Abstract
Mantle cell lymphoma (MCL) is a distinct clinicopathologic entity of non-Hodgkin's lymphoma, characterized by a monotonous proliferation of small to medium-sized lymphocytes with co-expression of CD5 and CD20, an aggressive and incurable clinical course, and frequent t(11;14)(q13;q32) translocation. We examined 151 cases of lymphoma with MCL morphology from a viewpoint of cyclin D1 overexpression, which is now easily detectable by immunohistochemistry. 128 cases (85%) showed positive nuclear staining for cyclin D1, while the remaining 23 (15%) were negative. Except for cyclin D1 immunohistochemistry, current diagnostic methods, including morphological and phenotypical examinations, could not make this distinction. Although both the cyclin D1-positive and -negative groups were characterized by male predominance, advanced stages of the disease, frequent extranodal involvement, and low CD23 reactivity, the cyclin D1-positive group showed a higher age distribution (P = .04), larger cell size (P = .02), higher mitotic index (P = .01), more frequent gastrointestinal involvement (P = .05), higher international prognostic index score (P = .05), and lower p27KIP1 expression (P < .0001). Of particular interest is that cyclin D1-positive MCL showed significantly worse survival than cyclin D1-negative lymphoma (5-year survival: 30% versus 86%, P = .0002), which was confirmed by multivariate analysis to be independent of other risk factors. These data suggest that cyclin D1-positive and -negative groups may represent different entities and that the former closely fits the characteristics of classical, typical MCL. We therefore propose that cyclin D1-positivity should be included as one of the standard criteria for MCL, and that innovative therapies for this incurable disease should be explored on the basis of the new criteria. (Blood. 2000;95:2253-2261
Mantle cell lymphoma (MCL) is a malignant proliferation of B cells in the mantle zone of lymphoid follicles.1 Since its initial recognition in the mid-1970s, this distinct entity has been described with various diagnostic terms, ie, lymphocytic lymphoma of intermediate differentiation by Berard,2 centrocytic lymphoma by Lennert,3 and mantle zone lymphoma by Weisenburger.4 This history reflects the process of identifying the relatively divergent histologic patterns (diffuse, nodular, and mantle zone patterns) of this entity, which may sometimes create diagnostic pitfalls even for expert pathologists. In 1992, Banks et al5 showed that these differently named lymphomas fell within the same entity and named it mantle cell lymphoma.
MCL is mostly characterized by a monotonous proliferation of small to medium-to-large lymphocytes with scant cytoplasm and slightly irregular contoured nuclei. Immunophenotypically, co-expression of CD5 and pan B-cell antigens (CD19, CD20, CD22, and CD24) are characteristic of MCL, though this feature is also observed in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). CD23, which is positive for CLL and generally negative for MCL, is a distinguishing feature, but some contradictory findings have been reported.6 The intensity of CD20 and immunoglobulin light chain expression, which is bright in MCL and dim in B-CLL/SLL, has also been shown to be useful for distinguishing MCL from B-CLL/SLL,7 but some exceptional cases have been identified.8 Clinically, patients with MCL are characterized by advanced age and male predominance, presentation at advanced stages (III and IV), and frequent involvement of bone marrow (BM), peripheral blood, and other extranodal sites.9-16Despite the use of combination chemotherapy for aggressive lymphoma, the median survival of patients has been only 3 to 4 years in most large-scale series. Thus, MCL is regarded as an incurable lymphoma.
Typical MCL contains a cytogenetic abnormality with t(11;14)(q13;q32) translocation, less commonly with t(11;22)(q13;q11),17which involves a rearrangement of the BCL-1 locus. The putative oncogene deregulated by this alteration has subsequently been identified as cyclin D1.18-21 Cyclin D1 belongs to the G1 cyclins and plays a key role in cell cycle regulation during the G1/S transition by cooperating with cyclin-dependent kinases (CDKs).22,23 Further evidence suggests that cyclin D1 can function as an oncogene, the overexpression of which may lead to growth advantage for tumor cells by way of cell cycle progression. Indeed, its overexpression has also been reported in various other human cancers, eg, esophageal, breast, and bladder carcinomas. Among hematolymphoid malignancies, cyclin D1 overexpression resulting from translocational activation has also been recognized in a subset of B-chronic lymphocytic leukemia (B-CLL), multiple myeloma, splenic marginal zone lymphoma, and hairy cell leukemia, though the relationship between them remains to be clarified. Because the breakpoint on chromosome 11q13 covers a wide range between 15 kb and more than 400 kb distance from the cyclin D1 gene, Southern blotting or polymerase chain reaction (PCR) analysis cannot detect all of t(11;14). Immunohistochemical detection of the cyclin D1 protein has been reported to correlate with overexpression of cyclin D1 mRNA.24 A previous preliminary study of ours demonstrated that the cyclin D1-positive MCL group, which comprises the majority of MCLs and pursues an aggressive clinical course, should be demarcated from the cyclin D1-negative group, which has a remarkably favorable prognosis.12 For the current study, we collected data on 151 patients with lymphomas with morphological features of MCL and retrospectively investigated incidence, clinicopathologic features and prognosis to clarify the significance of cyclin D1 overexpression for the diagnosis of MCL.
Patients and methods
Patients
One hundred fifty-one patients with low-grade B-cell lymphoma with MCL morphology were identified in the Aichi Cancer Center and other collaborating institutions. All were histologically reviewed by 3 independent pathologists (Y.Y., T.S., and S.N.), and those meeting the morphologic criteria of MCL5 25 in agreement were enrolled in this study. Excluded were those occasional patients whose primary diagnostic material was not optimal for the identification of features relevant to this series, including minute biopsy specimens, tissues with extensive necrosis, and tissue materials used for rapid frozen-section diagnosis. Patients with plasmacytoid features or those with pseudofollicular growth were also excluded from this study. On the other hand, phenotypically atypical patients (CD23-positive or CD20dim) or those with extranodal involvement (eg, orbit), which were encountered in both cyclin D1-positive and -negative groups, were included in this study for a precise comparison. Statistical analysis was performed with and without these atypical patients. The patients' records and clinical data were reviewed retrospectively.
Histopathology
The formalin-fixed paraffin-embedded sections were stained with hematoxylin and eosin, periodic acid-Schiff, Giemsa, and silver impregnation by Gomori. The histologic sections were further reviewed, with special attention to various histologic features, namely, the pattern of infiltration (diffuse or vaguely nodular), the presence or absence of germinal centers, the presence or absence of typical mantle zone pattern (ie, nodular growth pattern around residual germinal centers), the size of nuclei, the presence or absence of hyaline deposit around small blood vessels, and the mitotic index (per 20 high-power field [HPF]). These features were graded semiquantitatively (ie, absent, present, or abundant) when applicable.
Immunophenotyping
Immunohistochemical13 and flow-cytometric26analyses were performed as described previously. The monoclonal antibodies used were OKT11 (CD2), OKB7 (CD21), OKB22 (CD22), and OKT10 (CD38) (Ortho Diagnostics, Raritan, NJ); Leu4 (CD3), Leu3a (CD4), Leu1 (CD5), Leu2 (CD8), CALLA (CD10), LeuM1 (CD15), Leu12 (CD19), Leu16 (CD20), TCR1 (TCRαβ), and TCRδ1 (TCRγδ) (Becton Dickinson, Mountain View, CA); Tp120 (CD6) and Tp40 (CD7) (established in our laboratory); J5 (CD10), B4 (CD19), B1 (CD20), B2 (CD22), My9 (CD33), and NKH-1 (CD56) (Coulter, Hialeah, FL); MCS-2 (CD13) and H107 (CD23) (Nichirei, Tokyo, Japan); L26 (CD20), Tac (CD25), Ber-H2 (CD30), UCHL1 (CD45RO), anti-HLA-DR, anti-IgA, anti-IgG, anti-IgM, anti-IgD, anti-κ, and anti-λ (DAKO, Carpinteria, CA); and MT1/CD43 (Bio-Science Products, Emmenbrucke, Switzerland). Intensity of antigen expression by flow cytometry was defined as the ratio of the linearized peak fluorescence of the positive distribution to that of the isotype-matched control. The expression intensity of CD20 and immunoglobulin light chain (IgL) was compared with that of CD19 with the same fluorescein, and a higher intensity than that of CD19 was defined as “bright” and a lower intensity as “dim” as previously described. 7 8
Immunohistochemistry for cyclin D1 and cell cycle regulators
Formalin-fixed, paraffin-embedded 5-μm thick sections were stained with standard ABC methods, using diamino-bentizine as the visualizing substrate.27,28 Antigen retrieval for the staining of cyclin D1 and p27KIP1 was performed with microwave treatment in 0.01 mol/L citrate buffer (pH 6.8). The following antibodies were used: cyclin D1 (IBL, Gunma, Japan),27p27KIP1 (Transudation Laboratories, Lexington, KY), and the phosphorylated form of pRb (ppRb) at serine 780 (MBL, Nagoya, Japan).29 For the analysis of pRb, frozen sections of 35 available patients were fixed with paraformaldehyde for 15 minutes, followed by immunohistochemical staining as described above.
Cyclin D1 overexpression was defined as a definite positive reaction in the nuclei of lymphoma cells, as described previously.13For the examination of ppRb and p27KIP1, labeling indexes in the 4 most representative high-power fields were counted.
Statistical analysis
The chi-square test, the Fisher exact test, and Student ttest were used to examine the relation between any 2 factors. The Mann-Whitney U test was performed to compare graded factors. Actuarial survival curves were estimated with the Kaplan-Meier method and compared by means of the log-rank test. A Cox proportional hazard regression model was used to identify factors with a significant influence on survival. Data were analyzed with the SAS system (SAS Institute, Cary, NC).
Results
Cyclin D1 overexpression in MCL
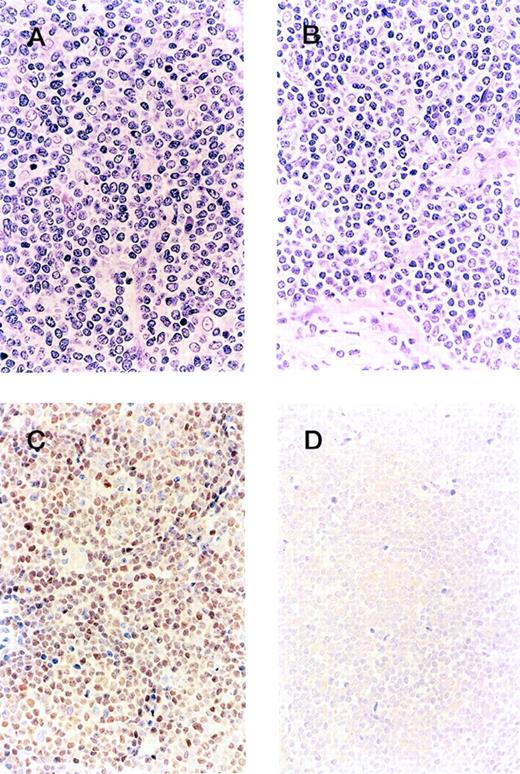
Among the 151 patients with MCL morphology, cyclin D1 overexpression was detected in 128 (85%). Representative morphology and immunohistochemical profiles of both cyclin D1-positive and -negative groups are shown in Figure 1.
Clinicopathologic boundary of mantle cell lymphoma. Although the morphology of the cyclin D1-positive group (A) and -negative group (B) is indistinguishable, the patient from the former group shows cyclin D1 overexpression (C), whereas the latter is negative for cyclin D1 (D).
Clinicopathologic boundary of mantle cell lymphoma. Although the morphology of the cyclin D1-positive group (A) and -negative group (B) is indistinguishable, the patient from the former group shows cyclin D1 overexpression (C), whereas the latter is negative for cyclin D1 (D).
Patient characteristics for cyclin D1-positive and -negative
The clinical features of the cyclin D1-postive and -negative groups were analyzed for comparison (Table 1). Those of the cyclin D1-negative patients are summarized in Table2 to facilitate evaluation. The patients of both groups showed male predominance, whereas the cyclin D1-positive group showed a higher age distribution (median age, 65 and 60 years, respectively; P = .04, Student t test). The majority of both groups presented with advanced stages (III or IV) of the disease (85% and 69%, respectively). Primary sites of involvement of stage I cases were Waldeyer ring (n = 2), stomach (n = 1), and lymph nodes (n = 3; neck, inguinal, and abdominal) for the cyclin D1-positive group, whereas they were orbit (n = 2), Waldeyer ring (n = 1), and subcutis (n = 1) for the cyclin D1-negative group. Extranodal involvement was frequently recognized in both groups (71% and 74%, respectively). BM and peripheral blood were the most frequent sites of extranodal involvement in both groups. The cyclin D1-positive group exhibited a significant preponderance of gastrointestinal tract involvement compared with the cyclin D1-negative group (27% versus 9%; P = .05), and the cyclin D1-negative group showed more frequent presentation or involvement of the orbit (2% versus 13%;P = .05). Serum LDH level and performance status tended to be higher for the cyclin D1-positive group, though the difference was not statistically significant. IPI score was higher for the cyclin D1-positive group (P = .04; Mann-Whitney U test).
Patient characteristics
| . | Cyclin D1-positive (n = 128) . | Cyclin D1-negative (n = 23) . | P . |
|---|---|---|---|
| Age (y), median (range) | 65 (36–81) | 60 (31–74) | .04 |
| Age >60 | 81 (63%) | 11 (48%) | |
| Sex (M/F) | 89/39 | 15/8 | .68 |
| Stage | |||
| I | 6 (5%) | 4 (17%) | |
| II | 13 (10%) | 3 (13%) | .46 |
| III | 32 (26%) | 3 (13%) | |
| IV | 74 (59%) | 13 (56%) | |
| Extranodal involvement | 87 (71%) | 17 (74%) | .80 |
| ≥2 sites | 43 (36%) | 8 (35%) | .88 |
| BM and/or PB | 61 (50%) | 12 (52%) | .82 |
| Spleen | 45 (36%) | 6 (26%) | .37 |
| Liver | 19 (15%) | 4 (17%) | .80 |
| GI tract | 34 (27%) | 2 (9%) | .05 |
| Waldeyer ring | 13 (10%) | 2 (9%) | .59 |
| Orbit | 3 (2%) | 3 (13%) | .05 |
| Serum LDH > normal | 38 (33%) | 4 (18%) | .14 |
| PS ≥2 | 24 (21%) | 2 (9%) | .16 |
| International prognostic index | |||
| Low | 28 (23%) | 9 (39%) | |
| Low-intermediate | 39 (32%) | 9 (39%) | .04 |
| High-intermediate | 31 (25%) | 4 (17%) | |
| High | 19 (15%) | 1 (4%) |
| . | Cyclin D1-positive (n = 128) . | Cyclin D1-negative (n = 23) . | P . |
|---|---|---|---|
| Age (y), median (range) | 65 (36–81) | 60 (31–74) | .04 |
| Age >60 | 81 (63%) | 11 (48%) | |
| Sex (M/F) | 89/39 | 15/8 | .68 |
| Stage | |||
| I | 6 (5%) | 4 (17%) | |
| II | 13 (10%) | 3 (13%) | .46 |
| III | 32 (26%) | 3 (13%) | |
| IV | 74 (59%) | 13 (56%) | |
| Extranodal involvement | 87 (71%) | 17 (74%) | .80 |
| ≥2 sites | 43 (36%) | 8 (35%) | .88 |
| BM and/or PB | 61 (50%) | 12 (52%) | .82 |
| Spleen | 45 (36%) | 6 (26%) | .37 |
| Liver | 19 (15%) | 4 (17%) | .80 |
| GI tract | 34 (27%) | 2 (9%) | .05 |
| Waldeyer ring | 13 (10%) | 2 (9%) | .59 |
| Orbit | 3 (2%) | 3 (13%) | .05 |
| Serum LDH > normal | 38 (33%) | 4 (18%) | .14 |
| PS ≥2 | 24 (21%) | 2 (9%) | .16 |
| International prognostic index | |||
| Low | 28 (23%) | 9 (39%) | |
| Low-intermediate | 39 (32%) | 9 (39%) | .04 |
| High-intermediate | 31 (25%) | 4 (17%) | |
| High | 19 (15%) | 1 (4%) |
BM, bone marrow; PB, peripheral blood; GI, gastrointestinal; PS, performance status.
Clinical and immunophenotypic profiles of cyclin D1-negative patients
| Patient no. . | Age (y)/ sex . | Sites of involvement . | Clinical stage . | LDH >normal . | PS > 1 . | Extranodal involvement >1 . | IPI . | CD5 . | CD10 . | CD23 . | sIgH . | sIgL . | Flow cytometry . | Therapy . | Effect . | Follow-up (mo) . | Outcome . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LN . | H/S . | BM . | PB . | Others . | CD20 . | Ig L . | ||||||||||||||||
| 1 | 48/F | + | −/− | + | − | IV A | + | − | − | LI | + | + | ND | − | λ | MC | CR | 56+ | Relapsed, alive in 2nd CR | |||
| 2 | 67/F | + | −/− | − | − | II A | + | − | − | LI | + | − | − | IgM | κ | CHOP | CR | 24+ | Alive in CR | |||
| 3 | 56/M | + | −/+ | + | + | IV A | − | − | + | LI | + | − | ND | ND | λ | MC | CR | 80+ | Alive in CR | |||
| 4 | 66/M | + | −/− | − | − | III A | − | − | − | LI | + | − | − | ND | λ | COP | NC | 69+ | Alive with disease | |||
| 5 | 73/M | − | −/− | − | − | Orbit | I E A | − | − | − | L | + | − | − | ND | κ | Radiation | CR | 13+ | Lost to follow-up in CR | ||
| 6 | 53/M | + | −/+ | − | − | WR, stomach, intestine | IV A | + | − | + | HI | + | − | − | ND | λ | MC | PR | 77 | Regrowth, DWD | ||
| 7 | 41/M | + | +/+ | + | + | IV B | − | − | + | LI | ND | ND | ND | ND | ND | Not given | — | — | Lost to follow-up | |||
| 8 | 67/F | + | −/− | − | − | III A | − | − | − | LI | + | ND | − | ND | ND | MC | PR | 12+ | Alive in PR | |||
| 9 | 74/F | + | −/− | − | − | Orbit | II E A | − | − | − | L | + | − | − | IgM | κ | Bright | Bright | Radiation | PR | 13+ | Alive in PR |
| 10 | 62/M | − | −/− | − | − | Orbit | I E A | − | − | − | L | + | − | − | IgM | λ | Bright | Bright | Radiation | CR | 58+ | Alive in CR |
| 11 | 60/M | + | −/− | − | − | II A | − | − | − | L | ND | ND | − | ND | ND | Radiation | CR | 79+ | Alive in CR | |||
| 12 | 61/F | + | +/+ | + | + | IV A | − | − | + | HI | + | ND | ND | IgM | κ | CHOP | PR | 12+ | Alive in PR | |||
| 13 | 62/M | + | −/+ | + | − | Small intestine, PE | IV A | − | − | + | HI | + | ND | − | ND | ND | MC | PR | 23+ | Alive in PR | ||
| 14 | 49/M | + | −/− | + | − | PE | IV A | − | − | + | LI | + | ND | − | ND | ND | CHOP | PR | 28+ | Regrowth, alive with disease | ||
| 15 | 62/M | + | −/− | − | − | III A | − | − | − | LI | + | − | + | IgM | λ | Dim | Dim | CY | NC | 49+ | Alive with disease | |
| 16 | 59/M | + | +/− | + | + | IV B | + | + | + | H | + | − | + | IgMD | λ | Dim | Bright | MC | CR | 60+ | Relapsed, alive with disease | |
| 17 | 53/M | + | −/− | + | − | IV A | − | + | − | LI | + | − | + | IgMD | λ | Bright | Bright | MC | CR | 81+ | Alive in CR | |
| 18 | 72/M | − | −/− | − | − | WR | I A | − | − | − | L | + | − | ND | IgM | κ | Radiation | CR | 83+ | Alive in CR | ||
| 19 | 74/F | + | −/− | + | − | IV A | − | − | + | HI | + | + | ND | IgM | κ | MC | CR | 150+ | Alive in CR | |||
| 20 | 54/M | + | −/− | + | + | IV A | − | − | − | L | + | − | + | IgMD | λ | Bright | Bright | CHOP | CR | 41+ | Alive in CR | |
| 21 | 57/F | − | −/− | − | − | Subcutis | I E A | − | − | − | L | ND | ND | ND | ND | ND | Resection + CHOP | CR | 47+ | Alive in CR | ||
| 22 | 32/M | + | +/+ | + | + | IV A | − | − | − | L | + | − | ND | IgMD | κ | MC | PR | 41+ | Alive with disease | |||
| 23 | 47/F | + | −/− | + | − | IV A | − | − | − | L | + | − | − | IgM | λ | Bright | Bright | MC | CR | 84 | Relapsed, DWD | |
| Patient no. . | Age (y)/ sex . | Sites of involvement . | Clinical stage . | LDH >normal . | PS > 1 . | Extranodal involvement >1 . | IPI . | CD5 . | CD10 . | CD23 . | sIgH . | sIgL . | Flow cytometry . | Therapy . | Effect . | Follow-up (mo) . | Outcome . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LN . | H/S . | BM . | PB . | Others . | CD20 . | Ig L . | ||||||||||||||||
| 1 | 48/F | + | −/− | + | − | IV A | + | − | − | LI | + | + | ND | − | λ | MC | CR | 56+ | Relapsed, alive in 2nd CR | |||
| 2 | 67/F | + | −/− | − | − | II A | + | − | − | LI | + | − | − | IgM | κ | CHOP | CR | 24+ | Alive in CR | |||
| 3 | 56/M | + | −/+ | + | + | IV A | − | − | + | LI | + | − | ND | ND | λ | MC | CR | 80+ | Alive in CR | |||
| 4 | 66/M | + | −/− | − | − | III A | − | − | − | LI | + | − | − | ND | λ | COP | NC | 69+ | Alive with disease | |||
| 5 | 73/M | − | −/− | − | − | Orbit | I E A | − | − | − | L | + | − | − | ND | κ | Radiation | CR | 13+ | Lost to follow-up in CR | ||
| 6 | 53/M | + | −/+ | − | − | WR, stomach, intestine | IV A | + | − | + | HI | + | − | − | ND | λ | MC | PR | 77 | Regrowth, DWD | ||
| 7 | 41/M | + | +/+ | + | + | IV B | − | − | + | LI | ND | ND | ND | ND | ND | Not given | — | — | Lost to follow-up | |||
| 8 | 67/F | + | −/− | − | − | III A | − | − | − | LI | + | ND | − | ND | ND | MC | PR | 12+ | Alive in PR | |||
| 9 | 74/F | + | −/− | − | − | Orbit | II E A | − | − | − | L | + | − | − | IgM | κ | Bright | Bright | Radiation | PR | 13+ | Alive in PR |
| 10 | 62/M | − | −/− | − | − | Orbit | I E A | − | − | − | L | + | − | − | IgM | λ | Bright | Bright | Radiation | CR | 58+ | Alive in CR |
| 11 | 60/M | + | −/− | − | − | II A | − | − | − | L | ND | ND | − | ND | ND | Radiation | CR | 79+ | Alive in CR | |||
| 12 | 61/F | + | +/+ | + | + | IV A | − | − | + | HI | + | ND | ND | IgM | κ | CHOP | PR | 12+ | Alive in PR | |||
| 13 | 62/M | + | −/+ | + | − | Small intestine, PE | IV A | − | − | + | HI | + | ND | − | ND | ND | MC | PR | 23+ | Alive in PR | ||
| 14 | 49/M | + | −/− | + | − | PE | IV A | − | − | + | LI | + | ND | − | ND | ND | CHOP | PR | 28+ | Regrowth, alive with disease | ||
| 15 | 62/M | + | −/− | − | − | III A | − | − | − | LI | + | − | + | IgM | λ | Dim | Dim | CY | NC | 49+ | Alive with disease | |
| 16 | 59/M | + | +/− | + | + | IV B | + | + | + | H | + | − | + | IgMD | λ | Dim | Bright | MC | CR | 60+ | Relapsed, alive with disease | |
| 17 | 53/M | + | −/− | + | − | IV A | − | + | − | LI | + | − | + | IgMD | λ | Bright | Bright | MC | CR | 81+ | Alive in CR | |
| 18 | 72/M | − | −/− | − | − | WR | I A | − | − | − | L | + | − | ND | IgM | κ | Radiation | CR | 83+ | Alive in CR | ||
| 19 | 74/F | + | −/− | + | − | IV A | − | − | + | HI | + | + | ND | IgM | κ | MC | CR | 150+ | Alive in CR | |||
| 20 | 54/M | + | −/− | + | + | IV A | − | − | − | L | + | − | + | IgMD | λ | Bright | Bright | CHOP | CR | 41+ | Alive in CR | |
| 21 | 57/F | − | −/− | − | − | Subcutis | I E A | − | − | − | L | ND | ND | ND | ND | ND | Resection + CHOP | CR | 47+ | Alive in CR | ||
| 22 | 32/M | + | +/+ | + | + | IV A | − | − | − | L | + | − | ND | IgMD | κ | MC | PR | 41+ | Alive with disease | |||
| 23 | 47/F | + | −/− | + | − | IV A | − | − | − | L | + | − | − | IgM | λ | Bright | Bright | MC | CR | 84 | Relapsed, DWD | |
LN, lymph node: H/S, hepatosplenic; BM, bone marrow; PB, peripheral blood; WR, Waldyer ring; PE, pleural effusion; PS, performance status; IPI, international prognostic index; L, low; LI, low-intermediate; HI, high-intermediate; H, high; ND, not done; sIg, surface immunoglobulin; IgL, immunoglobulin light chain; MC, multiple chemotherapy with doxorubicin; CHOP, cyclophosphamide, doxorubicin, vincristin, and predonisone; COP, cyclophosphamide, vincristin, and predonisone; CY, cyclophosphamide; CR, complete remission; PR, partial response; NC, no change; DWD, dead with disease.
Histologic features
In histologic terms, both cell size and mitotic index of the 2 groups were different (Table 3). In the cyclin D1-positive group, the majority (107 patients, 84%) featured a monotonous population of atypical small to medium-sized lymphoid cells with irregular and indented nuclei, whereas pleomorphic and blastic/blastoid variants were encountered in 10 (8%) and 11 patients (9%), respectively. Consequently, cyclin D1-positive MCL showed a larger cell size (P = .006) and higher mitotic index (P = .003). However, the distribution of growth patterns (mantle zone, nodal, or diffuse) revealed no remarkable differences between the 2 groups. The characteristic mantle zone pattern was also seen in cyclin D1-negative patients without identification of a proliferation center.
Histologic features of cyclin D1-positive and -negative patients
| . | Cyclin D1-positive (n = 128) Number (%) . | Cyclin D1-negative (n = 23) Number (%) . | P . |
|---|---|---|---|
| Morphology | |||
| Growth pattern | |||
| Typical mantle zone pattern | 22 (17) | 6 (26) | |
| Vaguly nodular | 48 (38) | 7 (30) | .57 |
| Diffuse | 58 (45) | 10 (43) | |
| Cell size | |||
| Small | 3 (2) | 2 (9) | |
| Intermediate | 59 (46) | 16 (70) | |
| Medium | 44 (34) | 5 (22) | .006 |
| Medium to large | 17 (13) | 0 | |
| Large | 5 (4) | 0 | |
| Morphologic variants | |||
| Blastic/blastoid | 11 (9) | 0 | .15 |
| Pleomorphic | 10 (8) | 0 | .18 |
| Residual follicular pattern | |||
| Naked germinal centers | 45 (35) | 11 (48) | .25 |
| Mitotic index | |||
| High (>25/20HPF) | 42 (33) | 1 (4) | .003 |
| Low (<24/20HPF) | 86 (67) | 22 (96) |
| . | Cyclin D1-positive (n = 128) Number (%) . | Cyclin D1-negative (n = 23) Number (%) . | P . |
|---|---|---|---|
| Morphology | |||
| Growth pattern | |||
| Typical mantle zone pattern | 22 (17) | 6 (26) | |
| Vaguly nodular | 48 (38) | 7 (30) | .57 |
| Diffuse | 58 (45) | 10 (43) | |
| Cell size | |||
| Small | 3 (2) | 2 (9) | |
| Intermediate | 59 (46) | 16 (70) | |
| Medium | 44 (34) | 5 (22) | .006 |
| Medium to large | 17 (13) | 0 | |
| Large | 5 (4) | 0 | |
| Morphologic variants | |||
| Blastic/blastoid | 11 (9) | 0 | .15 |
| Pleomorphic | 10 (8) | 0 | .18 |
| Residual follicular pattern | |||
| Naked germinal centers | 45 (35) | 11 (48) | .25 |
| Mitotic index | |||
| High (>25/20HPF) | 42 (33) | 1 (4) | .003 |
| Low (<24/20HPF) | 86 (67) | 22 (96) |
HPF, high-power field.
Phenotypic features
Immunophenotypically, most of the cyclin D1-positive and -negative patients showed similar profiles of CD5+, CD10−, CD19+, CD20+, and CD23− phenotypes, though 10 of the cyclin D1-positive patients (11%) and 4 of the cyclin D1-negative patients (27%) showed CD23 expression (Table 4). With the aid of flow cytometry, the intensity of CD20 and IgL expression was compared with that of CD19 in 40 of the cyclin D1-positive and 7 of the cyclin D1-negative patients. Bright expression of CD20 and IgL was recognized in most of the patients in both groups (Table 4), but 1 patient showed a dim expression of both CD20 and IgL, and 2 other patients in the cyclin D1-positive group showed bright CD20 and dim IgL expression. In the cyclin D1-negative group, 1 patient also displayed a dim expression of both antigens, and another patient displayed dim CD20 but bright IgL expression. Five other patients exhibited a bright expression of both CD20 and IgL.
Phenotypic and cytogenetic features
| . | Cyclin D1-positive . | Cyclin D1-negative . | P . |
|---|---|---|---|
| Immunophenotype | |||
| CD5 | 103/109 (94%) | 20/20 (100%) | .36 |
| CD20 | 101/101 (100%) | 17/17 (100%) | 1.00 |
| CD23 | 10/88 (11%) | 4/15 (27%) | .12 |
| κ:λ | 44:55 | 7:10 | .91 |
| Flow cytometry | |||
| CD20 bright:dim | 39:1 | 5:2 | .06 |
| IgL bright:dim | 36:3 | 6:1 | .50 |
| Cytogenetics | |||
| t(11;14)(q13;q32)+ | 184-150 | 0 | .09 |
| t(11;14)(q13;q32)− | 18 | 3 | |
| No metaphase | 12 | 2 | |
| Not examined | 80 | 13 |
| . | Cyclin D1-positive . | Cyclin D1-negative . | P . |
|---|---|---|---|
| Immunophenotype | |||
| CD5 | 103/109 (94%) | 20/20 (100%) | .36 |
| CD20 | 101/101 (100%) | 17/17 (100%) | 1.00 |
| CD23 | 10/88 (11%) | 4/15 (27%) | .12 |
| κ:λ | 44:55 | 7:10 | .91 |
| Flow cytometry | |||
| CD20 bright:dim | 39:1 | 5:2 | .06 |
| IgL bright:dim | 36:3 | 6:1 | .50 |
| Cytogenetics | |||
| t(11;14)(q13;q32)+ | 184-150 | 0 | .09 |
| t(11;14)(q13;q32)− | 18 | 3 | |
| No metaphase | 12 | 2 | |
| Not examined | 80 | 13 |
Including 1 patient with t(11;22)(q13;q11).
IgL, immunoglobulin light chain.
Cytogenetic and genetic studies
Cytogenetic profiles are summarized in Table 4. Exactly half of the cyclin D1-positive MCLs showed t(11;14)(q13;q32), and none of the cyclin D1-negative group did, though the number of informative patients was limited. Southern blotting identified the rearrangement of the BCL-1 locus in 8 of 15 cyclin D1-positive patients (2 with t(11;14) and 6 with no cytogenetic data), and Northern blotting detected cyclin D1 mRNA in 14 of 15 cyclin D1-positive patients (3 with t(11;14), 1 with normal karyotype, and 10 with no cytogenetic data) as described previously.24 In contrast, none of the 3 cyclin D1-negative patients (1 without t(11;14) and 2 with no cytogenetic data) showed any evidence of either rearrangement of BCL-1 or cyclin D1 mRNA.
Expression of cell cycle regulators
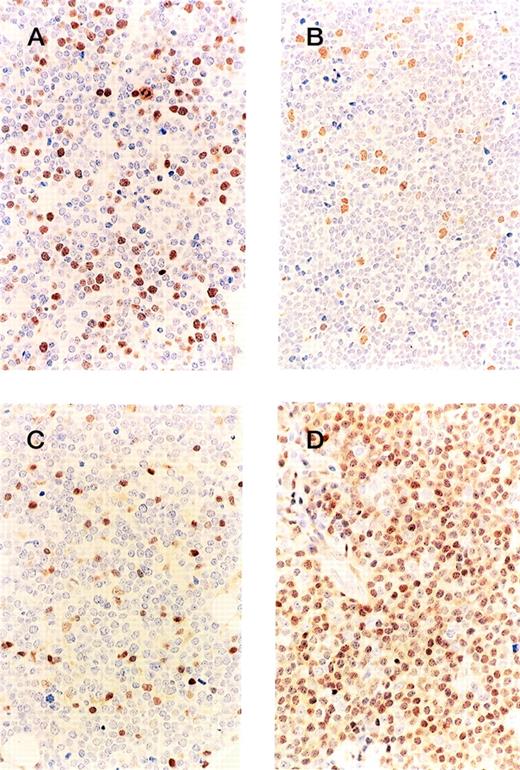
In both the cyclin D1-positive and -negative groups, the expression of ppRb was low throughout (Figures 2A, 2B), and no difference in expression was found between the 2 groups. Less than 20% of the tumor cells were positive for ppRb in all patients. However, the labeling index for p27KIP1expression (Figures 2C, 2D) showed a sharp division into 2 categories, very low (less than 10% positivity), and high (more than 60%). Twenty of the 23 cyclin D1-positive MCL patients exhibited no or a low expression of p27KIP1, and 10 of the 12 cyclin D1-negative patients exhibited a high expression of p27, thus showing a marked difference in expression (Table5, P < .0001).
Immunohistochemistry of cell cycle regulators. The labeling index of phosphorylated pRb is low for both the cyclin D1-positive (A) and -negative (B) groups, but the expression ofp27KIP1 in the cyclin D1-positive MCL (C) is lower than in the cyclin D1-negative group (D).
Immunohistochemistry of cell cycle regulators. The labeling index of phosphorylated pRb is low for both the cyclin D1-positive (A) and -negative (B) groups, but the expression ofp27KIP1 in the cyclin D1-positive MCL (C) is lower than in the cyclin D1-negative group (D).
Expression of cell-cycle regulators
| . | % . | Cyclin D1-positive (n = 23) . | Cyclin D1-negative (n = 12) . | P . |
|---|---|---|---|---|
| ppRb | 1–5 | 16 | 7 | .63 |
| 5–10 | 4 | 2 | ||
| 10–20 | 3 | 0 | ||
| p27KIP1 | >60 | 3 | 10 | <.0001 |
| <10 | 20 | 2 |
| . | % . | Cyclin D1-positive (n = 23) . | Cyclin D1-negative (n = 12) . | P . |
|---|---|---|---|---|
| ppRb | 1–5 | 16 | 7 | .63 |
| 5–10 | 4 | 2 | ||
| 10–20 | 3 | 0 | ||
| p27KIP1 | >60 | 3 | 10 | <.0001 |
| <10 | 20 | 2 |
Therapeutic response and prognosis
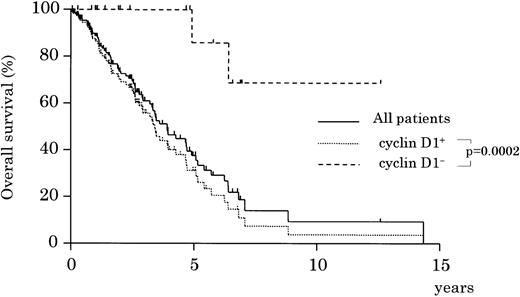
Of the patients with cyclin D1-positive MCL, 78% received chemotherapy containing doxorubicin, whereas 73% of the cyclin D1-negative group did. Other patients were treated with radiation or chemotherapy without doxorubicin. In both groups, approximately 40% (44% of cyclin D1-positive and 42% of cyclin D1-negative) attained complete remission. However, survival showed a significant difference between the 2 groups (Figure 3,P = .0002): the 5-year survival rate for the cyclin D1-positive group was only 30%, whereas that for the cyclin D1-negative group was 86% (Figure 3). Analysis after exclusion of patients with CD23+ phenotype or those with orbital involvement also resulted in a statistically significant difference in survival (P = .001). The survival rates were not different, however, for subgroups divided according to the choice of initial treatment, that is, chemotherapy with or without doxorubicin, or radiation (data not shown). Univariate analysis showed that patient's age, serum LDH level, stage, PS, and IPI at diagnosis were significant risk factors for survival (Table 6). Multivariate analysis confirmed that cyclin D1 status (positive or negative) was an independent factor for survival when compared with each of the risk factors and with the IPI risk category (Table 6); both types of analysis determined that cyclin D1 positivity was the highest risk factor (respective relative risks, 8.5 and 7.5). Analysis after the exclusion of patients with CD23+ phenotype or with orbital involvement also showed the statistical significance of cyclin D1 (respective relative risks, 6.5 [1.5-28.0, P = .01] and 7.4 [1.8-30.8, P = .005]).
Overall survival of cyclin D1-positive and -negative patients. The cyclin D1-positive group shows a significantly worse prognosis (P = .0002).
Overall survival of cyclin D1-positive and -negative patients. The cyclin D1-positive group shows a significantly worse prognosis (P = .0002).
Prognostic factors affecting overall survival
| Variables . | Unfavorable factor . | Univariate . | Multivariate . | |
|---|---|---|---|---|
| P . | Relative risk (CI) . | P . | ||
| Comparison with risk factors | ||||
| Cyclin D1 status | Positive | .0002 | 8.5 (2.0–36.3) | .004 |
| Age | >60 years | .03 | 2.5 (1.4-4.6) | .003 |
| LDH | >Normal | .009 | 1.4 (0.8-2.6) | ns |
| Stage | III/IV | .02 | 2.9 (1.0-8.4) | .05 |
| PS | 2–4 | .04 | 1.4 (0.7-2.7) | ns |
| Extranodal disease | ≥2 sites | ns | 1.1 (0.7-1.9) | ns |
| Comparison with IPI category | ||||
| Cyclin D1 status | Positive | .0002 | 7.5 (1.8-31.0) | .005 |
| IPI category | H/H-I | .0003 | 2.5 (1.5-4.2) | .0004 |
| Variables . | Unfavorable factor . | Univariate . | Multivariate . | |
|---|---|---|---|---|
| P . | Relative risk (CI) . | P . | ||
| Comparison with risk factors | ||||
| Cyclin D1 status | Positive | .0002 | 8.5 (2.0–36.3) | .004 |
| Age | >60 years | .03 | 2.5 (1.4-4.6) | .003 |
| LDH | >Normal | .009 | 1.4 (0.8-2.6) | ns |
| Stage | III/IV | .02 | 2.9 (1.0-8.4) | .05 |
| PS | 2–4 | .04 | 1.4 (0.7-2.7) | ns |
| Extranodal disease | ≥2 sites | ns | 1.1 (0.7-1.9) | ns |
| Comparison with IPI category | ||||
| Cyclin D1 status | Positive | .0002 | 7.5 (1.8-31.0) | .005 |
| IPI category | H/H-I | .0003 | 2.5 (1.5-4.2) | .0004 |
CI, confidence interval; H, high; H-I, high-intermediate; ns, not significant.
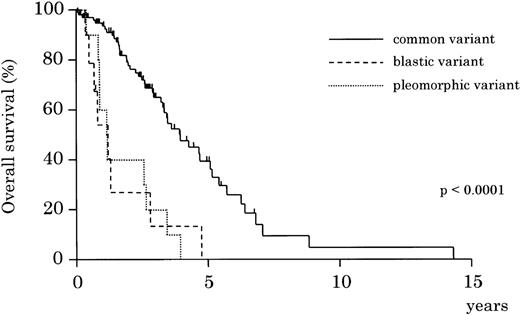
In the cyclin D1-positive group, blastic and pleomorphic variants followed a more aggressive clinical course than the common variant (Figure 4, P < .0001).
Overall survival by morphologic variants of cyclin D1-positive MCL. Blastic and pleomorphic variants show a significantly more aggressive clinical course than the common variant of MCL (P < .0001).
Overall survival by morphologic variants of cyclin D1-positive MCL. Blastic and pleomorphic variants show a significantly more aggressive clinical course than the common variant of MCL (P < .0001).
Discussion
In this study, we examined 151 patients of lymphomas with the morphologic features of MCL. Because of the clinicopathologic and significant prognostic differences between the cyclin D1-positive and -negative groups indicated by our results, the determination of cyclin D1 status is thought to be essential for the diagnosis of MCL. For the cyclin D1-positive group, the median survival period was 3.5 years, which was consistent with that for intermediate grade lymphomas as determined by the Working Formulation. However, there was no plateau in the survival curve, in clear contrast with that for other intermediate grade lymphomas. The incurable clinical course of the patients with cyclin D1-positive, but not cyclin D1-negative, lymphomas corroborated the characteristic behavior of the former type of lymphoma, which was almost the same as the reported prognosis for a smaller series of MCL.9-16 Those studies emphasized the overall poor outcome for MCL because of the predominance of cyclin D1-positive patients and overlooked the favorable clinical course of the cyclin D1-negative patients because there were so few.
From the viewpoint of lymphoma classification, cyclin D1-negative lymphoma with MCL morphology appears to pose a diagnostic problem because some difficulties remain in defining the classifications for low-grade B-cell lymphomas. Although the survival curve of cyclin D1-negative patients was similar to that of B-CLL/SLL, the morphologic and immunophenotypic features were different. This indicates that these lymphomas are indeed different from B-CLL/SLL. Although CD5 positivity is observed in only a few B-cell lymphoma entities, ie, MCL and CLL, CD23 is regarded as a useful marker of CLL and is generally negative for MCL.25 However, some anomalous patients have been reported in the literature, 6,14,16,30-36 and consensus criteria for the diagnosis of MCL established by the European Lymphoma Task Force do not require CD23 negativity for patients with typical cytology.37 In our series, most of the cyclin D1-positive and -negative patients were CD23−, but 14 patients were CD23+ (10 cyclin D1-positive and 4 cyclin D1-negative). When the focus is on CD23 expression, these CD23+ cyclin D1-negative patients could be B-CLL/SLL, and CD23+ cyclin D1-positive patients might be classified as cyclin D1-positive atypical CLL/SLL. However, none of the CD23+ patients showed histologic features contradictory to those described for MCL. These data suggest that CD23 is not a precise marker for the distinction of MCL and other types of low-grade B-cell lymphoma (ie, MCL-like B-cell lymphoma or SLL with plasmacytoid differentiation 6), though it is valid for a differential diagnosis of MCL and B-CLL/SLL. Therefore, to avoid a bias for patient selection we decided to include all patients with MCL morphology in this study. Prognostic significance of cyclin D1 expression was analyzed with an entire cohort of the patients and those without the atypical characteristics. Both types of analyses showed the same result—that cyclin D1 overexpression is a strong, independent, and significant prognostic factor for lymphoma with MCL morphology.
The intensity of CD20 and IgL expression has also been described as useful for distinguishing between MCL and B-CLL/SLL. In our series, most of the cyclin D1-positive and -negative patients showed a bright expression of CD20 and IgL, though the number of patients examined was limited. Two patients (1 patient each from the cyclin D1-positive and -negative groups) showed dim expression of both CD20 and IgL, and 3 patients (2 from the cyclin D1-positive and 1 from the cyclin D1-negative group) showed contradictory results for CD20 and IgL. Therefore, the intensity of CD20 and IgL was also found to have a limited value for differential diagnosis in our series. On the other hand, patients of CLL with variant morphology or variant immunophenotype (CD23-negative or CD20-bright) might be included with the cyclin D1-negative patients. Nevertheless, cyclin D1 is still a more reliable marker for differential diagnosis of MCL than morphology or immunophenotype.
Another concern is that some of the cyclin D1-negative patients could have marginal zone B-cell lymphomas (MZBCL). Ferry et al38have reported a series of CD5+ cyclin D1-negative MZBCL featuring an advanced disease stage, frequent orbital presentation, a propensity for BM involvement and relapse, and an indolent but recurrent clinical course. These findings appear to be similar to those for our 3 patients (patients 5, 9, and 10) of the cyclin D1-negative group, though our patients pursued a silent clinical course without BM involvement. In addition, our cyclin D1-positive MCL patients also showed orbital involvement, albeit infrequently. Ferry et al38 mentioned that CD5 might be a marker for MZBCL with persistent disease and dissemination to the marrow and other extranodal sites. Our patients, however, showed a similar phenotype but followed a stable course, resembling that of typical MZBCL. Although these points must be clarified, we want to emphasize that the term MCL should be restricted at least to cyclin D1-positive patients. The cyclin D1-negative group might be better termed “cyclin D1-negative MCL-like B-cell lymphoma” rather than “cyclin D1-negative MCL” as it is at present. Furthermore, though the oncogenic mechanism of cyclin D1 overexpression remains to be specified, the use of this molecular marker should lead to a better understanding of MCL and may contribute to a redefinition of the disease entity.
Cyclin D1 immunostaining is one of the methods for detecting pathogenic translocations of the BCL-1 locus, mostly t(11;14)(q13;q32). These translocations can also be detected by means of cytogenetic analysis, Southern blotting, or fluorescence in situ hybridization (FISH). Cytogenetic and Southern blotting analyses indicate that approximately 50% to 70% of MCL show this specific gene alteration (reviewed in Welsenburger and Armitage1). However, cytogenetic analysis is not always informative because of the low rate of proliferation in lymphoma cells. Interphase FISH techniques39 can be used to avoid such low proliferative activity, though FISH and genomic analyses are hampered by the large area within the BCL-1 locus in which the rearrangement breakpoint can be located.20,40-42 Cyclin D1 overexpression at the mRNA level can be detected by Northern blotting24,43,44 or by reverse transcription–polymerase chain reaction (RT-PCR) analysis and might be a reliable marker for the diagnosis of mantle cell lymphoma. However, mRNA is not always obtainable from all patients, and Northern blotting is sometimes complicated by RNA degradation in the specimens. The RT-PCR assay is likely to amplify faint cyclin D1 derived from contaminating nonneoplastic cells, thus necessitating special techniques such as competitive45 or quantitative PCR,46 which might make the diagnostic procedure more complicated. As a result, these methods are not convenient for routine diagnostic use.
Immunohistochemical detection of cyclin D1 overexpression is particularly important for clinical application because it is simple to use for a far larger number of patients than is possible with mRNA detection. Other investigators have also reported the usefulness of immunohistochemical cyclin D1 detection for the diagnosis of mantle cell lymphomas.12,47,48 However, previous studies, including ours, examined relatively small numbers of patients, and because cyclin D1-negative patients account for no more than 15% of all MCLs, they may be overlooked in studies with small cohorts. Even though the proportion of cyclin D1-negative patients is small, the prognosis for the 2 types is different enough so that failure to notice such patients can cause an inaccurate overall prognosis of MCL and thus might result in misinterpretation and misjudgment in clinical trials. A critical review of the patients, with knowledge of the type of cyclin D1 expression, also led to the identification of the broad morphologic spectrum underlying the category of cyclin D1-positive MCL, which has been previously addressed by Swerdlow et al.34 It is important to realize that those variations do not connote different disease entities because the REAL classification emphasizes that variations in histologic grade can be identified within individual disease entities. In our series, 21 patients (16%) with cyclin D1-positive MCL showed a morphology of blastic/pleomorphic variants accompanied by more aggressive clinical courses.14 49 Thus, the diversity of morphologies seen in different patients of cyclin D1-positive MCL was relatively broad, ranging from small cells with round or slightly irregular nuclei at one end of the spectrum to large transformed cells with small nucleoli at the other end.
The oncogenic role of cyclin D1 is considered to be the promotion of the cell cycle as a result of its overexpression. We therefore examined the upstream regulator and the downstream effector closely related to the cyclin D1 molecule and focused on the phosphorylation status at the residue of serine 780 of Rb, a site specifically phosphorylated by the cyclin D1-CDK complex.29 For the upstream regulator,p27KIP1 expression was examined.p27KIP1 is considered to be the main regulator of cellular responses to antimitogenic signals, and it is associated predominantly with the cyclin D-CDK4 complex.50,51 In normal lymph nodes, germinal center cells are positive for pRb but not for p27KIP1, which reflects their highly proliferative activity. In contrast, normal mantle cells are negative for pRb but positive for p27KIP1, indicating that they are in a cytostatic status. Cyclin D1-positive MCLs lackedp27KIP1 expression, as has been reported by other investigators,52 which suggests a growth advantage for lymphoma cells as compared with their normal counterparts. The cyclin D1-negative group maintained its p27KIP1expression, whereas that of its normal counterpart remains to be clarified. The differences in p27KIP1 expression as well as in mitotic index and cell size between the cyclin D1-positive and -negative groups offer further support to the idea that these 2 groups have different disease entities.
The MCL specimens showed no significant difference in the labeling index of ppRb between the cyclin D1-positive and -negative groups. This finding was supported by the relatively low percentage of S-phase fractions and the infrequent occurrence of mitotic figures in MCL and was comparable with the results obtained from Western blot analysis by other investigators.53,54 During the cell cycle progression from the G1 to the S phase, cyclin D1 binds to CDK4 to form a complex that facilitates pRb phosphorylation, resulting in the release of E2F, a transcription factor that drives the cell cycle. Therefore, the low phosphorylation status of pRb in cyclin D1-positive MCL does not correlate with the current speculation on the oncogenic role of cyclin D1 overexpression.55 Because the overexpression of ppRb is not essential for cell cycle progression, at least in MCLs, further studies to explain these functional inconsistencies are warranted.
An effective therapeutic approach for cyclin D1-positive MCL has not yet been established. In our series of 128 cyclin D1-positive patients with MCL, the dose intensity of chemotherapy or the use of Adriamycin did not influence the survival (data not shown), as has been reported for patients without cyclin D1 examination. There is thus a strong need to design new strategies for the treatment of MCLs. Myeloablative chemoradiotherapy with stem cell support has recently been proposed as a promising strategy,56,57 but this could not be confirmed by other investigators,58,59 even with ex vivo purging using anti-B-cell antibodies.60,61 A prospective trial with a longer follow-up for a larger number of patients is warranted. In the meantime, we recommend that attention be paid to cyclin D1 status for patient selection because our patients in the cyclin D1-negative group showed onset at a younger age. Other therapeutic approaches using new drugs such as anti-CD20 monoclonal antibody62 or purine analogues63 64 also deserve to be further evaluated on the basis of an accurate disease definition of and diagnostic criteria for MCL.
Acknowledgments
We thank the collaborators from the following institutions for providing the patients' data and specimens: Third Department of Internal Medicine, Akita University School of Medicine; Japanese Red Cross Nagoya First Hospital; Aichi Prefectural Hospital, Okazaki Municipal Hospital; First Department of Internal Medicine, Nagoya University School of Medicine; Atomic Disease Institute, Nagasaki University School of Medicine; Research Institute for Radiation Biology and Medicine, Hiroshima University School of Medicine; Second Department of Internal Medicine, Kyoto Prefectural University School of Medicine; Asahikawa Kosei Hospital; First Department of Internal Medicine, Saitama Medical School; Niigata Cancer Center Hospital; National Minami-Okayama Hospital; Departments of Hematology and Pathology, Kawasaki University School of Medicine; Tenri Hospital; Hamamatsu Medical Center; Miyagi Medical Cancer; Sapporo National Hospital; Ichinomiya Municipal Hospital.
Y.Y. and R.S. contributed equally to this study and should both be regarded as first authors.
Reprints:Shigeo Nakamura, Department of Pathology and Clinical Laboratories,
Aichi Cancer Center, 1-1 Kanokoden, Chikusa-ku, Nagoya 464-8681; e-mail: snakamur@aichi-cc.pref.aichi.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal