Abstract
The Epstein–Barr virus (EBV)-encoded latent membrane protein-1 induces NF-κB activity by targeting IκBα. To understand the role of NF-κB activation in EBV-related oncogenesis, we have subcloned mutated IκBα32/36A cDNA into a pHEBo vector containing doxycycline regulatory sequences and stably transfected this construct into a lymphoblastoid cell line. Two tightly regulated clones were obtained in which IκBα32/36A was inducible in a doxycycline dose-dependent manner. Levels of inducible IκBα32/36A peaked at day 2. Inhibition of NF-κB activity was closely correlated with levels of inducible IκBα32/36A. Levels of 3 well-known NF-κB-dependent genes, CD54, p105, and endogenous IκBα, were decreased when IκBα32/36A was induced, and the growth of IκBα32/36A-induced EBV-infected cells was slightly reduced. Loss of NF-κB activity was associated with decreased Bcl-2 protein levels. Finally, the induction of apoptosis was strongly increased in IκBα32/36A-overexpressing cells. Together these results show that it is possible to control IκBα32/36A levels, ie, NF-κB activity, in EBV-infected B-lymphocytes using a doxycycline-inducible vector. Moreover, our results indicate that NF-κB can protect EBV-infected cells from apoptosis by Bcl-2. Finally, our results suggest that a cellular model with doxycycline-inducible IκBα32/36A may be useful in the identification of genuine NF-κB target genes in EBV-infected B cells.
Introduction
Epstein–Barr virus (EBV)-related B-cell transformation involves cell reprogramming through the modulation of critical cellular transcription factors. Such dysregulation may be the result of direct interactions between viral proteins and cellular transcription factors and from a rerouting of defined cellular signal transduction pathways. For example, EBNA2 protein interacts directly with RBP-Jκ, an inhibitory transcription factor, converting it to a positive transcription regulator. Another well-documented example is the activation of both the AP-1 and the NF-κB transcription factors by LMP-1, which mimics a constitutively active receptor of the tumor necrosis factor (TNF) receptor family (see Mosialos1 and Manet et al2 for review).
In mammalian cells, nuclear NF-κB activity corresponds to heterodimers and homodimers between the 5 Rel/NF-κB proteins, including p50/p105 (NF-κB1), p65 (RelA), p52/p100 (NF-κB2), c-Rel, and RelB. These proteins share a common Rel homology domain, involved in both dimerization and DNA binding to the consensus κB site. NF-κB activation has been implicated in the positive regulation of a variety of genes of the immune response, such as i-NOS, IL-1, IL-2, IL-6, IL-8, tumor necrosis factor-α (TNF-α), major histocompatibility complex (MHC) class 1 and 2, CD25, CD54, and CD80, A20 protein, the proto-oncogene c-myc, and the Rel/NF-κB proteins itself, including NF-κB1, NF-κB2, c-Rel, and the inhibitor IκBα.3 Moreover, even though NF-κB has been associated with the induction of apoptosis in some cellular systems, there is increasing evidence for a role of NF-κB activation in the protection of cells against apoptosis.3,4
Rel/NF-κB complexes containing RelA or c-Rel are usually trapped within the cytoplasm of most cell types by interaction with inhibitory proteins called IκBα, IκBβ, and IκBε. Activation of NF-κB depends on the phosphorylation and degradation of IκBs, leading to the accessibility of nuclear transport signals and nuclear translocation of the RelA- and c-Rel-containing dimers. Various cell activation pathways such as the TNF-α, IL-1, PKC, and lipopolysaccharide signal transduction pathways converge at the IκB phosphorylation step through the activation of a specific, multisubunit complex called the IκB kinase (IKK) that contains at least 3 polypeptides directly involved in IκB phosphorylation: IKKα, IKKβ,5–8 and NEMO.9 IKKα and IKKβ are able to phosphorylate IκBα and IκBβ specifically on serine residues 32/36 and 19/23 respectively, whereas the NEMO polypeptide is thought to be involved in signal integration from a variety of signaling pathways that converge at IκB phosphorylation and NF-κB activation.
That constitutive NF-κB activation can contribute directly to cell transformation has been recently highlighted in the Tax model. Indeed, transfection of NEMO into mutant cells unable to activate NF-κB is associated with both the restoration of NF-κB activation and the transformation capacity of Tax.9,10 Various studies suggest a role of Rel/NF-κB in oncogenesis. For example, v-Rel, a virally truncated and mutated version of c-Rel, is responsible for fatal hematologic diseases in chicken and turkey, and transgenic mice expressing v-Rel have rapid T-cell lymphoblastic disorders.11–13 In humans, the chromosomal translocation t(10;14)(q24;q32) affects p52 and represents a recurrent chromosomal rearrangement found in certain lymphoid malignancies14–16 of B-cell origin. Amplification of the REL locus has been reported to be a feature of primary extranodal lymphoma.17
LMP-1, one of the major transforming proteins of EBV, contributes to enhanced NF-κB activation in EBV-infected cells.18 It is a transmembrane protein consisting of a short amino terminal cytoplasmic region, 6 transmembrane domains, and 200 cytoplasmic carboxyterminal amino acids. Deletion experiments revealed that the carboxyterminal cytoplasmic tail carries two domains, CTAR1 and CTAR2, collectively responsible for LMP-1 tumorigenesis and the activation of both NF-κB19,20 and JNK kinase.21 CTAR1 is responsible for approximately 30% and CTAR2 for approximately 70% of LMP1-associated NF-κB activation. The 6 transmembrane domains are required for LMP-1 aggregation and the consequent signal transduction that, through the aggregation of TRADD and TRAF molecules,22,23 allows LMP-1 to mimic a constitutively active receptor of the TNF receptor family. The CTAR1 and CTAR2 domains of LMP-1 contain binding sites for TRAF1 and TRADD molecules, respectively.24,25 Both adaptor proteins can themselves bind to TRAF2, which leads to NF-κB activation through the recruitment of the NF-κB-inducing kinase (NIK) and to subsequent IKK activation, which leads in turn to the phosphorylation and degradation of IκBs.26
The aim of this work was to design a cellular model that would allow us functionally to analyze the role of NF-κB and to identify putative NF-κB target genes in EBV-infected lymphocytes. For this purpose, we chose to inhibit Rel/NF-κB complexes specifically by stable transfection of the cDNA encoding for IκBα mutated on serine 32 and 36 (IκBα32/36A).27 Furthermore, to avoid selection bias for those clones able to escape the potentially deleterious effects of IκBα overexpression, dominant-negative IκBα32/36A was expressed in a conditional fashion from a doxycycline-inducible promoter.28 To prevent vector integration into the genomic DNA, which is difficult to obtain in lymphoblastoid cells and may give rise to position effects, we used the pHEBo backbone containing the EBV OriP sequences. The pHEBO construct allows the vector to replicate episomally in the presence of EBV-encoded nuclear protein-1 (EBNA1).29,30 Our results showed that it was possible to obtain cells in which IκBα32/36A is inducible after doxycycline treatment in a dose-responsive manner. Levels of IκBα32/36A induction were tightly correlated with those of NF-κB inhibition. In addition, we demonstrated that the inhibition of NF-κB was correlated with a decrease in CD54, p105/NF-κB1, and endogenous IκBα expression, implicating these genes as potentially NF-κB-regulated in EBV-infected cells. Finally, we showed that the loss of NF-κB activity in EBV-infected lymphocytes was associated with a significant decrease of Bcl-2 protein expression and with a dramatic increase in their sensitivity to apoptosis.
Materials and methods
Plasmid constructs
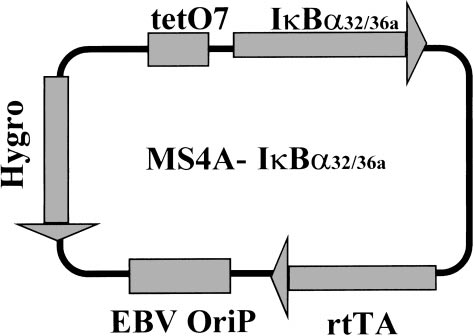
MS4A was derived by cloning the reverse tetracyclin repressor-transactivator protein (rtTA28) and a tetracycline regulatable promoter31 onto the pHEBo vector.29 To achieve tight regulation, the cytomegalovirus (CMV) promoter was replaced by the EBV–LMP2A promoter in which the endogenous EBNA2 response element was substituted by a heptamerized tet operator sequence. The IκBα32/36A cDNA was excised from pCMV–IκBα32/36A27 by HindIII and XbaI digestion and subcloned into the MS4A vector at the Sfi1 sites (MS4A–IκBα32/36A; see Figure 1). The 0.4SK-luc plasmid containing the IκBα promoter with 3 κB sites upstream of the luciferase gene was a kind gift of A. Israël (Institut Pasteur, Paris, France).32
Schematic diagram of the MS4A-IκBα32/36A vector. The different cassettes are indicated in grey boxes: tetO7, tetracycline-responsive elements; IκBα32/36A, c-DNA encoding for IκBα32/36A super-repressor; rtTA, cassette encoding for the doxycycline-responsive factor; EBV–OriP, EBV-OriP sequences; Hygro, cassette encoding for hygromycin resistance.
Schematic diagram of the MS4A-IκBα32/36A vector. The different cassettes are indicated in grey boxes: tetO7, tetracycline-responsive elements; IκBα32/36A, c-DNA encoding for IκBα32/36A super-repressor; rtTA, cassette encoding for the doxycycline-responsive factor; EBV–OriP, EBV-OriP sequences; Hygro, cassette encoding for hygromycin resistance.
Protein extracts and electromobility shift assay
Western blot
Our standard gel conditions consisted of 10% SDS–PAGE without stacking gel as described.36 Where indicated, a stacking gel (4% polyacrylamide, 125 mmol/L glycine, pH 6.8) was used according to standard procedures.37 Thirty micrograms protein was fractionated and electrotransferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA) 0.8 mA/cm2 with the Hoefer semidry apparatus (Pharmacia Biotech, Orsay, France). Antibodies used for Western blot analysis were MAD 10B for IκBα38 (generous gift of R. Hay, University of St Andrew, St Andrew, UK) diluted at 1/500, monoclonal antibody 124 for Bcl-2 (Dako, Trappes, France) diluted at 1/100, rabbit immune serum no. 1140 diluted at 1/1500 for p105 (generous gift of N. Rice, NCI, Frederick). Detection of the antigen–antibody complexes was performed using a horseradish peroxidase-coupled antimouse or antirabbit antibody (Biorad, Ivry/Seine, France) diluted at 1/10 000, followed by chemiluminescent visualization (Biolabs, Hitchin, UK).
Cell culture and cell transfection
One of our lymphoblastoid cell lines, named PRI, was cultured continuously in RPMI 1640 with standard concentrations of l-glutamine and antibiotics and 10% fetal calf serum. Transfected cells were cultured in the presence of hygromycin (Calbiochem, Meudon, France) at 300 μg/mL and 20% fetal calf serum. Twenty million cells were electroporated with 20 μg MS4A–IκBα32/36A at 250 V and 960 μF. After 4 days, hygromycin was added at 50 μg/mL. Hygromycin concentration was progressively increased up to 300 μg/mL during the first 2 weeks of selection. After 4 weeks of culture in the presence of hygromycin, transfected cells were plated at a limiting dilution on 96-well plates. Screening of clones was based on the inducibility of IκBα expression after doxycycline treatment. To induce IκB overexpression with doxycycline, cells were washed once in RPMI and resuspended in standard medium without hygromycin. Doxycycline (purchased from AP-HP, Paris, France and stored at −20°C at 1 mg/mL in water) was then added at concentrations ranging from 0.01 μg/mL to 8 μg/mL (usually 2 μg/mL). Western blot analysis of IκBα expression was performed 48 hours after doxycycline treatment. Transient transfections were conducted in triplicate with 20 μg 0.4SK-luc plasmid for 10 million cells. Cells were washed once in RPMI, resuspended in standard medium, and electroporated at 250 V and 960 μF. Then the cells were resuspended in standard medium at 106 cells/mL, left 1 hour at 37°C, washed once, and resuspended in standard medium without hygromycin and with or without doxycycline at 2 μg/mL. Luciferase assays were performed after 2 days (Promega, Madison, WI), according the manufacturer’s instructions.
Immunolabelings and flow cytometry analysis
Antibodies tested are listed in Table 1. The expression of cell-surface molecules was assessed by direct and indirect immunofluorescence staining according standard protocols, using a Coulter XL flow cytometer (Coulter, Margency, France).
Antibodies used
| Specificity . | Clone . | Dilution . | Supplier . | Fluorochrome . |
|---|---|---|---|---|
| CD10 | J5 | 1/20 | Coulter* | FITC |
| CD19 | B4:89D | 1/25 | Coulter | PE |
| CD20 | BLy.1 | 1/20 | Dako† | FITC |
| CD21 | B120 | 1/20 | Coulter | PE |
| CD23 | MHM6 | 1/20 | Dako | FITC |
| CD25 | 1HT44H3 | 1/20 | Coulter | FITC |
| CD30 | HRS4 | 1/30 | Immunotech | None |
| CD38 | T16 | 1/30 | Becton-Dickinson‡ | PE |
| CD39 | AC2 | 1/20 | Immunotech | None |
| CD44 | J173 | 1/20 | Immunotech | None |
| CD48 | J4.57 | 1/30 | Immunotech | None |
| CD54 | 84H10 | 1/10 | Immunotech | FITC |
| CD58 | AICD58 | 1/30 | Immunotech | None |
| CD71 | 37L | 1/20 | Coulter | FITC |
| CD80 | MAB1.4 | 1/20 | Immunotech | None |
| CD95 | CH-11 | 1/5 | Immunotech | None |
| IgM | AF6 | 1/20 | Dako | FITC |
| HLAI | B9.12.1 | 1/50 | Immunotech | None |
| HLAII | B8.12.2 | 1/50 | Immunotech | None |
| F(ab′)† | Polyclonal | 1/20 | Dako | FITC |
| F(ab′)† | Polyclonal | 1/20 | Dako | FITC |
| Specificity . | Clone . | Dilution . | Supplier . | Fluorochrome . |
|---|---|---|---|---|
| CD10 | J5 | 1/20 | Coulter* | FITC |
| CD19 | B4:89D | 1/25 | Coulter | PE |
| CD20 | BLy.1 | 1/20 | Dako† | FITC |
| CD21 | B120 | 1/20 | Coulter | PE |
| CD23 | MHM6 | 1/20 | Dako | FITC |
| CD25 | 1HT44H3 | 1/20 | Coulter | FITC |
| CD30 | HRS4 | 1/30 | Immunotech | None |
| CD38 | T16 | 1/30 | Becton-Dickinson‡ | PE |
| CD39 | AC2 | 1/20 | Immunotech | None |
| CD44 | J173 | 1/20 | Immunotech | None |
| CD48 | J4.57 | 1/30 | Immunotech | None |
| CD54 | 84H10 | 1/10 | Immunotech | FITC |
| CD58 | AICD58 | 1/30 | Immunotech | None |
| CD71 | 37L | 1/20 | Coulter | FITC |
| CD80 | MAB1.4 | 1/20 | Immunotech | None |
| CD95 | CH-11 | 1/5 | Immunotech | None |
| IgM | AF6 | 1/20 | Dako | FITC |
| HLAI | B9.12.1 | 1/50 | Immunotech | None |
| HLAII | B8.12.2 | 1/50 | Immunotech | None |
| F(ab′)† | Polyclonal | 1/20 | Dako | FITC |
| F(ab′)† | Polyclonal | 1/20 | Dako | FITC |
FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Coulter–Immunotech, Margency, France.
Dako, Trappes, France.
Becton–Dickinson, Erembodegem–Aalst, Belgium.
Cell-cycle analysis and apoptosis detection
Cell-cycle analysis and detection of apoptosis were performed by flow cytometry as described39 on the basis of BrdU incorporation into DNA and propidium iodide staining. Fluorescence analysis of chromatin condensation and fragmentation was conducted after cytocentrifugation of 50 000 cells and DNA staining with the Hoechst 33258 diluted at 20 μg/mL in phosphate-buffered saline. Staining of cells with fluorescein isothiocyanate-conjugated Annexin V (Pharmingen, San Diego, CA) was performed according to the manufacturer’s protocol.
Results
Characteristics of IκBα32/36A induction by doxycycline in lymphoblastoid cells transfected with the MSA4-IκBα32/36A vector
The MS4A-IκBα32/36A vector was generated by subcloning the IκBα cDNA mutated at positions encoding for the two serine 32 and 36 residues (IκBα32/36A)27 into the MS4A vector, whose transcriptional activity is positively regulated by the addition of doxycycline. The MS4A construct consists of a pHEBo backbone29 (with its EBV–OriP sequences) into which have been subcloned both a promoter carrying the tetO7 tetracycline responsive element and the cDNA encoding for rtTA protein28 (Figure 1).
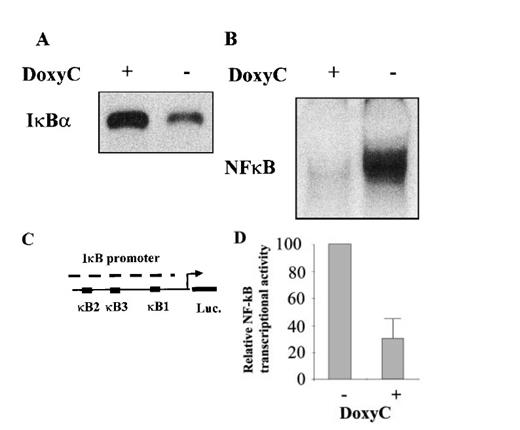
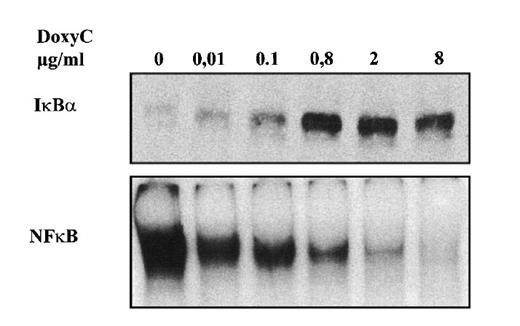
After transfection of cells with the MS4A-IκBα32/36A vector, cells were grown in the presence of hygromycin and cloned by limiting dilution. Twenty-four clones were screened by Western blotting for IκBα32/36A induction after 48 hours of doxycycline treatment at 2 μg/mL. Two clones were found to be IκBα32/36A inducible. Then the NF-κB activity of IκBα32/36A-inducible clones was assessed by both EMSA and luciferase assay after the transfection of 0.4SK-luc plasmid, which harbors the promoter of IκBα with its 3 κB sites.32 A typical result is illustrated in Figure 2, which shows that a significant induction of IκBα32/36A was correlated with a dramatic decrease of NF-κB activity in DNA binding and transcriptional activity. We next set up a dose–response experiment (Figure 3). Results demonstrated that IκBα32/36A was inducible by doxycycline in a dose-dependent manner and that the decrease of NF-κB binding activity was tightly correlated with the levels of IκBα32/36A induction. In the following experiment, induction of IκBα32/36A was performed at 2 μg/mL doxycycline because doxycycline concentrations greater than 5 μg/mL had been reported to be toxic. SDS–PAGE without a stacking gel36 did not allow the separation of endogenous IκBα from IκBα32/36A (Figures 2, 3). Endogenous IκBα could, however, be distinguished from the transfected gene product on the basis of its higher electrophoretic mobility if a stacking gel was used.27 A comparison of both gel conditions is depicted in Figure 4. Our results showed that there was no detectable leakage of the tet07 promoter (Figure 4). Furthermore, levels of endogenous IκBα decreased when IκBα32/36A expression was induced by doxycycline (Figure 4), suggesting that endogenous IκBα is a genuine target gene of NF-κB in EBV-infected cells.
Characterization of the IκBα32/36A inducibility after doxycycline treatment. (A) Western blot detection of IκBα protein in MS4A-IκBα32/36A-transfected cells with (+) or without (−) doxycycline at 2 μg/mL. (B) NF-κB binding activity assessed by EMSA in MS4A-IκBα32/36A-transfected cells with (+) or without (−) doxycycline at 2 μg/mL. (C) Schematic representation of the transcriptional regulatory element of the IκBα promoter with its 3 κB sites in front of the luciferase gene (construct 0.4SK-luc). (D) Relative NF-κB transcriptional activity in MS4A-IκBα32/36A-transfected cells with (+) or without (−) doxycycline at 2 μg/mL. Presented results correspond to the mean of 3 transfection experiments with the 0.4SK-luc construct. Each transfection experiment was conducted in triplicate.
Characterization of the IκBα32/36A inducibility after doxycycline treatment. (A) Western blot detection of IκBα protein in MS4A-IκBα32/36A-transfected cells with (+) or without (−) doxycycline at 2 μg/mL. (B) NF-κB binding activity assessed by EMSA in MS4A-IκBα32/36A-transfected cells with (+) or without (−) doxycycline at 2 μg/mL. (C) Schematic representation of the transcriptional regulatory element of the IκBα promoter with its 3 κB sites in front of the luciferase gene (construct 0.4SK-luc). (D) Relative NF-κB transcriptional activity in MS4A-IκBα32/36A-transfected cells with (+) or without (−) doxycycline at 2 μg/mL. Presented results correspond to the mean of 3 transfection experiments with the 0.4SK-luc construct. Each transfection experiment was conducted in triplicate.
Dose-responsive inducibility of IκBα32/36A by doxycycline. MS4A-IκBα32/36A-transfected cells were treated for 48 hours with various concentrations of doxycycline as indicated. Upper panel: Western blot detection of IκBα. Lower panel: NF-κB-binding activity assessed by EMSA.
Dose-responsive inducibility of IκBα32/36A by doxycycline. MS4A-IκBα32/36A-transfected cells were treated for 48 hours with various concentrations of doxycycline as indicated. Upper panel: Western blot detection of IκBα. Lower panel: NF-κB-binding activity assessed by EMSA.
Gel electrophoresis with or without stacking gel followed by Western blot analysis of IκBα32/36A. MS4A-IκBα32/36A-transfected cells were (lanes 1 and 3) or were not (lanes 2 and 4) treated with doxycycline for 48 hours. SDS–PAGE and Western blot detection of IκBα was performed without (lanes 1 and 2) or with (lanes 3 and 4) stacking gel.
Gel electrophoresis with or without stacking gel followed by Western blot analysis of IκBα32/36A. MS4A-IκBα32/36A-transfected cells were (lanes 1 and 3) or were not (lanes 2 and 4) treated with doxycycline for 48 hours. SDS–PAGE and Western blot detection of IκBα was performed without (lanes 1 and 2) or with (lanes 3 and 4) stacking gel.
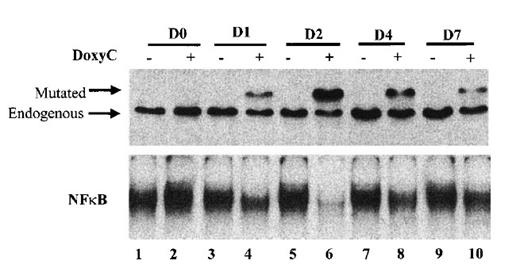
Finally, we established the kinetics of IκBα32/36A induction. As shown in Figure 5, IκBα32/36A induction was highest after 48 hours of doxycycline treatment at 2 μg/mL. Levels of IκBα32/36A slowly decreased at day 4 and day 7. Kinetics of the inhibition of NF-κB binding activity strictly correlated with the kinetics of IκBα32/36A induction. Therefore, phenotypic analysis of doxycycline-treated cells was systematically performed at day 2.
Kinetics of IκBα32/36A inducibility after doxycycline treatment. Cells were (+) or were not (−) treated with doxycycline at 2 μg/mL. Times of protein extraction are indicated at the top of the figure (D0: protein extraction was performed immediately after doxycycline treatment; D1, D2, D4, and D7: protein extraction was performed immediately after 1, 2, 4, and 7 days of doxycycline treatment, respectively). Upper panel: Western blot detection of IκBα after SDS–PAGE with stacking gel; mutated and endogenous IκBα are indicated by the arrow. Lower panel: NF-κB-binding activity assessed by EMSA.
Kinetics of IκBα32/36A inducibility after doxycycline treatment. Cells were (+) or were not (−) treated with doxycycline at 2 μg/mL. Times of protein extraction are indicated at the top of the figure (D0: protein extraction was performed immediately after doxycycline treatment; D1, D2, D4, and D7: protein extraction was performed immediately after 1, 2, 4, and 7 days of doxycycline treatment, respectively). Upper panel: Western blot detection of IκBα after SDS–PAGE with stacking gel; mutated and endogenous IκBα are indicated by the arrow. Lower panel: NF-κB-binding activity assessed by EMSA.
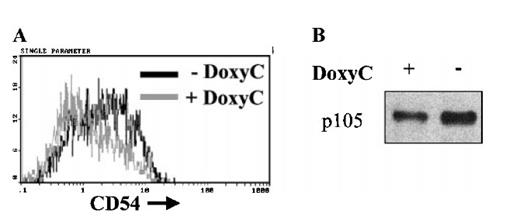
CD54, p105, and IκBα are putative target genes of NF-κB in EBV infected B cells
Loss of NF-κB activity in the presence of IκBα32/36A was associated with down-regulation of the transcriptional activity of the IκBα promoter (Figure 2D) and resulted in a significant decrease of endogenous IκBα levels (Figures 4, 5). This strongly implicates IκBα to be a genuine target gene of NF-κB in EBV-infected cells. To further evaluate the function of the induced IκBα32/36A protein, we analyzed the expression of some genes that may be positively regulated by NF-κB. Expression of CD54 and p105 was decreased in these cells after 48 hours of doxycycline treatment at 2 μg/mL (Figure 6). These results suggested that the CD54 and p105 genes may also be genuine target genes of NF-κB in EBV-immortalized lymphoblastoid cells.
Down-regulation of CD54 and p105 expression after induction of IκBα32/36A. Levels of CD54 membrane expression were assessed by flow cytometry on cells treated (+ DoxyC) or not (− DoxyC) with doxycycline at 2 μg/mL for 48 hours (A). Levels of p105 expression were assessed by Western blot on cells treated (+) or not (−) with doxycycline at 2 μg/mL for 48 hours (B).
Down-regulation of CD54 and p105 expression after induction of IκBα32/36A. Levels of CD54 membrane expression were assessed by flow cytometry on cells treated (+ DoxyC) or not (− DoxyC) with doxycycline at 2 μg/mL for 48 hours (A). Levels of p105 expression were assessed by Western blot on cells treated (+) or not (−) with doxycycline at 2 μg/mL for 48 hours (B).
Functional consequences of the IκBα32/36A induction in lymphoblastoid cells
Because NF-κB has been reported to protect cells from apoptosis,40–42 and because LMP-1 transfection has been reported to enhance Bcl-2 expression in B cells,43,44 we examined the relationship between the inducible inhibition of NF-κB and any changes in cell proliferation sensitivity to apoptosis. Proliferation of doxycycline-treated cells was decreased slightly in terms of the growth kinetics of cells in culture and the fraction of cells in S-phase, as estimated by flow cytometry after BrdU incorporation (not shown).
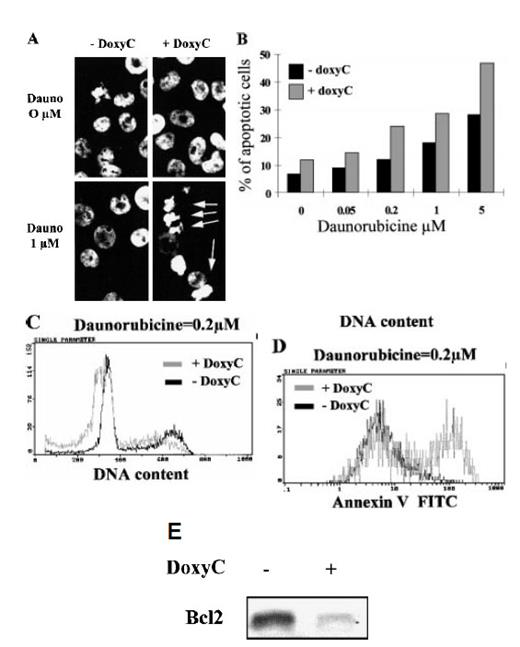
Spontaneous apoptosis did not increase significantly in doxycycline-treated cells. However, treatment of cells with daunorubicine, a DNA-intercalating chemotoxic drug that induces apoptosis, clearly showed that IκBα32/36A overexpression (ie, the loss of NF-κB activity) can sensitize EBV-infected B cells to apoptosis, as revealed by chromatin condensation and DNA fragmentation (Figures 7A, 7B), sub-G1 peak induction (Figure 7C), and annexin-V cell-surface fixation (Figure 7D). Finally, we found that the loss of NF-κB binding activity was correlated with a dramatic decrease of Bcl-2 expression within 48 hours in EBV-infected B cells (Figure 7E).
Induction of apoptosis and expression of Bcl-2 in MS4A-IκBα32/36A-transfected cells. (A) Analysis of nuclear condensation after Hoechst 33258 staining. Cells were (+ DoxyC) or were not (− DoxyC) pretreated with doxycycline at 2 μg/mL for 24 hours. Daunorubicine (Dauno) was added for 18 hours at the concentrations indicated. (B) Dose response to apoptosis induction by daunorubicine in cells pretreated (+ DoxyC) or not (− DoxyC) with doxycycline at 2 μg/mL for 24 hours. Percentages of apoptotic cells were assessed by counting cells with apoptotic nuclei under fluorescence microscope after Hoechst 33258 staining. (C) Flow cytometry analysis of DNA content after propidium iodide staining of the DNA of cells pretreated (+ DoxyC) or not (− DoxyC) with doxycycline at 2 μg/mL for 24 hours and then treated with daunorubicine at 0.2 μmol/L for 18 hours. (D) Annexin V staining of cells pretreated (+ DoxyC) or not (− DoxyC) with doxycycline at 2 μg/mL for 24 hours and then treated with daunorubicine at 0.2 μmol/L for 18 hours. (E) Bcl-2 expression of cells treated (+) or not (−) with doxycycline at 2 μg/mL for 48 hours was assessed by Western blot.
Induction of apoptosis and expression of Bcl-2 in MS4A-IκBα32/36A-transfected cells. (A) Analysis of nuclear condensation after Hoechst 33258 staining. Cells were (+ DoxyC) or were not (− DoxyC) pretreated with doxycycline at 2 μg/mL for 24 hours. Daunorubicine (Dauno) was added for 18 hours at the concentrations indicated. (B) Dose response to apoptosis induction by daunorubicine in cells pretreated (+ DoxyC) or not (− DoxyC) with doxycycline at 2 μg/mL for 24 hours. Percentages of apoptotic cells were assessed by counting cells with apoptotic nuclei under fluorescence microscope after Hoechst 33258 staining. (C) Flow cytometry analysis of DNA content after propidium iodide staining of the DNA of cells pretreated (+ DoxyC) or not (− DoxyC) with doxycycline at 2 μg/mL for 24 hours and then treated with daunorubicine at 0.2 μmol/L for 18 hours. (D) Annexin V staining of cells pretreated (+ DoxyC) or not (− DoxyC) with doxycycline at 2 μg/mL for 24 hours and then treated with daunorubicine at 0.2 μmol/L for 18 hours. (E) Bcl-2 expression of cells treated (+) or not (−) with doxycycline at 2 μg/mL for 48 hours was assessed by Western blot.
Discussion
The relationship between LMP-1 and NF-κB activation has been extensively studied, and every step between the autoaggregation of LMP-1 and the activation of the IKK has been documented. LMP-1, acting through TRAF molecules, activates several signal transduction pathways including the NF-κB,45 JNK,21 and ras-MAPK46 activation cascades. Therefore, to address the question of the specific role of NF-κB in EBV-infected B lymphocytes, our strategy was to specifically inhibit NF-κB in EBV-infected B cells.
Using the doxycycline-inducible system, we obtained EBV-immortalized B lymphocytes in which it was possible to inhibit NF-κB. To our knowledge, this is the first model that allows one to study the role of NF-κB in EBV-infected B lymphocytes. Establishment of stably transfected cells is often a critical step in the analysis of the function of a particular protein. In current stable transfection protocols, a major bias is introduced by the fact that selection takes place while the transfected gene of interest is expressed. If that particular gene is toxic or induces cell-cycle arrest, establishment of transfected clones is either impossible or will lead to the selection of clones which, through mutation, have escaped the effect of the protein to be studied. To circumvent this problem, various groups have worked on and developed inducible vectors. Fusion of the protein of interest to the hormone binding domain of the estrogen or glucocorticoid receptor has provided a powerful tool with which to study the induction of proliferation, differentiation, and apoptosis.47–49 However, in this system, interference of estrogen with NF-κB cannot be excluded. Indeed, a physical association between the activated glucocorticoid receptors and p65 has been reported,50 and estrogen molecules may interact directly with glucocorticoid receptors.51 However, the tetracycline/doxycycline inducible system originally designed by Bujard and collaborators31,52 has proven itself useful for the analysis of the function of proteins whose continuous expression may be deleterious for the cell or may prevent selection in vitro.53 The system described by Gossen and Bujard31 and Gossen et al52 consists of two plasmids: one encodes the tetracycline repressor-VP16 or the reversed tetracycline repressor-VP16 transactivator fusion protein; the other plasmid encodes the gene of interest under the control of a tetracycline-regulatable promoter. The major difficulties of this system are that cells must be stably transfected twice and that the level of expression is highly variable, depending on the site of vector integration into the genomic DNA—a phenomenon known as position effect.
To circumvent these problems, we cloned both the doxycycline-dependent transcription factor rtTA and the tetracycline-regulatable sequences onto the same derived episomal vector (Schuhmacher, manuscript in preparation). pHEBo vectors can be easily transfected into EBV-infected B lymphocytes and maintained episomally. They do not exhibit position effects and, therefore, show little variation in the expression of the transfected gene among different, stably transfected cells.54,55 We have cloned the mutated IκBα32/36A cDNA into this vector and have established stably transfected EBV-immortalized cells that indeed express the mutated IκBα32/36A cDNA in a doxycycline dosage-dependent, inducible fashion. Furthermore IκBα32/36A was functional in terms of the inhibition of NF-κB binding, NF-κB transcriptional activity, and NF-κB-dependent gene expression such as that of endogenous IκBα, p105, and CD54. We obtained a tight correlation between doxycycline concentrations in the medium, levels of IκBα32/36A protein induction, and levels of NF-κB binding inhibition. These results indicated that IκBα32/36A protein levels directly correlated with the level of active rtTA molecules.
Conditional gene expression systems have also proven useful in the analysis of the role of LMP-1 in EBV-driven proliferation. A conditional LMP-1 gene has been introduced into a mini-EBV background56 haboring a tetR–KRAB chimeric repressor cassette and used to immortalize primary B cells.57 Inducible loss of LMP-1 expression was associated with the proliferative arrest of these cells. Reinduction of LMP-1 expression was associated with the reentry of the cells into the cell cycle and the reactivation of the JNK1 and NF-κB signal transduction pathways. These results do not clarify to which extent either JNK1 or NF-κB activation contributes to cellular proliferation. In our cellular model, the inducible loss of NF-κB was associated with only minor effects on the cell cycle in terms of DNA synthesis and cell growth in culture (not shown). These data could appear to suggest that continuous LMP-1-induced NF-κB activation was not required for proliferation and that proliferation would be caused by the activation of a different LMP-1-mediated signal transduction pathway. However, this interpretation is not conclusive because analysis of the kinetics of NF-κB inhibition has revealed a gradual decrease in the level of mutated IκBα32/36A protein over time. This decrease in protein level was associated with a concomitant, gradual increase of NF-κB DNA binding activity to considerable, albeit lower, levels than in the absence of doxycycline. An autoregulatory loop between NF-κB and endogenous IκBα is well established32 but was not expected for the mutated IκBα32/36A in EBV-infected cells. Several possibilities may account for this phenomenon. For example, levels of rtTA–VP16 fusion protein may be down-regulated after IκBα32/36A overexpression. In fact, a search of cryptic κB sites in the MS4A-IκBα32/36A vector revealed the presence of 3 κB consensus sequences (GGGRNNYYCC) within the CMV promoter of the rtTA gene. On the other hand, the accumulation of IκBα32/36A-mutated protein may allow its phosphorylation on tyrosine 4258 or on its caboxyterminal end,59 promoting its proteolytic degradation. This suggests that over a longer period of time, a new equilibrium is reached in the transfected cells between the inhibition of NF-κB by the mutated IκBα32/36A and the activation of NF-κB by LMP-1, resulting in significant and stable levels of NF-κB activation, though at a lower level than in absence of doxycycline. A relationship between NF-κB activation and cyclin D1 expression was reported recently.60 Therefore, our results do not answer whether EBV-immortalized B cells can in fact proliferate in the absence of NF-κB over a longer period of time. Furthermore, our results do not exclude a role for NF-κB in the initial steps of EBV immortalization of primary B cells. Indeed, it is noteworthy that acetylsalicylic acid, which inhibits NF-κB, can block EBV-induced B-cell proliferation in vitro.61
A series of recent publications points to a relationship between NF-κB activity and the protection of cells from apoptosis.40–42,62,63 These results have been obtained in various cell types, including EBV-negative B-cells and EBV-infected lymphoblastoid cells.63–65 Here we show that the induction of IκBα32/36A protein is associated with the sensitization of cells to apoptosis in EBV-infected B-cells. Furthermore, we show that the loss of NF-κB is correlated with a significant decrease of the anti-apoptotic protein Bcl-2. Induction of Bcl-2 by EBV in B cells has already been demonstrated.43,44 Transfection of these cells by LMP-1 is associated with an increase in NF-κB activity and the subsequent overexpression of Bcl-2.44 A correlation between LMP-1 and Bcl-2 expression has been noted in AIDS-related primary brain lymphomas.66 Antisense oligonucleotides against LMP-1 suppress Bcl-2 expression in EBV-infected B cells.67 Bcl-2 induction by LMP-1 may be a feature exclusive to B cells. Rowe et al44 have shown that the transfection of LMP-1 into non-B-cell lines is associated with NF-κB activation and CD54 up-regulation without any effect on Bcl-2. Furthermore, even in B cells, Bcl-2 up-regulation by LMP-1 does not seem to be mediated directly by NF-κB because after the transfection of LMP-1, NF-κB activation and CD54 occurred simultaneously whereas Bcl-2 induction was delayed.44 Therefore, to date, a direct causal relationship between NF-κB activation and Bcl-2 up-regulation by LMP-1 has not been determined. Our results clearly show that the loss of NF-κB is associated with a down-regulation of Bcl-2. This suggests that Rel/NF-κB complexes participate in the positive control of Bcl-2 gene expression in EBV-infected B cells, most likely through intermediate target gene expression.
It is noteworthy that protection against apoptosis through NF-κB activation does not seem to be restricted to a particular cell type. A recent report62 has shown that NF-κB’s anti-apoptotic effect is mediated by the up-regulation of TRAF1, TRAF2, c-IAP1, and c-IAP2 molecules in the HT1080 fibrosarcoma cell line, resulting in the inhibition of caspase-8 activation. No up-regulation of Bcl-2 by NF-κB activation was noted in this non-B-cell line. Caspase-8 activation and Bcl-2 down-regulation are initial steps in the cascade of events leading to programmed cell death. The caspase-8 cascade is activated after the aggregation of death-domain proteins at the membrane in response to external stimuli (see Vaux and Korsmeyer68 for review). It would be interesting to verify whether EBV-mediated NF-κB activation leads to the control of TRAF and c-IAP gene expression in our cellular model. Bcl-2 down-regulation seems to be provoked by endogenous cell damage, such as DNA strand breaks after x-ray irradiation. Apoptosis induced by x-ray irradiation is mediated by p53 (see Levine69 for review). P53 exerts both positive transcriptional control on the Bax gene70 and negative control of Bcl-2 gene transcription.71 LMP-1 is responsible for the up-regulation of the A20 protein, a 790-amino acid protein with a unique zinc finger motif.18 A20 gene expression is up-regulated by NF-κB in response to TNF-α and protects cells against the cytotoxic effect of TNF-α.72 LMP-1-mediated protection of cells from apoptosis occurs through A20 overexpression, which in turn inhibits p53.73 Therefore, it can be hypothesized that the loss of NF-κB in EBV-infected cells could be responsible for the down-regulation of A20 gene transcription, thus allowing p53 to inhibit Bcl-2 gene expression and to activate the transcription of Bax. This in turn results in the down-regulation of Bcl-2 gene expression and a sensitization of cells to apoptosis.
One of the next tasks to further our understanding of the role of NF-κB in EBV-related lymphomagenesis will be the identification of NF-κB target genes. MS4A-IκBα32/36A-transfected cells may allow the identification of such genes in EBV-infected B cells. Using bidimensional gel electrophoresis, we are performing a comparative analysis of MS4A–IκBα32/36A-transfected lymphoblas-toid cells either overexpressing or not expressing IκBα32/36A. Initial results show that at least 15 cytosolic polypeptides are down-regulated when NF-κB is inhibited. Thus, MS4A–IκBα32/36A-transfected B-lymphoblastoid cells may be a useful and valuable cellular model to help us in our search for genuine NF-κB target genes in the context of EBV-transformed B lymphocytes.
We thank Hermann Bujard and Manfred Gossen for the gift of plasmids and for sharing their results before publication. We also thank R. Hay (University of St. Andrews, UK) and N. Rice (NCI, Frederick) for the kind gift of monoclonal antibodies and immune sera. We thank Alain Israël (Institut Pasteur, Paris, France) for the kind gift of the 0.4SK-luc plasmid and Charles Hall for critically reading the manuscript.
Supported by a EU grant (Pathogenesis of EBV-related lymphoproliferative disorders in immunocompromised patients contract CHRX-CT94-0651) to M.R. and G.W.B. Partially supported by ANRS (contrat 98017), Association pour la Recherche sur le Cancer, Villejuif, France; contrat 9348, Comité Départemental de la Ligue contre le Cancer; BMBF (01 GE 9609/3); Die Deutsche Forschungsgemeinschaft; and Fonds der Chemischen Industrie. J.F. was supported by a grant from the Ligue Départementale Contre le Cancer (Seine Saint Denis).
References
Author notes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal