Abstract
The treatment of advanced Hodgkin's disease (HD) with chemotherapy (CTx) alone or combined modality treatments has been controversial. In 1989, we designed a randomized study to compare 2 cycles of CTx to (sub)total nodal irradiation (RTx) as consolidation treatments for patients with stage IIIB/IV HD in complete remission (CR) or good partial response after 6 cycles of CTx. A total of 559 patients were randomized to receive 6 cycles of MOPP/ABV (mechlorethamine, vincristine, procarbazine, prednisone/Adriamycin [doxorubicin], bleomycin, vinblastine) hybrid (n = 266) or ABVPP (n = 267). After induction treatment, 418 patients could be evaluated for the consolidation phase. With a median follow-up of 48 months, the 5-year disease-free survival estimates were 80% for 8 cycles of MOPP/ABV, 82% for 6 cycles of MOPP/ABV plus RTx, 68% for 8 cycles of ABVPP, and 75% for 6 cycles of ABVPP plus RTx (P = .01). The 5-year disease-free survival estimates did not differ between CTx and RTx, 74% and 79%, respectively (P = .07). After MOPP/ABV, the 5-year overall survival estimates did not differ between CTx and RTx, 85% and 88%, respectively (P = .2). After ABVPP, the 5-year survival estimates were 94% for CTx and 78% for RTx (P = .002). These results showed that RTx was not superior to CTx consolidation after doxorubicin-induced CR for patients with advanced HD. Because of the uncertainty of obtaining a prolonged second remission for patients relapsing after CTx and RTx and the possible long-term effects of RTx, we prefer 8 cycles of CTx as standard treatment when a CR has been achieved after 6 cycles.
The optimal therapeutic strategy for patients with advanced Hodgkin's disease (HD) remains undefined. Major advances have been achieved with the use of chemotherapy (CTx) combined or not with radiation therapy. However, despite complete remission (CR) rates ranging from 80% to 97%, up to 40% of CR patients will subsequently relapse.1-5 In an attempt to minimize the risk of relapse after CR following induction CTx, adjuvant radiotherapy (RTx) was evaluated in uncontrolled or retrospective studies6,7 and randomized clinical trials.5,8-13 In the late 1980s, the role of RTx remained controversial, and its advantage in terms of survival was not demonstrated.8 Ten years later, the potential benefit of combined treatment over CTx alone has not yet been definitively demonstrated. Trials that compared additional RTx versus no further treatment failed to demonstrate a benefit in terms of survival5; 2 studies showed an advantage of combined treatment only for disease control, with significant reduction of nodal infield relapses9 or prolonged failure-free survival.10 Trials that compared additional RTx versus additional CTx did not show any advantage of RTx in terms of survival.8,11-13 More recently, a meta-analysis of trials with parallel RTx/CTx design showed that the use of RTx was associated with significantly poorer overall survival.14That study brought to light the importance of taking into consideration the risk for disease-unrelated fatal events.
The objectives of the study were to compare 2 doxorubicin-containing regimens and to compare RTx versus CTx as consolidation treatments for patients who had achieved a CR or a partial response (PR) of at least 75% after 6 cycles of chemotherapy. When this trial was initiated, response to initial CTx was known to be a prognostic factor, and investigators of the Groupe d'études des Lymphomes de l'Adulte opted for a strategy including early salvage therapy for poor responders. As a consequence, this trial included salvage therapy for patients who did not achieve a response of at least 75% after induction CTx or who relapsed after CR. This paper reports an interim analysis of the results of the first-line therapy.
Patients and methods
Study design
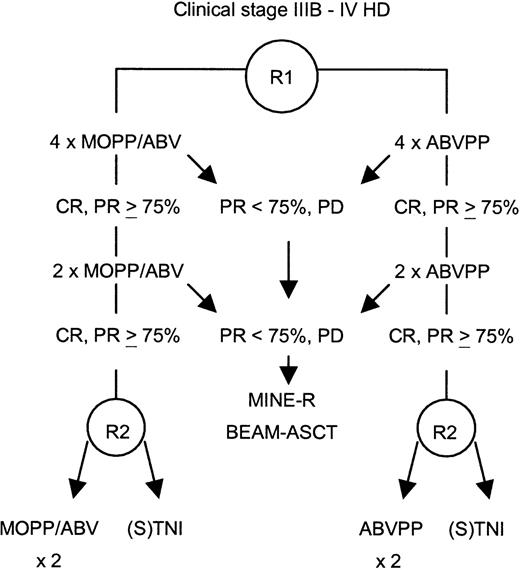
This study was a prospective, randomized trial. Randomization was stratified according to participating centers for the induction arm—MOPP/ABV (mechlorethamine, vincristine, procarbazine, prednisone/Adriamycin [doxorubicin], bleomycin, vinblastine) hybrid or ABVPP—and according to participating centers and induction regimen for the consolidation treatment (Figure 1). The primary objective of the study was to detect a 10% difference between the 2 consolidation treatment procedures on the basis of a projected 80% 2-year disease-free survival rate in the CTx consolidation arm (α risk of 0.05 and β risk of 0.10) for patients achieving CR or PR of at least 75% after induction CTx. Secondary objectives included assessment of responses, overall survival, toxicities of the 2 induction regimens, and results of salvage therapy with high-dose CTx for patients not achieving CR or PR of at least 75% after induction CTx.
Study design.
CR indicates complete remission; PR, partial response; PD, progressive disease; R, randomization; ASCT, autologous stem cell transplantation; (S)TNI, (sub)total nodal irradiation.
Study design.
CR indicates complete remission; PR, partial response; PD, progressive disease; R, randomization; ASCT, autologous stem cell transplantation; (S)TNI, (sub)total nodal irradiation.
Patient eligibility
Previously untreated patients were eligible for the H89 trial if they had biopsy-confirmed HD, were between 15 and 65 years old, and were classified as being Ann Arbor stage IIIB/IV. Patients were excluded for the following reasons: human immunodeficiency virus infection, major organ failure, or previous cancer (except in situ cervical carcinoma or skin epithelioma). The study protocol was approved by the Ethics Committee of Saint-Louis Hospital, Paris, and all patients gave their written informed consent.
Diagnostic studies
Initial staging procedures included physical examination; complete blood count with leukocyte differential; serum lactate dehydrogenase (LDH) level expressed as a percentage of the maximum normal value; erythrocyte sedimentation rate (ESR); renal and liver function tests; posteroanterior and lateral chest radiographs; bone marrow biopsy; and chest, abdominal, and pelvic computed tomography (CT) scans. Bipedal lymphangiogram was recommended and performed in 65% of the patients. Bone scintigraphy was performed in 57% of the patients. Only 4% of the patients underwent a staging laparotomy as part of the diagnostic procedure. Mediastinal masses were classified as large when the ratio of the largest transverse diameter of the mass to the transverse diameter of the thorax at the level of T5-6 (MT ratio) was at least 0.33, as determined on a chest radiograph. Patients with MT ratios of more than 0.45 were considered to have very large mediastinal masses. Patients with any mass more than 10 cm or an MT ratio of more than 0.45 were considered to have bulky disease. Performance status was based on the Eastern Cooperative Oncology Group (ECOG) scale (0 from 4).
A panel of hematopathologists from the Groupe d'études des Lymphomes de l'Adulte reevaluated the histologic slides of 93% of the patients; the diagnoses of the treating institution were used for the remaining patients. Immunophenotyping was performed on deparaffinized tissue sections using a panel of monoclonal antibodies (CD45, CD15, CD30, EMA, BNH9, CD20, CD3, CD45RO).
Treatment
The patients were randomly assigned to 1 of 2 CTx regimens: MOPP/ABV hybrid or ABVPP. MOPP/ABV hybrid CTx was given according to the University of British Columbia regimen.4 In an attempt to reduce alkylating agents, the ABVPP regimen was previously developed11 and consisted of doxorubicin, 30 mg/m2 intravenously (IV), on day 1; bleomycin, 5 mg/m2IV, on days 1 and 8; vinblastine, 5 mg/m2 IV, on days 1 and 8; procarbazine, 100 mg/m2, on days 1-14; and prednisone, 40 mg/m2, on days 1-14. Cycles were repeated every 28 days if the absolute neutrophil count was more than 1.6 × 109/L and the platelet count was more than 125 × 109/L. The ratio of actual/planned full-dose CTx for 3 drugs (nitrogen mustard, procarbazine, and doxorubicin) was calculated. The average percentage was used to describe the amount of consolidation CTx given. A reduction of doses was defined as an actual/planned ratio of less than 0.85.
Patients in CR or PR of at least 75% after 6 cycles were randomized for consolidation treatment, either 2 more cycles of the same CTx, or RTx. RTx was delivered to mantle field, paraaortic, and spleen areas (subtotal nodal irradiation) or inverted Y and spleen areas (total nodal irradiation) only in the case of iliac or inguinal involvement. A total of 30 Gy were delivered to all the volumes, plus 5 Gy to the initially involved areas, and an additional 5 Gy to the site of a residual mass after CTx, given in 2-Gy fractions, 5 fractions per week. Patients not in CR or PR of at least 75% after 6 cycles were not randomized for consolidation treatment. PR patients with 50% to 75% reduction of their tumor masses after 4 to 6 cycles and documented active disease (by biopsy or gallium-67 scan) and patients who did not respond or progress under treatment received salvage therapy with the MINE regimen: mitoguazone, ifosfamide, vinorelbine (Navelbine, Pierre-Fabre Medicament, Boulogne, France), and etoposide; 2 to 3 cycles followed by BEAM: carmustine, etoposide, cytarabine, melphalan; and autologous stem cell transplant.15
Evaluation of response and follow-up
Patients were clinically evaluated after 4 and 6 cycles of CTx and at completion of treatment. Routine staging procedures included hematologic and blood biochemistry studies, ESR measurement, chest radiograph, and plain film of the abdomen when a lymphangiogram had been performed initially. CT scans that were abnormal at diagnosis were repeated after 4 cycles of CTx, after 6 cycles in the case of a PR, and at the completion of treatment. When a previous bone marrow biopsy had been abnormal, the bone marrow was reassessed after 4 cycles. Most patients with bulky mediastinal disease at diagnosis and PR of less than 75% after initial CTx had a gallium scan or lymph node biopsy, if necessary. Patients were reevaluated every 3 months during years 1 and 2, every 4 months during year 3, every 6 months during years 4 and 5, and then yearly.
CR was defined as the disappearance of all clinical evidence of HD and the normalization of all laboratory values, radiographic findings, and bone marrow biopsy. PR at least 75% was defined as a reduction of at least 75% in the largest diameter of each measurable site of disease, the normalization of systemic symptoms and laboratory values or the persistence of minor abnormalities, and the normalization of bone marrow biopsy. PR of 50% to less than 75% was defined as a reduction of at least 50% in the largest diameter of each measurable site. The disease was considered to be stable when tumor regression was less than 50% or bone marrow involvement persisted after 4 cycles of CTx. Progression was defined as an increase of tumor size or evidence of new sites of involvement.
Statistical analyses
All analyses were performed on an intention-to-treat basis. The stopping date was set at June 1, 1998. Patient characteristics, response rates, and toxicities were compared with χ2tests. Survival duration was calculated from the time of the first randomization (induction treatment) to the stopping date, date of death, or date of last follow-up evaluation when the stopping date had not yet been reached. Disease-free survival was calculated as the interval between the date of achieving CR and the stopping date, relapse, death, or date of last follow-up evaluation when the stopping date had not yet been reached. Event-free survival was measured as the interval between the date of the first randomization and the stopping date, progression, death, or date of last follow-up evaluation when the stopping date had not yet been reached. The event-free survival analysis included patients with stable disease, patients who progressed under treatment, partial responders (all patients who had received salvage therapy), all complete responders who subsequently relapsed, and deaths from all causes. Disease-free survival, event-free survival, and overall survival were estimated using the Kaplan-Meier method16 and were compared according to treatment group by log-rank tests17 at the P=.05 significance level. All statistical analyses were carried out with use of SAS 6.12 software (SAS Institute, Cary, NC). Relative risks were estimated using the Cox regression model.18 All regression models were restricted to the patients with complete datasets.
Results
Patient characteristics
Between July 1, 1989, and December 1, 1996, 559 patients were enrolled for the study; 278 patients were assigned to receive MOPP/ABV and 281 to the ABVPP induction regimen. Twenty-four were considered ineligible for analysis: 11 who were not properly enrolled (8 with clinical stage IIA, IIB, or IIIA disease; 2 aged 14 and 68 years; and 1 with an abnormal left ventricular ejection fraction and a contraindication to receive doxorubicin) and 13 whose histologic diagnoses were changed after reevaluation. Two patients could not be evaluated because of missing information on treatment; these patients were excluded from the final analysis. Thus, the study population consisted of 533 patients, including 266 in the MOPP/ABV group and 267 in the ABVPP group. The median age of the 352 (66%) men and 181 (34%) women was 32 years. The histologic classification distribution was as follows: 79% nodular sclerosis, 10% mixed cellularity, 2% lymphocyte depletion, and 9% unclassifiable due to unsatisfactory quality of material available for reevaluation. A total of 214 (40%) patients entering this study were classified as having HD stage IIIB (including 9 with stage IIIEB), 62 (12%) as having stage IVA, and 257 (48%) with stage IVB. The clinical characteristics of the patients at presentation and according to treatment arm are listed in Table1. These characteristics did not differ between the 4 treatment arms. According to the international prognostic score for advanced HD, the distribution of patients in each risk group was as follows: 90% for patients with a score of 2 or above, 61% for patients with a score of 3 or above, and 34% for patients with a score of 4 or above. The mediastinum was involved in 78% of the patients (n = 416), and the MT ratio was at least 0.33 in 15% (n = 82). Among the patients, 48% had pulmonary hilar involvement, 26% had lung involvement, and 16% had liver involvement.
Characteristics of all 533 eligible patients and of 418 patients achieving CR or PR of at least 75% after induction CTx and randomized for consolidation treatment
| . | Eligible patients . | Patients randomized for consolidation treatment . | |||
|---|---|---|---|---|---|
| MOPP/ABV × 8 . | ABVPP × 8 . | MOPP/ABV × 6 + RTx . | ABVPP × 6 + RTx . | ||
| No. of patients | 533 | 92 | 116 | 114 | 96 |
| Male gender (%) | 66 | 73 | 68 | 68 | 67 |
| Age | |||||
| Median (y) | 32 | 32 | 32 | 32 | 32 |
| ≥45 y (%) | 23 | 21 | 16 | 17 | 22 |
| Stage | |||||
| IIIB (%) | 40 | 52 | 41 | 39 | 46 |
| IVA (%) | 12 | 10 | 11 | 13 | 10 |
| IVB (%) | 48 | 38 | 48 | 48 | 44 |
| Mediastinum (%) | |||||
| Uninvolved | 22 | 24 | 25 | 18 | 16 |
| MT ratio <0.33 | 63 | 65 | 59 | 63 | 74 |
| 0.33 ≤ MT ≤ 0.45 | 6 | 4 | 8 | 9 | 4 |
| MT >0.45 | 9 | 7 | 8 | 10 | 6 |
| Tumor >10 cm (%) | 24 | 25 | 28 | 22 | 26 |
| Inguinal involvement (%) | 19 | 13 | 19 | 22 | 26 |
| Extranodal sites ≥2 (%) | 27 | 21 | 22 | 25 | 23 |
| Bone marrow involvement (%) | 20 | 12 | 16 | 20 | 18 |
| ECOG performance status ≥2 (%) | 12 | 8 | 10 | 11 | 11 |
| Hemoglobin <10.5 g/L (%) | 29 | 24 | 34 | 21 | 22 |
| LDH ratio >1 (%) | 44 | 36 | 40 | 42 | 45 |
| Albumin <40 g/L (%) | 23 | 20 | 30 | 22 | 22 |
| WBC ≥15 × 109/L (%) | 25 | 21 | 25 | 19 | 27 |
| Lymphocyte count <0.6 × 109/L or <8% of WBC (%) | 13 | 8 | 16 | 12 | 8 |
| International prognostic score (%) | |||||
| 0 | 2 | 0 | 4 | 0 | 2 |
| 1 | 8 | 10 | 11 | 11 | 6 |
| 2 | 29 | 31 | 25 | 37 | 32 |
| 3 | 28 | 30 | 27 | 24 | 27 |
| 4 | 19 | 21 | 22 | 15 | 22 |
| ≥5 | 14 | 8 | 11 | 13 | 11 |
| . | Eligible patients . | Patients randomized for consolidation treatment . | |||
|---|---|---|---|---|---|
| MOPP/ABV × 8 . | ABVPP × 8 . | MOPP/ABV × 6 + RTx . | ABVPP × 6 + RTx . | ||
| No. of patients | 533 | 92 | 116 | 114 | 96 |
| Male gender (%) | 66 | 73 | 68 | 68 | 67 |
| Age | |||||
| Median (y) | 32 | 32 | 32 | 32 | 32 |
| ≥45 y (%) | 23 | 21 | 16 | 17 | 22 |
| Stage | |||||
| IIIB (%) | 40 | 52 | 41 | 39 | 46 |
| IVA (%) | 12 | 10 | 11 | 13 | 10 |
| IVB (%) | 48 | 38 | 48 | 48 | 44 |
| Mediastinum (%) | |||||
| Uninvolved | 22 | 24 | 25 | 18 | 16 |
| MT ratio <0.33 | 63 | 65 | 59 | 63 | 74 |
| 0.33 ≤ MT ≤ 0.45 | 6 | 4 | 8 | 9 | 4 |
| MT >0.45 | 9 | 7 | 8 | 10 | 6 |
| Tumor >10 cm (%) | 24 | 25 | 28 | 22 | 26 |
| Inguinal involvement (%) | 19 | 13 | 19 | 22 | 26 |
| Extranodal sites ≥2 (%) | 27 | 21 | 22 | 25 | 23 |
| Bone marrow involvement (%) | 20 | 12 | 16 | 20 | 18 |
| ECOG performance status ≥2 (%) | 12 | 8 | 10 | 11 | 11 |
| Hemoglobin <10.5 g/L (%) | 29 | 24 | 34 | 21 | 22 |
| LDH ratio >1 (%) | 44 | 36 | 40 | 42 | 45 |
| Albumin <40 g/L (%) | 23 | 20 | 30 | 22 | 22 |
| WBC ≥15 × 109/L (%) | 25 | 21 | 25 | 19 | 27 |
| Lymphocyte count <0.6 × 109/L or <8% of WBC (%) | 13 | 8 | 16 | 12 | 8 |
| International prognostic score (%) | |||||
| 0 | 2 | 0 | 4 | 0 | 2 |
| 1 | 8 | 10 | 11 | 11 | 6 |
| 2 | 29 | 31 | 25 | 37 | 32 |
| 3 | 28 | 30 | 27 | 24 | 27 |
| 4 | 19 | 21 | 22 | 15 | 22 |
| ≥5 | 14 | 8 | 11 | 13 | 11 |
Induction treatment
After 6 cycles of induction CTx, 267 patients (50%) achieved CR, 193 patients (36%) achieved PR of at least 75%, 23 patients (4%) achieved PR of 50% to less than 75%, 36 patients (7%) failed to respond or progress, and 14 (3%) died during or after these 6 cycles. Response rates did not differ between the 2 induction regimens of CTx: among patients treated with MOPP/ABV, CR or PR of at least 75% was obtained in 51% and 34%, respectively; among patients treated with ABVPP, CR or PR of at least 75% was obtained in 49% and 39%, respectively.
Consolidation treatment
A total of 115 patients were not randomized to receive the consolidation treatment for the following reasons: early death (n = 11); PR to induction CTx, which required salvage therapy (n = 59); treatment-related toxicity (n = 13); underlying diseases (n = 3); patient refusal (discontinuation of treatment after induction, n = 3; randomization refusal in RTx arm, n = 4); and protocol violation (n = 22). With few exceptions, these latter 22 patients received consolidation treatment with CTx or RTx. A total of 418 patients were randomized: 208 to consolidation CTx and 210 to RTx. However, 400 of these 418 patients (96%) received the consolidation treatment according to randomization. Among the 208 patients randomized to CTx, 205 actually received CTx; 3 patients refused the treatment; 29% of the patients (50 of 175 assessable patients) received consolidation CTx with a reduction of doses. Among these patients, 5 died: 3 of progressive disease and 2 of treatment-related causes. Among the 210 patients randomized to RTx, 195 patients received this therapy and 15 did not for the following reasons: patient refusal (n = 6), protocol violation (n = 3), and progressive disease immediately after randomization (n = 6). A total of 183 patients (87%) received RTx to the planned volumes (mantle field, paraaortic, and spleen areas, n = 96; mantle field, inverted Y, and spleen areas, n = 87). The planned volumes of RTx were partially delivered in 12 patients (mantle, n = 8; sub-diaphragmatic, n = 4) for the following reasons: protocol violation of RTx (n = 5), progressive disease during RTx (n = 4), premature termination at the patient's request (n = 2), and toxic death (n = 1). The planned radiation doses were not completed in 31 patients (15%) who experienced hematologic toxicity. Finally, 152 patients (72%) received radiation according to the assigned volumes and doses, and 58 patients (28%) did not. Among these 58 patients, 18 died: 13 of progressive disease, 2 of treatment-related causes, 2 of second cancers, and 1 of intercurrent disease. After consolidation treatment, 394 of 418 randomized patients (94%) achieved CR, 2 patients achieved PR, 19 patients suffered early disease progression, and 3 patients died during consolidation RTx. The CR rate by treatment arms (induction CTx plus consolidation treatment) is shown in Table 2.
Estimated outcomes of 418 patients achieving CR or PR of at least 75% after induction CTx and randomized for consolidation treatment
| Treatment . | Patients at risk (%) . | CR rate (%) . | 5-year disease-free survival*,† . | 5-year event-free survival‡ . | 5-year overall survival† . |
|---|---|---|---|---|---|
| MOPP/ABV × 8 | 92 | 91 | 80 ± 4 | 74 ± 5 | 85 ± 4 |
| ABVPP × 8 | 116 | 99 | 68 ± 5 | 67 ± 5 | 94 ± 3 |
| MOPP/ABV × 6 + RTx | 114 | 95 | 82 ± 5 | 78 ± 5 | 88 ± 4 |
| ABVPP × 6 + RTx | 96 | 91 | 75 ± 5 | 66 ± 5 | 78 ± 4 |
| Total | 418 | 94 | 75 ± 2 | 62 ± 2 | 79 ± 2 |
| Treatment . | Patients at risk (%) . | CR rate (%) . | 5-year disease-free survival*,† . | 5-year event-free survival‡ . | 5-year overall survival† . |
|---|---|---|---|---|---|
| MOPP/ABV × 8 | 92 | 91 | 80 ± 4 | 74 ± 5 | 85 ± 4 |
| ABVPP × 8 | 116 | 99 | 68 ± 5 | 67 ± 5 | 94 ± 3 |
| MOPP/ABV × 6 + RTx | 114 | 95 | 82 ± 5 | 78 ± 5 | 88 ± 4 |
| ABVPP × 6 + RTx | 96 | 91 | 75 ± 5 | 66 ± 5 | 78 ± 4 |
| Total | 418 | 94 | 75 ± 2 | 62 ± 2 | 79 ± 2 |
Values are means ± SE.
394 patients in CR were analyzed.
P = .01 for the comparison among the 4 treatment arms.
P = .07 for the comparison among the 4 treatment arms.
Disease-free survival
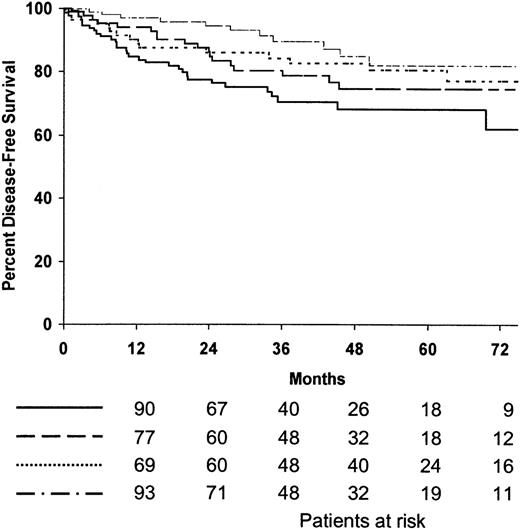
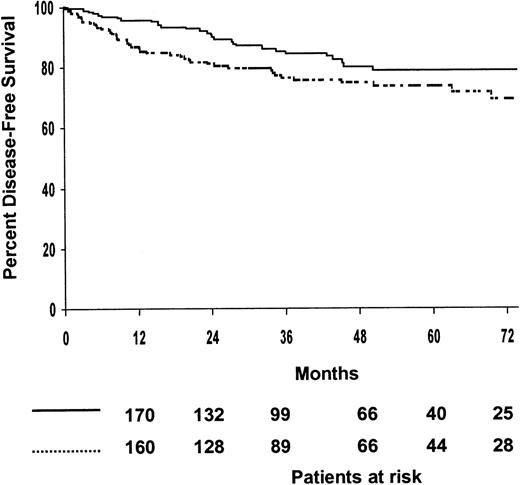
Among the 394 patients who achieved CR, 75% were predicted to be alive without disease after 5 years (95% confidence interval [CI], 70%-80%). The 5-year disease-free survival estimates for patients treated with MOPP/ABV or ABVPP were 81% (95% CI, 75%-87%) and 71% (95% CI, 65%-77%), respectively (P = .009). The 5-year disease-free survival estimates for the 4 treatment arms are shown in Table 2, and disease-free survival curves are shown in Figure2; the 4 treatment arms differed significantly (P = .01). The 5-year disease-free survival estimates of patients treated with consolidation CTx or RTx were 74% (95% CI, 68%-80%) and 79% (95% CI, 73%-85%), respectively; no difference was observed between the 2 consolidation arms when randomized patients were stratified according to the induction regimen (P = .07) (Figure 3). The predicted 5-year disease-free survival estimate was 75% (95% CI, 71%-79%) for the 102 patients with bulky disease who achieved CR and 76% (95% CI, 68%-84%) for the 292 patients without bulky disease who achieved CR (P = .7). No difference was observed between the 2 consolidation arms when randomized patients with bulky disease were stratified as a function of the regimen (P = .5). According to univariate analysis, 2 factors adversely affected disease-free survival: hemoglobin less than 10.5 g/L (P = .01) and inguinal involvement (P = .05).
Estimated disease-free survival according to treatment arms.
(·····) MOPP/ABV, 8 cycles (patients at risk, n = 84; relapse or death, n = 15; 5-year estimate, 80%); () ABVPP, 8 cycles (patients at risk, n = 115; relapse or death, n = 31; 5-year estimate, 68%); (—·—·) MOPP/ABV, 6 cycles plus RTx (patients at risk, n = 108; relapse or death, n = 11; 5-year estimate, 82%); (———) ABVPP, 6 cycles plus RTx (patients at risk, n = 7; relapse or death, n = 18; 5-year estimate, 75%).P = .01.
Estimated disease-free survival according to treatment arms.
(·····) MOPP/ABV, 8 cycles (patients at risk, n = 84; relapse or death, n = 15; 5-year estimate, 80%); () ABVPP, 8 cycles (patients at risk, n = 115; relapse or death, n = 31; 5-year estimate, 68%); (—·—·) MOPP/ABV, 6 cycles plus RTx (patients at risk, n = 108; relapse or death, n = 11; 5-year estimate, 82%); (———) ABVPP, 6 cycles plus RTx (patients at risk, n = 7; relapse or death, n = 18; 5-year estimate, 75%).P = .01.
Estimated disease-free survival according to consolidation treatments.
(····) Chemotherapy (patients at risk, n = 199; relapse or death, n = 46; 5-year estimate, 74%); () radiotherapy (patients at risk, n = 195; relapse or death, n = 29; 5-year estimate, 79%). P = .07.
Estimated disease-free survival according to consolidation treatments.
(····) Chemotherapy (patients at risk, n = 199; relapse or death, n = 46; 5-year estimate, 74%); () radiotherapy (patients at risk, n = 195; relapse or death, n = 29; 5-year estimate, 79%). P = .07.
Event-free survival
The overall event-free survival probability for the entire population was 62% at 5 years (95% CI, 58%-66%). The 5-year event-free survival estimate for patients treated with MOPP/ABV or ABVPP was 66% (95% CI, 60%-72%) and 56% (95% CI, 50%-62%), respectively (P = .1). Predicted event-free survival did not differ significantly between the 4 treatment arms (Table 2;P = .07).
Overall survival
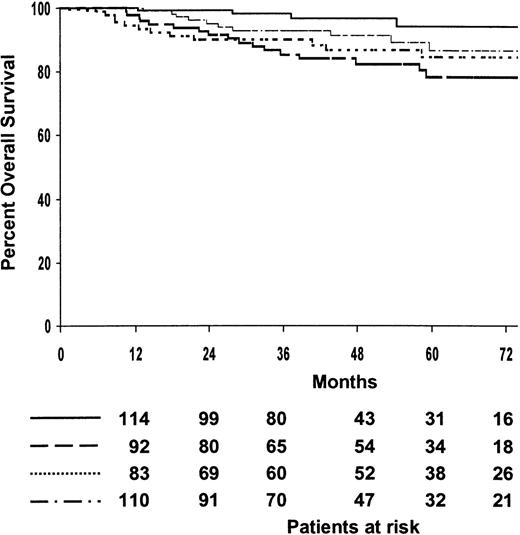
The median follow-up of patients who survived was 48 months (range, 8-109 months), with 79% of them predicted to be alive at 5 years (95% CI, 75%-84%). The 5-year survival estimate of patients treated with MOPP/ABV or ABVPP was 79% (95% CI, 73%-85%) and 80% (95% CI, 74%-86%), respectively (P = .4). The 5-year survival estimates for the 4 treatment arms are shown in Table 2, and overall survival curves are shown in Figure 4. The 4 treatment arms differed significantly (P = .01). After patients were analyzed according to induction treatment arms, no significant difference was observed for survival after 8 cycles of MOPP/ABV or 6 cycles of MOPP/ABV plus RTx, with a 5-year survival probabilities of 85% (95% CI, 77%-93%) and 88% (95% CI, 80%-96%), respectively (P = .2). In contrast, survival differed significantly after 8 cycles of ABVPP or 6 cycles of ABVPP plus RTx, with predicted 5-year survival estimates of 94% (95% CI, 88%-100%) and 78% (95% CI, 68%-88%), respectively (P = .002). The estimated 5-year survival was 80% (95% CI, 72%-88%) for the 137 patients with bulky disease and 80% (95% CI, 76%-84%) for the 396 patients without bulky disease (P = .9). Ninety patients died (49 in the MOPP/ABV group and 41 in the ABVPP group). The major causes of death were HD progression (47%) and treatment-related complications (23%) during initial treatment or salvage therapy. The causes of death are given in detail in Table 3. Intercurrent causes of death were as follows: infection, 3; respiratory insufficiency, 1; suicide, 1; urologic fistula, 1; unknown, 6.
Estimated overall survival according to treatment arms.
(····) MOPP/ABV, 8 cycles (patients at risk, n = 92; deaths, n = 12; 5-year estimate, 85%); () ABVPP, 8 cycles (patients at risk, n = 116; deaths, n = 4; 5-year estimate, 94%); (—·—·) MOPP/ABV, 6 cycles plus RTx (patients at risk, n = 114; deaths, n = 9; 5-year estimate, 88%); (———) ABVPP, 6 cycles plus RTx (patients at risk, n = 96; deaths, n = 17; 5-year estimate, 78%).P = .01.
Estimated overall survival according to treatment arms.
(····) MOPP/ABV, 8 cycles (patients at risk, n = 92; deaths, n = 12; 5-year estimate, 85%); () ABVPP, 8 cycles (patients at risk, n = 116; deaths, n = 4; 5-year estimate, 94%); (—·—·) MOPP/ABV, 6 cycles plus RTx (patients at risk, n = 114; deaths, n = 9; 5-year estimate, 88%); (———) ABVPP, 6 cycles plus RTx (patients at risk, n = 96; deaths, n = 17; 5-year estimate, 78%).P = .01.
Causes of death as a function of randomized-or-not consolidation treatment
| . | Patients not randomized to consolidation treatment (n) . | Patients randomized to consolidation treatment (n) . | |
|---|---|---|---|
| CTx . | RTx . | ||
| Patients at risk | 115 | 208 | 210 |
| Died | 47 | 16 | 27 |
| Causes of death | |||
| Related to HD | |||
| Failure to achieve CR | 25 | 3 | 8 |
| After relapse | 1 | 2 | 3 |
| Related to initial treatment | 9 | 2 | 3 |
| Related to salvage therapy | 2 | 2 | 3 |
| Second cancer | |||
| Solid tumor | 3 | 2 | 3 |
| Non-Hodgkin's lymphoma | 1 | 1 | 3 |
| Acute leukemia | 2 | — | — |
| Intercurrent disease | 4 | 4 | 4 |
| . | Patients not randomized to consolidation treatment (n) . | Patients randomized to consolidation treatment (n) . | |
|---|---|---|---|
| CTx . | RTx . | ||
| Patients at risk | 115 | 208 | 210 |
| Died | 47 | 16 | 27 |
| Causes of death | |||
| Related to HD | |||
| Failure to achieve CR | 25 | 3 | 8 |
| After relapse | 1 | 2 | 3 |
| Related to initial treatment | 9 | 2 | 3 |
| Related to salvage therapy | 2 | 2 | 3 |
| Second cancer | |||
| Solid tumor | 3 | 2 | 3 |
| Non-Hodgkin's lymphoma | 1 | 1 | 3 |
| Acute leukemia | 2 | — | — |
| Intercurrent disease | 4 | 4 | 4 |
Univariate analysis identified the following parameters as adversely influencing overall survival: age of at least 45 years (P = .0001), performance status of at least 2 (P = .0001), at least 2 extranodal sites (P = .0001), serum albumin level less than 40 g/L (P = .0001), hemoglobin level less than 10.5 g/L (P = .0001), lymphocyte count less than 0.6 × 109/L or less than 8% of white blood cells (WBCs) (P = .003), bone marrow involvement (P = .004), systemic symptoms (P = .007), LDH level above normal (P = .01), inguinal involvement (P = .02), WBC of at least 15 × 109/L (P = .03). When these parameters were entered in a Cox model, only age (P = .0001), serum albumin (P = .04), and performance status (P = .04) remained statistically significant. A multivariate analysis including the variables of the international prognostic score for advanced HD19 and the consolidation treatment was undertaken to interpret the difference between survival rates after ABVPP regimen and consolidation treatment. Regression analysis using a Cox model according to the induction regimen for overall survival showed that, for patients treated with ABVPP who were subsequently randomized to receive RTx, the relative risk estimate was 4.37 times higher than that for patients treated with ABVPP who were then randomized to received CTx (P = .01; 95% CI, 1.39%-13.7%). For patients treated with MOPP/ABV, RTx provided no significant benefit (relative risk = 0.75; P = .55; 95% CI, 0.30%-1.89%). These results suggested an interaction between the induction regimen and consolidation treatment. A Cox model including the interaction variable confirmed a significant qualitative interaction between induction and consolidation treatments (likelihood ratio test,P = .03).
Toxicity
The short-term adverse events of induction and consolidation treatments are summarized in Table 4. During RTx, thrombocytopenia (less than 50 × 109/L) was seen in 22% of the patients, resulting in a temporary postponement (2-8 weeks) of treatment for 23% of the patients and discontinuation for 16% of patients who did not receive the planned treatment. Fourteen patients suffered grade 5 (fatal) toxicities (ABVPP group, n = 6; MOPP/ABV group, n = 8). Eleven patients died during induction CTx (ABVPP group, n = 3; MOPP/ABV group, n = 8), and 3 patients died during RTx after ABVPP. Among the 14 treatment-related deaths, 10 resulted from infection and 12 occurred in patients at least 45 years old.
Toxicity of induction and consolidation treatments
| Toxicity . | WHO grade . | Patients with adverse events (%) . | |||
|---|---|---|---|---|---|
| Induction . | Consolidation . | ||||
| MOPP/ABV . | ABVPP . | Chemotherapy . | RTx . | ||
| Patients assessable | 208/2384-150 | 210/2464-150 | 150/1834-150 | 177 | |
| Neutropenia | 3-4 | 54 | 42 | 33 | 154-151 |
| Thrombocytopenia | 3-4 | 9 | 3 | 7 | 22 |
| Anemia | 3-4 | 11 | 2 | 4 | 3 |
| Infection | 1-2 | 22 | 21 | 10 | 9 |
| 3-4 | 10 | 7 | 2 | 1 | |
| Nausea/vomiting | 3-4 | 9 | 7 | 3 | 11 |
| Peripheral neuropathy | 1-2 | 24 | 11 | 10 | — |
| 3 | 2 | 1 | — | — | |
| Alopecia | 3-4 | 18 | 14 | — | — |
| Toxicity . | WHO grade . | Patients with adverse events (%) . | |||
|---|---|---|---|---|---|
| Induction . | Consolidation . | ||||
| MOPP/ABV . | ABVPP . | Chemotherapy . | RTx . | ||
| Patients assessable | 208/2384-150 | 210/2464-150 | 150/1834-150 | 177 | |
| Neutropenia | 3-4 | 54 | 42 | 33 | 154-151 |
| Thrombocytopenia | 3-4 | 9 | 3 | 7 | 22 |
| Anemia | 3-4 | 11 | 2 | 4 | 3 |
| Infection | 1-2 | 22 | 21 | 10 | 9 |
| 3-4 | 10 | 7 | 2 | 1 | |
| Nausea/vomiting | 3-4 | 9 | 7 | 3 | 11 |
| Peripheral neuropathy | 1-2 | 24 | 11 | 10 | — |
| 3 | 2 | 1 | — | — | |
| Alopecia | 3-4 | 18 | 14 | — | — |
Number of patients assessable for hematologic/nonhematologic toxicity.
Percentage of leukopenic patients is given for RTx consolidation.
At the time of the analysis, 22 patients had developed a second malignancy. Six second cancers were diagnosed within 12 months after the initial diagnosis of HD (3 with thyroid cancer, 1 with lung cancer, 1 with colon cancer, and 1 with myelodysplastic syndrome) and had no influence on treatment decisions for 4 of them. Sixteen other second cancers were diagnosed 14 to 75 months after the initial diagnosis (3 with colorectal cancer, 3 with lung cancer, 2 with head and neck cancer, 1 with thyroid cancer, 4 with acute myeloid leukemia, 2 with non-Hodgkin's lymphoma, and 1 with cutaneous epithelioma). Acute myeloid leukemia occurred 27, 34, 40, and 54 months after the initial diagnosis; 3 of these patients were alive without disease at the end of the study period, and 1 patient died of veno-occlusive disease after allogeneic bone marrow transplant. The second malignancy rate was 3% for the 14 patients who had received the planned treatment (ABVPP, n = 9, and MOPP/ABV, n = 5; CTx alone, n = 7, and RTx, n = 7). Other complications were severe infections in 11 cases, prolonged cytopenia in 10, dyspnea or functional pulmonary impairment in 10, myocardial infarction in 2, angina pectoris in 3, clinical hypothyroidism in 6, femoral necrosis in 4, and peripheral neuropathy in 4.
Discussion
This randomized study does not demonstrate any advantage of RTx over CTx as consolidation treatment at the time of CTx-induced CR or good PR in patients with stage IIIB/IV HD. After MOPP/ABV regimen, similar disease-free survival and overall survival rates were observed in both CTx and RTx consolidation arms. After the ABVPP regimen, no statistically significant difference could be detected, in terms of disease-free survival, between the consolidation treatments; however, an interaction between induction and consolidation treatments was significant for overall survival. The comparison between 2 doxorubicin-containing regimens showed that ABVPP was inferior to MOPP/ABV in terms of disease-free survival, but no differences were found in terms of event-free survival and overall survival.
Our study contributes to the understanding of CTx versus RTx as consolidation treatment for patients with advanced-stage HD who responded to a doxorubicin-containing regimen. After the MOPP/ABV regimen, the 5-year disease-free survival estimates were similar in both CTx and RTx arms. The lower disease-free survival estimates after ABVPP alone, compared to combined modality treatments, do not reflect an advantage of RTx but, rather, the effect of the ABVPP regimen given alone. Indeed, the disease-free survival estimate after 6 cycles of ABVPP plus RTx was similar to that of the MOPP/ABV regimen with CTx or RTx as consolidation. After 8 cycles of ABVPP, the estimated disease-free survival was lower than those for the 3 other treatment arms. The role of the induction regimen suggested by our results may be explained by the superiority of the 7-drug regimen over the 4-drug regimen. With respect to the endpoint overall survival, no significant difference could be detected between CTx and RTx consolidation for patients treated with MOPP/ABV. In contrast, patients who received ABVPP and RTx consolidation had poorer overall survival than those treated with ABVPP alone. The initial randomization between 2 induction chemotherapy regimens resulting in a 4-arm study and the outcome of patients treated with ABVPP alone confound the interpretation of results related to the comparison between RTx and CTx consolidation treatments. A significant qualitative interaction between the induction and consolidation treatments was found for overall survival, with significantly better overall survival only for the group that received ABVPP alone. The interaction indicates that the effectiveness of consolidation treatment varies according to the induction regimen. A Cox model including the interaction variable proved that RTx offers no survival benefit over CTx consolidation after doxorubicin-induced CR for patients with advanced HD.
The choice of (sub)total nodal irradiation was based on our previous experience with stage IIIB or IV HD20 and the results of retrospective studies.7 Ten years after our study was initiated, we consider that (sub)total nodal irradiation, which concerns a larger volume than involved-field irradiation, can no longer be recommended because of its long-term toxicity, particularly the risk of second malignancies.
The results of this randomized study support previously published data comparing radiation with further CTx as consolidation. Trials that compared 6 cycles plus low-dose involved-field radiation and CTx alone (8-12 cycles) failed to demonstrate the superiority of combined treatment.12,13 Trials that compared CTx, in most cases 6 cycles, followed by extended-field radiation (30-40 Gy) or CTx alone (10-12 cycles), showed no advantage of radiotherapy with respect to overall survival.8,9 Another study showed a benefit in terms of overall survival after CTx alone for stage IIIB patients.11 Our CTx-alone arm consisted of fewer CTx cycles than other trials that compared CTx plus extended-field radiation. Our results indicate that 8 cycles of MOPP/ABV are equivalent to 6 cycles followed by RTx for patients who achieved CR after 6 cycles, and they suggest that 8 cycles of a doxorubicin-containing regimen are sufficient. Moreover, prolongation of the duration of CTx beyond 2 or 3 cycles after apparent CR did not improve tumor control and overall survival for patients with advanced disease.21 The possibility of treating patients with stage IIIB or IV HD with 6 cycles of a doxorubicin-containing regimen remains to be demonstrated. The Cancer and Leukemia Group B study compared 6 and 12 cycles of CVPP (CCNU, vinblastine, procarbazine, prednisone) and found no statistical difference for disease-free survival and overall survival.13 However, 6 versus 8 cycles were not compared in a randomized study. Most randomized trials that compared CTx alone and the same CTx plus additional radiation used 6 cycles of MOPP or MOPP variants.5,9,10 A meta-analysis of the additional radiation-design trials showed improved tumor-control rates after radiotherapy but no difference with respect to overall survival.14 Other observations were consistent with an increased risk of relapse when treatment was discontinued in patients who had achieved CR after 6 cycles of a doxorubicin-containing regimen.5,12,22 The use of 6 cycles has not yet been supported by clinical trial data and cannot be considered as standard treatment for patients with advanced HD. Our policy is in agreement with the conclusion of the recent meta-analysis, which defined 8 cycles as appropriate for CTx administered alone.14 However, we strongly recommend early restaging after induction CTx to attempt to treat poor responders with salvage therapy and patients achieving CR with consolidation CTx.
Finally, we conclude that there are no major differences between RTx and consolidation CTx for patients with advanced HD who have achieved a response of at least 75% after 6 cycles of a doxorubicin-containing regimen. The results of our trial, the uncertainty of attaining a prolonged second remission for patients who relapse after CTx and RTx, and the possible long-term effects of radiotherapy lead us to prefer 8 cycles of a doxorubicin-containing regimen as standard treatment for patients with stage IIIB or IV Hodgkin's lymphomas when CR has been obtained after 6 cycles.
Acknowledgments
The authors thank the following clinicians and pathologists who actively participated in this study: C. Allard, M. André, R. Angonin, D. Assouline, B. Audhuy, G. Auzanneau, J.C. Barats, Y. Bastion, P. Bensimon, F. Berger, P. Bey, P. Biron, M. Blanc, M. Bontemps, D. Bordessoule, A. Bosly, K. Bouabdallah, S. Boucheron, T. Bouillet, O. Boulat, P. Brice, J. Brière, J.C. Brouet, D. Caillot, C. Carrie, R.O. Casasnovas, S. Castaigne, G. Catanzano, D. Cazals-Hatem, F. Charlotte, A.M. Chesneau, B. Christian, J.P. Clauvel, B. Coiffier, P. Colin, F. Cosnard, M.F. D'Agay, J. d'Anjou, E. Deconinck, A. Delannoy, M. Delos, H. Demeaux, A. de Mascarel, T. De Revel, A. Devidas, J. Diebold, M. Diviné, C. Doyen, M. Dray, G. Dupont, B. Dupriez, E. Dupuy, C. Duval, B. Duvert, M. Echard, J.C. Eisenmann, J.M. Emberger, P. Fargeot, J.P. Fermand, A. Ferrant, D. Fière, M. Flesch, M. Floiras, J. Frija, N. Froment, J. Gabarre, P. Gaulard, H. Gautier, J.P. Gérard, C. Gisselbrecht, H. Guy, D. Guyotat, C. Haioun, Hamels, C. Hennequin, M. Janvier, J. Jaubert, R. Jeandel, Y. Kerneis, J.P. Knop, F. Kohser, C. Lavignac, P. Lederlin, R. Lefur, E. Legouffe, R. Leloup, M. Lenoble, P. Maingon, G. Marit, J.P. Marolleau, C. Martin, C. Maylin, V. Meignin, C. Merignargues, J.L. Michaut, J.M. Micléa, P. Mineur, M. Monconduit, P. Morel, F. Morvan, G. Nédellec, H. Noel, E. Oksenhendler, P.Y. Peaud, T. Petrella, C. Platini, J.P. Pollet, M. Raphael, M. Raymond-Gelle, O. Reman, P. Renaudier, M. Resbeut, F. Reyes, B. Richard, M. Rochet, B. Roullet, J.F. Rossi, C. Rozec, A. Rozenbaum, B. Salles, G. Salles, H. Schill, M. Simon, P. Solal-Céligny, E. Solary, A. Stamattoullas, P. Thirion, A. Thyss, J.D. Tigaud, H. Tilly, J. Troncy, L. Vandenbossche, B. Velay, M. Vincent, L. Voillat, and J.M. Zini. We are indebted to Catherine Balmale for assistance with data management, Nicolas Mounier for helpful comments on the statistical analyses, and Janet Jacobson for editing the English article.
Reprints:Christophe Fermé, Groupe d'études des Lymphomes de l'Adulte, Centre Hayem, Hôpital Saint-Louis, 1 avenue C. Vellefaux, 75475 Paris Cedex 10, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.