total number of RU per liver at day 14 and 16 of gestation was 10- and 33-fold higher than that of RU at day 12 of gestation.
Number of hematopoietic stem cells in the fetal liver at days 12 and 16 of gestation
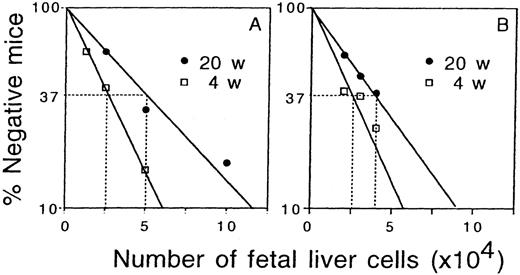
To gain further insight into the mechanism of an increase in the total stem cell activity of the fetal liver, a limiting dilution-type assay was performed with fetal liver cells at days 12 and 16 of gestation. Three different numbers of fetal liver cells for each gestation day were mixed with 2 × 105 bone marrow cells, and transplanted into irradiated adult recipients. Peripheral blood cells of the recipients were analyzed 4 and 20 weeks after transplantation to examine whether there was a significant contribution by the fetal liver-derived cells (more than 1% chimerism). Figure3 shows the result of in vivo limiting dilution analysis. The frequency of CRU was determined according to Poisson statistics as demonstrated in Figure 3. The frequency of CRU in day 12 fetal liver was similar to that in day 16 fetal liver both at 4 and 20 weeks after transplantation (Table3). The absolute numbers of CRU in the liver at 4 and 20 weeks after transplantation showed 29-fold and 38-fold increases from days 12 to 16 of gestation.
In vivo limiting dilution analysis of repopulating cells.
A group of irradiated mice were transplanted with varying numbers of fetal liver cells at day 12 (A) and day 16 (B) of gestation. At 4 and 20 weeks after transplantation peripheral blood cells were analyzed for the presence of fetal liver-derived cells. The number of recipients that survived until the time of analysis were 6 to 13 mice and 8 to 13 mice per group for day 12 and day 16 fetal liver cell transplantation. Mice that did not contain more than 1% of fetal liver-derived cells in a total of fetal liver- and competitor-derived cells were considered to be negative.
In vivo limiting dilution analysis of repopulating cells.
A group of irradiated mice were transplanted with varying numbers of fetal liver cells at day 12 (A) and day 16 (B) of gestation. At 4 and 20 weeks after transplantation peripheral blood cells were analyzed for the presence of fetal liver-derived cells. The number of recipients that survived until the time of analysis were 6 to 13 mice and 8 to 13 mice per group for day 12 and day 16 fetal liver cell transplantation. Mice that did not contain more than 1% of fetal liver-derived cells in a total of fetal liver- and competitor-derived cells were considered to be negative.
Number of repopulating cells and mean activity of stem cell (MAS)
| Gestation . | Frequency of CRU (×10−4) . | No of CRU Per Liver . | MAS . | |||
|---|---|---|---|---|---|---|
| Posttransplantation . | Posttransplantation . | Posttransplantation . | ||||
| 4 wk . | 20 wk . | 4 wk . | 20 wk . | 4 wk . | 20 wk . | |
| Day 12 | 1/2.5 | 1/5.0 | 80/liver | 40/liver | 0.16 | 1.25 |
| (1/1.7-1/4.2) | (1/3.1-1/7.7) | |||||
| Day 16 | 1/2.6 | 1/3.9 | 2308/liver | 1538/liver | 0.18 | 1.06 |
| (1/1.6-1/4.1) | (1/2.3-1/6.3) | |||||
| Gestation . | Frequency of CRU (×10−4) . | No of CRU Per Liver . | MAS . | |||
|---|---|---|---|---|---|---|
| Posttransplantation . | Posttransplantation . | Posttransplantation . | ||||
| 4 wk . | 20 wk . | 4 wk . | 20 wk . | 4 wk . | 20 wk . | |
| Day 12 | 1/2.5 | 1/5.0 | 80/liver | 40/liver | 0.16 | 1.25 |
| (1/1.7-1/4.2) | (1/3.1-1/7.7) | |||||
| Day 16 | 1/2.6 | 1/3.9 | 2308/liver | 1538/liver | 0.18 | 1.06 |
| (1/1.6-1/4.1) | (1/2.3-1/6.3) | |||||
The frequencies of competitive repopulating units (CRU) in fetal liver cells at days 12 and 16 of gestation were obtained by limiting dilution analysis as shown in Figure 3 and expressed as mean (95% confidence range). On the basis of the mean frequencies, the total numbers of CRU per liver were calculated. The mean activity of stem cell (MAS) represents the number of RU per CRU.
To estimate repopulating activity of a stem cell, we introduced a novel unit, mean activity of stem cell (MAS), which can be obtained by calculating the number of RU divided by CRU for the same number of cells. MAS indicates the average repopulating ability of individual stem cell. MAS of day 12 fetal liver cells was similar to that of day 16 fetal liver cells at the same time point after transplantation (Table 3).
Discussion
The hematopoietic stem cell activity in the fetal liver at successive stages of development was measured in a quantitative manner using unfractionated fetal liver cells. Both total RU16 and CRU17 per liver were taken into account for evaluation of this activity. Given the numbers of RU and CRU, the mean activity of stem cell (MAS = RU/CRU) is proposed to compare the repopulating ability of individual CRU on average between different sources of cells. In all experiments, 2 × 105 bone marrow cells of 8- to 10-week-old B6 mice were used as competitor cells to ensure the survival of the recipients and to obtain a constant repopulating ability to compete. In addition, by means of Ly5.1-, Ly5.2-, and F1-type cells, a competition between test donor and competitor cells was assessed more accurately in different recipients regardless of a variation in the amount of residual host cells on analysis. The Ly5.1 and Ly5.2-negative cells, such as red blood cells contaminated in samples, were also excluded from the analysis.
The total stem cell activity per liver from day 12 of gestation to the day before birth was estimated (Table 2 and Figure 2). Stem cell activity in day 11 fetal liver has been described.6However, we could not detect it in our assay system. The number of cells transplanted may not be sufficient to repopulate bone marrow against 2 × 105 bone marrow competitor cells. It is conceivable that the level of stem cell activity in day 11 fetal liver is much lower than that in day 12.22 23
RU at 4 weeks after transplantation successively increased along with day of gestation. This kinetics resembles that of in vivo and in vitro colony-forming cells in the fetal liver.1,8 It is assumed that myeloid lineage-committed precursor cells were mostly responsible for repopulating activity at 4 weeks after transplantation. On the other hand, RU at 20 weeks after transplantation exhibited an increase in the fetal liver until day 16 of gestation during the embryonic life. We interpreted that the number of RU at 4 and 20 weeks after transplantation represented the short- and long-term repopulating abilities of a cell population.24 A 32- and 33-fold increase was observed for short- and long-term repopulating abilities from days 12 to 16 of gestation. Because the frequencies of CRU remained rather constant during this period, the total numbers of short- and long-term CRU per liver showed a 29- and 38-fold increase, which corresponded well with increases in the total RU. Thus, an increase in CRU mostly accounts for increase in RU in this study.
The observation that RU at 20 weeks is higher than that at 4 weeks after transplantation at all gestation days examined supports the notion that stem cells in fetal liver have a higher intrinsic capacity for self-renewal than do those in bone marrow.12 These data indicate that RU at 4 weeks after transplantation may not reflect actual stem cell activity in the case of fetal liver stem cells. These findings may reflect a unique property of fetal liver–derived stem cells in that they do not efficiently generate as many myeloid precursor cells in a short-term as do bone marrow stem cells. However, they are able to repopulate more intensely in multilineage later on. The MAS at short- and long-term remained similar between days 12 and 16 of gestation. On average, therefore, the repopulating ability of each CRU did not change from days 12 to 16. It is concluded that major part of hematopoietic precursor and stem cell expansion takes place in the fetal liver by day 16 of gestation.
It has been shown that the hematopoietic activity in the AGM region and yolk sac is maximal at day 11 of gestation, and rapidly ceases thereafter.3,23,25 Our results support the notion that hematopoietic stem cells migrate into the liver mostly between days 11 and 12. An increase in the number of hematopoietic stem cells up to 38-fold between days 12 and 16 is best explained by self-renewal of these cells in the fetal liver, because no other active site for hematopoiesis in this period has been described. Furthermore, in utero transplantation of fetal liver cells suggested self-renewal of hematopoietic stem cells in the liver.26However, there still remains a possibility that prestem cells migrating from the AGM region and yolk sac give rise to stem cells in the liver in their differentiation process. These prestem cells may be detectable in the conditioned newborn recipients, but not in the irradiated adult mice.7
RU at 20 weeks after transplantation showed a peak at day 16 of gestation. The decline in RU after day 16 may suggest a massive exit of stem cells from the liver to either spleen or bone marrow. A less likely alternative is an abrupt reduction in self-renewal of hematopoietic stem cells, due to the deterioration of hematopoietic microenvironment. This deterioration may also induce a rapid decrease of MAS or apoptosis of hematopoietic stem cells after day 17 of gestation.
Acknowledgments
We thank K. Shimada for helpful discussion, K. Fujii for advice in statistical analysis, and M. Onodera and A. Shibuya for critical reading of the manuscript.
Supported by grants from CREST of Japan Science and Technology Corporation, the Ministry of Education, Science, Sports and Culture in Japan, the Agency for Science and Technology, and the Japan Society for the Promotion of Science JSPS-RFTF96I00202.
Reprints:Hiromitsu Nakauchi, Department of Immunology, Institute of Basic Medical Sciences, University of Tsukuba and CREST (JST), 1-1-1 Tennodai, Tsukuba, 305-8575 Japan; e-mail address:nakauchi@md.tsukuba.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal