Abstract
B lymphocyte production in murine bone marrow is negatively regulated by sex steroids and the aim of this study was to identify early hormone sensitive checkpoints. Estrogen (E2) treatment reduced cμ+ pre-B cells, a change that occurred concomitantly with decreased Ig gene rearrangements and rag-1 transcripts. Estrogen decreased B lineage precursors in Ig transgenic mice, demonstrating that hormonal regulation is independent of the recombination process. B lineage precursors in Bcl-2 transgenic mice were resistant to estrogen treatment, suggesting that life/death decisions are involved in hormonal regulation. A previously uncharacterized population of CD43−cμ− B lineage precursors was identified in normal, Ig transgenic, and RAG−/− mice after estrogen treatment, revealing that down-regulation of CD43 can occur independent of Ig heavy chain expression. These cells expressed transcripts for both tdt andbcl-2, characteristics of early B-cell precursors. BrdU incorporation analysis revealed that the mitotic activity of early B-lineage cells is reduced in hormone-treated mice. We conclude that sex steroids modulate the production of B-lineage cells by influencing the differentiation, proliferation, and survival of early B-cell precursors. These findings are informative about mechanisms of hormonal regulation, as well as the significance of some differentiation-related events.
B-cell progenitors within bone marrow progress through developmental stages characterized by changes in surface and cytoplasmic molecules, responsiveness to growth factors, and status of Ig gene rearrangements.1-5 Although a wealth of information is accumulating about these events, important questions remain about which are intrinsic to differentiating lymphocytes and which are regulated by extrinsic factors. Gene targeting has been used to identify transcription factors, cytokines, Ig gene products, and survival genes that are essential for commitment of cells to, and progression within, the B lineage.6-8 However, this experimental approach has primarily defined molecules which act in a positive way and the role of negative regulators in modulating numbers of B lymphocytes produced in the marrow remains poorly resolved. A major goal of this study was to identify critical events in B lymphopoiesis that are sensitive to a known negative regulator, 17-β estradiol (E2).9
Steady state B lymphopoiesis is believed to be influenced by interactions between lymphoid precursors and marrow stromal cells, as well as by the availability of stimulatory and inhibitory regulators. Under certain experimental conditions, IL-1, IL-3, IL-4, IL-10, γ-interferon, α/β interferon, TGF-β, and GM-CSF are capable of suppressing B lymphopoiesis.10-12 However, it has not been established whether most of these factors are involved in normal steady state regulation of B-cell production. The negative effects of sex steroids on B lymphopoiesis were initially suggested by the observation that a selective reduction in pre-B cells occurred during pregnancy.13 This was reflected by reduced numbers of IL-7 responding cells in bone marrow and diminished lymphocyte proliferation as ascertained by BrdU incorporation. In contrast, percentages of mature recirculating B lymphocytes, as well as myeloid and erythroid progenitors in marrow, were not reduced. Systemic levels of estrogen and progesterone increase during pregnancy, and we found that sustained exposure to estrogen caused a reduction of IL-7 responsive cells and pre-B cells in the marrow, as well as newly made B cells in the periphery.9 Additional observations suggested that sex steroids participate in normal, steady state control of B lymphopoiesis. Hormone-deficient hypogonadal (HPG) or castrated mice were found to have elevated numbers of B-cell precursors, and estrogen replacement reduced them to within the normal range.14-16 A recent study revealed that multiple estrogen and androgen receptors can mediate these responses.17 Although changes in systemic levels of estrogen can profoundly alter the numbers of new B lymphocytes generated in the marrow, the mechanism is largely unknown.
We have now identified hormone sensitive stages in early B-cell development by controlled exposure of normal mice, Ig rearrangement deficient mice, Ig transgenic mice, and Bcl-2 transgenic mice to estrogen. We documented significant changes in B lineage precursors, including decreased rag-1 gene expression, frequency of Ig gene rearrangements, and numbers of cμ+ pre-B cells. However, we also found that E2 treatment had dramatic effects on Ig transgenic mice and immunodeficient RAG−/− mice. Hormone treatment significantly reduced the mitotic activity of B lineage precursors, causing the accumulation of noncycling cells. Overexpression of Bcl-2 in the B lineage abrogated hormonal regulation. We conclude that estrogen regulates the production of B lineage lymphocytes by influencing critical early events associated with the differentiation, proliferation, and survival of early precursors.
Material and methods
Animals
BALB/c, RAG-2−/− (purchased from Taconic Farms, Germantown, NY), and Eμ-bcl-2 (line 36) mice were bred and maintained in our laboratory animal resource facility.18 Bcl-2 transgenic mice were identified by flow cytometric analysis using the antibody to human Bcl-2, 6C8 (PharMingen, San Diego, CA) diluted in 0.03% saponin, and revealed by FITC conjugated goat antihamster Ig (Caltag, Burlingame, CA). Nontransgenic littermates were used as controls. RAG-1−/−(S), 3-83μκ transgenic, RAG-1−/−/ human μ transgenic and the appropriate controls mice have been previously described.19,20 RAG-1−/−(M) mice were purchased from Jackson Labs (Bar Harbor, ME).21
Hormone treatment in vivo
Estrogen treatment causes an osteosclerotic response in the marrow,22 therefore all data are reported as percentages. Implantation of pellets containing 0.05 to 0.1 mg of 17-β estradiol (E2) reduced CD45R+ B-cell precursors and IL-7 responding cells, but allowed sufficient progenitors to remain so that populations of early cells could be analyzed.
Time-release pellets of E2 were purchased from Innovative Research of America (Sarasota, FL) and implanted subcutaneously with a sterile trochar. Mice given 0.05 mg dose E2 were analyzed after 14 days, whereas those given 0.1 mg were analyzed after 7 days.
Immunofluorescent staining and cell sorting
Cell surface staining.
Cells were harvested from femurs and tibias from individual mice and suspended in staining buffer (phosphate-buffered saline without Ca2+ and Mg2+ with 3% heat-inactivated fetal bovine serum and 0.1% sodium azide), as previously described.13 Antibodies used in these analyses were the following: CD45R (RA3-6B2), CD45R (14.8), CD24 (M1/69 or 30F1), BP-1, CD43 (S7), CD19 (1D3), CD25 (7D4) were purchased from PharMingen, and SB/199 FITC (IL-7R α) was purified and labeled in our lab. Ultra-avidin Texas Red (Leinco, Mallwin, MO) or Streptavidin Cychrome (PharMingen) were used to reveal biotinylated reagents.
Cytoplasmic staining for μ.
After surface staining, bone marrow cells were incubated in 4% paraformaldehyde in PBS- (pH 7.4) for 10 minutes on ice, followed by permeabilization for 20 minutes at room temperature in PBS containing 0.2% Tween 20. Mouse cells were then incubated with a FITC-labeled polyclonal Goat antimouse IgM (Southern Biotechnology, Birmingham, AL) or Rat antimouse IgM antiserum (Zymed, San Francisco, CA). Intracellular staining with the appropriate isotype control was used to set gates to identify positively staining cells. Human μ transgene expression was detected using affinity purified goat antihuman IgM F(ab')2 FITC (Southern Biotechnology). Cells were kept on ice until analyzed.
Cell sorting.
Bone marrow cells from control or E2-treated mice were harvested and enriched for lymphoid cells by incubating cells with Gr-1, Ter119 (PharMingen), and Mac-1 (10 × concentrated culture supernatant, hybridoma from American Type Culture Collection, Logan, UT) monoclonal antibodies, followed by washing with staining buffer. Cells were then incubated with goat antirat IgG coated magnetic beads (PerSeptive Diagnostics, Framingham, MA), followed by magnetic separation to deplete myeloid and erythroid cells. Lymphoid enriched cells were then incubated with the appropriate combinations of antibodies, which allowed identification of early populations.2 Cells were sorted on the FACStarPlus cell sorter.
Ig gene rearrangement
Lymphoid enriched marrow cells were resuspended in PCR lysis buffer (10 mmol/L Tris, pH 8.4, 50 mmol/L KCl, 2 mmol/L MgCl2, 0.5% NP-40, 0.5% Tween-20, 40 μg/mL proteinase K) and incubated overnight at 50°C. The proteinase K was inactivated by incubation at 95°C for 10 minutes and the DNA used directly for PCR. Two microliters of template was used neat, diluted 1/10, and 1/100. 50 μL PCR reactions were performed using template, 625 ng of each primer, 5 μL of 10 × buffer, 2.0 mmol/L MgCl2, 100 μg/mL of bovine serum albumin (BSA), 10 μmol dNTPs, and 2.5 U Taq DNA polymerase (FisherBiotech, Pittsburgh, PA). Twenty-five cycles of amplification were performed consisting of 1 minute at 95°C, 45 seconds at 63°, and 2 minutes at 72°C, followed by a single incubation at 72° for 10 minutes. Ten microliters of the PCR reaction was then electrophoresed through a 1.4% agarose gel in Tris Borate EDTA (TBE) buffer. The gel was transferred to a nylon membrane (MSI, Westboro, MA), baked at 80° C for 2 hours, then cross-linked with ultraviolet (UV) light for 5 minutes. The membranes were probed with a 32P-labeled JH34probe23 and exposed overnight on Kodak Scientific Imaging Film (Eastman Kodak, Rochester, NY). The primers used were alpha actin,2 DHL(5′), and J3(3′) to detect DH-JH,24 Q52 and 7183(5′ primers used separately)3 and J3(3′) to detect VH-DJH rearrangements, and Mu0(5′) and J1(3′)24 to detect germline alleles.
RT-PCR analysis of gene expression
For RNA isolation, sorted cells were washed and resuspended in Trizol Reagent (Gibco-BRL). RNA was prepared and resuspended in DEPC-treated water. Total RNA was treated with Dnase I (Gibco-BRL) to remove contaminating genomic DNA, and cDNA was made using oligo-dT (Gibco-BRL) and Moloney murine leukemia virus reverse transcriptase (Gibco-BRL). RNA representing 104 cell equivalents was used neat, diluted 1:5, or 1:25 per RT-PCR reaction. The conditions for RT-PCR were 95°C for 1 minute, 63°C for 45 seconds, 72°C for 1.5 minutes for 35 cycles, followed by a 15 minutes incubation at 72°C. All RT-PCR reactions were performed using previously published primer sequences.3 One-fifth of the PCR product was electrophoresed in a 1.2% agarose gel in Tris Acetate EDTA (TAE) buffer, transferred onto nylon membranes (MSI, Westboro, MA) and hybridized using random primed probes (Boehringer Mannheim, Germany). The blots were prehybridized for 1 to 2 hours then hybridized overnight at 42°C using reagents purchased from 5′-3′, Inc. (Boulder, CO). After washing, the blots were exposed from 2 hours to overnight on Kodak Scientific Imaging Film.
BrdU treatment in vivo and analysis
Mice were treated with 0.1 mg pellets of E2 for 6 or 7 days. RAG-2−/− mice were treated with 0.1 mg pellets of E2 for 10 days. BrdU at 1 mg/mL (+5% glucose to overcome taste aversion) was given in drinking water for the last 3 days of treatment. Bone marrow was harvested and analyzed for BrdU uptake as previously described.13
Results
Estrogen treatment reduces early cμ+ pre-B cells
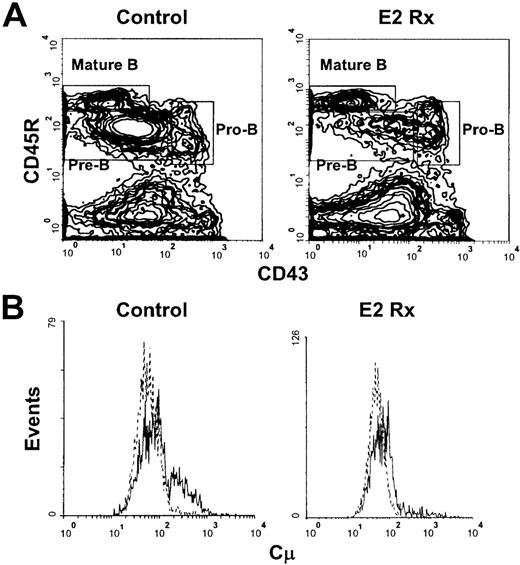
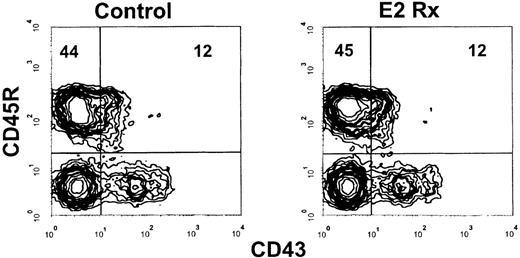
Our previous studies revealed that sustained exposure to17—β estradiol selectively reduced the production of new B lymphocytes in murine bone marrow. However, the doses of estrogen used severely depleted all B-lineage precursors, making analysis of early hormone sensitive events difficult. We found that sustained exposure to 0.05 to 0.1 mg of estrogen (2.4-4.8 μg of hormone released per day) allowed sufficient early precursor populations to remain so that characterization of early hormone sensitive events could be performed. Percentages of total pro-B cells identified as CD45R+CD43+ were similar in E2-treated mice, compared with controls with this dose of hormone (9.0% ± 1.8% vs 8.4% ± 1.6%, respectively, and Figure1A), whereas their progeny, the small pre-B cells, were diminished (34.8% ± 5.5% vs 14.8% ± 3.3% in controls vs E2 treated, respectively, Figure 1A). Recirculating CD45RhisIgM+ cells (Mature B cells, Figure 1A) were unaffected by this dose of E2. Similar results were obtained using CD19, a more B-lineage restricted marker, to evaluate the effects of this dose of hormone (summarized in Table1).
Early pre-B cells are depleted in estrogen-treated BALB/c mice.
(A) B-lineage populations in bone marrow from control and E2-treated mice were revealed by surface staining with antibodies to CD45R and CD43. Mature B cells were identified as CD45RhiCD43−. (B) Cells within the CD45R+CD43+ gate were then analyzed for cμ heavy chains (solid lines) as compared with staining with a normal goat IgG control (dotted lines). The histograms depict 50 000 events collected within lymphocyte light scatter gates and are representative of results obtained from 7 individual control and 9 estrogen-treated mice.
Early pre-B cells are depleted in estrogen-treated BALB/c mice.
(A) B-lineage populations in bone marrow from control and E2-treated mice were revealed by surface staining with antibodies to CD45R and CD43. Mature B cells were identified as CD45RhiCD43−. (B) Cells within the CD45R+CD43+ gate were then analyzed for cμ heavy chains (solid lines) as compared with staining with a normal goat IgG control (dotted lines). The histograms depict 50 000 events collected within lymphocyte light scatter gates and are representative of results obtained from 7 individual control and 9 estrogen-treated mice.
Estrogen alters B lineage precursor populations in normal and mutant mice
| Mouse . | N . | CD19+CD43+ . | CD19+CD43− . |
|---|---|---|---|
| BALB/c ctr | 5 | 6.0 ± 1.4* | 36.5 ± 10.9 |
| BALB/c E2 | 5 | 5.7 ± 2.0 | 15.9 ± 3.5 |
| 3-83 μκ ctr | 2 | 2.2 ± 0.7 | 21.9 ± 1.5 |
| 3-83 μκ E2 | 2 | 1.0 ± 0.1 | 8.8 ± 0.1 |
| RAG-1−/− hu μ ctr | 1 | 1.2 | 30.9 |
| RAG-1−/−hu μ E2 | 1 | 0.2 | 7.4 |
| RAG-1−/− ctr | 2 | 21.1 ± 5.3 | 2.4 ± 0.7 |
| RAG-1−/− E2 | 2 | 10.7 ± 0.8 | 15.9 ± 0.1 |
| RAG-2−/− ctr | 3 | 12.6 ± 2.5 | 1.4 ± 0.3 |
| RAG-2−/−E2 | 3 | 7.0 ± 1.2 | 7.8 ± 2.3 |
| Mouse . | N . | CD19+CD43+ . | CD19+CD43− . |
|---|---|---|---|
| BALB/c ctr | 5 | 6.0 ± 1.4* | 36.5 ± 10.9 |
| BALB/c E2 | 5 | 5.7 ± 2.0 | 15.9 ± 3.5 |
| 3-83 μκ ctr | 2 | 2.2 ± 0.7 | 21.9 ± 1.5 |
| 3-83 μκ E2 | 2 | 1.0 ± 0.1 | 8.8 ± 0.1 |
| RAG-1−/− hu μ ctr | 1 | 1.2 | 30.9 |
| RAG-1−/−hu μ E2 | 1 | 0.2 | 7.4 |
| RAG-1−/− ctr | 2 | 21.1 ± 5.3 | 2.4 ± 0.7 |
| RAG-1−/− E2 | 2 | 10.7 ± 0.8 | 15.9 ± 0.1 |
| RAG-2−/− ctr | 3 | 12.6 ± 2.5 | 1.4 ± 0.3 |
| RAG-2−/−E2 | 3 | 7.0 ± 1.2 | 7.8 ± 2.3 |
Data reflect percentages ± SD of bone marrow cells within lymphocyte light scatter gates representing B-lineage precursor populations. The CD19+CD43− population in both BALB/c and 3-83 μκ mice is inclusive of sIgM+cells. N denotes the number of animals analyzed. The RAG-1−/− mice in this table were on the same background strain as the RAG-1−/− hu μ transgenic. Similar results were obtained with RAG-1−/−(M) and RAG-1−/−(S) mice (Figure 8 and data not shown).
Early cμ+ pre-B cells within the CD45R+CD43+ compartment have been identified.4,5 25 To determine whether the loss of pre-B cells initiated before down-regulation of CD43, early CD45R+CD43+ B cell precursors were isolated from marrows of control and estrogen-treated mice and analyzed for cμ protein. We found that estrogen treatment reduced percentages of cμ+ cells within the CD45R+CD43+compartment (10.0% ± 3.5% vs 25.6% ± 9.4% in estrogen treated vs controls, respectively, Figure 1B). Therefore, loss of pre-B cells in hormone-treated animals initiates before down-regulation of CD43 in the B lineage.
Estrogen treatment reduces Ig heavy chain gene rearrangements
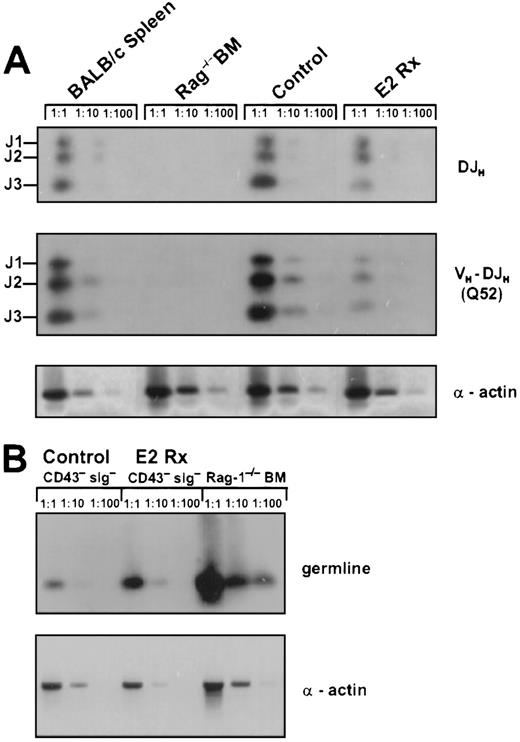
The reduction of early cμ+ pre-B cells in E2-treated animals could be the result of diminished Ig gene rearrangement. Therefore, we isolated lymphocytes from marrows of individual control and hormone-treated animals and compared incidences of DH-JH and VH-DJH gene rearrangements by semiquantitative PCR. We found that DH-JH rearrangements in hormone treated animals were slightly, but consistently reduced compared with control mice (Figure 2A). Next, we examined VH-DJH gene rearrangements to the Q52 and 7183 (not shown) V region families. We found a 75% decrease in VH-DJH rearrangements, involving Q52 and 7183 V genes (Figure 2A and data not shown). Therefore, the hormone could regulate B-cell production by influencing the recombination process itself, the proliferation of cells having undergone successful recombination events, or cμ+ pre-B cells could be particularly sensitive to E2 or a hormone-induced negative regulator.
Estrogen treatment reduces Ig gene rearrangements in bone marrow.
(A) DNA samples representing equivalent numbers of bone marrow lymphocytes from control and E2-treated mice were evaluated for DH-JH and VH-DJH gene rearrangements by PCR and specific products were labeled with respect to rearrangements to J1, J2, or J3. DH-JHrearrangements were detected with a primer that recognizes 9 of 10 known D minigenes and a J3 primer.24VH-DJH rearrangements were detected using primers specific for the Q52 V region family and a J3 primer.3 24 Genomic DNA was serially diluted 1:1,1:10,1:100 for semiquantative analysis and 25 cycles of amplification was performed. Alpha actin was used as a control for genome representation. Densitometry confirmed that equal amounts of DNA were compared. These data are representative of results obtained in 3 independent experiments. (B) CD45R+CD43−sIg− B-lineage precursors in estrogen-treated mice are enriched for cells with germline Ig genes. CD45R+CD43− sIg− cells were sorted from control or estrogen-treated mice. PCR was performed with genomic DNA, using primers that amplify segments normally deleted during Ig gene rearrangement (see “Materials and Methods”). DNA was amplified for 30 cycles and alpha actin was used as a control for genome representation. These data were obtained by pooling cells from 4 control and 4 estrogen-treated mice. RAG-1−/−bone marrow was used as a control for germline DNA. Cells sorted from control and estrogen-treated mice were 99% and 100% pure, respectively, based on postsort analyses.
Estrogen treatment reduces Ig gene rearrangements in bone marrow.
(A) DNA samples representing equivalent numbers of bone marrow lymphocytes from control and E2-treated mice were evaluated for DH-JH and VH-DJH gene rearrangements by PCR and specific products were labeled with respect to rearrangements to J1, J2, or J3. DH-JHrearrangements were detected with a primer that recognizes 9 of 10 known D minigenes and a J3 primer.24VH-DJH rearrangements were detected using primers specific for the Q52 V region family and a J3 primer.3 24 Genomic DNA was serially diluted 1:1,1:10,1:100 for semiquantative analysis and 25 cycles of amplification was performed. Alpha actin was used as a control for genome representation. Densitometry confirmed that equal amounts of DNA were compared. These data are representative of results obtained in 3 independent experiments. (B) CD45R+CD43−sIg− B-lineage precursors in estrogen-treated mice are enriched for cells with germline Ig genes. CD45R+CD43− sIg− cells were sorted from control or estrogen-treated mice. PCR was performed with genomic DNA, using primers that amplify segments normally deleted during Ig gene rearrangement (see “Materials and Methods”). DNA was amplified for 30 cycles and alpha actin was used as a control for genome representation. These data were obtained by pooling cells from 4 control and 4 estrogen-treated mice. RAG-1−/−bone marrow was used as a control for germline DNA. Cells sorted from control and estrogen-treated mice were 99% and 100% pure, respectively, based on postsort analyses.
Expression of IL-7Rα has been shown to be important for differentiation of B lineage cells and VH-DJHrearrangements to distal VH gene families are impaired in mice lacking this receptor. A flow cytometric analysis of IL-7Rα expression on bone marrow cells from hormone-treated animals revealed no reduction in percentages of CD45R+IL-7R+cells (4.7% ± 1.1% vs 6.9% ± 0.8% in control vs E2 treated, respectively). Therefore, E2 does not inhibit B-cell differentiation by diminishing expression of the IL-7Rα chain.
Rag-1 transcripts are reduced in hormone-treated animals.
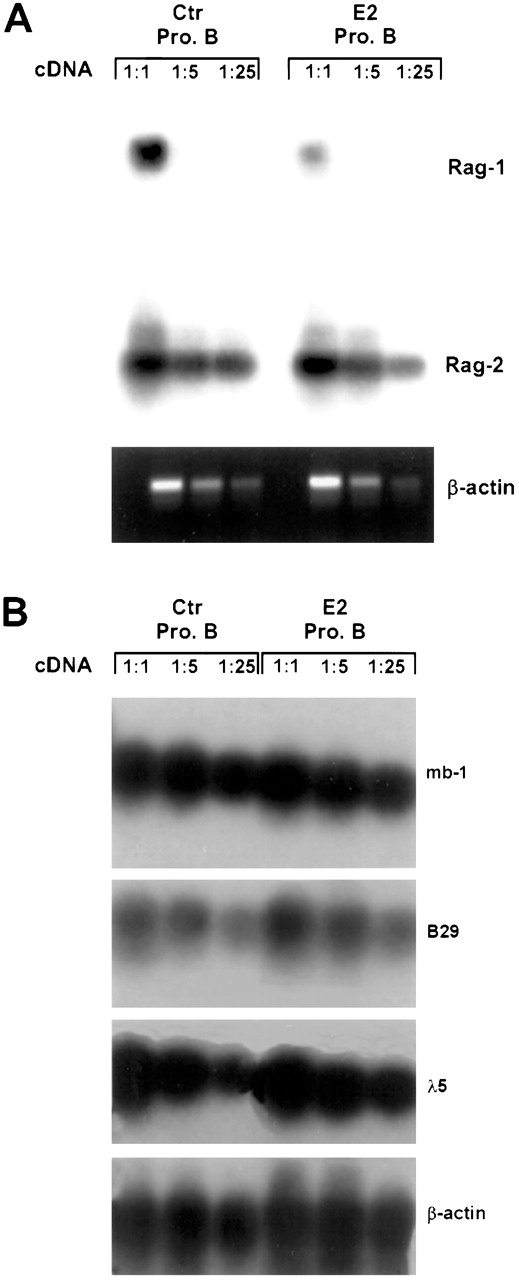
The above findings demonstrate that cells within, and prior to, the pre-B compartment are hormone sensitive. Therefore, we sorted early pro-B cells (CD45R+CD43+CD24+BP-1−) from control and estrogen-treated mice and used RT-PCR to evaluate critical genes expressed at this stage.3 Althoughrag-2 transcripts were essentially normal in cells from estrogen-treated mice, rag-1 transcripts were substantially reduced (Figure 3A). Estrogen had no discernable influence on expression of mb-1,B29, λ5, tdt, or VpreB (Figure 3B and data not shown). Taken together, this analysis suggests that particular events in early B-lineage differentiation, and primarily those associated with Ig gene rearrangements, are subject to hormonal regulation.
RAG-1 expression is preferentially suppressed in estrogen-treated mice.
Early pro-B cells (CD45R+CD43+CD24+BP-1−sIgM−) were sorted from control and E2-treated BALB/c mice. Total RNA representing 104 cell equivalents was reverse transcribed into cDNA, followed by RT-PCR using previously published primers. The data represent autoradiographs of PCR products after membrane transfer and hybridization with appropriate32P-labeled probes or ethidium-stained bands. Similar results were obtained in 3 independent experiments.
RAG-1 expression is preferentially suppressed in estrogen-treated mice.
Early pro-B cells (CD45R+CD43+CD24+BP-1−sIgM−) were sorted from control and E2-treated BALB/c mice. Total RNA representing 104 cell equivalents was reverse transcribed into cDNA, followed by RT-PCR using previously published primers. The data represent autoradiographs of PCR products after membrane transfer and hybridization with appropriate32P-labeled probes or ethidium-stained bands. Similar results were obtained in 3 independent experiments.
B cells from Ig transgenic mice are estrogen sensitive.
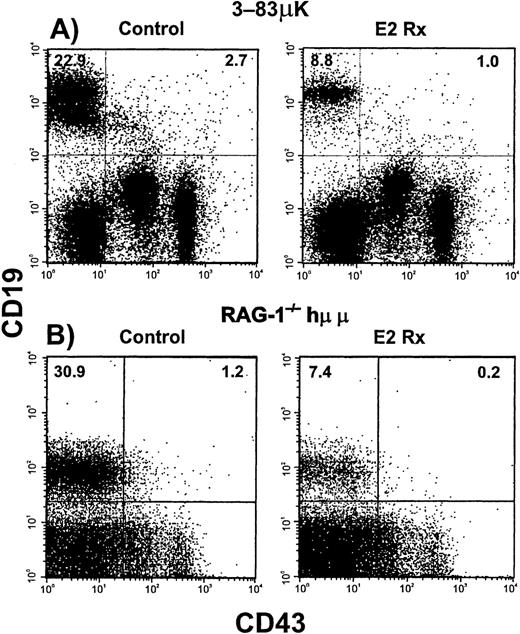
The above findings raised the possibility of an early, hormone sensitive, control point that determines how many B-cell precursors are produced. We speculated that expression of Ig heavy and light chain transgenes, that are known to suppress endogenous VH-DJH gene rearrangements,20 might make B lymphopoiesis hormone resistant. Analysis of the pro-B compartment in hormone-treated 3-83 μκ Ig transgenic mice revealed a significant decrease in percentages of B-lineage precursors starting as early as the CD19+CD43+ stage and extending into the pre-B stage (CD19+CD43−sIgM−, Figure 4A and summarized in Table 1). Incidences of IL-7 responsive precursors in transgenic mice were comparable to those in normal C57BL/6 mice and these declined an average of 92% with estrogen treatment. Percentages of newly made IgM+IgD− B cells declined more than 80% in estrogen-treated Ig transgenic mice (16.1% ± 2.5% in control vs 2.5% ± 0.1% in hormone treated), whereas percentages of recirculating IgM+IgD+ B cells did not change appreciably (3.5% ± 0.3% in controls vs 2.9% ± 0.6% in hormone treated). Therefore, enforced expression of Ig heavy and light chains did not alter sensitivity of the B lineage to this hormone.
B-lineage cells in Ig transgenic mice are sensitive to estrogen.
Flow cytometry was used to analyze bone marrow cells from untreated (Control) and E2-treated Ig transgenic mice. Results obtained with murine μ heavy chain plus κ light chain (3-83μκ) transgenic mice are shown in Panel A. Panel B shows a similar experiment performed with immunodeficient mice bearing a human μ transgene (RAG-1−/−/Hu μ). Incidences of Pro-B cells are given in the upper right quadrants, whereas incidences of more differentiated cells are indicated in the upper left quadrants. The data are summarized in Table 1.
B-lineage cells in Ig transgenic mice are sensitive to estrogen.
Flow cytometry was used to analyze bone marrow cells from untreated (Control) and E2-treated Ig transgenic mice. Results obtained with murine μ heavy chain plus κ light chain (3-83μκ) transgenic mice are shown in Panel A. Panel B shows a similar experiment performed with immunodeficient mice bearing a human μ transgene (RAG-1−/−/Hu μ). Incidences of Pro-B cells are given in the upper right quadrants, whereas incidences of more differentiated cells are indicated in the upper left quadrants. The data are summarized in Table 1.
Although introduction of Ig transgenes has been shown to suppress VH-DJH Ig gene rearrangement events, endogenous DH-JH rearrangements occur normally. The above results demonstrate that early CD19+ CD43+precursors are sensitive to hormonal regulation. Introduction of a human heavy chain transgene inrag−/− mice (RAG-1−/−/hu μ) allows progression to the small, CD43−, pre-B cell stage.19 26Estrogen treatment in this model caused a 70% reduction in CD19+CD43− cells (7.4%, treated vs 30.9%, control; Figure 4B). There was a corresponding reduction in numbers of IL-7 responding precursors from 93 to 15 colony forming cells per 105 cultured marrow cells. From these data we conclude that hormonal regulation of B lymphopoiesis is independent of the recombination process.
B lymphopoiesis is resistant in Bcl-2 transgenic mice.
Diminished output of B-lineage cells in E2-treated animals might result from decreased production and/or survival of lymphocyte progenitors. Bcl-2 is known to be essential for the production of lymphocytes and is expressed in early lymphocyte precursors.8 27 We found that total CD45R+ cells were not reduced in E2 treated Bcl-2 transgenic mice (59.0% ± 1.5% versus 55.6% ± 1.5% in E2 versus control mice, respectively, and Figure 5), nor was the distribution of cells in the B-lineage based on differential expression of sIgM (data not shown). In addition, percentages of CD19+ B-cell precursors were not affected by hormone treatment (data not shown). Early cμ+ pre-B cells are substantially altered by estrogen in normal mice (Figure 1B and see Figure 7 below). However, percentages of both CD43+ and CD43− pre-B cells remained unchanged in Bcl-2 transgenic mice after exposure to hormone (19.1% ± 4.9% vs 25.7% ± 8.9% CD43+ pre-B cells and 87.0% ± 2.0% vs 89.4% ± 2.1% CD43− pre-B cells in control and estrogen treated, respectively). Therefore, constitutive expression of Bcl-2 in the B lineage abrogated hormonal control of B lymphopoiesis.
B-cell precursors in Bcl-2 transgenic mice are resistant to hormone treatment.
Bcl-2 transgenic mice were treated with E2 for 14 days before bone marrow harvest and analysis by flow cytometry.
B-cell precursors in Bcl-2 transgenic mice are resistant to hormone treatment.
Bcl-2 transgenic mice were treated with E2 for 14 days before bone marrow harvest and analysis by flow cytometry.
Estrogen treatment reduces the mitotic activity of B-cell precursors.
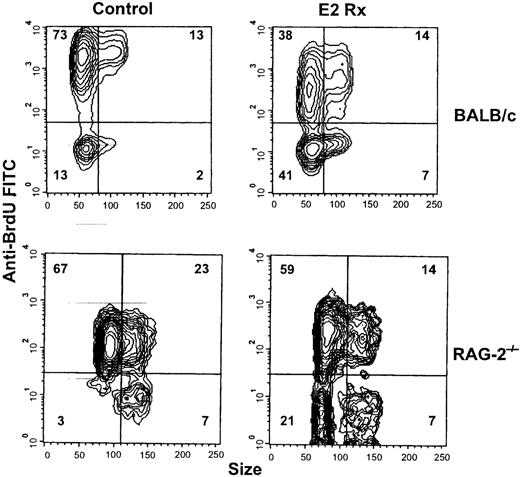
Bcl-2 has an influence on cell cycle progression, in addition to its function as a survival protein.28 29 B-cell precursors normally undergo proliferative expansion and cell cycle–related genes could represent hormone sensitive targets. To evaluate this possibility, BALB/c mice were given BrdU in drinking water for the last 3 days of an E2 treatment course. In control animals, an average of 84.8% ± 0.8% of the B-lineage lymphocytes incorporated BrdU, whereas only 53.6% ± 3.0% had undergone division during this interval in E2-treated mice (Figure 6, upper contour plots). Essentially all the unlabeled cells in both groups were small as assessed by low-angle light scatter. A 3-fold increase in small noncycling lymphocytes was documented in hormone-treated bone marrow (13.4% ± 0.7% in control vs 39.9% ± 2.4% in estrogen-treated animals). Furthermore, there was a conspicuous population of very heavily labeled small lymphocytes in control, but not estrogen-treated animals. These cells presumably represent the normal product of multiple cell divisions occurring during the 3-day interval. Supporting results were obtained when CD45R+CD43+ and CD45RdullCD43− B-lineage precursors were sorted from control and E2-treated animals, fixed, and evaluated with respect to cell cycle status. Percentages of cells in the S+ G2/M stages of the cell cycle were reduced approximately 50% after estrogen exposure (data not shown).
Estrogen reduces mitotic activity of B cell precursors.
BALB/c and RAG-2−/− mice were given hormone implants and BrdU (1 mg/mL +5% glucose) was administered in the drinking water for the last 3 days of treatment. CD45R+cells were then analyzed with respect to size (forward angle light scatter) versus BrdU uptake. The data are representative of results obtained with 5 control and 8 estrogen-treated BALB/c and 3 control and 3 estrogen-treated RAG-2−/− mice.
Estrogen reduces mitotic activity of B cell precursors.
BALB/c and RAG-2−/− mice were given hormone implants and BrdU (1 mg/mL +5% glucose) was administered in the drinking water for the last 3 days of treatment. CD45R+cells were then analyzed with respect to size (forward angle light scatter) versus BrdU uptake. The data are representative of results obtained with 5 control and 8 estrogen-treated BALB/c and 3 control and 3 estrogen-treated RAG-2−/− mice.
Similar experiments conducted with RAG−/− mice revealed that the mitotic activity of B-cell precursors is hormone sensitive from an early stage of differentiation. Although 3 days of BrdU exposure labeled 90.3% ± 1.2% of the pro-B cells in control RAG−/− mice, this value was reduced to 70.5% ± 2.9% with estrogen treatment (Figure 6). Particularly noteworthy was the 7-fold increase in small BrdU− cells that resulted from hormone elevation. These represent cells that remained out of cycle throughout the 3-day interval. Expansion of early B-cell precursors is required to maintain normal lymphopoiesis, and we now show that estrogen treatment reduces proliferation of B-lineage precursors before and independent of Ig gene rearrangement.
A unique population of B-lineage cells accumulates in estrogen-treated mice.
Our analysis of estrogen-treated animals revealed a greater degree of change in cμ+ pre-B cells than in total B-lineage precursors. As previously reported, almost all (93% ± 2%) of the CD45RdullCD43− precursors in normal mice are small, pre-B cells. In contrast, only 60% ± 6% of the cells with these surface characteristics were cμ+ in estrogen-treated BALB/c mice (Figure 7A). The decrease in cμ+ pre-B cells is independent of Ig gene rearrangement, as similar results were obtained with a hormone-treated RAG-1−/−/hu μ Ig transgenic mouse (Figure7A). Loss of CD43 is generally held to represent a late event in B lymphopoiesis and roughly corresponds to the transition from large to small pre-B cell stages.2 30 Therefore, it initially seemed likely that the conspicuous CD43−cμ− cells in estrogen-treated animals were defective pre-B cells that had somehow traversed this critical checkpoint. Uniform expression of CD19, small size, and high levels of BP-1 antigen would also be consistent with that interpretation (see Figure 9 below).
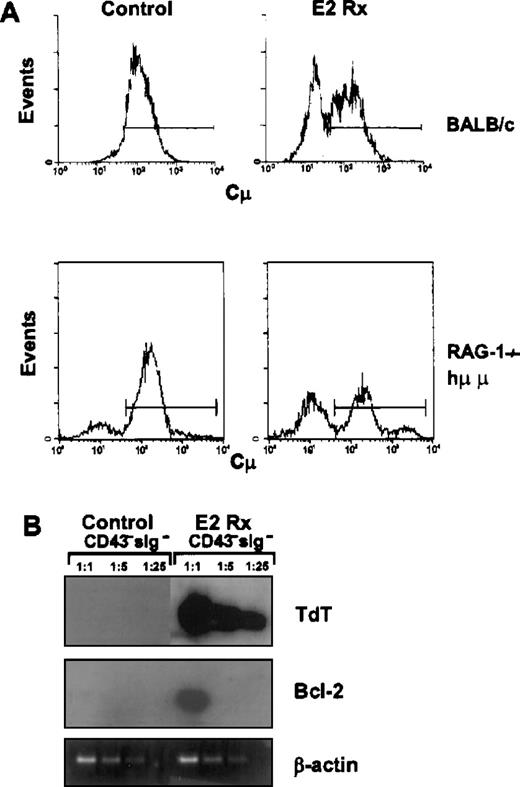
A normally rare population of B-cell precursors accumulates in estrogen-treated mice.
CD45R+CD43− sIgM− cells from control or E2-treated BALB/c mice were sorted, fixed and made permeable as described in the “Materials and Methods.” The sorted cells were then incubated with an antimouse IgM to detect cμ expression (Panel A). In this example, 40% of the CD43−cells in hormone-treated mice were cμ−compared with 7% in the control animals. Panel B shows a typical RT-PCR analysis of tdt and bcl-2 gene expression in CD45R+CD43− sIgM− cells sorted from control (bone marrow pooled from 3 mice) or E2-treated animals (bone marrow pooled from 5 mice).
A normally rare population of B-cell precursors accumulates in estrogen-treated mice.
CD45R+CD43− sIgM− cells from control or E2-treated BALB/c mice were sorted, fixed and made permeable as described in the “Materials and Methods.” The sorted cells were then incubated with an antimouse IgM to detect cμ expression (Panel A). In this example, 40% of the CD43−cells in hormone-treated mice were cμ−compared with 7% in the control animals. Panel B shows a typical RT-PCR analysis of tdt and bcl-2 gene expression in CD45R+CD43− sIgM− cells sorted from control (bone marrow pooled from 3 mice) or E2-treated animals (bone marrow pooled from 5 mice).
More detailed analysis revealed that the CD45R+CD43− cμ− cells lacked CD25, a marker normally acquired in conjunction with successful Ig gene rearrangement (Figure 9, below).4,31Down-regulation of tdt represents another hallmark in B-lineage progression.1,3 32Tdt is normally found in cells before Ig gene rearrangement and expression ceases with appearance of μ heavy chains. RT-PCR analyses of sorted CD45RdullCD43− sIgM−lymphocytes revealed persisting high levels of tdt transcripts in cells from E2-treated animals (Figure 7B). Additional evidence of the early stage of differentiation represented by these cells was expression of transcripts for bcl-2 (Figure 7B).
Ig heavy chain gene rearrangement status represents a definitive characteristic of late stage precursors.3,5 33 Therefore, we sorted B-lineage precursors from control and E2-treated animals and assessed germline IgH gene segments (Figure 2B). Nonrearranged IgH segments were increased approximately 2-fold in CD43−lymphocytes from hormone-treated animals. This is consistent with the appearance of μ− cells, persisting tdtmRNA, and the decreased abundance of IgH gene rearrangement products in these lymphocytes (Figure 2B). Thus, many of the CD43− μ− precursors had not initiated Ig heavy chain gene rearrangement.
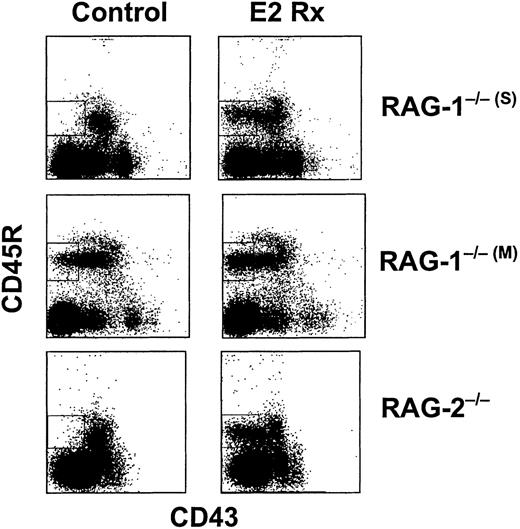
The identification of CD45R+CD43−μ− cells in normal mice treated with estrogen led us to study mice that cannot initiate Ig gene rearrangement. In contrast to what we observed with normal or Ig transgenic mice, sustained exposure to estrogen did not reduce percentages of total CD19+ B-lineage precursors in RAG−/− mice (Table 1). In addition, markedly reduced CD43 expression was observed in 3 different lines of RAG−/− mice after hormone treatment. This population became especially conspicuous because small CD43− cells are normally rare in these animals (Figure8).19 26The CD43− lymphocytes were small in size, expressed CD19 as well as BP-1, and lacked both CD25 and cytoplasmic μchains (Figure 9 and data not shown). The CD45R+BP-1+CD43− cells were isolated from estrogen-treated RAG-2−/− mice and compared with CD43− cells from untreated normal mice by RT-PCR. Transcripts corresponding to tdt andbcl-2 were abundant (Figure 7B and data not shown). Therefore, CD43− precursors that accumulated in hormone-treated RAG−/− and BALB/c mice were similar in many respects.
Normally rare B-lineage cells accumulate in hormone-treated RAG−/− mice.
Mice were treated with E2 for 14 days before bone marrow harvest and analysis by flow cytometry. The boxed regions contain B-cell precursors that accumulate in hormone-treated animals.
Normally rare B-lineage cells accumulate in hormone-treated RAG−/− mice.
Mice were treated with E2 for 14 days before bone marrow harvest and analysis by flow cytometry. The boxed regions contain B-cell precursors that accumulate in hormone-treated animals.
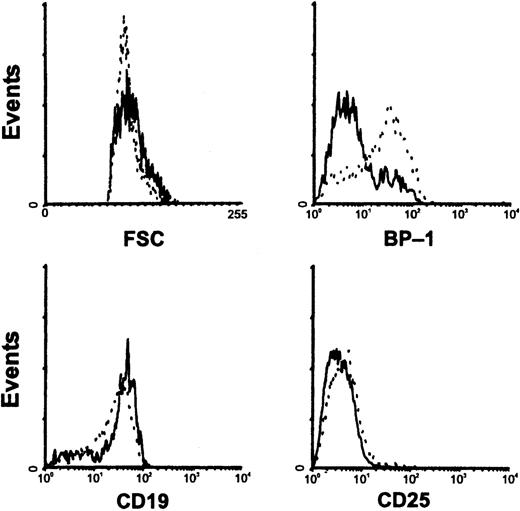
Characterization of CD45R+CD43− cells in RAG-1−/−(M) mice.
Bone marrow cells from control (solid lines) or E2-treated (hatched lines) RAG-1−/−(M) mice were harvested and compared by flow cytometry with respect to size and expression of B-lineage surface markers.
Characterization of CD45R+CD43− cells in RAG-1−/−(M) mice.
Bone marrow cells from control (solid lines) or E2-treated (hatched lines) RAG-1−/−(M) mice were harvested and compared by flow cytometry with respect to size and expression of B-lineage surface markers.
In the course of these studies, an interesting observation was made with 1 line of RAG-1 targeted mice (RAG-1−/−(M)).21 These animals had a conspicuous population of CD43 low to negative cells without exposure to hormone (Figure 8). They differed from CD43− cells found in estrogen-treated mice in that more were large in size and BP-1 antigen expression tended to be low (Figure 9). Estrogen treatment of RAG-1−/−(M) mice resulted in the appearance of small CD45R+CD43−CD19+CD25−BP-1+lymphocytes (Figures 8 and 9). The 2 lines of RAG-1 gene targeted animals are both on a C57BL/6 background, but were produced with different gene targeting strategies.19 21
We conclude that the accumulation of CD43−μ− cells in response to estrogen does not require Ig gene rearrangement. These unusual cells probably derive from a relatively early stage of differentiation because they expressbcl-2. Expression of CD25 and down-regulation of tdt, represent events linked with successful IgH gene rearrangement and do not occur in these normally rare progenitors. There is acquisition of BP-1 and loss of CD43, although these changes were previously thought to be exclusively associated with B-lineage progression.
Discussion
The production of new B lymphocytes in murine bone marrow is suppressed during pregnancy or after sustained exposure to estrogen, and augmented in mice deficient in sex steroids.9,13-15 17These data suggest that sex steroids are involved in normal steady state regulation of B lymphopoiesis. We now show that experimental manipulation of estrogen-influenced numbers of undifferentiated precursors, Ig gene rearrangements, and proliferative activity. E2-induced population changes were blocked by expression of a Bcl-2 transgene. Observations made with Ig transgenic and RAG deficient mice demonstrated that estrogen influenced some events that precede and are independent of Ig gene rearrangement. A normally rare population of early lymphocytes accumulated in estrogen-treated mice. These findings are informative about potential mechanisms for hormonal control of B lymphopoiesis, as well as the sequence and significance of some differentiation related changes.
B-lineage precursors in the bone marrow have been developmentally ordered by changes in display of cell surface and cytoplasmic markers, changes in patterns of gene expression, status of Ig gene rearrangements, and dependence on stromal cells for proliferation and survival.1-5 Any of these events could represent potential targets of hormone-mediated regulation. CD19+CD43+ pro-B cells were depleted in most animal models studied, revealing that hormonal regulation initiates early in the B lineage (summarized in Table 1). Therefore, we examined the consequences of estrogen treatment on critical events in B-cell development, including those associated with the differentiation, survival, and proliferation of early B-lineage precursors.
Changes at the early pro-B stage could reflect decreased expression of genes required for recombination events and expression of the pre-B receptor. Therefore, we characterized pro-B cells from control and hormone-treated animals with respect to expression of critical early genes, Ig gene rearrangements, and cμ protein. Numbers of cμ+ cells were reduced in the CD45R+CD43+ compartment and we found a concomitant reduction inrag-1 transcripts and VH-DJH Ig gene rearrangements (Figures 1 to 3). In contrast, transcripts corresponding to other antigen receptor components, including Ig-α, Ig-β, λ5, and VpreB, were unaffected (Figure 3 and data not shown). Experiments with 2 types of Ig transgenic mice demonstrated that events that precede, or are in addition to, Ig gene rearrangement are hormone sensitive. Rearranged μ transgene products suppress endogenous Ig gene rearrangements in normal mice and alter pro-B population sizes.34 Regardless, estrogen treatment dramatically reduced numbers of B-lineage cells in such animals (Table1 and Figure 4). Furthermore, similar changes were observed in RAG-1−/−/hu μ mice that are incapable of endogenous Ig gene rearrangements and where a μ transgene is known to be expressed from a very early stage.35 Finally, none of the changes we observed resulted from down-regulation of the IL-7 receptor on B lineage precursors. Taken together, these results demonstrate that early differentiation related events in B lymphopoiesis are subject to hormonal regulation.
Recent studies suggest that estrogen can influence levels of Bcl-2 in nonlymphoid tissues such as brain neurons and breast cancer cells.36-38 Up-regulation of Bcl-2 by estrogen may account for the resistance of some breast cancers to antitumor drugs.39 Overexpression of Bcl-2 in the B lineage protects precursors from apoptosis induced by growth factor deprivation, ionizing radiation, and the glucocorticoid hormone dexamethasone.40 We found that the same transgene provided remarkable protection from estrogen for both pro-B and pre-B cells, as well as IL-7 responding precursors (Figure 5 and data not shown). In addition, we found that the CD43− cμ-cells in hormone-treated mice expressed Bcl-2 transcripts, possibly explaining their persistance with estrogen treatment. These data suggest that hormonal regulation involves life/death decisions in early B lineage cells.
Proliferative expansion at discrete stages must be sustained to maintain steady state production of B cells.1,41-44Interestingly, a Bcl-2 transgene is known to affect the mitotic activity of early B-lineage cells.28 BrdU incorporation and cell cycle analysis revealed that estrogen treatment dramatically reduced the mitotic activity of B-lineage precursors in normal and RAG−/− mice (Figure 6). Early CD43+CD45R+ cells have long-term proliferation capacity on stromal cells, and upon differentiation, give rise to precursors that can respond to IL-7 alone. Diminished mitotic activity of these early precursors in estrogen-treated animals would provide fewer cells undergoing Ig gene rearrangement, less IL-7 responding cells, cμ+ pre-B cells, and immature B lymphocytes. We documented all of these changes in hormone-treated animals and previously in stromal cell cocultures in which numbers of recovered lymphocytes were greatly reduced in the presence of estrogen.45 In addition, diminished mitotic activity would also result in less Ig transgene expressing precursors, as we observed (Figure 4). BrdU uptake was not evaluated in Ig transgenic mice because early μ expression is known to alter the kinetics of B-lymphocyte development.34 Thus, estrogen also regulates B lymphopoiesis via its influence on cell proliferation. The effect is lineage specific and involves precursors from an early stage.
Overall percentages of phenotypically defined “pro-B cells” were not reduced in hormone-treated BALB/c mice, largely because of an accumulation of CD45R+CD19+BP-1+CD25−cμ− B-cell precursors. Most of their properties, such as continued expression of bcl-2 and tdt, along with the presence of these cells in RAG−/−mice, are consistent with a relatively early stage of B-lineage development.1 32 Our studies also indicate that these cells are noncycling and have at least some IgH genes in germline configuration. This normally rare population of cells may be positioned near a hormone-regulated control point in B-lineage differentiation.
Expression of a rearranged μ heavy chain as part of a pre-B receptor complex and signaling via associated Ig-α/Ig-β molecules are necessary and sufficient for progression of cells in the B lineage.19,26,46 Subsequent events include a short period of rapid proliferation, exit from the cell cycle, reduction in cell size, loss of CD43, gain of CD25, and initiation of Ig light chain gene rearrangement.30,47 Virtually all of the CD45R+CD43− lymphocytes in normal bone marrow contain μ heavy chains of immunoglobulin (Figure 7A). For this reason, loss of CD43 is widely used as a hallmark for transition from the pro-B/large pre-B to small pre-B cell stages. However, we provide evidence that down-regulation of CD43 is not dependent on μ chain expression. Hormone treatment induced the accumulation of small, non-cycling cells and a conspicuous population of CD43− cells in the absence of Ig gene rearrangement. Our results show that down-regulation of CD43 is not coupled to expression of CD25, and CD25 to be a more valid indication of successful IgH gene rearrangement at the pro-B/pre-B transition.4 31 A tendency for down-regulation of CD43 in the absence of Ig gene rearrangement was found in 1 of 2 lines of RAG-1 targeted mice (Figure 8). Unlike the CD43−cμ− cells that accumulated in normal or RAG−/− mice after estrogen treatment, the CD43− cells in RAG-1−/−(M)were large in size and expressed low densities of BP-1. This finding is of unknown significance and may reflect differences in gene targeting strategies. However, it again brings into question the validity of CD43 as a “differentiation” marker in all circumstances.
These findings also provided insight into another “differentiation” related change. BP-1 acquisition has been used to discriminate late pro-B cells from earlier precursors, a transition that corresponds to completed IgH rearrangements (either successful or abortive).3,5 However, the CD43− cells that accumulated in estrogen-treated animals expressed high levels of BP-1, although they were immature in all other respects (see above). Other studies suggest that BP-1 expression may merely reflect the period of exposure of precursors to IL-7.48
Our observations raise many issues concerning regulation of normal B lymphopoiesis. An early, hormone-sensitive, control point appears to exist in murine bone marrow. Ongoing studies should reveal which cells in murine marrow possess relevant hormone receptors and address questions about transcription factors and cell cycle regulators known to be estrogen regulated in other cell types.45,49-52 It will be particularly important to learn whether parallels exist for humans, given the widespread therapeutic use of sex steroids, their agonists, and antagonists. Furthermore, a mechanistic connection might be sought between our findings and the well-known influences of estrogen on autoimmune diseases.53-55
Acknowledgments
We are grateful to Drs Chris Roman and David Baltimore for RAG-1−/−(s) mice, Dr David Nemazee for providing the 3-83 μκ Ig transgenic mice, Dr. Richard Hardy for providing RAG-1−/−and RAG-1−/−/Hu μ Ig transgenic mice, and Drs Alan Harris and Suzanne Cory for providing the Bcl-2 transgenic mice. We acknowledge the expert technical assistance provided by Karla Garrett and expert flow cytometry assistance provided by Jim Henthorn and Viji Dandapani. Finally, we appreciate the secretarial assistance provided by Shelli Wasson.
Supported by grant AI 20069 from the National Institutes of Health.
Reprints:Paul W. Kincade, Immunobiology and Cancer Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: paul-kincade@omrf.ouhsc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal