Abstract
T-cell receptor (TCR)-mediated apoptosis, also known as activation-induced cell death (AICD), plays an important role in the control of immune response and in the development of T-cell repertoire. Mechanistically, AICD has been largely attributed to the interaction of Fas ligand (Fas-L) with its cell surface receptor Fas in activated T cells. Signal transduction mediated by the integrin family of cell adhesion receptors has been previously shown to modulate apoptosis in a number of different cell types; in T cells, integrin signaling is known to be important in cellular response to antigenic challenge by providing a co-stimulatory signal for TCR. In this study we demonstrate that signaling via the collagen receptor 2β1 integrin specifically inhibits AICD by inhibiting Fas-L expression in activated Jurkat T cells. Engagement of the 2β1 integrin with monoclonal antibodies or with type I collagen, a cognate ligand for 2β1, reduced anti-CD3 and PMA/ionomycin-induced cell death by 30% and 40%, respectively, and the expression of Fas-L mRNA by 50%. Further studies indicated that the 2β1-mediated inhibition of AICD and Fas-L expression required the focal adhesion kinase FAK, a known component in the integrin signaling pathways. These results suggest a role for the 2β1 integrin in the control of homeostasis of immune response and T-cell development.
Programmed cell death, or apoptosis, plays an important role in the regulation of cellular homeostasis and provides protection against inflammation and cancer. In T lymphocytes, T-cell receptor (TCR)–mediated apoptosis has been shown to be critical for the negative selection of immature T cells within the thymus and in maintaining the peripheral tolerance of mature T cells.1This so-called activation-induced cell death (AICD) has been largely attributed to the interaction of Fas ligand (Fas-L) with its receptor Fas (CD95),2 and it has been shown to occur in both T-cell clones and T-cell lines, as well as in T-cell hybridomas.3,4 Stimulation via TCR results in transcriptional activation of the genes for Fas-L and Fas.2Subsequent ligation of Fas receptor with Fas-L on the cell surface induces receptor aggregation, which in turn results in the recruitment of the adaptor protein FADD and FLICE (caspase-8) and in the activation of downstream caspases that lead to irreversible cell damage.5-7 As exemplified by the mutant lpr andgld mice, loss of function of the Fas/Fas-L pathway leads to abnormal lymphoproliferation and generalized lymphoproliferative disease.2
Integrins are α-β heterodimeric membrane proteins that mediate cell adhesion to the surrounding extracellular matrix (ECM). In addition to their mechanistic role in anchoring cells to the substratum, integrins have the capacity to elicit a wide variety of intracellular signals that regulate cell behavior.8 It is known that several cell types, such as epithelial and endothelial cells, undergo apoptosis on loss of integrin-mediated cell attachment, a process referred to as anoikis.9 Among the signaling molecules involved in integrin-mediated cell survival is the focal adhesion kinase FAK, which becomes activated after integrin ligation and may in turn activate downstream survival pathways such as those composed of phosphatidylinositol 3′-kinase (PI 3-kinase) and the serine/threonine kinase Akt.9
Lymphocytes express several members of the β1 subfamily of integrins, which mediate cell attachment, spreading and migration on ECM.10 The role of β1 integrins in T-cell costimulation and activation is well documented; several studies have shown that engagement of β1 integrins on activated T cells leads to increased cell proliferation11,12 and tyrosine phosphorylation of intracellular proteins such as FAK.13,14 Recent evidence suggests that signals via integrins may modulate T-cell apoptosis, either by providing a survival signal or by enhancing or inducing a death signal. For example, the α5β1 integrin has been shown to mediate protective effects provided by TGFβ1 in CD8-positive T cells against AICD,15 whereas coligation of the α4β1 integrin and TCR rescues human thymocytes from steroid-induced apoptosis.16 In antigen-specific T-cell clones, AICD is enhanced by the coligation of TCR and the α4β1 and αLβ2 (LFA-1) integrins.17 Further, fibronectin has been shown to induce apoptosis in several hematopoietic cell lines, including T lymphocytes.18 Despite these findings, the exact role of integrin signaling in AICD and the mechanisms underlying these effects are unknown.
In this study, we have evaluated the involvement of integrins in the modulation of TCR-induced apoptosis in Jurkat T cells, which is a widely used model system in the studies for AICD. We found that interaction of collagen type I with its receptor α2β1 integrin and ligation of α2β1 with agonists such as activating antibodies inhibited CD3- and PMA/ionomycin-induced apoptosis in T cells. α2β1-mediated inhibition of AICD was accompanied by a reduction in the Fas-L mRNA expression. Ligand binding of α2β1 did not affect Fas-mediated or cycloheximide-induced apoptosis, suggesting that α2β1 signaling specifically inhibits apoptosis induced on TCR stimulation. Overexpression of FRNK, a dominant-negative acting form of FAK, abrogated the effects of α2β1 integrin on both Fas-L expression and AICD, demonstrating that FAK is required for α2β1-mediated inhibition of AICD. Thus, engagement of the collagen receptor α2β1 integrin on T cells may be involved in the regulation of the homeostasis of immune response.
Materials and methods
Cells, antibodies, and other reagents
Human Jurkat T-cell line was obtained from ATCC and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mmol/L glutamine and 100 U/mL of penicillin and streptomycin (= complete medium). Cells were passaged 3 times a week to prevent spontaneous apoptosis. Anti-CD3 monoclonal antibody (mAb) Hit3a and isotype-matched control antibodies were from Pharmingen (San Diego, CA). Mouse antihuman Fas antibodies (CH-11 and UB2) were from Kamiya Biomedicals (Seattle, WA) and anti-Fas-L antibody was from Transduction Laboratories (Lexington, KY). Blocking anti-α1 (FB12) and anti-α2 (PIE6) integrin antibodies, as well as activating anti-β1 (B44) and anti-α2β1 (JSB2) mAbs, were from Chemicon (Temecula, CA). PMA was from Sigma and ionomycin was from Calbiochem (San Diego, CA). Purified bovine type I collagen and mouse laminin were from Becton Dickinson (Bedford, MA) and Gibco-BRL (Grand Island, NY), respectively. Human fibronectin was obtained from Finnish Red Cross and poly-l-lysine from Sigma.
Activation-induced cell death and determination of apoptosis
Cells were suspended at 0.5 × 106 cells/mL in complete medium, plated on wells of 96-well plates in triplicate samples (0.5 × 105 cells per well), and incubated with or without stimuli for 24 hours. For TCR stimulation, wells had been precoated with 50 μg/mL of anti-CD3 antibody in phosphate-buffered saline (PBS) overnight. PMA and ionomycin stimulations were carried out at concentrations of 50 ng/mL and 0.5 μg/mL, respectively. Activating anti-integrin antibodies and their isotype-matched controls were immobilized on plates at a concentration of 50 μg/mL. ECM proteins fibronectin, laminin and collagen I and the nonintegrin ligand poly-l-lysine were used in the soluble form at concentrations ranging from 25 to 100 μg/mL. Inhibitory anti-integrin antibodies were used at a concentration of 25 μg/mL. The percentage of cell death was calculated by using trypan blue dye exclusion (total number of blue cells/total number of cells × 100). Apoptotic cell death was monitored by measuring DNA fragmentation using the Cell Death Detection ELISA kit according to manufacturer's instructions (Boehringer Mannheim, Indianapolis, IN). In some of the experiments, apoptosis was also determined by propidium iodide and FACScan® analysis (Becton Dickinson). Briefly, stimulated cells were washed twice with ice-cold PBS and 5 μg/mL propidium iodide was added to the cells followed by immediate FACScan® analysis. Apoptotic cells were identified as those taking up the dye.
Plasmids and transient transfections
Plasmids encoding the wild-type form of FAK and its dominant-negative acting form known as FRNK (FAK-related nonkinase) have been described in Whitney et al19 and Zhao et al,20 respectively. The 1.2 kb human Fas-L promoter in front of the Luciferase reporter gene becomes transactivated after TCR activation and has been described previously.21 Cells were transfected by electroporation using a BTX electroporator 600 (BTX, San Diego, CA) according to the manufacturer's protocol. Briefly, 20 million cells in log-phase growth were harvested, washed twice with PBS, and resuspended in 0.4 mL of complete medium containing 50 μg of total plasmid DNA. Electroporation was carried out at the settings of 1600 μF, 150 V, and 72 ohms. Cotransfection with the pCMV β-galactosidase plasmid was used to normalize the transfection efficiency. Forty-eight hours after transfection, viable cells were recovered by Ficoll-Hypaque (Sigma) density gradient centrifugation. The cells were then washed 3 times in PBS and used for subsequent cell death experiments.
Luciferase assays
After transfection as above, cells were harvested and stimulated with either anti-CD3 or with PMA/ionomycin for 24 hours. Luciferase activity was measured by using the Luciferase Assay System (Promega) according to the manufacturer's protocol and a monolight 2010 luminometer.
Cell adhesion assays
A 96-well microtiter plate (TC plate, flat bottom, Falcon) was coated with 20 μg/mL of collagen I, fibronectin or poly-l-lysine in PBS, pH 7.4 for 2 hours at 37°C. The wells were then washed with PBS and blocked for 1 hour at 37°C with 1% bovine serum albumin (BSA) (Sigma). Five × 104 cells in 0.1 mL of complete medium were added to the wells. The cells were then incubated for 1 hour at 37°C in the presence or absence of anti-CD-3 or PMA/ionomycin. In some of the experiments, 25 μg/mL of blocking anti-α1 and –α2 integrin antibodies were added to the cells 30 minutes before cellular activation. After incubation, the cells were washed gently 3 times in PBS and fixed with 1% glutaraldehyde, 0.5% sucrose in PBS for 15 minutes at room temperature. After 2 washes with PBS, the cells were stained with 0.5% Crystal violet in 20% methanol/PBS for 30 minutes at room temperature. The cells were then washed 5 times with PBS and the cell-bound stain was resolubilized in methanol and absorbance was measured at 600 nm.
Flow cytometric analysis
1 × 106 cells in 0.1 mL of PBS containing 1% FCS and 0.2% sodium azide (FACS buffer) were incubated with 10 μg/mL of anti-Fas mAb UB2 or isotype-matched control antibodies for 30 minutes at 4°C. The cells were washed 3 times in PBS and incubated with phycoerethryn-conjugated goat antimouse IgG secondary antibodies at a dilution of 1:100 (Jackson ImmunoResearch Laboratories, West Grove, PA) in 0.1 mL of FACS buffer for another 30 minutes. The cells were washed 3 times in PBS and analyzed with a FACScan® (Becton Dickinson).
Cytotoxicity assay
Lysis of the Hut-78 lymphoma cell line, which is sensitive to Fas-mediated cell death, was used as an indicator of Fas-L surface expression. Hut-78 cells were grown in complete RPMI 1640 medium and were labeled with 51Cr as previously described.22 The labeled cells were used as a target for Jurkat cells that had been previously stimulated with 50 μg/mL of anti-CD3 for 8 hours to allow for Fas-L expression. Stimulation was carried out in the presence or absence of 50 μg/mL of poly-l-lysine or collagen I. Various concentrations of Jurkat cells were cocultured with 51Cr-labeled Hut-78 for 12 hours, and the release of51Cr was determined with a gamma counter. Spontaneous release of 51Cr was also determined. The percentage of specific cell lysis was calculated as previously described.22
Immunoprecipitation and immunoblotting
Jurkat cells (5 × 106/0.5 mL serum-free medium) were washed twice with RPMI without serum and stimulated or not with anti-CD3, collagen I, or combination of the two for 10 minutes. Cell lysate preparations, immunoprecipitations with anti-FAK antibody (Transduction Laboratories), as well as immunoblotting with HRPO-conjugated anti-phosphotyrosine py20 antibody (Transduction Laboratories) and with the anti-FAK antibody, followed by enhanced chemiluminescence detection (Pierce, Rockford, IL) were carried out as by Vuori and Ruoslahti.23
Results
Engagement of β1 integrins decreases activation-induced cell death in Jurkat cells
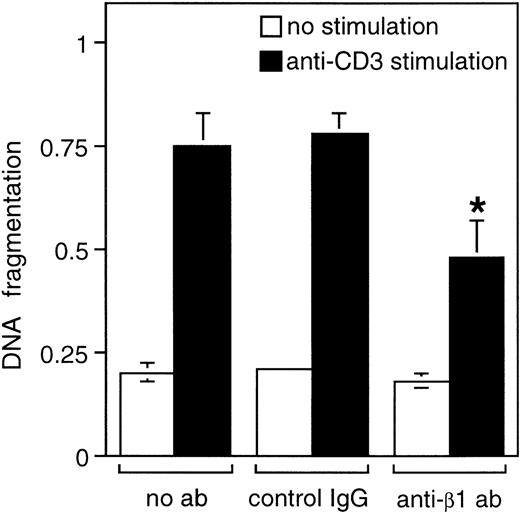
The β1 integrin-positive human leukemic Jurkat T cell line is a widely used cellular model for activation-induced cell death, or AICD, as activation of these cells via TCR leads to up-regulation of Fas-L mRNA and subsequently to AICD and apoptosis.3 Stimulation of T cells with PMA and ionomycin mimics the effects of TCR ligation, and similarly induces apoptosis in a Fas-dependent manner.24 To examine the role of β1 integrin signaling in activation-induced cell death in human T lymphocytes, we used the Jurkat cell model and studied the effect of β1 integrin engagement on CD3-and PMA/ionomycin-induced apoptosis. As shown in Figure1, Jurkat T cells undergo apoptosis on activation of TCR by anti-CD3 antibodies. Addition of activating anti-β1 integrin antibodies, together with anti-CD3 antibody, reduced anti-CD3-induced apoptosis, as evidenced by the 25% inhibition of DNA fragmentation. In contrast, isotype-matched control antibodies had no effect on anti-CD3-induced apoptosis. Similar inhibitory effects by anti-β1 integrin antibodies were observed on PMA/ionomycin-induced apoptosis (data not shown).
β1 integrin signaling inhibits AICD in Jurkat cells.
Jurkat cells were stimulated with or without 50 μg/mL of immobilized anti-CD3 antibody for 24 hours in the presence or absence of 50 μg/mL of immobilized anti-β1 or IgG control antibodies. Apoptosis was determined by DNA fragmentation analysis as described in “Materials and methods.” The results are mean of 3 independent experiments, each done in a duplicate. *P < .05 between anti-CD3-stimulated control and anti-β1 antibody samples.
β1 integrin signaling inhibits AICD in Jurkat cells.
Jurkat cells were stimulated with or without 50 μg/mL of immobilized anti-CD3 antibody for 24 hours in the presence or absence of 50 μg/mL of immobilized anti-β1 or IgG control antibodies. Apoptosis was determined by DNA fragmentation analysis as described in “Materials and methods.” The results are mean of 3 independent experiments, each done in a duplicate. *P < .05 between anti-CD3-stimulated control and anti-β1 antibody samples.
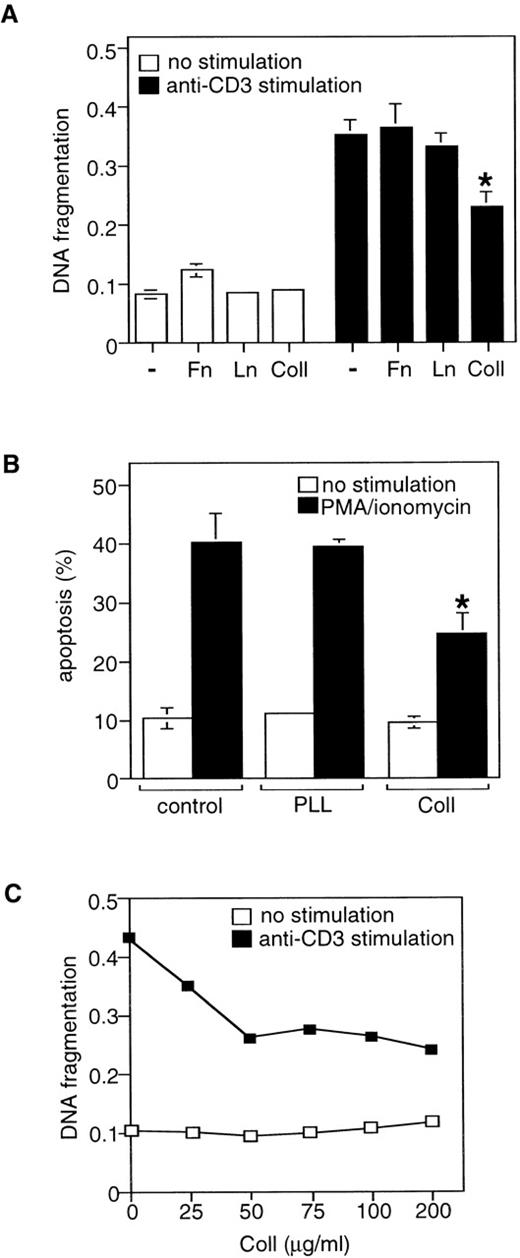
The β1 integrin subunit is shared by several ECM receptors on Jurkat T cells, and we next determined which ECM protein(s) can mimic the effects of the activating anti-β1 integrin antibodies on AICD. Jurkat cells were stimulated with apoptotic anti-CD3 or PMA/ionomycin stimuli in the presence of 75 μg/mL of various purified ECM proteins, such as fibronectin, laminin, and collagen I, and cell death was determined 24 hours later. The nonintegrin ligand poly-l-lysine was used as a control. Cell treatment with collagen I, but not with fibronectin, laminin or poly-l-lysine, protected Jurkat T cells from AICD; DNA fragmentation was inhibited by 32% (Figure2A) and the number of viable cells was increased by 38% in collagen I-treated cells (Figure 2B). Further studies indicated that the effects of collagen I on AICD were dose-dependent (Figure 2C). Preincubation of Jurkat cells for 1, 3, or 6 hours with collagen I before the exposure of the cells to anti-CD3 did not increase cell survival compared with when cells had been treated with collagen I and anti-CD3 at the same time (data not shown). Together, these results indicate that β1 integrin signaling through the collagen I integrin receptors provides a survival signal in T lymphocytes by inhibiting AICD and apoptosis.
Collagen type I inhibits AICD in Jurkat cells.
(A) Jurkat cells were stimulated with or without immobilized anti-CD3 antibody for 24 hours in the presence or absence of 50 μg/mL of the indicated ECM proteins. Apoptosis was determined by DNA fragmentation analysis. Fn, fibronectin; Ln, laminin; Coll, collagen I. *P < .05 between anti-CD3-stimulated control and collagen-treated samples. (B) Cells were stimulated with or without PMA/ionomycin for 24 hours in the presence or absence of 50 μg/mL of collagen I (Coll I) or poly-l-lysine (PLL). Cell death was determined by propidium iodide uptake as described in “Materials and methods.” *P < .05 between PMA/ionomycin-stimulated control and collagen-treated samples. (C) Dose-response effect of collagen I on AICD. The results are representative of 3 independent experiments.
Collagen type I inhibits AICD in Jurkat cells.
(A) Jurkat cells were stimulated with or without immobilized anti-CD3 antibody for 24 hours in the presence or absence of 50 μg/mL of the indicated ECM proteins. Apoptosis was determined by DNA fragmentation analysis. Fn, fibronectin; Ln, laminin; Coll, collagen I. *P < .05 between anti-CD3-stimulated control and collagen-treated samples. (B) Cells were stimulated with or without PMA/ionomycin for 24 hours in the presence or absence of 50 μg/mL of collagen I (Coll I) or poly-l-lysine (PLL). Cell death was determined by propidium iodide uptake as described in “Materials and methods.” *P < .05 between PMA/ionomycin-stimulated control and collagen-treated samples. (C) Dose-response effect of collagen I on AICD. The results are representative of 3 independent experiments.
A specific role for the 2β1 integrin in activation-induced cell death
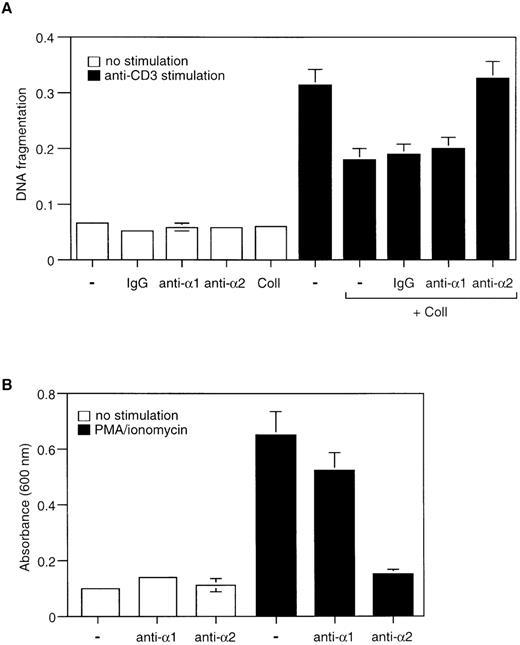
Collagen I can bind to several members of the β1 integrin family, at least 2 of which, the α1β1 or α2β1 integrins, are known to be expressed in Jurkat cells. Previous studies have demonstrated that the α2β1 integrin is likely to be the functional receptor for collagen I in these cells.25 26 To confirm and extend these findings, Jurkat cells were stimulated with PMA/ionomycin in the presence of soluble collagen I (75 μg/mL) and blocking antibodies directed against α1β1 or α2β1 integrins; isotype-matched antibodies were used as a control. As shown in Figure3A, the protective effects of collagen I on AICD were completely abrogated by anti-α2β1 blocking antibodies, whereas blocking anti-α1β1 antibodies and the isotype-matched control antibodies had no effect on collagen I-mediated protection from apoptosis. These results indicate that the effects of collagen I on AICD are likely to be mediated through its interaction with the α2β1 integrin receptor. These results were supported by the finding that the blocking anti-α2β1 antibody inhibits the adhesion of PMA/ionomycin-activated Jurkat cells to collagen I-coated wells by 85%, whereas the blocking anti-α1β1 antibody did not have any effect (Figure 3B). In conclusion, our results demonstrate that activation of Jurkat cells induces cell adhesion to collagen I via the α2β1 integrin and that the collagen I-α2β1 integrin interaction inhibits AICD.
Collagen I-induced inhibition of AICD in Jurkat cells is mediated by the 2β1 integrin.
(A) Jurkat cells were stimulated with or without anti-CD3 and collagen I in the presence or absence of inhibitory antibodies against α1 and α2 integrin subunits or control antibodies (IgG). The anti-integrin antibodies were used at 25 μg/mL and were added 30 minutes before plating the cells on anti-CD3–coated wells. The cells were then cultured for 24 hours and cell death was determined by DNA fragmentation assay. (B) Adhesion assay of Jurkat cells on immobilized collagen I. Cells were activated or not with PMA/ionomycin and plated on wells that had been coated with collagen I and were allowed to attach for 1 hour at 37°C. 25 μg/mL of inhibitory anti-α1 and anti-α2 integrin antibodies were added to the wells as indicated in the figure. Adhesion was quantified as described in “Materials and methods.” The results are representative of 3 different experiments performed in triplicates.
Collagen I-induced inhibition of AICD in Jurkat cells is mediated by the 2β1 integrin.
(A) Jurkat cells were stimulated with or without anti-CD3 and collagen I in the presence or absence of inhibitory antibodies against α1 and α2 integrin subunits or control antibodies (IgG). The anti-integrin antibodies were used at 25 μg/mL and were added 30 minutes before plating the cells on anti-CD3–coated wells. The cells were then cultured for 24 hours and cell death was determined by DNA fragmentation assay. (B) Adhesion assay of Jurkat cells on immobilized collagen I. Cells were activated or not with PMA/ionomycin and plated on wells that had been coated with collagen I and were allowed to attach for 1 hour at 37°C. 25 μg/mL of inhibitory anti-α1 and anti-α2 integrin antibodies were added to the wells as indicated in the figure. Adhesion was quantified as described in “Materials and methods.” The results are representative of 3 different experiments performed in triplicates.
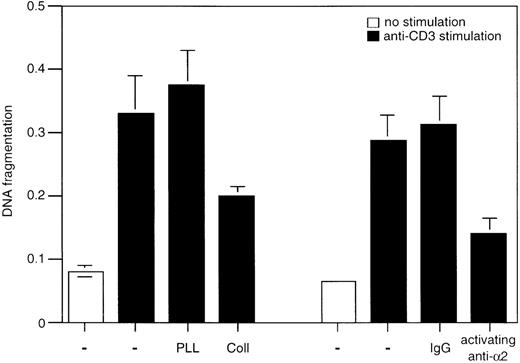
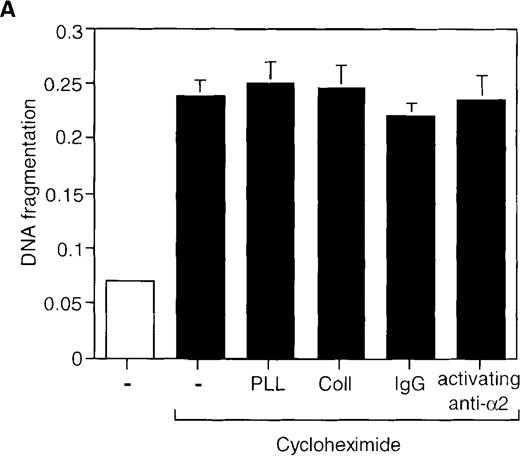
To gain more insight into the protective function of α2β1, we studied whether direct activation of this integrin by the activating anti-α2β1 mAb JSB2, which is known to transduce intracellular signals in Jurkat cells,27 could also block AICD. Indeed, CD3-induced cell death was inhibited by 40% in the presence of the anti-α2β1 mAb JSB2, whereas its isotype-matched control antibody did not have any effect (Figure 4). Thus, signals via the α2β1 integrin can rescue T lymphocytes from AICD. We next investigated whether α2β1 signaling can inhibit other forms of apoptosis in Jurkat cells and analyzed the effects of the JSB2 antibody and collagen I on cycloheximide-induced apoptosis. Treatment of Jurkat cells with cycloheximide (20 μg/mL) for 6 hours induced significant cell death that was not abrogated by either collagen I or JSB2 (Figure 5A); similar results were obtained with cycloheximide concentrations ranging from 5 to 20 μg/mL (data not shown). These findings demonstrate that signaling via the α2β1 integrin is unable to inhibit apoptosis induced by cycloheximide, and suggest that the ability of α2β1 to inhibit AICD in Jurkat cells is specific so that engagement of this integrin does not result in a general inhibitory effect on cell death.
Effect of 2β1 antibody cross-linking on AICD.
Jurkat cells were cultured for 24 hours in wells that had been coated with anti-CD3 and with 50 μg/mL of activating anti-α2β1 antibody JSB2 or with control antibodies (IgG). 50 μg/mL of poly-l-lysine or collagen I was added to some of the wells as indicated in the figure. DNA fragmentation was determined as previously described.
Effect of 2β1 antibody cross-linking on AICD.
Jurkat cells were cultured for 24 hours in wells that had been coated with anti-CD3 and with 50 μg/mL of activating anti-α2β1 antibody JSB2 or with control antibodies (IgG). 50 μg/mL of poly-l-lysine or collagen I was added to some of the wells as indicated in the figure. DNA fragmentation was determined as previously described.
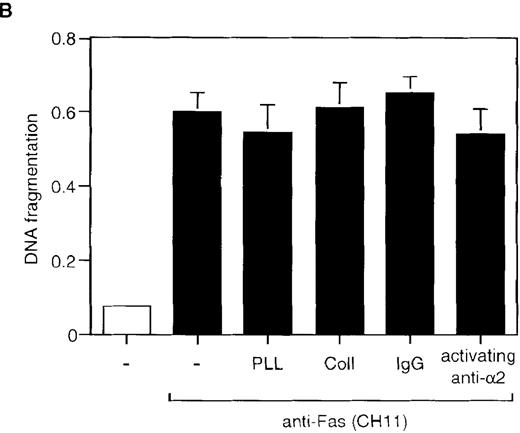
Engagement of the 2β1 integrin does not inhibit cycloheximide- or Fas-induced apoptosis in Jurkat cells.
Jurkat cells were stimulated with 20 μg/mL of cycloheximide for 6 hours (A) or with 1 μg/mL of anti-Fas antibody CH-11 for 24 hours (B) in the presence or absence of 50 μg/mL of collagen I or poly-l-lysine, or 50 μg/mL of immobilized activating anti-α2β1 antibody JSB2 or control antibodies. Apoptosis was determined by DNA fragmentation as previously described.
Engagement of the 2β1 integrin does not inhibit cycloheximide- or Fas-induced apoptosis in Jurkat cells.
Jurkat cells were stimulated with 20 μg/mL of cycloheximide for 6 hours (A) or with 1 μg/mL of anti-Fas antibody CH-11 for 24 hours (B) in the presence or absence of 50 μg/mL of collagen I or poly-l-lysine, or 50 μg/mL of immobilized activating anti-α2β1 antibody JSB2 or control antibodies. Apoptosis was determined by DNA fragmentation as previously described.
Collagen prevents activation-induced cell death by inhibiting Fas ligand mRNA expression
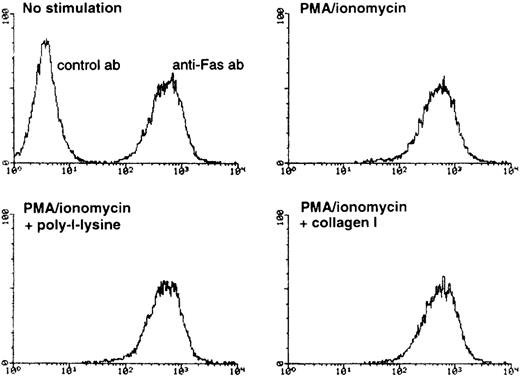
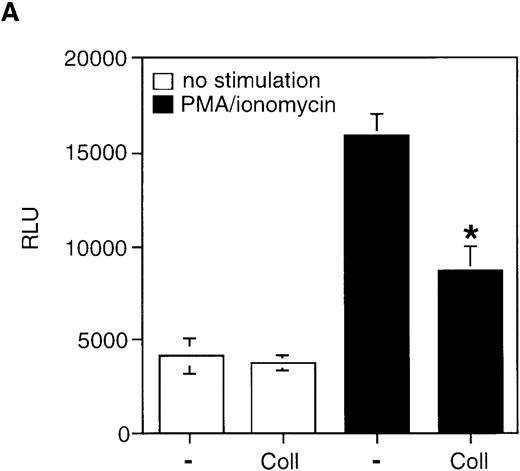
It has been previously demonstrated that AICD proceeds via induction of Fas-L mRNA expression and subsequent Fas/Fas-L interaction on the surface of activated T cells.2 3 Therefore, the observed inhibition of AICD by collagen I could conceivably be mediated by at least 3 nonexclusive mechanisms: by inhibition of Fas-L expression, by inhibition of Fas receptor expression, or by inhibition of apoptotic signaling events downstream of Fas ligation. To study the latter possibility, we analyzed whether Fas-mediated cell death can be blocked by α2β1 signaling. When anti-Fas was used at 1 μg/mL, neither collagen I (100 μg/mL) nor activating anti-α2β1 mAb JSB2 blocked anti-Fas-induced cell death (Figure 5B), suggesting that α2β1 integrin does not interfere with apoptotic signals propagated by Fas ligation. We next established that the α2β1 signaling does not affect the levels of Fas receptor on the cell surface; FACS® analysis demonstrated that after 8 hours of PMA/ionomycin treatment, the constitutive levels of Fas receptor on the cell surface remained unchanged in the presence and absence of α2β1 ligation (Figure6). Similar results were obtained after 24 hours of stimulation with anti-CD3 or with PMA/ionomycin (data not shown). Finally, we determined the effect of α2β1 ligation on Fas-L expression in activated T cells. The transcription of the Fas-L gene was evaluated by using Fas-L promoter construct in a reporter gene transactivation assay. Also, the expression of Fas-L on the surface of CD3-activated Jurkat cells was assessed by functional analysis using51Cr-labeled Hut-78 as a target population. We found that collagen I inhibits the transactivation of Fas-L promoter induced by anti-CD3 and by PMA/ionomycin, as evidenced by a 50% inhibition of Fas-L promoter-driven luciferase activity by collagen I (50 μg/mL), but not by poly-l-lysine (50 μg/mL) in activated Jurkat cells (Figure7A). Further, α2β1 signaling was found to inhibit the expression of Fas-L on the surface of activated Jurkat cells, most likely because of the inhibition of Fas-L mRNA expression. As shown in Figure 7B, stimulation of Jurkat cells with anti-CD3 resulted in Fas-L expression on the cell surface as evidenced by the ability of stimulated Jurkat cells to induce apoptosis in Fas-sensitive Hut78 cells. In the presence of collagen I, but not of poly-l-lysine, the ability of anti-CD3-stimulated Jurkat cells to induce cytotoxicity in Hut-78 cells was inhibited by 35%, suggesting that Fas-L expression is inhibited by α2β1 signaling in activated Jurkat cells (Figure 7B). Similar results were obtained when the protein expression levels of Fas-L were analyzed by immunoblot analysis; treatment of PMA/ionomycin-stimulated Jurkat cells with 50 mg/mL of collagen I inhibited Fas-L protein expression levels by 40% (data not shown). In conclusion, collagen I appears to prevent CD3-mediated AICD by inhibiting the induction of Fas-L expression in T cells.
FACS analysis of Fas antigen expression on the surface of Jurkat cells.
Jurkat cells were treated for 8 hours with PMA/ionomycin in the presence or absence of 100 μg/mL of poly-l-lysine or collagen I. The cells were harvested and stained as described in “Methods.” The upper left panel represents negative and positive staining of unstimulated Jurkat cells with an isotype-matched control antibody and anti-Fas antibody, respectively. The 3 other panels show antiFas staining in stimulated Jurkat cells that had been treated with PMA/ ionomycin, poly-l-lysine and collagen I as indicated. X axis, relative fluorescence intensity; Y axis, cell number.
FACS analysis of Fas antigen expression on the surface of Jurkat cells.
Jurkat cells were treated for 8 hours with PMA/ionomycin in the presence or absence of 100 μg/mL of poly-l-lysine or collagen I. The cells were harvested and stained as described in “Methods.” The upper left panel represents negative and positive staining of unstimulated Jurkat cells with an isotype-matched control antibody and anti-Fas antibody, respectively. The 3 other panels show antiFas staining in stimulated Jurkat cells that had been treated with PMA/ ionomycin, poly-l-lysine and collagen I as indicated. X axis, relative fluorescence intensity; Y axis, cell number.
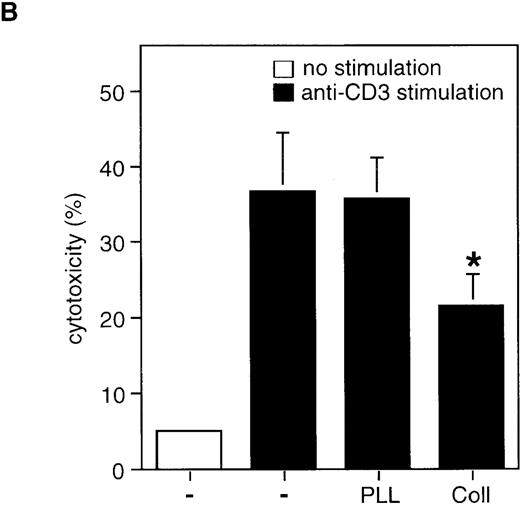
Collagen I inhibits AICD by modulating Fas-L expression.
(A) Collagen I inhibits transcriptional activity of the Fas-L promoter in activated Jurkat cells. Jurkat cells were transiently transfected with luciferase reporter construct containing the 1.2 kb Fas-L promoter together with a pCMVβ-Gal plasmid encoding β-galactosidase. 48 hours after transfection, the cells were stimulated with or without PMA/ionomycin in the presence or absence of 50 μg/mL of poly-l-lysine and collagen I. The relative luciferase activity (RLU) was determined 18 hours after stimulation. *P < .05 between PMA/ionomycin-stimulated control and collagen-treated samples. (B) Collagen inhibits Fas-L expression on the cell surface as determined by a cytotoxicity assay. Jurkat cells were stimulated with 50 μg/mL of anti-CD3 for 6 hours to allow for Fas-L expression. As indicated, 50 μg/mL of collagen I or poly-l-lysine was added. The cells were harvested, washed twice with PBS, and incubated with51Cr-labeled Hut-78 cells for 12 hours. The percentage of cytotoxicity was determined as described in “Materials and methods.” The results are mean of 2 experiments performed in triplicates. *P < .05 between anti-CD3-stimulated control and collagen-treated samples.
Collagen I inhibits AICD by modulating Fas-L expression.
(A) Collagen I inhibits transcriptional activity of the Fas-L promoter in activated Jurkat cells. Jurkat cells were transiently transfected with luciferase reporter construct containing the 1.2 kb Fas-L promoter together with a pCMVβ-Gal plasmid encoding β-galactosidase. 48 hours after transfection, the cells were stimulated with or without PMA/ionomycin in the presence or absence of 50 μg/mL of poly-l-lysine and collagen I. The relative luciferase activity (RLU) was determined 18 hours after stimulation. *P < .05 between PMA/ionomycin-stimulated control and collagen-treated samples. (B) Collagen inhibits Fas-L expression on the cell surface as determined by a cytotoxicity assay. Jurkat cells were stimulated with 50 μg/mL of anti-CD3 for 6 hours to allow for Fas-L expression. As indicated, 50 μg/mL of collagen I or poly-l-lysine was added. The cells were harvested, washed twice with PBS, and incubated with51Cr-labeled Hut-78 cells for 12 hours. The percentage of cytotoxicity was determined as described in “Materials and methods.” The results are mean of 2 experiments performed in triplicates. *P < .05 between anti-CD3-stimulated control and collagen-treated samples.
Focal adhesion kinase FAK is required for the inhibition of activation-induced cell death and Fas ligand expression by collagen I
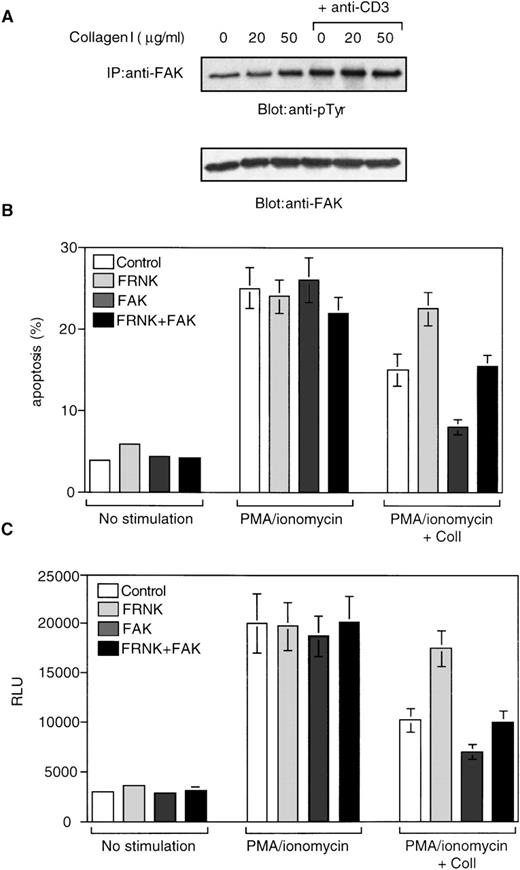
Previous studies have demonstrated that activation and tyrosine phosphorylation of FAK is crucial for cell survival signals downstream of integrins in multiple cellular systems.9 Also, it has been demonstrated that stimulation via CD3 and integrins results in a rapid (within 5 minutes) tyrosine phosphorylation of FAK and the phosphorylation levels are maintained high up to 2 hours in Jurkat cells.28 29 To determine whether FAK is involved in α2β1-mediated inhibition of AICD, we first studied whether FAK becomes tyrosine-phosphorylated in response to α2β1 ligation in Jurkat cells. As shown in Figure 8A, low, 20 μg/mL concentration of collagen I had no significant effect on the basal tyrosine phosphorylation level of FAK. Densitometry analysis demonstrated a 2-fold increase in FAK phosphorylation on stimulation of cells with higher, 50 μg/mL concentration of collagen I. A similar 2-fold induction in FAK phosphorylation was observed when cells were stimulated with 20 μg/mL anti-CD3 antibody. Simultaneous stimulation of cells with 20 μg/mL of anti-CD3 and 50 μg/mL of collagen I resulted in a 3-fold induction in FAK phosphorylation (Figure 8A). These results demonstrate that α2β1 ligation either alone or in combination with anti-CD3 increases FAK tyrosine phosphorylation in Jurkat cells.
FAK is phosphorylated in response to 2β1 ligation and mediates 2β1-induced inhibition of AICD and Fas-L expression.
(A) The cells were stimulated or not with collagen I at 20 and 50 μg/mL either alone or in combination with 20 μg/mL of soluble anti-CD3 for 10 minutes. The cells were washed and lysed and subjected to immunoprecipitation with anti-FAK antibodies. The immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine antibodies (top panel) and with antibodies against FAK (lower panel). (B) Jurkat cells were cotransfected with a plasmid encoding the dominant-negative form of FAK (FRNK), wild-type form of FAK (FAK), combination of FRNK- and FAK-encoding plasmids, or a control plasmid, together with a plasmid encoding GFP as described in “Materials and methods.” Viable cells were recovered 48 hours later by Ficoll gradient and stimulated with or without PMA and ionomycin in the presence or absence of 50 μg/mL collagen I for 24 hours. The cells were then washed and propidium iodide was added for 15 minutes on ice, and apoptosis was analyzed by FACScan®. The analysis was carried out on the double positive cell population for propidium iodide and fluorescent GFP. (C) Jurkat cells were cotransfected with plasmids encoding FRNK, FAK, Fas-L promoter reporter construct, and β-galactosidase. After 48 hours, the cells were washed and treated with the various stimuli as in (B) and luciferase activity (RLU) was determined after 18 hours.
FAK is phosphorylated in response to 2β1 ligation and mediates 2β1-induced inhibition of AICD and Fas-L expression.
(A) The cells were stimulated or not with collagen I at 20 and 50 μg/mL either alone or in combination with 20 μg/mL of soluble anti-CD3 for 10 minutes. The cells were washed and lysed and subjected to immunoprecipitation with anti-FAK antibodies. The immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine antibodies (top panel) and with antibodies against FAK (lower panel). (B) Jurkat cells were cotransfected with a plasmid encoding the dominant-negative form of FAK (FRNK), wild-type form of FAK (FAK), combination of FRNK- and FAK-encoding plasmids, or a control plasmid, together with a plasmid encoding GFP as described in “Materials and methods.” Viable cells were recovered 48 hours later by Ficoll gradient and stimulated with or without PMA and ionomycin in the presence or absence of 50 μg/mL collagen I for 24 hours. The cells were then washed and propidium iodide was added for 15 minutes on ice, and apoptosis was analyzed by FACScan®. The analysis was carried out on the double positive cell population for propidium iodide and fluorescent GFP. (C) Jurkat cells were cotransfected with plasmids encoding FRNK, FAK, Fas-L promoter reporter construct, and β-galactosidase. After 48 hours, the cells were washed and treated with the various stimuli as in (B) and luciferase activity (RLU) was determined after 18 hours.
The C-terminal domain of FAK is expressed in some cells as an alternatively spliced form known as FRNK (FAK-related nonkinase), which is devoid of any tyrosine kinase activity.30 Exogenous expression of FRNK has been shown to interfere with the endogenous FAK signaling in a dominant-negative fashion,31 32 and therefore provides an efficient tool to dissect the signaling pathways downstream of FAK. Accordingly, we investigated next the role of FAK in collagen I–induced inhibition of AICD by exogenous expression of FRNK in Jurkat cells. The cells were transiently transfected with plasmids encoding FRNK or control plasmids, together with a plasmid encoding the green fluorescent protein (GFP) to allow detection of transfected cells. The ability of collagen I to protect the cells from AICD was then assessed. As shown in Figure 8B, expression of FRNK per se had no effect on the viability of Jurkat cells. The protective effects of collagen I on AICD, however, were abrogated by 85% in cells that had been transfected with FRNK, but not in cells that had been transfected with control plasmids. Hence, these results suggest that FAK has an important role in mediating the protective effects of α2β1 on AICD. In support of this, we found that exogenous expression of wild-type FAK increased the capacity of collagen I to protect the cells from AICD without affecting the overall viability of Jurkat cells (Figure 8B). In addition, coexpression of wild-type FAK with FRNK completely rescued the effect of FRNK on collagen-mediated cell survival, confirming that exogenously expressed FRNK specifically interferes with FAK signaling in Jurkat cells (Figure 8B).
We also examined whether exogenous expression of FRNK influences collagen I–induced inhibition of Fas-L promoter activity in activated Jurkat cells. Cells were transfected with a Fas-L reporter gene plasmid together with an FRNK encoding or control plasmid. As shown in Figure8C, exogenous expression of FRNK abrogated the inhibitory effects of collagen I on PMA/ionomycin-induced Fas-L promoter activity by 80%, without affecting the basal Fas-L promoter activity. Exogenous expression of wild-type FAK in turn enhanced the ability of collagen to inhibit Fas-L promoter activity, and coexpression of wild-type FAK with FRNK abolished the inhibitory effect of FRNK on α2β1 signaling. Exogenous expression of FAK did not have any effect on the basal Fas-L promoter activity (Figure 8C). Together, these results indicate that FAK is required for the inhibition of both Fas-L promoter activity and AICD in activated Jurkat cells by α2β1 signaling.
Discussion
Integrin signaling has been shown to protect various cell types from apoptosis and to play an important role in providing costimulatory signals in T cells. In agreement with a previous report,33we demonstrate here that treatment of Jurkat T cells with an activating anti-β1 antibody inhibits AICD, suggesting that signaling via β1 integrin(s) interferes with signals for AICD in T lymphocytes. We have extended these studies and identified α2β1 as the β1 integrin that is responsible for these effects, at least in Jurkat T cells. An α2β1 integrin ligand collagen I and activating antibodies against α2β1 inhibited AICD, but had no effect on cycloheximide-induced cell death. We further show that activation of Jurkat cells induces cell binding to collagen I via α2β1 integrin, which subsequently results in the inhibition of AICD. Together, these results indicate that α2β1 may have an important role the regulation of AICD and in the maintenance of the homeostasis of the immune system.
Our results demonstrate that only α2β1, but not the other β1 integrins that act as receptors for, eg, laminin and fibronectin, signal for inhibition of AICD in Jurkat cells. As a matter of fact, fibronectin, which interacts mainly with the α4β1 integrin in Jurkat cells (data not shown and Mobley et al34) slightly enhanced AICD at later time points (48 hours) (data not shown). These results are in agreement with a previous report demonstrating that in antigen-specific T-cell clones, stimulation of α4β1 with its ligand VCAM-1 synergizes with TCR signals in inducing apoptosis.17Interestingly, it has been demonstrated that the protective effects of TGFβ1 on cell survival in CD8+ T cells are mediated through another fibronectin receptor, the α5β1 integrin.15 These observations suggest that T cells may respond differentially to their tissue microenvironment, depending on the expression profile and activation state of the integrin receptors on the cell surface. Similarly, the cells appear to use different ECM receptors to perform different biologic functions. The exact mechanisms by which integrins link to specific intracellular signaling pathways to create the appropriate cellular responses are unknown at present.
Although the in vivo importance of our findings remains to be determined, the results reported here nevertheless add to the notion that integrins not only modulate T-cell activation and migration but they also regulate lymphocyte apoptosis. The α2β1 integrin is also known as VLA-2 for “very late antigen-2,” and, unlike some other VLA-denoted integrins, it is considered as a “true” very late T-cell activation antigen as it appears 2 to 4 weeks after in vitro activation of T lymphocytes.35 Our results suggest that a function for the α2β1 integrin may be to inhibit AICD during the late stages of T-cell activation. Collagen type I is a very abundant extracellular matrix protein in connective tissues of skin, tendon, and bone, and it is the main collagen type produced by dermal fibroblasts.36 It can be envisioned that T lymphocytes may encounter collagen I during the immune response in tissues that are rich in collagen I; this may occur, for example, during the elimination of infected cells on viral infections. The α2β1 integrin-collagen I interaction may facilitate the development of memory cells in these situations. Also, expression levels of collagen type I are upregulated in certain inflammatory and autoimmune disease conditions, such as systemic sclerosis.37 Thus, the α2β1 integrin-collagen I interaction may also play a role in chronic inflammatory diseases: interaction of activated T cells with collagen I could result in hyperactivation of T cells due to inhibition of apoptosis, which in turn may lead to an uncontrolled inflammatory response and tissue damage. Along these lines, CD44, which is another cell adhesion molecule and which also protects T cells from AICD,38 has been linked to the development of T-cell memory. It has been shown that only CD44 positive cells survive and develop into T memory cells during peripheral T lymphocyte depletion.39 Inhibition of AICD via β1 integrins may also have a role in promoting activation of autoreactive lymphocytes in various autoimmune and inflammatory diseases; in rheumatoid arthritis, the ratio of α1β1 and α2β1 integrins on synovial fluid T lymphocytes is associated with stages of T-cell activation.40 These observations indicate that adhesion molecules may be important in controlling lymphocyte homeostasis and peripheral depletion by modulating apoptotic signals triggered via TCR and perhaps also via other membrane receptors.
Our results demonstrate that ligation of the α2β1 integrin inhibits AICD by inhibiting transcriptional activation of the Fas-L gene. In previous studies, the mechanism(s) by which integrins or other adhesion molecules modulate survival and apoptosis in T cells has not been addressed. In contrast to its effects on Fas-L expression, α2β1 ligation had no effect on Fas antigen expression or on Fas antigen-mediated activation of cell death pathways. We found, however, that when the stimulatory anti-Fas CH-11 mAb was used at lower doses (0.1 μg/mL), ligation of α2β1 was able to inhibit Fas-mediated cell death by 30% (data not shown), suggesting that signaling via α2β1 may interfere with Fas-signaling pathway under some conditions. Our results are similar to the previous reports that demonstrate that CD28, CD2, and TGFβ1 signaling inhibits AICD by inhibiting Fas-L expression.22,41,42 Together, these results suggest that modulation of Fas-L expression may be a critical checkpoint in AICD. Stimulation of T cells via TCR on the cell surface transduces a series of intracellular signals that eventually lead to Fas-L expression and AICD.43 One explanation for the observed inhibitory effects of α2β1 ligation on Fas-L expression could be the sequestration of the TCR signaling complex at the cell surface. This is unlikely to be the case, however, as we found that α2β1 ligation also inhibits PMA/ionomycin-induced AICD by the same mechanism. Expression of Fas-L downstream of TCR is regulated by transcription factors such as NF-κB, c-myc, NF-AT, and Egr-3,21,41,42,44-46 and it has been shown that TGFβ1 inhibits Fas-L expression and subsequent AICD via down-regulation of c-Myc.42 We are currently exploring the possibility that α2β1 signaling functions by inhibiting the activity of one of these transcription factors.
Our studies identify a functional role for the focal adhesion kinase FAK in T cells, as we find that FAK is a crucial component in the α2β1 signaling pathways that mediate inhibition of Fas-L expression and AICD. Our results are in agreement with the role of FAK in protecting against anoikis and serum deprivation-induced cell death in adherent cells.47,48 Along these lines, it was reported previously that β1 integrin signaling increases expression of FAK mRNA, which in part may be responsible for the protective signaling effects of β1 integrins in T-cells.33 Also, FAK has been found to become proteolytically cleaved during Fas-mediated apoptosis in Jurkat cells.49 Together, these findings contribute to the emerging picture that FAK is a key element in cell survival not only in adherent cells but also in nonadherent cells such as T lymphocytes. In agreement with previous publications,28,29we found that CD3 occupancy also causes FAK phosphorylation. At present, the significance of this finding is unknown. Our results suggest that FAK activation does not partake in modulation of cell death directly downstream of CD3, as expression of dominant-negative FRNK had no effect on CD3-induced Fas-L expression and apoptosis (Figure 8). One possibility is that FAK would play a role in modulating cytoskeletal responses downstream of CD3. Indeed, recent findings indicate that the docking protein Cas-L, which is one of the FAK targets in T cells, mediates cell migratory events in response to CD3 ligation.50 Because FAK is promoting cell survival downstream of α2β1 integrin, it seems paradoxical that FAK is also activated on TCR ligation. However, it is not uncommon that a signaling molecule that promotes cell survival would become activated also downstream of stimuli that lead to apoptosis. For example, activation of PI 3-kinase, which mediates cell survival downstream of a number of cell surface receptors,51 also becomes activated on TCR ligation.52 Furthermore, activation of NF-κB is known to promote cell survival, but NF-κB nevertheless is also activated on TNFα-induced cell death.53 The mechanisms by which FAK transduces the protective effects of integrin signaling to the regulation of Fas-L expression and AICD are unknown. It should be noted that activation and tyrosine phosphorylation of FAK is not specific for α2β1 integrin ligation in Jurkat cells: stimulation of α4β1 integrin (data not shown, and Maguire et al29) signaling pathways also leads to FAK phosphorylation. It is therefore possible that additional specific signals triggered by α2β1 ligation contribute to the inhibition of AICD. In this regard, it has been shown that cross-linking of α2β1 with activating antibodies induces tyrosine phosphorylation of several intracellular proteins and leads to accumulation of active p21 Ras molecules in Jurkat cells.27Further elucidation of the mechanisms by which α2β1 signaling inhibits Fas-L transcription and AICD is likely to provide new important insights into the role of integrin signaling in the regulation of T-cell homeostasis in normal and pathologic conditions.
Acknowledgments
We are grateful to Dr Douglas Green (La Jolla Institute for Allergy and Immunology, San Diego, CA) and Dr Jun-Lin Guan (Cornell University, NY) for providing the reporter gene construct of human Fas-L promoter and the dominant-negative FRNK plasmid, respectively. We also thank Dr Reem Al-Daccak (Laval University, Quebec, Canada) for helpful discussions.
Supported by NIH grants CA71560 and CA76037 (to K.V.). K.V. is a PEW scholar in biomedical sciences.
Reprints:Kristiina Vuori, Cancer Research Center, The Burnham Institute, 10901 N Torrey Pines Rd, La Jolla, CA 92037; e-mail:kvuori@burnham-inst.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal