Abstract
Sickle red blood cells (RBC) become dehydrated as a consequence of potassium loss. This process depends at least partly on deoxygenation and may be influenced by the presence of oxygenation/deoxygenation cycles and the frequency of cycling. In this study, sickle RBC were subjected to approximately 180 oxygenation/deoxygenation cycles during 4 hours to evaluate RBC dehydration with cycle periods more similar to in vivo cycles than those in previous studies. A continuous-flow, steady-state apparatus circulated a dilute RBC suspension through gas-permeable silicone tubing with segments that were exposed to either nitrogen or ambient oxygen. The percentage of sickling and partial pressure of oxygen were measured by means of sampling ports in the deoxygenation and oxygenation regions. The density increase (dehydration) of young (transferrin receptor-positive) and mature (transferrin receptor-negative) RBC and the requirements for calcium and chloride were evaluated. Density increase correlated with the percentage of sickled cells at the deoxygenation sampling port and was observed only in the presence of calcium, thereby implicating the calcium-dependent potassium channel (Gardos pathway). Density increase was not dependent on the presence of chloride, making it unlikely that KCl cotransport was an important pathway under these conditions.
Dehydration of sickle red blood cells (RBC), which is caused by intracellular depletion of potassium (K+), appears to be an important pathophysiologic factor in sickle cell disease.1,2 Both mature and immature (transferrin receptor-positive [TfR+]) sickle RBC are found in the abnormally dense fractions, indicating that loss of K+ is a rapid process for at least some cells.3,4 The K+ efflux that mediates sickle RBC dehydration and shifts in cell density may occur through 2 high-capacity pathways, KCl cotransport and the calcium (C++)-dependent K+channel.1 A relatively nonselective increase in cation permeability also occurs as a direct result of sickling. Although cation fluxes due to this deoxygenation-induced mechanism are most likely too small to cause measurable density changes directly during short in vitro experiments, this pathway is thought to be responsible for the influx of Ca++ that activates the C++-dependent K+ channel if intracellular Ca++ exceeds a threshold value of approximately 40 nmol/L.5 6
Previous studies showed that KCl cotransport was active in sickle RBC at low pH (maximal at pH 6.8) and under hypotonic conditions.7 There is also evidence that sickle RBC KCl cotransport activity is dependent on oxygen in a complex way, with higher activity at a low and high partial pressure of oxygen (pO2) and lower activity at an intermediate pO2.8 This activity may contribute to the dehydration of sickle RBC, especially younger cells, under oxygenated conditions. Apovo et al9 and Franco et al10found that during relatively slow oxygenation/deoxygenation cycles, both KCl cotransport and the Ca++-dependent K+channel were important in the dehydration of sickle RBC but that hydration changes during continuous deoxygenation depended only on the presence of Ca++. Horiuchi and Asakura11 studied a reticulocyte-enriched light fraction subjected to deoxygenation cycles and found that extracellular Ca++ was required for net cation depletion and density increase. However, the role of KCl cotransport was not investigated.
The goal of the research presented here was to determine the pathway or pathways responsible for dehydration of mature (transferrin receptor-negative [TfR−]) sickle RBC and immature (TfR+) sickle RBC during a large number of relatively fast oxygenation/deoxygenation cycles (180 cycles at 80 seconds per cycle). The contribution of the Ca++-dependent K channel to sickle RBC dehydration was determined by performing experiments with and without Ca++. KCl cotransport was evaluated in experiments with and without chloride (Cl−).
Materials and methods
Informed consent was obtained under a protocol approved by the University of Cincinnati Institutional Review Board. Subjects (genotype, βSβS) had not undergone transfusion for at least 3 months and were in a clinically stable condition. Nine experiments were conducted. Blood was drawn from the subjects into heparin-coated vacuum containers. After overnight storage in plasma at 4°C, RBC were washed 3 times with the appropriate buffer and suspended to a concentration of 50%. The buffers used were HBIS (135 mmol/L of sodium chloride [NaCl], 20 mmol/L of HEPES, 5 mmol/L of KCl, 1 mmol/L of dibasic sodium phosphate [Na2HPO4], 1.5 mmol/L of calcium chloride, 1 mmol/L of magnesium chloride, and 10 mmol/L of glucose; 290-300 mOsm/kg, pH 7.4, at 37°C); HBIN, which was the same as HBIS except that Cl− was replaced with nitrate (NO3−); ethylene glycol tetraacetic acid (EGTA), which was Ca++-free HBIS with 0.1 mmol/L of EGTA; and phosphate-buffered saline (145 mmol/L of NaCl, 1.4 mmol/L of sodium biphosphate, and 8.3 mmol/L of Na2HPO4; pH 7.4).
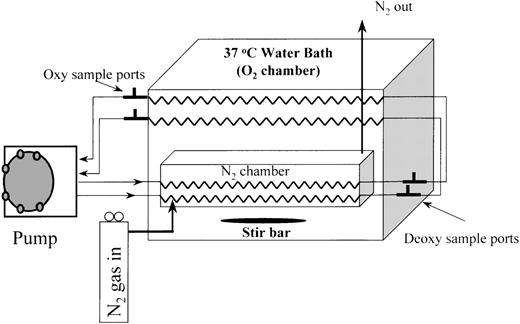
Oxygenation/deoxygenation continuous-circulation system
The in vitro continuous-circulation system for RBC oxygenation/deoxygenation (Figure 1) used gas-permeable silicone tubing to transfer oxygen. For deoxygenation, the tubing passed though a cylindrical gas-filled chamber that was flushed with nitrogen. This cylinder was submerged in a temperature-regulated (37°C) water bath that was continuously stirred. For oxygenation, the gas-permeable tubing passed through water-bath fluid that was exposed to ambient air. A peristaltic pump circulated the RBC suspension through the oxygenation and deoxygenation segments, which were connected by gas-impermeable tubing containing oxygenation and deoxygenation sampling ports (Figure 1). The total volume of the RBC suspension was 7.2 mL and the flow rate was 5.5 mL/min, yielding an average cycle time of approximately 80 seconds, with 40 seconds in the deoxygenator and 22 seconds in the oxygenator. The suspension spent an additional 11 seconds in the oxygenation state in the pump and associated tubing and 7 seconds in the deoxygenation state in the connecting tubing. The sampling ports, which consisted of 3-way valves, allowed samples (200 μL) to be withdrawn as fresh buffer was drawn into the system, thereby preserving the system volume. The presence of parallel circuits permitted 2 RBC suspensions to be cycled simultaneously. The calculated maximum shear stress on the cells was < 1 dyne/cm2, which is much less than the threshold value (≈100 dynes/cm2) for shear-dependent K+-transport activation in normal RBC.12
Two-channel fast oxygenation/deoxygenation circulation system.
Oxygenated blood is pumped through the nitrogen chamber, outside the water bath to access ports, to the oxygenator, and back to the pump.
Two-channel fast oxygenation/deoxygenation circulation system.
Oxygenated blood is pumped through the nitrogen chamber, outside the water bath to access ports, to the oxygenator, and back to the pump.
In most experiments, the gas-permeable silicone tubing had an inner diameter (ID) of 2 mm and an outer diameter (OD) of 3 mm (Specialty Manufacturing, Saginaw, MI). In some experiments, however, thinner-walled silicone tubing (1.5-mm ID × 2-mm OD) was used to provide a greater difference in pO2 values between the oxygenation and deoxygenation states. The length of the tubing with the smaller ID was increased to provide the same volume and circulation time as the larger-ID tubing. The interaction between the oxygenation and deoxygenation segments is complex, and the pO2 values depend not only on tubing-wall thickness but also on hematocrit, flow rate, and the relative lengths of tubing in the oxygenator and deoxygenator. In preliminary experiments, values for these variables that provided an adequate pO2 range while minimizing the oxygenation/deoxygenation cycle time were determined.
The fast-cycle apparatus used in these experiments has several advantages in addition to approximating in vivo kinetics more closely than methods using slower cycles. There is no air–liquid interface, which may cause lysis and lead to concentration of salts during long incubation periods. Also, because the cells are in a flow system and subjected to shear forces that tend to align them with the flow axis, waves of polymer formation tend to be aligned in the same direction, resulting in uniform, severely elongated sickle forms.
Experimental protocol
Buffer alone was circulated for approximately 20 minutes before 0.14 mL of the 50% RBC suspension was added during a 1.5-minute period to distribute the cells evenly. The RBC suspensions (1%) were cycled for 4 hours. Oxygenation and deoxygenation samples were taken for pO2 measurements (Blood Gas Analyzer, model 1304; Instrumentation Laboratories, Lexington, MA) at 30 minutes, 2 hours, and 4 hours to verify pO2 stability. After 4 hours of cycling, the 1% cell suspensions were centrifuged and the optical density (415 nm) of the supernatants was determined. This value was always < 5% of the corresponding optical density for a lysed 1% cell suspension. A simultaneous oxygenated control was obtained by adding RBC (1% final concentration) to the same buffers used for oxygenation/deoxygenation cycling and incubating the mixture for 4 hours in a temperature-controlled (37°C) water bath. In 2 control experiments, cells were circulated without exposure to nitrogen, so that no oxygenation/deoxygenation cycling occurred.
Approximately 20 minutes before the end of the cycle period, 200 μL of cell suspension was removed and transferred, without exposure to the atmosphere, to deoxygenated PBS containing 10% formalin. The percentage of sickle RBC was determined by using Nomarsky optics to assess duplicate, coded samples. A RBC was considered sickled if it had at least 1 pointed projection. In these preparations, TfR+and TfR− cells were not distinguished, and all data on cell sickling included both cell types.
After removal from the apparatus, the RBC suspensions were centrifuged and the osmolality of the supernatant was determined. For each experiment, the osmolality was found to be unchanged, thus eliminating the possibility of a leak in the apparatus during cycling. All further processing was done under ambient, oxygenated conditions. The cells were washed once with HBIS and resuspended to a final RBC concentration of 40%. An aliquot was placed in 10% buffered formalin to measure the percentage of sickle RBC after reoxygenation.
Discontinuous arabinogalactan density gradients were prepared as previously described4 by using densities of 1.086, 1.094, and 1.103 g/mL and a cushion > 1.15 g/mL. Ten microliters of each 40% cell suspension, including 2 cycled test samples and 2 noncycled controls, were separated at 22°C into 4 density fractions in a microultracentrifuge (Airfuge; Beckman, Palo Alto, CA). After the RBC in each fraction were counted (CBC-5 counter; Coulter, Hialeah, FL), the cells were allowed to react with mouse monoclonal fluorescein isothiocyanate-conjugated anti-TfR (Dako, Carpenteria, CA) and an appropriate idiotypic control antibody. The percentage of TfR+ cells in each fraction was determined by flow cytometry (XL-MCL flow cytometer, Coulter) as previously described.4
TfR+ cells are a well-defined population that normally (ie, in the absence of nucleated erythroid cells) contains the youngest erythroid cells in the circulation. TfR− cells represent a wide range of cell age, from reticulocytes that no longer have detectable TfR to the oldest cells in the circulation. Usually about one half of sickled reticulocytes are TfR+. Therefore, in a typical patient with 10% reticulocytes, about 5% of the TfR− population are reticulocytes and 95% are mature RBC.
After the percentage of total TfR+ cells in each fraction was determined, a density score (range, 100-400) was calculated to characterize the density distribution of the RBC suspension numerically.4 Density shift was defined as the difference between the density score of the cycled RBC suspension and the density score of the paired noncycled control RBC suspension. The role of Ca++ in mediating the density shift of the cells was evaluated by comparing the density shifts in parallel flow channels in buffers containing either 1.5 mmol/L Ca++ or no Ca++ (0.1 mmol/L EGTA). Activation of KCl cotransport was determined by comparing buffers that contained either Cl− or NO3−in parallel flow chambers in the presence of Ca++. The KCl cotransport pathway is dependent on the presence of Cl−, whereas the Ca++-dependent K+ channel is inactive in the absence of Ca++.
Results
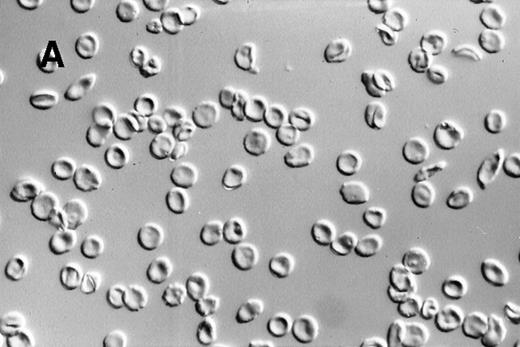
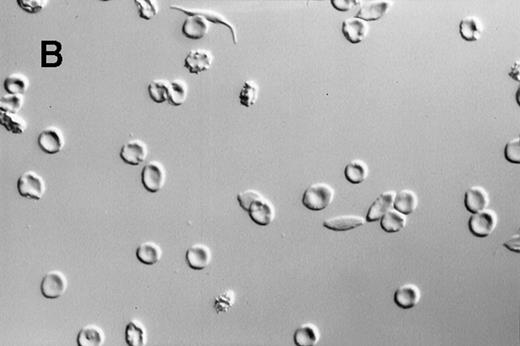
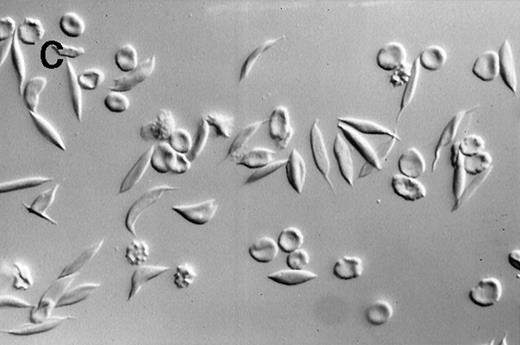
A dilute suspension of sickle RBC (Figure2) was repetitively cycled every 80 seconds between oxygenation and deoxygenation states in a continuous-flow, steady-state apparatus. The oxygenation and deoxygenation pO2 values stabilized within 30 minutes after the RBC were added and remained constant thereafter. The pO2 ranges were 25 to 37 mmHg in the deoxygenation port and 55 to 80 mmHg in the oxygenation port. Because of the variability in pO2 values and differences in RBC properties among patients, there was a wide range in the percentage of sickle RBC: 12% to 92% at the deoxygenation port and 4% to 59% at the oxygenation port. Morphologic sickling was uniform, with essentially all the sickle RBC extending in 2 directions along the same axis (Figure 2C). These cells appeared to be morphologically similar to those in venous blood samples and were distinctly different from the forms with more random polymer orientation observed with deoxygenation in nonflow systems.13 The cycling-induced sickling was reversible, as indicated by an almost complete lack of sickle forms after the cycled cells were washed in oxygenated buffer (Figure 2D).
Photomicrographs of fixed sickle red blood cells from a typical experiment.
(A) Results after 4 hours of incubation at 37°C under oxygenation conditions. (B) Photomicrograph obtained from the oxygenation port after 3.67 hours of cycling. (C) Photomicrograph obtained from the deoxygenation port after 3.67 hours of cycling. (D) Results after 4 hours of cycling followed by oxygenation.
Photomicrographs of fixed sickle red blood cells from a typical experiment.
(A) Results after 4 hours of incubation at 37°C under oxygenation conditions. (B) Photomicrograph obtained from the oxygenation port after 3.67 hours of cycling. (C) Photomicrograph obtained from the deoxygenation port after 3.67 hours of cycling. (D) Results after 4 hours of cycling followed by oxygenation.
After 4 hours of cycling, the cells were separated by density under oxygenated conditions. An example of the distribution histograms for cell density of control (incubated 4 hours under oxygenated conditions) and cycled cells, with and without Ca++, is shown in Figure3. Density distributions and scores are given for the total RBC population and for the TfR+ and TfR− subpopulations. In the presence of Ca++, the average density of all RBC increased during cycling compared with the density in the paired noncycled controls. In the absence of Ca++, there was no change in the density score. As shown in Figure 3, there was a greater shift among TfR+ cells than among TfR− cells in the presence of Ca++. Cells that were circulated without oxygenation/deoxygenation cycling, either with or without Ca++, had no change in density score for either TfR− or TfR+ cells compared with incubated, noncirculated controls.
Typical cell-density distributions for control cells (incubated 4 hours under oxygenation conditions) and for cells that were cycled for 4 hours.
The cycled samples with and without calcium were run concurrently in the 2 channels of the oxygenation/deoxygenation apparatus.
Typical cell-density distributions for control cells (incubated 4 hours under oxygenation conditions) and for cells that were cycled for 4 hours.
The cycled samples with and without calcium were run concurrently in the 2 channels of the oxygenation/deoxygenation apparatus.
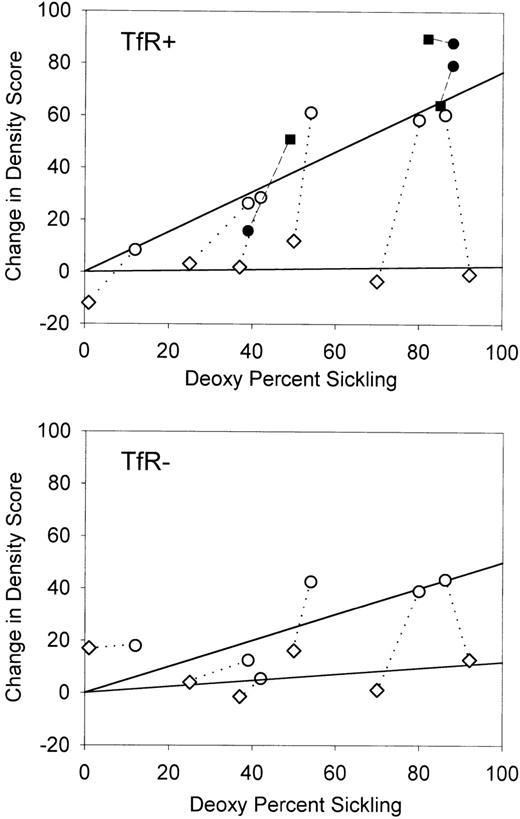
It was expected that the amount of cycle-dependent dehydration would be a function of the degree of sickling, which varied widely among experiments. Figure 4 shows the change in density score as a function of sickling (on the deoxygenation side) after 4 hours of oxygenation/deoxygenation cycling. The trend lines were drawn by using only these experiments, with a direct comparison of the effect of Ca++. For both TfR+ and TfR− cells, there was a linear relation between the percentage of the sickle cells and the change in density in the presence of Ca++. As was found in previous studies with slower oxygenation/deoxygenation cycles,10 young (TfR+) cells had a greater density shift than mature (TfR−) cells. In the absence of Ca++, no consistent density shifts were observed, even with a high percentage of sickle RBC. This lack of shift in the absence of Ca++ indicates that under these conditions there is little or no dehydration that can be attributed to activation of KCl cotransport.
Change in density score (DS) compared with the percentage of deoxygenation sickling for transferrin receptor-positive and transferrin receptor-negative cells in the presence and absence of extracellular calcium.
The change in DS is defined as the DS after 4 hours of oxygenation/deoxygenation cycling minus the DS of the appropriate control incubated for 4 hours under oxygenation conditions. Open symbols indicate experiments with (○) or without (⋄) calcium. Shaded symbols indicate experiments with (•) or without (▪) chloride in the presence of calcium. Symbols connected by a dotted or broken line indicate paired experiments.
Change in density score (DS) compared with the percentage of deoxygenation sickling for transferrin receptor-positive and transferrin receptor-negative cells in the presence and absence of extracellular calcium.
The change in DS is defined as the DS after 4 hours of oxygenation/deoxygenation cycling minus the DS of the appropriate control incubated for 4 hours under oxygenation conditions. Open symbols indicate experiments with (○) or without (⋄) calcium. Shaded symbols indicate experiments with (•) or without (▪) chloride in the presence of calcium. Symbols connected by a dotted or broken line indicate paired experiments.
Additional experiments (shaded symbols in Figure 4) compared NO3− and Cl− buffers in parallel flow channels in the presence of Ca++. For TfR+ cells, which have a high potential KCl cotransport activity and therefore represent a sensitive test for activation of this pathway, there was clearly no Cl− dependency for the observed density shift. These results confirm that the KCl cotransport pathway was not active under these conditions.
Discussion
Two major pathways have been proposed to mediate sickle RBC dehydration: the Ca++-dependent K channel, which appears to be activated by a sickling-induced increase in Ca++permeability, and KCl cotransport, which is thought to participate in the volume regulation of young erythroid cells but may be overactive in sickle cells.
Our study showed that the Ca++-dependent K channel, but not KCl cotransport, is activated in sickle RBC by fast oxygenation/deoxygenation cycling at physiologic pH and osmolality and that the resulting density shift is proportional to the percentage of sickle cells. This is the first demonstration of sickle cell dehydration mediated by oxygenation/deoxygenation cycles with periods approaching those in vivo. As in previous studies with slower cycles, the young TfR+ cells had a greater density shift than the more mature RBC in the same cycled sample. Therefore, it appears that the Ca++-dependent K channel is activated by both continuous10 and cyclic deoxygenation conditions and that either slow10 or fast cycles are effective in both mature and immature sickle RBC. The greater density change of TfR+cells could be due to either a quantitative or qualitative difference in sickling or to a greater sensitivity to sickling-induced dehydration in these cells. In the presence of Ca++, dehydration was independent of Cl−. Therefore, under fast-cycle conditions, there was no evidence of KCl cotransport activation.
In young erythroid cells, KCl cotransport may be activated in vitro by hypotonic swelling or low pH. However, the mechanism responsible for activation of this pathway in vivo has not been determined. There is evidence that cyclic deoxygenation can serve as an activating stimulus, but this now appears to depend on the rate of cycling employed. Data from Apovo et al9 and our laboratory10 showed that under relatively slow cyclic, but not continuous, deoxygenation conditions, KCl cotransport was activated. To explain the difference between continuous and slow cyclic deoxygenation, we developed a model in which activation depends on both the phosphorylation state of the cell and the concentration of free magnesium ([Mg++]).10,14 Jennings and Schulz15 showed that KCl cotransport activation is associated with protein dephosphorylation, and deoxygenation results in a general RBC protein dephosphorylation.16 However, there is also an increase in [Mg++] on deoxygenation, since 2,3-diphosphoglycerate is bound to deoxyhemoglobin rather than Mg++. Because [Mg++] inhibits KCl cotransport,17-19 for the cotransporter to be active there must be both dephosphorylation and low [Mg++], two conditions not present during either continuous oxygenation or continuous deoxygenation. When cells are cycled between oxygenation and deoxygenation, however, it is possible that these two conditions exist during part of the cycle.
It is reasonable to assume that changes in [Mg++] are fast and follow the oxygenation/deoxygenation state of the hemoglobin. However, enzyme-mediated changes in phosphorylation state may be slower. In fact, a delay time of several minutes for volume-stimulated KCl cotransport has been observed in sickle RBC.20 On the basis of the slow-cycle data, we hypothesized that immediately after reoxygenation there is a period during which [Mg++] is low but rephosphorylation lags behind, allowing both conditions to be present simultaneously. It is during this phase of the cycle that KCl cotransport may be active. This hypothesis is supported by recent studies14 in which the ionophore A23187 and 0.15 mmol/L of extracellular [Mg++] was used to clamp the intracellular [Mg++] at oxygenated levels during continuous deoxygenation of a light fraction of sickle RBC. This prevented the usual increase in [Mg++] and resulted in a significant increase in deoxygenation-induced, chloride-dependent potassium flux (KCl cotransport). Therefore, these experiments showed that deoxygenation, presumably through dephosphorylation, could activate the cotransporter if [Mg++] is maintained at a low level.
The fast-cycle experiments described here examined this question from another viewpoint. If KCl cotransport is active during slow cycling only during the first part of the oxygenation phase, then it might be expected that increasing the number of cycles in a given period would result in greater activation, since the transition would occupy a greater fraction of the cycle time. However, this assumes that there is sufficient time during the deoxygenation phase for dephosphorylation to occur. As noted above, evidence from studies in cells in which KCl cotransport was activated by swelling indicated that dephosphorylation takes minutes.20 If this were also true of activation by deoxygenation, cells would not remain in the deoxygenation state for a sufficient time during fast cycles for subsequent activation upon oxygenation.
Although KCl cotransport was not active in our fast-cycle experiments, there is good evidence that this pathway contributes to sickle RBC dehydration in vivo. Oral administration of magnesium, an inhibitor of this pathway, increases intracellular magnesium levels in sickle RBC and has a beneficial effect on sickle cell hydration.21Furthermore, rehydrated dense TfR+ sickle cells have been found to have higher KCl cotransport activity than TfR+sickle cells that remained light in vivo,4suggesting that this pathway contributes to the fast dehydration that occurs in some young sickle cells. KCl cotransport may be activated if sickle cells are subjected to slower-than-normal deoxygenation cycles in vivo. For example, sickle reticulocytes adhere to postcapillary venules,22 presumably as a result of their high number of adhesion molecules.23 24 The retention of these cells in the circulation may be an important factor in activation of sickle RBC KCl cotransport in vivo.
Supported by National Institutes of Health grant RO1 HL51174 and Cincinnati Comprehensive Sickle Cell Center grant HL57614.
Reprints:Anthony J. McGoron, Florida International University Biomedical Engineering Institute, 10555 W Flagler St, Miami, FL 33174; e-mail: mcgoron@eng.fiu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal