Abstract
In vitro studies have shown that damaged red cells and apoptotic cells are efficiently phagocytosed by scavenger receptors from macrophages, even under non-opsonizing conditions. Damaged red blood cells are in vivo effectively removed from the blood circulation, but the responsible receptor systems are largely unknown. We used a murine model in which 51Cr-labeled oxidized red blood cells were injected intravenously, and the cellular uptake sites and the potential involvement of scavenger receptors were analyzed. The decay of damaged red cells was rapid (more than 50% removed within 10 minutes after injection), whereas native red cells were not cleared. The main site of uptake of damaged red cells was the liver Kupffer cells, which contained 24% of the injected dose at 10 minutes after injection. The blood decay and liver uptake were inhibited by typical ligands for scavenger receptors, such as polyinosinic acid, liposomes containing phosphatidylserine, oxidized low-density lipoprotein, and fucoidan, but not by polyadenosinic acid or liposomes without phosphatidylserine. Mice lacking scavenger receptors class A type I and II showed no significant decrease in the ability to take up damaged red cells from the circulation. We conclude that Kupffer cells are mainly responsible for the removal of damaged red cells from the blood circulation, a process mediated by polyinosinic acid- and phosphatidylserine-sensitive scavenger receptors, different from scavenger receptor class A type I and II. Our data indicate that scavenger receptors, as pattern-recognizing receptors, play an important role in vivo in the removal of apoptotic, damaged, or other unwanted cells from the blood circulation.
It is generally accepted that recognition of aged, infected, or damaged red blood cells (RBCs) is mediated by pathways that include altered carbohydrate moieties of membrane proteins,1,2 adherence of antiband 3 autoantibodies,3,4 or the loss of membrane phospholipid asymmetry.5-7 To what extent these changes are involved in providing a signal for erythrophagocytosis in vivo is not clear. During normal aging of RBCs, auto-oxidative damage occurs to lipid and protein components in the membrane.8-10 Previous in vitro studies showed that the binding and phagocytosis of RBCs, which were oxidatively damaged (OxRBC), can be inhibited by ligands for macrophage scavenger receptors.11,12 Oxidized low-density lipoprotein (OxLDL), but not native or acetylated low-density lipoprotein (LDL), inhibited the binding of OxRBC to isolated murine peritoneal macrophages by approximately 80%. Other scavenger receptor ligands, such as fucoidan, polyinosinic acid (poly I), and liposomes containing phosphatidylserine (PS), exhibited similar inhibitory properties, and it was thus suggested that OxRBC are a possible ligand for scavenger receptors. This suggestion is consistent with the hypothesis that scavenger receptors, as pattern-recognizing receptors, are involved in the innate immune system in which macrophages play a role in the discrimination between “self” and “non-self.”13 14 The removal of apoptotic, damaged, and other unwanted cells from the blood circulation or tissues is important for homeostasis, and any impairment of this process could potentially result in chronic inflammation.
Apart from scavenger receptors, numerous other receptors have been described that may participate in recognition and phagocytosis by macrophages. The macrophage mannose receptor recognizes exposed mannose residues of plasma membrane proteins,15 and F and complement receptors16 are responsible for the uptake of opsonized particles. However, many studies have shown that even under nonopsonizing conditions, damaged cells, such as apoptotic cells or senescent erythrocytes, are efficiently taken up by macrophages. This recognition can be mediated through progressive exposure of PS on the outer leaflet of the plasma membrane during aging, damage, or apoptosis.17-21
The potential function and quantitative role of scavenger receptors for the removal of modified cells in vivo has not been studied, and our data are aimed to show the potential relevance of the earlier in vitro data for the in vivo situation. In vivo, many different receptors or different cell types in various organs may operate simultaneously, and their activity will depend on receptor expression levels, on cellular localization, as well as on the presence of serum components or extracellular matrix. The present studies were undertaken to analyze the cell type(s) responsible for the in vivo uptake of OxRBC and the potential involvement in vivo of scavenger receptors. In addition to normal mice, scavenger receptor class A (SRA) knock out mice were utilized to verify to what extent this well-characterized receptor system might be responsible for OxRBC removal.
Materials and methods
Materials
51Cr-sodium chromate was from Amersham (Buckinghamshire, UK), CuSO4, L-ascorbic acid, poly I, polyadenosinic acid (poly A), fucoidan, collagenase (type IV), bovine serum albumin (BSA, fraction V), 3,3′-diaminobenzidine, Mayer's haematoxylin solution, and cholesterol were from Sigma (St. Louis, MO). Bovine brain PS and egg yolk phosphatidylcholine (PC) were purchased from Fluka (Buchs, Switzerland); Hepes was from Biosolve (Valkenswaard, The Netherlands), and EDTA was from Merck (Darmstadt, Germany).
Mice
Six- to 10-week-old male ICR mice with a weight between 26 and 32 grams were used. In some experiments, these mice were compared with male SV129 × ICR mice of the approximate age and weight. The latter lack expression of SRA I and II by disruption of exon 4 of the gene and were a kind gift from Dr Suzuki et al.22 Mice were bred in our local facility and kept under low pathogen conditions with free access to food and drink.
RBC preparation
Venous blood was drawn from a male, wild-type ICR mouse with 10 U/mL heparin. The RBC pellet was washed 4 times with sterile phosphate-buffered saline (PBS) and kept as a 20% hematocrit suspension at 4°C. RBCs were used within 3 days of storage. Labeling was carried out with 50 μCi51Cr-sodium chromate per mL of a 10% hematocrit RBC suspension in sterile PBS for 30 minutes at 37°C while gently shaking. RBCs were washed at least 4 times to remove free label and immediately incubated with 200 μmol/L CuSO4 in PBS containing 5 mmol/L ascorbic acid for 90 minutes at 37°C. Oxidation by Cu2+/ascorbic acid leads to extensive membrane crosslinking, as shown by SDS/PAGE, which is comparable to treatment of RBCs with 1 mmol/L glutaraldehyde or 200 μmol/L CuSO4plus 10 mmol/L H2O2.11 The RBCs showed a 4-fold increase in methemoglobin, as compared with untreated cells.17 Peroxide treatment or aldehyde treatment was shown earlier to enhance phagocytosis of RBCs.23-25 After oxidation, RBCs were washed once with PBS/5 mmol/L EDTA and once with PBS alone. A volume of 300 μL of a 10% hematocrit suspension was counted on a gamma counter (5550 Minaxi gamma, Packard, Downers Grove, IL) with a window set for 51Cr (200-400 KeV) to calculate the total injected dose afterward.
Liver uptake, blood decay, and tissue distribution of RBCs in mice
Mice were anesthetized with a subcutaneous injection of a mixture of Nimatek (112.5 mg ketamine/kg body weight), Hypnorm (1.125 mg fluanisone/kg and 0.035 mg fentanyl citrate/kg), and Thalamonal (1.6 mg droperidol/kg and 0.032 mg fentanyl/kg). The abdomens were opened, and 200 μL of a 10% hematocrit suspension of 51Cr-labeled RBCs (± 3.5 × 108 RBCs) were injected via the cavernous vein or the tail vein with or without preinjection of competitors. At the indicated times, liver lobules were tied off, excised, and weighed. The amount of liver tissue tied off during the experiment did not exceed 10% of the total liver weight. Blood samples (<50 μL) were taken, and radioactivity was measured. At 30 minutes, the mice were killed and tissues were excised and weighed to determine the tissue distribution. The radioactivity in liver and other tissue samples was corrected for blood present at the time of sampling, according to earlier determinations with 125I-BSA in these mice.26 The total blood volume was calculated from the distribution of 51Cr-labeled native RBCs (n = 5), since there was no detectable uptake of native RBCs in any tissue.
Isolation of liver cells and microscopy
At 30 minutes after injection of an unlabeled, Ox RBC suspension, the liver was pre-perfused at 37°C for 10 minutes with 142 mmol/L NaCl/6.7 mmol/L KCl/6.7 mmol/L Hepes, pH 7.4, at a flow rate of 14 mL/min and subsequently with 100 mL of collagenase buffer (67 mmol/L NaCl/6.7 mmol/L KCl/4.8 mmol/L CaCl2/67 mmol/L Hepes/2% BSA) containing 20 mg collagenase type VI, pH 7.6. Parenchymal, endothelial, and Kupffer cells were isolated by differential centrifugation and counterflow elutriation, as described earlier for rats.27 The different cell fractions were stained with 3,3′-diaminobenzidine for endogenous peroxidase activity and analyzed by light microscopy. The percentage of cells containing RBCs was determined by counting. To characterize the nonparenchymal cells, cytospins of each cell fraction were prepared. Cytospins were fixed with cold acetone and stained with haematoxylin solution.
Macrophage depletion
Liposomes containing dichloromethylene diphosphonate (DMDP) were a kind gift from Dr N. van Rooijen (Vrije Universiteit, Amsterdam). A volume of 200 μL liposome suspension (± 5 mg DMDP/mL) was injected into the tail vein 48 hours prior to the injection of RBCs. This treatment has been shown to deplete the liver and the spleen of most of the present macrophages.28 Control mice had the same volume of sterile PBS injected.
Liposomes
Unilamellar liposomes were prepared with egg yolk PC, bovine brain PS, and cholesterol in a phospholipid-to-cholesterol molar ratio of 2:1. Lipids in chloroform were mixed and evaporated under nitrogen. The lipid film was resuspended in sterile PBS at a concentration of 3 mmol/L total lipid and sonicated for 30 minutes with an MSE soniprep 150 (amplitude 16 μ) at 52°C under a constant stream of argon. The average particle size (47.0 nm for PC/cholesterol liposomes and 55 nm for PS/PC/cholesterol) and homogeneity were measured by photon correlation spectroscopy (System 4700 C, Malvern Instruments, Malvern, UK).
Lipoprotein preparation
LDL was isolated from serum from healthy volunteers by differential ultracentrifugation. Oxidation of LDL (0.1 mg/mL) was carried out with 10 μmol/L CuSO4 in PBS for 18 hours. The preparation was concentrated by speed vacuum rotation, and the protein concentration was measured by the method of Lowry.29
Statistical analysis
Data are shown as mean (± SEM). Statistical significance was calculated with 1-way analysis of variance.
Results
RBC clearance and tissue distribution
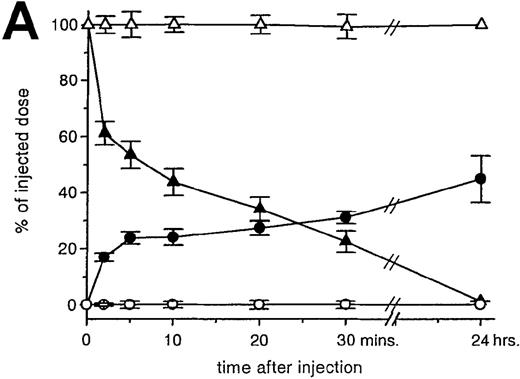
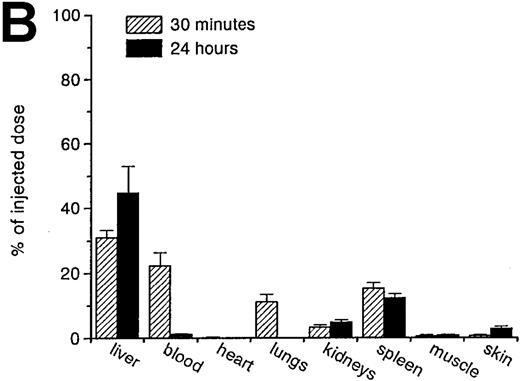
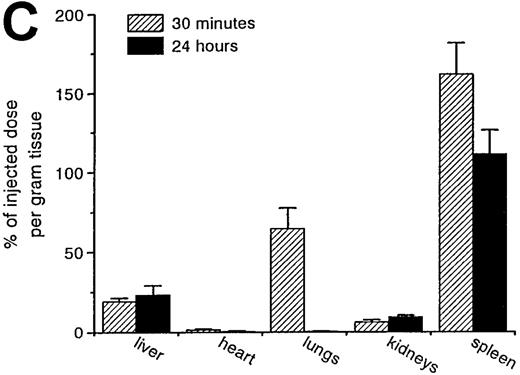
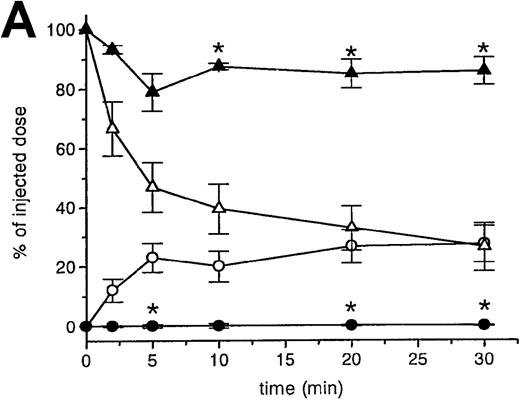
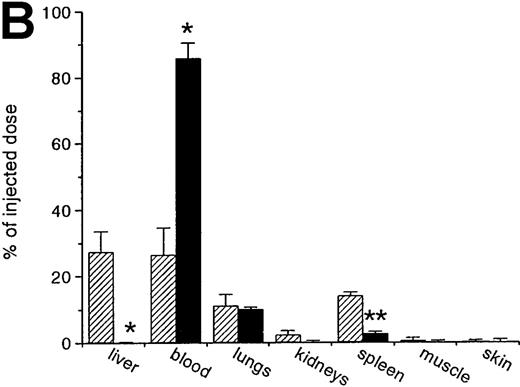
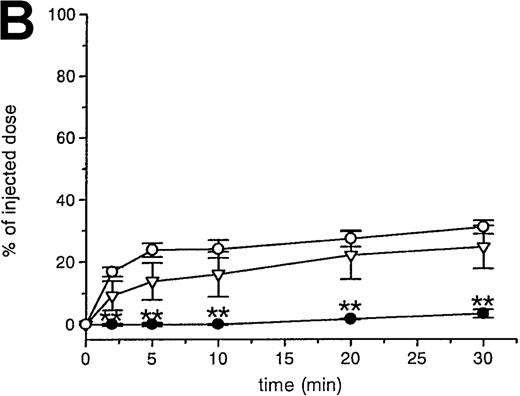
RBCs were isolated, labeled with 51Cr-sodium chromate, and injected into recipient mice via the cavernous vein or the tail vein. Without oxidative treatment, 51Cr-labeled RBCs were not taken up by any tissue, and, even at 24 hours after injection, the injected dose could be completely recovered from the blood. However, introduction of RBCs treated with CuSO4 and ascorbic acid resulted in a rapid blood decay and uptake by various tissues (Figure1 A-C), mainly by the liver and spleen. At 30 minutes after injection, more than 70% of the injected OxRBC were removed from the circulation, whereby 31% is taken up by the liver and 15% by the spleen. The relatively high association of OxRBC with the lungs at 30 minutes after injection appeared to be transient, since at 24 hours, a low activity was observed. Injection of OxRBC into the portal vein as route of entry resulted in an increase in liver uptake and a concomitant decrease in the lungs, which indicates that part of the uptake in the lungs is due to temporary trapping of the cells in the capillary bed (data not shown).
Uptake, decay, and tissue distribution of red blood cells (RBCs).
(A) Liver uptake (circles) and blood decay (triangles) of native (open symbols) and oxidized (closed symbols) RBCs (OxRBC). At t = 0, a single bolus of 200 μL 10% hematocrit RBC suspension was injected, and at various times after injection a liver lobule and blood sample were taken. (B) Tissue distribution of OxRBC at 30 minutes (hatched bars) and 24 hours (black bars) after injection, expressed as percent of injected dose. (C) Specific uptake of OxRBC per gram tissue at 30 minutes (hatched bars) and 24 hours (black bars) after injection, expressed as percent of injected dose per gram wet tissue weight. All figures: native RBC: mean of 5 ± SEM; OxRBC: mean of 6 ± SEM. The total body recovery for OxRBC of 6 independent determinations at 30 minutes after injection was 86.4 ± 4.3%.
Uptake, decay, and tissue distribution of red blood cells (RBCs).
(A) Liver uptake (circles) and blood decay (triangles) of native (open symbols) and oxidized (closed symbols) RBCs (OxRBC). At t = 0, a single bolus of 200 μL 10% hematocrit RBC suspension was injected, and at various times after injection a liver lobule and blood sample were taken. (B) Tissue distribution of OxRBC at 30 minutes (hatched bars) and 24 hours (black bars) after injection, expressed as percent of injected dose. (C) Specific uptake of OxRBC per gram tissue at 30 minutes (hatched bars) and 24 hours (black bars) after injection, expressed as percent of injected dose per gram wet tissue weight. All figures: native RBC: mean of 5 ± SEM; OxRBC: mean of 6 ± SEM. The total body recovery for OxRBC of 6 independent determinations at 30 minutes after injection was 86.4 ± 4.3%.
Cellular distribution of OxRBC in liver
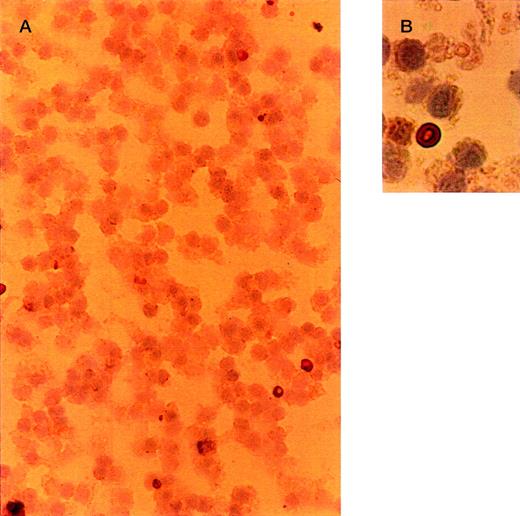
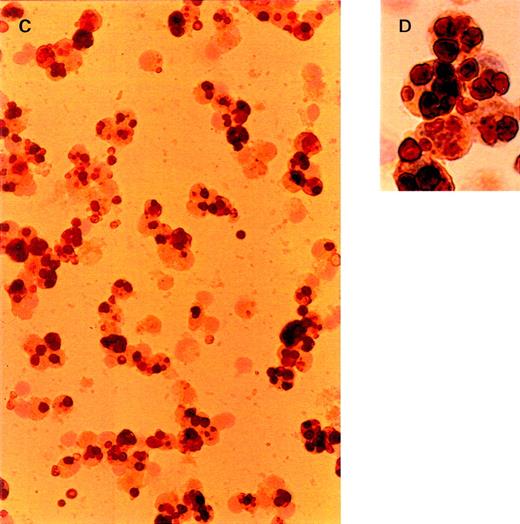
To determine which cell type is responsible for the liver uptake of OxRBC in vivo, we isolated nonparenchymal liver cells at 30 minutes after injection of OxRBC. The micrographs in Figure2 represent different cell fractions after isolation of nonparenchymal liver cells and separation into Kupffer cells and endothelial cells by counterflow elutriation. All fractions were stained with 3,3′-diaminobenzidine for endogenous peroxidase and counterstained with haematoxylin. RBCs are dark brown and clearly visible within the cells. Liver endothelial cells, which elute in the elutriation system at a flow rate of 26 mL/min, did not contain OxRBC (2.5 ± 2.1% of cells positive for OxRBC, mean of 4 counts of a representative experiment), even though they are in close contact with the circulating blood. At a flow rate of 75 mL/min, small and large Kupffer cells elute in the elutriation system, and it is in this cell fraction that associated RBCs can be seen (65 ± 3.6% of cells positive for OxRBC). The microscopic observations thus indicate that Kupffer cells and not endothelial cells internalize OxRBC in the liver. Parenchymal cells did not show any association with OxRBC (data not shown).
Light microscopy of nonparenchymal liver cells for the presence of oxidatively damaged red blood cells at 30 minutes after injection.
Cells were isolated as described in “Materials and methods.” Cytospins were fixed in acetone, stained with 3,3′-diaminobenzidine for endogenous peroxidase activity, and counterstained with hematoxylin. Red blood cells are dark brown. (A, B) Cells eluted at a flow rate of 26 mL/min (liver endothelial cells). (C, D) Cells eluted at a flow rate of 75 mL/min (Kupffer cells). Objective magnification ×40 (A, C); ×100 (B, D). The procedure was repeated 3 times and gave very similar results.
Light microscopy of nonparenchymal liver cells for the presence of oxidatively damaged red blood cells at 30 minutes after injection.
Cells were isolated as described in “Materials and methods.” Cytospins were fixed in acetone, stained with 3,3′-diaminobenzidine for endogenous peroxidase activity, and counterstained with hematoxylin. Red blood cells are dark brown. (A, B) Cells eluted at a flow rate of 26 mL/min (liver endothelial cells). (C, D) Cells eluted at a flow rate of 75 mL/min (Kupffer cells). Objective magnification ×40 (A, C); ×100 (B, D). The procedure was repeated 3 times and gave very similar results.
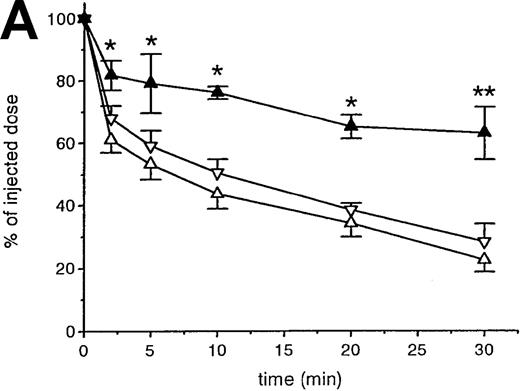
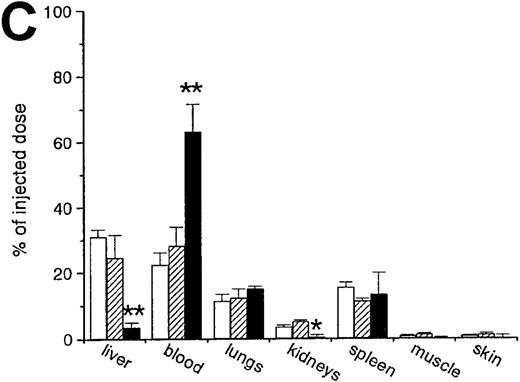
A second approach to analyze the involvement of macrophages in the removal of OxRBC was taken by using liposomes containing DMDP, an agent that leads to the depletion of macrophages in various tissues. This method was originally developed and established by Van Rooijen et al.28 Because these liposomes are fairly large (>200 nm), they are predominantly taken up by phagocytes of the mononuclear phagocytic system and cannot pass vascular barriers. When the liposomes are administered intravenously, mainly Kupffer cells and spleen macrophages are targeted and depleted. It appears that macrophage depletion nearly completely abolished the rapid clearance of OxRBC because of the lack of uptake by the liver and the spleen (Figure3A and B).
The effect of macrophage depletion on the liver uptake and blood decay of oxidatively damaged red blood cells (OxRBC).
Liposomes containing dichloromethylene diphosphonate (L-DMDP) were injected 45 hours prior to the injection of OxRBC. (A) Liver uptake (circles) and blood decay (triangles) of OxRBC after pretreatment with L-DMDP (closed symbols) or after injection of phosphate-buffered saline (PBS) (open symbols) as control. (B) Tissue distribution of OxRBC after injection of L-DMDP (black bars) or PBS (hatched bars). Tissues were taken at 30 minutes after injection of OxRBC and shown are the mean of 2 ± SEM. *P < .05; **P < .005.
The effect of macrophage depletion on the liver uptake and blood decay of oxidatively damaged red blood cells (OxRBC).
Liposomes containing dichloromethylene diphosphonate (L-DMDP) were injected 45 hours prior to the injection of OxRBC. (A) Liver uptake (circles) and blood decay (triangles) of OxRBC after pretreatment with L-DMDP (closed symbols) or after injection of phosphate-buffered saline (PBS) (open symbols) as control. (B) Tissue distribution of OxRBC after injection of L-DMDP (black bars) or PBS (hatched bars). Tissues were taken at 30 minutes after injection of OxRBC and shown are the mean of 2 ± SEM. *P < .05; **P < .005.
As a control for a possible effect of the liposomes on endothelial cells, we determined the uptake of 125I-tyramine cellobiose–labeled acetylated low-density lipoprotein (125I-TC-AcLDL) by liver endothelial cells in vivo with and without pretreatment with DMDP-liposomes. With preadministration of DMDP-liposomes, liver endothelial cells took up 7.2 ± 0.41% of the injected dose of 125I-TC-AcLDL per mg of cell protein, whereas without DMDP-liposomes this value was 6.99 ± 1.63% of the injected dose per mg of cell protein. These results are very comparable with those previously reported for these mice (7.24% ± 0.11 of injected dose/mg cell protein)30 and show that under these conditions endothelial cells do function normally.
Effect of PS liposomes and other scavenger receptor ligands on OxRBC removal in vivo
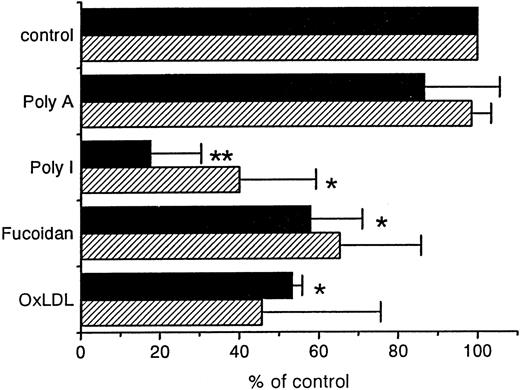
Previous in vitro studies showed that liposomes containing PS strongly inhibit the uptake of OxRBC by peritoneal macrophages.17 31 We determined whether PS liposomes injected in vivo, 2 minutes prior to the injection of OxRBC, would affect the blood removal and the liver uptake of OxRBC. We found that the rate of blood decay is decreased considerably by the presence of liposomes containing PS (Figure 4A). Liposomes without PS (PC liposomes) had no significant effect on the blood removal. The uptake of 51Cr-OxRBC by the liver was inhibited by more than 80%, whereas PC liposomes were much less effective (Figure 4B and C). We also examined the effect of other ligands for scavenger receptors such as poly I, fucoidan, and OxLDL, on the liver and spleen uptake (Figure 5). Of the ligands tested, poly I showed a highly significant inhibition, both on the clearance of OxRBC from the blood circulation (not shown) and on the uptake by the liver and spleen, whereas poly A was ineffective. OxLDL was also effective in inhibiting both liver and spleen uptake of OxRBC by about 50%, similar to fucoidan (Figure 5).
Effect of phosphatidylserine (PS) and phosphatidylcholine (PC) liposomes.
(A) The effect of PS and PC liposomes on the liver uptake and blood decay of oxidatively damaged red blood cells (OxRBC). A volume of 200 μL of a 3.0 mmol/L PC liposome suspension (open triangles) or PS liposome suspension (closed triangles) was injected 2 minutes prior to the injection of OxRBC. Blood samples were taken at the indicated times. (B) Liver uptake of OxRBC after preinjection of PC liposomes (open circles) or PS liposomes (closed circles). (C) Tissue distribution of OxRBC at 30 minutes after injection with prior injection of PC liposomes (hatched bars) or PS liposomes (black bars). In all graphs, control values without treatment are shown for reference (open inverted triangles and open bars). Shown are the means of 3 ± SEM for PC or PS liposomes. *P < .05; **P < .005.
Effect of phosphatidylserine (PS) and phosphatidylcholine (PC) liposomes.
(A) The effect of PS and PC liposomes on the liver uptake and blood decay of oxidatively damaged red blood cells (OxRBC). A volume of 200 μL of a 3.0 mmol/L PC liposome suspension (open triangles) or PS liposome suspension (closed triangles) was injected 2 minutes prior to the injection of OxRBC. Blood samples were taken at the indicated times. (B) Liver uptake of OxRBC after preinjection of PC liposomes (open circles) or PS liposomes (closed circles). (C) Tissue distribution of OxRBC at 30 minutes after injection with prior injection of PC liposomes (hatched bars) or PS liposomes (black bars). In all graphs, control values without treatment are shown for reference (open inverted triangles and open bars). Shown are the means of 3 ± SEM for PC or PS liposomes. *P < .05; **P < .005.
The effect of polyanions, fucoidan, or oxidized low-density lipoprotein (OxLDL) on the liver and spleen uptake of oxidatively damaged red blood cells (OxRBC).
Liver (black bars) and spleen (hatched bars) uptake after injection of OxRBC. At 2 minutes before the injection of OxRBC, the indicated inhibitors were injected at the following amounts: polyadenosinic acid and polyinosinic acid: 200 μg (n = 3 ± SEM); fucoidan: 15 mg (n = 2 ± SEM); OxLDL: 150 μg protein (n = 2 ± SEM). *P < .05; **P < .001.
The effect of polyanions, fucoidan, or oxidized low-density lipoprotein (OxLDL) on the liver and spleen uptake of oxidatively damaged red blood cells (OxRBC).
Liver (black bars) and spleen (hatched bars) uptake after injection of OxRBC. At 2 minutes before the injection of OxRBC, the indicated inhibitors were injected at the following amounts: polyadenosinic acid and polyinosinic acid: 200 μg (n = 3 ± SEM); fucoidan: 15 mg (n = 2 ± SEM); OxLDL: 150 μg protein (n = 2 ± SEM). *P < .05; **P < .001.
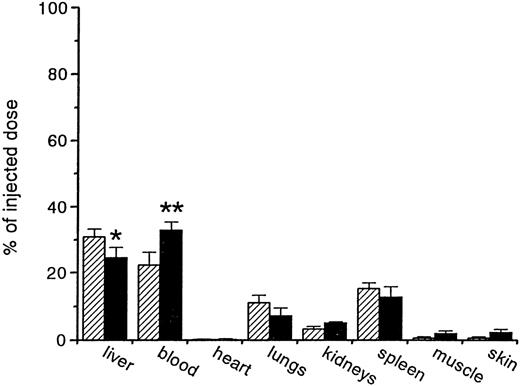
Tissue distribution of OxRBC in SRA knockout mice
From the competition experiments, we learned that typical scavenger receptor ligands could efficiently compete for the uptake of OxRBC in vivo. However, no definite conclusions regarding the identity of the particular types of scavenger receptors involved could be drawn. There is considerable overlap in ligand binding properties among the various types of scavenger receptors. To determine whether the lack of SRA on Kupffer cells influenced the uptake of OxRBC, we compared the in vivo fate of OxRBC in SRA knockout mice and in control mice. In Figure6, it is shown that there was no significant difference in the tissue distribution of 51Cr-labeled OxRBC between SRA knockout and control animals. Furthermore, no difference in the rate of blood decay nor the liver uptake was detected up to 30 minutes after injection (data not shown). These data indicate that in vivo, similar to in vitro, SRA is not responsible for the interaction between OxRBC and macrophages, pointing to other types of scavenger receptors as the primary site.
Tissue distribution of oxidatively damaged red blood cells (OxRBC).
Tissue distribution of OxRBC at 30 minutes after injection in wild-type (hatched bars) and scavenger receptor class A (SRA) knockout (black bars) mice. Shown are the means (n = 6 for wild-type mice, n = 3 for SRA knockout mice) ± SEM. *P = .13; **P = .11.
Tissue distribution of oxidatively damaged red blood cells (OxRBC).
Tissue distribution of OxRBC at 30 minutes after injection in wild-type (hatched bars) and scavenger receptor class A (SRA) knockout (black bars) mice. Shown are the means (n = 6 for wild-type mice, n = 3 for SRA knockout mice) ± SEM. *P = .13; **P = .11.
Discussion
The removal of damaged and dying cells from the blood circulation and tissues is important for the maintenance of cellular homeostasis. The recognition of damaged cells by different macrophage populations has mainly been studied by in vitro experiments, but information on their removal from the blood circulation and their tissue fate is lacking. To determine the relevance of the in vitro observation for the in vivo situation, we used mice in which51Cr-labeled oxidized RBCs were injected intravenously, and the organ and cellular uptake sites were quantitatively analyzed (the recovery of the injected label was 86.4 ± 4.3%). In addition, the characteristics of the site(s) responsible for OxRBC removal were analyzed by in vivo competition studies with PS and PC liposomes, OxLDL, fucoidan, and polyanions. Furthermore, the potential involvement of the likely candidate receptor, SRA type I,II, was analyzed by using mice deficient for this particular receptor. On injection into mice, 51Cr-labeled murine OxRBC were rapidly removed from the blood circulation, whereas native RBCs, as anticipated, remained in the circulation. Within 10 minutes after injection, more than 50% of the injected OxRBC were removed from the blood circulation and 24% were found in the liver. At 30 minutes, 31% of the injected cells were present in the liver and 15% in the spleen. It thus appears that the liver is the most important organ for OxRBC clearance, showing a 2-fold higher uptake than the spleen. However, it must be realized that, on a weight basis, the spleen uptake greatly exceeds the liver uptake per gram tissue, a finding reminiscent of the clearance of PS-bearing red cells.32 Light microscopic analysis of the isolated liver cells at 30 minutes after injection of OxRBC indicated that 65% of the isolated Kupffer cells had ingested 1 or more RBC, and there was no evidence for uptake of OxRBC by liver endothelial cells. When Kupffer cells were depleted from the mice by pretreatment with DMDP-liposomes, no uptake of OxRBC occurred by the liver and only very little by the spleen. These data indicate that Kupffer cells and spleen macrophages are largely responsible for the removal of OxRBC from the blood circulation.
In vitro data with peritoneal macrophages have indicated that the interaction of oxidatively damaged erythrocytes with macrophages is mediated by a macrophage receptor with specificity for OxLDL.11 17 To analyze whether specific receptors on Kupffer cells and spleen macrophages are involved in the removal of OxRBC in intact mice, we determined whether ligands for scavenger receptors interfere with the biological fate of the cells. It appears that the liver uptake of OxRBC is competed for by typical ligands for scavenger receptors, namely poly I, liposomes containing PS, fucoidan, and OxLDL. PS liposomes efficiently blocked the liver uptake of OxRBC compared with control liposomes. This blockage led to a significant inhibition of the removal of OxRBC from the blood circulation. Remarkably, the spleen uptake of the OxRBC was not influenced by the preinjection of PS liposomes. This finding indicates that different receptor system(s) are responsible for the removal of OxRBC by the liver and by the spleen. In contrast, preinjection of poly I, fucoidan, and OxLDL significantly inhibited uptake of OxRBC by both liver and spleen. Taken together, these results indicate that, in vivo, specific receptors with scavenger receptor-like properties are responsible for the removal of OxRBC from the blood circulation by liver Kupffer cells and spleen macrophages.
Among the various scavenger receptors,33-40 the SRA was the first to be fully characterized. The availability of SRA knockout mice made it possible to evaluate the importance of this receptor for the removal of OxRBC from the blood circulation. Apparently this receptor plays only a minor role, if any, in this process, since both the blood decay and the uptake by liver and other tissues of OxRBC in the control and knockout animals were largely similar. This finding is in agreement with in vitro data, showing that acetylated LDL, a typical ligand for SRA, does not compete for the binding of OxRBC by peritoneal macrophages.11 31
We have concentrated on the possibility that scavenger receptors are involved in the process of damaged cell removal, since some scavenger receptors show binding of anionic phospholipids41,42 and the exposure of PS on the outer leaflet of the plasma membrane is thought to be an important recognition site for removal of apoptotic cells by macrophages. It seems unlikely that MARCO, a scavenger receptor that is structurally related to SRA, would be involved, since it is not expressed on Kupffer cells in a nonactivated state.38 Another receptor recently implicated in the recognition of apoptotic cells is CD14, the LPS-receptor.43 Previous studies found that the 61D3 antibody, which has since been shown to be directed against CD14, inhibited the binding of apoptotic cells21 and phospholipid symmetric RBCs44 to macrophages. Because fucoidan45 and phospholipids46 are also competitors for binding of LPS to CD14, this receptor is, therefore, a candidate for the recognition of OxRBC. The general availability of the CD14 knockout mice will be needed to unequivocally prove or disapprove this possibility. The class B scavenger receptors, SR-BI and CD36, could also be involved, since they both have affinity for anionic phospholipids41,42 and CD36 recognizes RBCs infected withPlasmodium falciparum47,48 and apoptotic cells.49,50 Recently a lectin-like receptor for OxLDL, LOX-1, was cloned from endothelial cells,33 and it was shown to bind and phagocytose aged RBCs and apoptotic cells in vitro through a PS-dependent mechanism.51 It appears that LOX-1 is not only expressed by endothelial cells but also by differentiated monocytes.52 The selective uptake of OxRBC by liver Kupffer cells observed in our study, however, requires the expression of a specific receptor on Kupffer cells, which has not been reported for LOX-1 so far.
In conclusion, our data show that Kupffer cells are mainly responsible for the removal of OxRBC from the blood circulation and that the removal of OxRBC by Kupffer cells and spleen macrophages can be completely blocked in vivo by substrates for scavenger receptors. This blockade indicates that the recognition system for OxRBC on Kupffer cells and spleen macrophages shows scavenger receptor-like properties, although the studies with SRA knockout mice indicate a mechanism that is not dependent on SRA. We are currently investigating whether the in vivo clearance of apoptotic lymphocytes follows the same patterns as described here for OxRBC. Because the clearance of effete cells is of great importance for tissue homeostasis, it is possible that more than one member of the scavenger receptor family is involved in this pathway. Further characterization of the relative importance of the individual members of this family for the removal of aged cells forms an interesting future challenge.
Acknowledgments
We are grateful to Dr S. R. Sambrano (University of California, San Francisco) and to Dr D. Steinberg (University of California, San Diego) for helpful discussion and critical reading of the manuscript. We thank Dr N. van Rooijen for kindly providing DMDP-liposomes.
Reprints:Theo J. C. van Berkel, Division of Biopharmaceutics, Leiden/Amsterdam Center for Drug Research, University of Leiden, P.O. Box 9503, 2300 RA Leiden, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal