Abstract
In a 20-year period, 223 patients (median age, 64.8 years) with myelofibrosis with myeloid metaplasia (MMM) had therapeutic splenectomy at our institution. Primary indications for surgery were transfusion-dependent anemia (45.3%), symptomatic splenomegaly (39.0%), portal hypertension (10.8%), and severe thrombocytopenia (4.9%). Operative mortality and morbidity rates were 9% and 31%, respectively. The 203 survivors of surgery had a median postsplenectomy survival time (PSS) of 27 months (range, 0-155). Among preoperative variables, thrombocytopenia (platelet count less than 100 × 109/L) and nonhypercellular bone marrow were identified as independent risk factors for decreased PSS. Durable remissions in constitutional symptoms, transfusion-dependent anemia, portal hypertension, and severe thrombocytopenia were achieved in 67%, 23%, 50%, and 0% of the patients, respectively. Histologic or cytogenetic features of bone marrow obtained before splenectomy did not predict a response in cytopenias. After splenectomy, substantial enlargement of the liver and marked thrombocytosis occurred in 16.1% and 22.0% of the patients, respectively. The thrombocytosis was associated with an increased risk of perioperative thrombosis and decreased PSS. The rate of blast transformation (BT) was 16.3%, and the risk of BT was higher in the presence of increased spleen mass and preoperative thrombocytopenia. However, the PSS of patients with BT was not significantly different from that of patients without BT. We conclude that presplenectomy thrombocytopenia in MMM may be a surrogate for advanced disease and is associated with an increased risk of BT and inferior PSS. However, the development of BT after splenectomy may not affect overall survival and does not undermine the palliative role of the procedure for the other indications.
The term “myelofibrosis with myeloid metaplasia” (MMM) includes agnogenic, postthrombocythemic, and postpolycythemic myeloid metaplasia.1 The disorder is a clonal stem cell disease in which reactive bone marrow fibrosis develops, mediated by megakaryocyte-derived fibrogenic cytokines.2-4 Replacement of normal hematopoietic tissue with collagen fibrosis contributes to the underlying ineffective hematopoiesis and erythroid hypoplasia.5,6 Morphologic and kinetic data indicate that bone marrow fibrosis in MMM is accompanied by extramedullary hematopoiesis, which causes progressive enlargement of the spleen and liver.7 The resultant organomegaly causes pain and early satiety and promotes weight loss, portal hypertension, and profound fatigue. Furthermore, splenomegaly leads to exacerbation of cytopenias through sequestration and destruction of hematopoietic elements.
The reported median survival time of patients with MMM ranges from 3 to 8 years.5,8-11 Treatment is largely palliative and has not been shown to improve survival.12 Anemia and thrombocytopenia may improve transiently with drug therapy. Androgen preparations and corticosteroids are the most effective agents, whereas the benefit of α-interferon and erythropoietin is limited.13-18 Early results from the use of allogeneic bone marrow transplantation are promising.19,20 Nevertheless, the mainstays of current therapy are transfusions of red blood cells and administration of hydroxyurea to reduce spleen size.21
Although bone marrow erythroid hypoplasia and ineffective hematopoiesis are primarily responsible for the cytopenias occurring in MMM,6 cytopenias in chronic myeloproliferative diseases are exacerbated by marked splenomegaly, even in the absence of associated myelofibrosis.22 Removal of the enlarged spleen in patients with MMM has been shown to decrease plasma volume and peripheral hemolysis and perhaps to increase erythropoietic activity of the bone marrow.23 Although hematopoietic stem cells circulate in the peripheral blood of patients with MMM, they are usually absent in splenic tissue, which may instead facilitate their differentiation.24 Additional evidence suggests that, in this situation, the hematopoietic role of the spleen may be limited to the expansion of committed progenitors and not necessarily their terminal differentiation.25-29 Therefore, a direct reduction of excess splenic tissue in patients with MMM may have a favorable effect on peripheral cytopenia. This reduction can be accomplished by either external beam irradiation30 or splenectomy.31-44
Removal of the spleen as a therapeutic intervention in MMM has been investigated. Before 1940, the procedure was considered dangerous and reportedly had an operative mortality rate of 30% to 50%.37,45 Recently, however, improvements in surgical technique, patient selection, and perioperative care have made splenectomy in patients with MMM a safer and more accepted practice, with reported perioperative mortality rates of 7% to 15%.31-34,38 On the other hand, splenectomy has not been shown to improve overall survival of patients with MMM,46and the palliative benefit of the procedure should be weighed against the risk of perioperative and long-term complications.
In this report, we describe a single-institution experience with 223 consecutive cases of therapeutic splenectomy in patients with MMM. We provide overall operative mortality and morbidity rates, estimates of postsplenectomy survival and complication rates, and analysis of benefits and prognostic factors.
Patients and methods
Patients
After approval for this study was obtained from our institutional review board, the study patients were identified through the use of a comprehensive institutional database of medical diagnoses and procedures. The medical records of all patients with MMM who had splenectomy during the period of 1976 through 1996 were reviewed. The diagnosis of MMM was confirmed on the basis of traditional diagnostic criteria that included bone marrow fibrosis associated with splenomegaly and leukoerythroblastosis.47 48 Patients with bone marrow fibrosis due to other clonal or nonclonal disorders were excluded. These included patients with myelodysplastic syndrome, acute myelofibrosis, or chronic myelogenous leukemia.
Pertinent preoperative variables were recorded for all patients (Table1). In addition, postoperative variables were recorded 1, 6, and 12 months after splenectomy and at the time of the latest contact with the patient. Patients were categorized according to 1 of 4 primary indications for splenectomy: (1) symptomatic splenomegaly (severe mechanical discomfort or pain, with or without constitutional symptoms); (2) severe anemia (hemoglobin level less than 90 g/L or a need for transfusion of red blood cells); (3) symptoms and signs of portal hypertension; and (4) severe thrombocytopenia (platelet count less than 20 × 109/L). Patients were assigned a prognostic score based on the findings of Dupriez et al.8 A score of 0 was assigned for a hemoglobin level of more than 100 g/L and a white blood cell count between 4 × 109/L and 30 × 109/L, a score of 1 was assigned for either a hemoglobin level of less than 100 g/L or a white blood cell count of more than 30 × 109/L or less than 4 × 109/L, and a score of 2 was assigned if both the hemoglobin and white blood cell values were in those aberrant ranges.
Preoperative clinical variables in 223 patients with MMM who underwent therapeutic splenectomy
| Variable . | Value . |
|---|---|
| Age, median (range), y | |
| At diagnosis of MMM | 61.4 (18.6-82) |
| At time of splenectomy | 64.8 (18.6-82) |
| Sex: male/female, no. (%) | 121 (54.3)/102 (45.7) |
| Type of MMM, no. (%) of patients | |
| AMM | 159 (71.3) |
| PPMM | 48 (21.5) |
| PTMM | 16 (7.2) |
| Palpable spleen size, median (range), cm below LCM | 20 (0-32) |
| Spleen mass, median (range), g | 2700 (380-7735) |
| Palpable liver size, median (range), cm below RCM | 3 (0-15) |
| Ascites, no. (%) of patients | |
| Yes | 26 (11.7) |
| No | 197 (88.3) |
| Red blood cell transfusion requirement, no. (%) of patients | |
| Yes | 75 (33.6) |
| No | 148 (66.4) |
| No. of transfusion units/mo, median (range) | 2 (1-8) |
| Hemoglobin, median (range), g/L | 104 (49-178) |
| White blood cell count, median (range), × 109/L | 12.5 (0.9-163) |
| Platelet count, median (range), × 109/L | 140 (2-770) |
| Circulating blasts, median (range), % | 1 (0-14) |
| Alkaline phosphatase, median (range), U/L | 204 (85-787) |
| Aspartate aminotransferase, median (range), U/L | 26 (10-139) |
| Time from BM biopsy to splenectomy, median (range), mo (n = 206) | 1.2 (0-139) |
| Presplenectomy cellularity, no. (%) of patients (n = 199) | |
| Hypocellular | 50 (25.1) |
| Normocellular | 13 (6.5) |
| Hypercellular | 136 (68.4) |
| Presplenectomy % of myeloblasts in marrow, no. (%) of patients (n = 177) | |
| < 5% | 164 (92.7) |
| ≥ 5% | 13 (7.3) |
| Presplenectomy fibrosis grade on reticulin staining, no. (%) of patients (n = 162) | |
| 1 | 8 (4.9) |
| 2 | 40 (24.7) |
| 3 | 70 (43.2) |
| 4 | 44 (27.2) |
| Time from BM cytogenetic study to splenectomy, median (range), mo (n = 62) | 1 (0-72) |
| Normal cytogenetic results, no. (%) of patients | 27 (43.5) |
| Abnormal cytogenetic results, no. (%) of patients | 35 (56.5) |
| Constitutional symptoms, no. (%) of patients | |
| Fatigue | 218 (97.8) |
| Night sweats | 62 (27.8) |
| Fever | 22 (9.9) |
| Bone pain | 10 (4.5) |
| Weight loss, median (range), kg | 4.5 (0-13.6) |
| Indication for surgery, no. (%) of patients | |
| Anemia | 101 (45.3) |
| Symptomatic splenomegaly | 87 (39.0) |
| Portal hypertension | 24 (10.8) |
| Severe thrombocytopenia | 11 (4.9) |
| Time from diagnosis to splenectomy, median (range), mo (n = 223) | 24.7 (0-385) |
| Follow-up period after diagnosis, median (range), mo (n = 223) | 50.3 (0.3-479) |
| Follow-up period after splenectomy, median (range), mo (n = 201) | 14.4 (0.2-157) |
| Variable . | Value . |
|---|---|
| Age, median (range), y | |
| At diagnosis of MMM | 61.4 (18.6-82) |
| At time of splenectomy | 64.8 (18.6-82) |
| Sex: male/female, no. (%) | 121 (54.3)/102 (45.7) |
| Type of MMM, no. (%) of patients | |
| AMM | 159 (71.3) |
| PPMM | 48 (21.5) |
| PTMM | 16 (7.2) |
| Palpable spleen size, median (range), cm below LCM | 20 (0-32) |
| Spleen mass, median (range), g | 2700 (380-7735) |
| Palpable liver size, median (range), cm below RCM | 3 (0-15) |
| Ascites, no. (%) of patients | |
| Yes | 26 (11.7) |
| No | 197 (88.3) |
| Red blood cell transfusion requirement, no. (%) of patients | |
| Yes | 75 (33.6) |
| No | 148 (66.4) |
| No. of transfusion units/mo, median (range) | 2 (1-8) |
| Hemoglobin, median (range), g/L | 104 (49-178) |
| White blood cell count, median (range), × 109/L | 12.5 (0.9-163) |
| Platelet count, median (range), × 109/L | 140 (2-770) |
| Circulating blasts, median (range), % | 1 (0-14) |
| Alkaline phosphatase, median (range), U/L | 204 (85-787) |
| Aspartate aminotransferase, median (range), U/L | 26 (10-139) |
| Time from BM biopsy to splenectomy, median (range), mo (n = 206) | 1.2 (0-139) |
| Presplenectomy cellularity, no. (%) of patients (n = 199) | |
| Hypocellular | 50 (25.1) |
| Normocellular | 13 (6.5) |
| Hypercellular | 136 (68.4) |
| Presplenectomy % of myeloblasts in marrow, no. (%) of patients (n = 177) | |
| < 5% | 164 (92.7) |
| ≥ 5% | 13 (7.3) |
| Presplenectomy fibrosis grade on reticulin staining, no. (%) of patients (n = 162) | |
| 1 | 8 (4.9) |
| 2 | 40 (24.7) |
| 3 | 70 (43.2) |
| 4 | 44 (27.2) |
| Time from BM cytogenetic study to splenectomy, median (range), mo (n = 62) | 1 (0-72) |
| Normal cytogenetic results, no. (%) of patients | 27 (43.5) |
| Abnormal cytogenetic results, no. (%) of patients | 35 (56.5) |
| Constitutional symptoms, no. (%) of patients | |
| Fatigue | 218 (97.8) |
| Night sweats | 62 (27.8) |
| Fever | 22 (9.9) |
| Bone pain | 10 (4.5) |
| Weight loss, median (range), kg | 4.5 (0-13.6) |
| Indication for surgery, no. (%) of patients | |
| Anemia | 101 (45.3) |
| Symptomatic splenomegaly | 87 (39.0) |
| Portal hypertension | 24 (10.8) |
| Severe thrombocytopenia | 11 (4.9) |
| Time from diagnosis to splenectomy, median (range), mo (n = 223) | 24.7 (0-385) |
| Follow-up period after diagnosis, median (range), mo (n = 223) | 50.3 (0.3-479) |
| Follow-up period after splenectomy, median (range), mo (n = 201) | 14.4 (0.2-157) |
AMM indicates agnogenic myeloid metaplasia; PPMM, postpolycythemic myeloid metaplasia; PTMM, postthrombocythemic myeloid metaplasia; LCM, left costal margin; RCM, right costal margin; and BM, bone marrow.
Improvement in constitutional symptom status was defined as a more than 50% improvement in fatigue, a more than 50% recovery of preoperative weight loss, or disappearance of night sweats and fever. All complications occurring within the first 45 days after surgery were classified as perioperative complications. Accelerated hepatomegaly was defined as an increase in palpable liver size that extended more than 6 cm below the right costal margin (or a 6-cm increase from the baseline location in patients with palpable hepatomegaly before splenectomy). Extreme thrombocytosis was defined as a postsplenectomy platelet count of at least 600 × 109/L (or a platelet count of at least 1000 × 109/L in patients who had thrombocytosis before splenectomy). Leukemic transformation was confirmed by demonstration of more than 30% blasts in the bone marrow.
Statistical analysis
Postsplenectomy survival was defined as the interval from splenectomy to death or latest contact. In the analysis of postsplenectomy survival, an event was defined as death from any cause, unless otherwise indicated. Multiple preoperative clinical variables were studied for their influence on both perioperative complications and overall postsplenectomy survival. Univariate analyses to assess the independent effects of categorical preoperative variables on postsplenectomy survival were performed by creating survival curves with use of the Kaplan-Meier method and comparing these with 2-sided log-rank statistics. Univariate analysis to assess the independent effects of continuous preoperative variables on postsplenectomy survival was done with the Cox proportional hazards method. For analyses other than postsplenectomy survival, univariate logistic regression models were used to assess the association between binary dependent variables and continuous variables.
The Spearman rank correlation coefficient was used for a pair-wise comparison of the strength of the association between variables. A series of multivariate Cox proportional hazards regression models were generated to assess whether various factors had a significant effect on overall postsplenectomy survival and perioperative survival. Multivariate logistic regression models were generated to assess whether various factors had a significant effect on bleeding, thrombosis, leukemia, hepatomegaly, and thrombocytosis. The multivariate model-building process involved examining models proposed by forward-selection and backward-elimination procedures as well as models identified by the score method that contained 2, 3, and 4 variables. The findings from the score method were compared with those from the forward-selection and backward-elimination model-building procedures to identify subsets of factors with possible clinical relevance. All data were analyzed by using SAS software (SAS, Cary, NC).
Results
Patient characteristics
A total of 223 patients with MMM had therapeutic splenectomy at our institution from 1976 through 1996. Table 1 shows their clinical characteristics at the time of splenectomy. Most patients (71.3%) had agnogenic myeloid metaplasia; the rest had either postpolycythemic myeloid metaplasia or postthrombocythemic myeloid metaplasia. The most common indications for surgery were anemia (45.3%) and symptomatic splenomegaly (39.0%). Risk stratification according to the Dupriez-based prognostic scoring system both at the time of initial diagnosis and at the time of splenectomy is shown in Table2. Splenectomy was performed a median of 24.7 months (range, 0-385) after the initial diagnosis. The interval was significantly shorter in patients with a prognostic score of 2 at diagnosis than in those with a score of zero or 1. The median (range) intervals from diagnosis to splenectomy, according to indication for surgery, were 21.7 months (0-184) for anemia, 29.5 months (0.1-385) for symptomatic splenomegaly, 24.3 months (0.1-97) for severe thrombocytopenia, and 52.9 months (0.5-188) for portal hypertension (P = .69).
Prognostic stratification at initial diagnosis and at time of splenectomy and its influence on time to splenectomy and survival in 223 patients with MMM
| Dupriez score* . | No.of patients . | Median time to splenectomy, mo . | P value . | Median survival after diagnosis, mo . | P value . | Median survival after splenectomy, mo . | P value . |
|---|---|---|---|---|---|---|---|
| At diagnosis | |||||||
| 0 | 95 | 30 | 90 | 26 | |||
| 1 | 88 | 24 | 66 | 22 | |||
| 2 | 36 | 11 | < .01 | 62 | .41 | 24 | .90 |
| Total | 219 | ||||||
| At splenectomy | |||||||
| 0 | 51 | NA | 99 | 26 | |||
| 1 | 109 | NA | 69 | 24 | |||
| 2 | 62 | NA | 62 | 0.61 | 26 | .86 | |
| Total | 222 |
| Dupriez score* . | No.of patients . | Median time to splenectomy, mo . | P value . | Median survival after diagnosis, mo . | P value . | Median survival after splenectomy, mo . | P value . |
|---|---|---|---|---|---|---|---|
| At diagnosis | |||||||
| 0 | 95 | 30 | 90 | 26 | |||
| 1 | 88 | 24 | 66 | 22 | |||
| 2 | 36 | 11 | < .01 | 62 | .41 | 24 | .90 |
| Total | 219 | ||||||
| At splenectomy | |||||||
| 0 | 51 | NA | 99 | 26 | |||
| 1 | 109 | NA | 69 | 24 | |||
| 2 | 62 | NA | 62 | 0.61 | 26 | .86 | |
| Total | 222 |
NA indicates not applicable.
A Dupriez score of 0 was assigned for a hemoglobin level greater than 100 g/L and a white blood cell count between 4 × 109/L and 30 × 109/L, a score of 1 for either a hemoglobin level less than 100 g/L or a white blood cell count greater than 30 × 109/L or less than 4 × 109/L, and a score of 2 if both the hemoglobin level and white blood cell count were in the aberrant ranges.
Perioperative complications
Sixty-eight patients (30.5%) had perioperative complications in the first 45 days after splenectomy; 14.8% had bleeding, 8.5% had infection, and 7.2% had thrombosis (Table3). The complications occurred a median of 3 days postoperatively and were fatal in 19 patients. Causes of perioperative death (20 patients) included bleeding in 10 patients (4.5%), infection in 6 (2.7%), thrombosis in 3 (1.3%), and acute myeloid leukemia in 1. Univariate analysis was done to determine whether any preoperative clinical variables were predictive of perioperative bleeding or thrombosis (Table4). Thrombocytopenia (platelet count < 100 × 109/L) was the only preoperative variable that was significantly correlated with postoperative thrombosis. Severe thrombocytopenia (platelet count < 50 × 109/L) and bone marrow hypocellularity or normocellularity were significantly associated with a worse perioperative survival (Table 5). The findings were similar on multivariate analysis.
Perioperative complications in 223 patients with MMM who underwent therapeutic splenectomy
| Complication . | No. (%) of patients . |
|---|---|
| Total nonfatal complications | 68 (30.5) |
| Bleeding | 33 (14.8) |
| Infection | 19 (8.5) |
| Thrombosis | 16 (7.2) |
| Total fatal complications | 20 (8.9) |
| Bleeding | 10 (4.5) |
| Infection | 6 (2.7) |
| Thrombosis | 3 (1.3) |
| Acute leukemia | 1 (0.4) |
| Complication . | No. (%) of patients . |
|---|---|
| Total nonfatal complications | 68 (30.5) |
| Bleeding | 33 (14.8) |
| Infection | 19 (8.5) |
| Thrombosis | 16 (7.2) |
| Total fatal complications | 20 (8.9) |
| Bleeding | 10 (4.5) |
| Infection | 6 (2.7) |
| Thrombosis | 3 (1.3) |
| Acute leukemia | 1 (0.4) |
Univariate analysis of preoperative variables as possible prognostic factors for 2 perioperative complications of splenectomy in 223 patients with MMM
| Variable . | Bleeding . | Thrombosis . |
|---|---|---|
| Indication for surgery | .06 | .06 |
| Type of MMM | .30 | .97 |
| Hemoglobin <90 g/L | .19 | .14 |
| White blood cell count | .06 | .45 |
| Platelet count <100 × 109/L | .38 | .02 |
| Bone marrow cellularity | .51 | .55 |
| Bone marrow blast percentage | 1.0 | 1.0 |
| Fibrosis grade on reticulin staining | .79 | .38 |
| Presplenectomy cytogenetic findings | .49 | .10 |
| Splenic mass | .28 | .17 |
| Presence or absence of circulating blasts | .76 | .89 |
| Red cell-transfusion requirement | .65 | .39 |
| Variable . | Bleeding . | Thrombosis . |
|---|---|---|
| Indication for surgery | .06 | .06 |
| Type of MMM | .30 | .97 |
| Hemoglobin <90 g/L | .19 | .14 |
| White blood cell count | .06 | .45 |
| Platelet count <100 × 109/L | .38 | .02 |
| Bone marrow cellularity | .51 | .55 |
| Bone marrow blast percentage | 1.0 | 1.0 |
| Fibrosis grade on reticulin staining | .79 | .38 |
| Presplenectomy cytogenetic findings | .49 | .10 |
| Splenic mass | .28 | .17 |
| Presence or absence of circulating blasts | .76 | .89 |
| Red cell-transfusion requirement | .65 | .39 |
All values are P values.
Univariate analysis of the correlation between preoperative variables and perioperative and overall postsplenectomy survival in 223 patients with MMM
| Preoperative variable . | Perioperative survival . | Overall postsplenectomy survival . |
|---|---|---|
| Type of MMM | .66 | .55 |
| Indication for surgery | .55 | .16 |
| Red cell-transfusion requirement | .99 | .77 |
| Hemoglobin <90 g/L | .13 | .78 |
| Platelet count <100 × 109/L | .07 | .05 |
| Platelet count <50 × 109/L | .02 | .0001 |
| Presence of circulating blasts | .83 | .70 |
| White blood cell count | .43 | .97 |
| Leukemic transformation | NA | .79 |
| Bone marrow cellularity | .004 | .006 |
| Bone marrow blast count | .61 | .55 |
| Fibrosis on reticulin staining | .67 | .55 |
| Presplenectomy cytogenetic findings | .20 | .14 |
| Splenic mass | .84 | .74 |
| Dupriez prognostic score5-150 | NA | .86 |
| Preoperative variable . | Perioperative survival . | Overall postsplenectomy survival . |
|---|---|---|
| Type of MMM | .66 | .55 |
| Indication for surgery | .55 | .16 |
| Red cell-transfusion requirement | .99 | .77 |
| Hemoglobin <90 g/L | .13 | .78 |
| Platelet count <100 × 109/L | .07 | .05 |
| Platelet count <50 × 109/L | .02 | .0001 |
| Presence of circulating blasts | .83 | .70 |
| White blood cell count | .43 | .97 |
| Leukemic transformation | NA | .79 |
| Bone marrow cellularity | .004 | .006 |
| Bone marrow blast count | .61 | .55 |
| Fibrosis on reticulin staining | .67 | .55 |
| Presplenectomy cytogenetic findings | .20 | .14 |
| Splenic mass | .84 | .74 |
| Dupriez prognostic score5-150 | NA | .86 |
All values are P values. NA indicates not applicable.
See Table 1.
Benefit analysis
Palliative outcome varied according to the primary indication for surgery (Table 6). All evaluable patients who underwent splenectomy for symptomatic splenomegaly had relief from mechanical discomfort. In addition, an improvement in constitutional-symptom status was observed in 94%, 80%, and 67% of patients at 1, 6, and 12 months, respectively, after splenectomy. Among patients who had splenectomy for anemia, 37.6% had a quantitative improvement that consisted of either having no further need for red blood cell transfusions or achieving a durable increase of more than 10 g/L in hemoglobin level. Of the 75 patients who were dependent on red blood cell transfusions at the time of surgery, 30% became independent of transfusions by 6 months postoperatively, and the benefit was sustained in 23% at latest follow-up. Twenty-four patients underwent splenectomy because of symptomatic portal hypertension, and an overall improvement was observed in 67% of these patients at 6 months and in 50% at 12 months after splenectomy. This improvement included a substantial reduction in ascites or bleeding from gastrointestinal varices. No durable benefit was observed in patients who had splenectomy for severe thrombocytopenia. Postsplenectomy improvement in anemia was not influenced by presplenectomy bone marrow cellularity, degree of collagen fibrosis, or the presence of cytogenetic abnormalities.
Palliative effect of splenectomy in 223 patients with MMM evaluated at various times postoperatively
| Variable . | 1 Month . | 6 Months . | 12 Months . | Latest contact . |
|---|---|---|---|---|
| Constitutional symptoms improved, % of patients | 94 | 80 | 67 | 44.2 |
| Patients who became red cell transfusion–independent, % (n = 75) | 30 | 30 | 23 | 23 |
| Change in monthly red cell transfusion requirement, median (range), units | −2 (−8 to 0) | −2 (−4 to +2) | −2 (−7 to +2) | 0 (−7 to +4) |
| Portal hypertension improved, % of patients (n = 24) | 84.6 | 66.7 | 50 | 50 |
| Severe thrombocytopenia improved, % of patients (n = 11) | 54 | 25 | 0 | 0 |
| Variable . | 1 Month . | 6 Months . | 12 Months . | Latest contact . |
|---|---|---|---|---|
| Constitutional symptoms improved, % of patients | 94 | 80 | 67 | 44.2 |
| Patients who became red cell transfusion–independent, % (n = 75) | 30 | 30 | 23 | 23 |
| Change in monthly red cell transfusion requirement, median (range), units | −2 (−8 to 0) | −2 (−4 to +2) | −2 (−7 to +2) | 0 (−7 to +4) |
| Portal hypertension improved, % of patients (n = 24) | 84.6 | 66.7 | 50 | 50 |
| Severe thrombocytopenia improved, % of patients (n = 11) | 54 | 25 | 0 | 0 |
Leukemic conversion
After a median follow-up of 14.4 months (range, 0.23-157) after splenectomy, blast transformation developed in 33 patients (16.3% of those who survived splenectomy). Blast transformation occurred a median of 4.9 years after the initial diagnosis of MMM (range, 0.25-12.6) and 1.9 years after splenectomy (range, 0.13-7.9). The overall postsplenectomy survival in patients in whom blast transformation developed was not significantly different from that in those who did not have leukemic transformation (median survival, 25.6 compared with 23.9 months; P = .79). Univariate analysis of preoperative clinical characteristics revealed a significant association between blast transformation on one hand and an increased splenic mass and severe thrombocytopenia (platelet count less than 50 × 109/L) on the other (Table 7). However, the presence of either cytogenetic abnormalities or a bone marrow blast percentage more than or equal to 5% was not predictive of blast transformation. The findings were similar on multivariate analysis.
Univariate analysis of correlation between preoperative variables and long-term complications in 223 patients with MMM who underwent splenectomy
| Variable . | Blast transformation . | PSH . | PST . |
|---|---|---|---|
| Indication for surgery | .88 | .67 | .42 |
| Type of MMM | .72 | .84 | .92 |
| Hemoglobin < 90 g/L | .84 | .91 | .51 |
| White blood cell count | .61 | .49 | .72 |
| Platelet count < 100 × 109/L | .31 | .20 | .00017-150 |
| Platelet count < 50 × 109/L | .02 | .92 | .0097-151 |
| Splenic mass | .03 | .19 | .72 |
| Presence of circulating blasts | .81 | .57 | .64 |
| Bone marrow cellularity | .39 | .55 | .71 |
| Bone marrow blast percentage | .38 | 1.0 | 1.0 |
| Fibrosis grade on reticulin staining | .60 | .85 | .30 |
| Presplenectomy cytogenetic abnormalities | .29 | 1.0 | .77 |
| Red cell-transfusion requirement | .71 | .91 | .56 |
| Variable . | Blast transformation . | PSH . | PST . |
|---|---|---|---|
| Indication for surgery | .88 | .67 | .42 |
| Type of MMM | .72 | .84 | .92 |
| Hemoglobin < 90 g/L | .84 | .91 | .51 |
| White blood cell count | .61 | .49 | .72 |
| Platelet count < 100 × 109/L | .31 | .20 | .00017-150 |
| Platelet count < 50 × 109/L | .02 | .92 | .0097-151 |
| Splenic mass | .03 | .19 | .72 |
| Presence of circulating blasts | .81 | .57 | .64 |
| Bone marrow cellularity | .39 | .55 | .71 |
| Bone marrow blast percentage | .38 | 1.0 | 1.0 |
| Fibrosis grade on reticulin staining | .60 | .85 | .30 |
| Presplenectomy cytogenetic abnormalities | .29 | 1.0 | .77 |
| Red cell-transfusion requirement | .71 | .91 | .56 |
All values are P values. PSH indicates postsplenectomy palpable hepatomegaly (liver palpable greater than 6 cm below the RCM); and PST, postsplenectomy thrombocytosis (platelet count greater than 600 × 109/L).
Platelet count greater than 100 × 109/L.
Platelet count greater than 50 × 109/L.
Postsplenectomy enlargement of the liver and thrombocytosis
Postsplenectomy hepatomegaly (palpable liver extending more than 6 cm below the right costal margin) was observed in 36 patients (16.1%), and in 25 of these patients, the palpable liver extended 10 cm below the right costal margin. Univariate analysis of preoperative clinical characteristics revealed no significant predictors of postsplenectomy hepatomegaly (Table 7). Furthermore, the development of postsplenectomy hepatomegaly did not result in inferior survival. Postsplenectomy thrombocytosis (platelet count greater than 600 × 109/L) was observed in 49 patients (22.0%), 13 (5.8%) of whom had a platelet count of more than 1000 × 109/L. Preoperative platelet counts of more than 50 × 109/L were predictive of postsplenectomy thrombocytosis (Table 7). Postsplenectomy thrombocytosis was associated with increased perioperative thrombosis (P = .05) and decreased overall survival (P = .01). Of the 49 patients with postsplenectomy thrombocytosis, 9 (18%) died of either thrombosis (6 patients who had stroke, pulmonary embolus, or portal vein thrombosis) or gastrointestinal bleeding (3 patients).
Survival
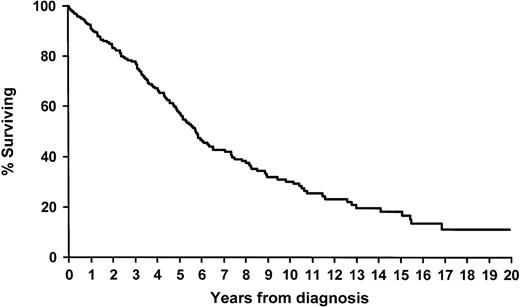
The median overall survival time was 5.8 years (range, 0-12.9) after the initial diagnosis of MMM and 2 years (range, 0-12.9) after splenectomy (Figure 1 and2). Prognostic stratification at either diagnosis or splenectomy did not reveal any significant overall or postsplenectomy survival differences (Table 2). At latest contact, a total of 132 patients (59.1%) had died. Of these, 20 (15.2%) died of perioperative complications. Causes of death not related to surgery included acute leukemia in 29 patients (22%), bleeding in 16 (12%), thrombosis in 11 (8%), liver failure in 9 (7%), infection in 6 (5%), other malignant diseases in 7 (5%), and cardiac events in 6 (5%). One patient died of cardiac extramedullary hematopoiesis. The immediate cause of death in the other patients could not be determined accurately.
Overall survival after initial diagnosis of MMM of 223 consecutively treated patients who had therapeutic splenectomy.
Overall survival after initial diagnosis of MMM of 223 consecutively treated patients who had therapeutic splenectomy.
Survival after splenectomy of 223 consecutively treated patients with MMM who had therapeutic splenectomy.
Survival after splenectomy of 223 consecutively treated patients with MMM who had therapeutic splenectomy.
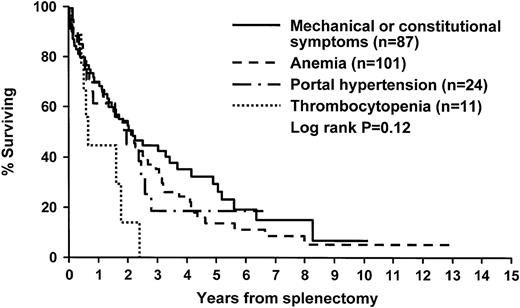
The particular subtype of MMM did not have a significant influence on either overall or postsplenectomy survival. Similarly, the postsplenectomy survival of patients with different indications for surgery was similar, although there was a trend toward inferior survival in patients with severe thrombocytopenia (Figure3). Univariate analysis involving several preoperative characteristics identified preoperative thrombocytopenia and bone marrow hypocellularity or normocellularity as the only variables predictive of decreased postsplenectomy survival (Table 5 and Figure 4). In a multivariate proportional hazards regression model, both these variables were found to be independently significant (P = .0003 and P = .01, respectively). On the other hand, postsplenectomy thrombocytosis (platelet count greater than 600 × 109/L) was associated with decreased postsplenectomy survival (P = .01).
Survival after splenectomy of patients with MMM, according to the indication for splenectomy.
Survival after splenectomy of patients with MMM, according to the indication for splenectomy.
Survival after splenectomy of patients with MMM, according to presplenectomy bone marrow cellularity.
Survival after splenectomy of patients with MMM, according to presplenectomy bone marrow cellularity.
Discussion
Our series of 223 patients with MMM who underwent splenectomy represents the largest single-institution experience compiled. In contrast to previous observations,34 we found that overall and postsplenectomy survival time and surgical outcome were similar among patients with agnogenic myeloid metaplasia, postpolycythemic myeloid metaplasia, and postthrombocythemic myeloid metaplasia. In general, our results confirm a high incidence of perioperative mortality (9%) and morbidity (30.5%). Incidence rates improved substantially in reports that appeared after 1940 compared with those published earlier.37 Since then, however, the reported operative mortality rates of 7% to 15% have not changed much.31-34,38 Bleeding, infection, and thrombosis are the leading causes of perioperative mortality and morbidity. In the current study, preoperative thrombocytopenia was significantly associated with perioperative thrombosis and decreased survival. In addition, presplenectomy bone marrow hypocellularity or normocellularity was also independently associated with a worse perioperative survival. However, both this study and a previous multicenter study involving 71 patients did not find any other preoperative clinical characteristics to be helpful in predicting surgical outcome.32 The paradoxic association of preoperative thrombocytopenia with postoperative thrombosis suggests the possibility that affected patients had occult disseminated intravascular coagulopathy. All of our patients, however, were systematically evaluated for the presence of this condition, and previous studies found that disseminated intravascular coagulopathy was associated with increased postsplenectomy hemorrhage but not thrombosis.13
Despite the relatively high rate of perioperative complications, a substantial number of patients with MMM had a durable benefit from splenectomy. Success was most likely in patients with mechanical symptoms from massive splenomegaly and profound constitutional symptoms. In particular, performance status, stamina, and body weight improved in most of our patients. Consistent with previous observations,13,32,37 less than one-half of our patients with anemia had a durable remission. Moreover, presplenectomy bone marrow cellularity or degree of collagen fibrosis did not predict a response in patients with anemia. Other benefits of splenectomy included alleviation of symptoms and signs of portal hypertension due to increased portal flow; however, portal hypertension caused by intrahepatic or portal vein obstruction may require portal-systemic shunt surgery.49 Splenic irradiation used as an alternative to splenectomy may provide relief from mechanical symptoms.30 However, the benefit is transient, and life-threatening cytopenias may occur in up to 44% of patients. Furthermore, operative risk may be increased in patients who may subsequently undergo splenectomy. Therefore, irradiation is reserved for patients who are poor candidates for surgery.
Substantial postsplenectomy hepatomegaly occurred in 16% of our patients. Previously reported incidence rates for this disorder range from 16% to 24%, with the range reflecting differences among studies in the selection of evaluable patients and the follow-up period.31,32,34 In the current study, the development of postsplenectomy hepatomegaly was not predicted by preoperative clinical variables and did not affect survival after splenectomy. Others32 found a significant correlation between transfusion-dependent anemia as a indication for surgery and the subsequent development of postsplenectomy hepatomegaly. Extramedullary hematopoiesis is the primary cause of postsplenectomy hepatomegaly and may coexist with additional histologic features of periportal fibrosis, hemosiderosis, microvascular portal vein thrombosis, and hepatic nodular regenerative hyperplasia.50,51 Nevertheless, postsplenectomy hepatomegaly seldom progresses to liver failure and is not a major cause of death. Liver failure was the direct cause of only 7% of the deaths in our series and of zero to 20% of deaths in other series.32,50 Treatment with hydroxyurea or 2-chlorodeoxyadenosine may be considered in patients in whom postsplenectomy hepatomegaly develops.52
In the present study, the incidence of extreme thrombocytosis after splenectomy was 22%. Incidence rates in other series vary from 18% to 50%, and this wide range may reflect differences in the timing and aggressiveness of myelosuppressive therapy.31,32,34,35Postsplenectomy thrombocytosis has been associated with the occurrence of thrombotic and hemorrhagic events.31,32,34,35 Similarly, we found significant associations between postsplenectomy thrombocytosis on one hand and perioperative thrombosis and worse postsplenectomy survival on the other. In our study, a preoperative platelet count of more than 50 × 109/L was predictive of postsplenectomy thrombocytosis, whereas others32 found an increased thrombohemorrhagic risk with preoperative platelet counts of more than 200 × 109/L. Of note, the actual incidence of postsplenectomy thrombosis may be higher than what is recognized clinically; thus, its overall impact on postsplenectomy survival remains undefined.53 Although increased platelet hyperaggregability may contribute to perioperative thrombosis, the high incidence of perioperative bleeding may not allow the prophylactic use of acetylsalicylic acid.54 However, preoperative institution of prophylactic platelet-lowering therapy may reduce the incidence and severity of postsplenectomy thrombocytosis and any thrombosis associated with it.
Overall median survival time in MMM has not changed in recent years and ranges from approximately 3 to 8 years.5,8-12,55,56 Various prognostic factors have been used to identify a group of patients with relatively long (greater than 10 years) or short (less than 2 years) survival.5,8,11,57,58 Because of the retrospective nature of studies involving patients with MMM who have undergone splenectomy, the effect of the procedure on overall survival may not be assessed accurately. In reports on cohorts that included both patients who had undergone splenectomy and patients who had not, overall survival after the time of diagnosis was not stated to be different in the 2 groups.8,59 Similarly, when patients with early-stage disease who had undergone splenectomy were compared with historical controls who received medical therapy, overall survival was not significantly different.46 However, most patients with MMM who are offered therapeutic splenectomy have advanced-stage disease and may also have disease characteristics not recognized by current prognostic criteria. Nevertheless, the observed overall median survival of 5.8 years in our study compares favorably with the corresponding values for high-risk disease and suggests the lack of an adverse effect on survival.
Reported rates of postsplenectomy survival and blast transformation vary widely because of disparities in sample size, patient selection, the definition of blast transformation used, and duration of follow-up. Also, perioperative deaths may or may not be included in the survival analysis. In our group of 223 patients, the median postsplenectomy survival time and the incidence of blast transformation among the 203 survivors of surgery were 27 months and 16%, respectively. Our results compare favorably with those of an analysis of 321 published cases that reported a median postsplenectomy survival of 13 months and a blast transformation rate of 11.2%.37 In contrast, a multicenter Italian study involving 71 patients reported a median postsplenectomy survival time of 55 months among the 65 patients who survived surgery.32 The superior survival time in the Italian study may be related partly to the younger age of the study population59 (median of 55 years compared with 65 years in the current study). This may also account for the higher proportion of deaths from leukemia in the Italian study (43% compared with 22% in the current study and 11.2% in previous series).37Similarly, the relatively lenient criteria used to define blast transformation and the longer follow-up period may account for the higher incidence of blast transformation (26% compared with 16% in the present series). Still, concern has been raised about the leukemogenic potential of splenectomy in MMM.
The Italian study59 compared 87 patients with MMM who had undergone splenectomy with a cohort of 462 patients who had not had the procedure and reported crude incidence rates of blast transformation of 26% and 12%, respectively, in the 2 groups. To adjust for clinical differences between the 2 groups, the authors applied statistical models of multivariate analysis (Cox proportional hazards model and recursive partitioning) and found splenectomy to be an independent risk factor for blast transformation. Despite the complementary role of the 2 multivariate models, however, both are based on consideration of known risk factors and may not adjust for hidden confounders. The fact that current prognostic criteria were not validated by the results in our patient cohort suggests that imminent splenectomy identifies a biologically different subset of patients.5,8,57 Furthermore, histopathologic studies of the spleen have suggested that subclinical blast transformation may influence the need for splenectomy and therefore account for the apparent increase in blast transformation.60 Other studies, however, did not find increased leukemic conversion rates after splenectomy,8 and our experience and that of others37 suggest that postsplenectomy survival may not be affected by the development of blast transformation. Therefore, an appropriate surgical recommendation should not be waived for fear of increased risk of leukemia and compromised survival.
The prognostic value of bone marrow histologic features and cytogenetic profile in assessing postsplenectomy outcome was examined in the current study. It should be noted, however, that because particular studies were not always performed either in all the patients or at the time of splenectomy, the statistical findings are not conclusive. Nevertheless, the presence of bone marrow hypercellularity was significantly associated with both an overall and a postsplenectomy survival advantage, and this finding is consistent with previously described associations between inferior survival and bone marrow hypocellularity in MMM.11 Both bone marrow hypocellularity or normocellularity and presplenectomy thrombocytopenia were independently associated with worse postsplenectomy survival and may be surrogates for either advanced or aggressive disease. In contrast, we did not detect significant correlations between the degree of collagen fibrosis and postsplenectomy outcome. This finding is also consistent with previous studies that showed a lack of significant association between bone marrow fibrosis and overall survival in MMM.5Finally, although abnormal cytogenetic findings have been associated with poor survival in agnogenic myeloid metaplasia,2 their detection in bone marrow obtained from patients before splenectomy in our study did not predict either worse survival or a higher risk of leukemic transformation.
In summary, the current study demonstrated that a durable benefit can be achieved in most patients with MMM who have splenectomy for the treatment of severe constitutional symptoms associated with marked splenomegaly and portal hypertension. In such patients, the improvement in quality of life may justify the relatively high risk of operative death.33 In contrast, severe thrombocytopenia may not be improved by splenectomy, and thrombocytopenia generally may be a surrogate for advanced disease with a poor surgical outcome. The selection of splenectomy to treat transfusion-dependent anemia should be based on the individual case and may be reserved for patients in whom drug therapy has failed and whose quality of life is compromised by the need for frequent transfusions. In addition, the superior postsplenectomy survival that was observed in patients with hypercellular bone marrow histologic features may allow consideration of bone marrow cellularity as an additional selection factor for splenectomy in patients with anemia. The currently available information indicates that splenectomy does not appear to either retard or hasten the progression of MMM. Similarly, whether or not the procedure increases the rate of blast transformation may not have major clinical relevance because overall survival may not be affected and the palliative benefit may outweigh any excess risk. Prophylactic myelosuppressive therapy may decrease the risk of perioperative thrombosis associated with postsplenectomy thrombocytosis and improve overall survival.
Murray N. Silverstein died on September 15, 1998.
Reprints:Ayalew Tefferi, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal