Abstract
Vascular endothelial growth factor (VEGF) signaling is required for both differentiation and proliferation of vascular endothelium. Analysis of differentiated embryonic stem cells with one or both VEGF-A alleles deleted showed that both the differentiation and the expansion of endothelial cells are blocked during vasculogenesis. Blood island formation was reduced by half in hemizygous mutant VEGF cultures and by 10-fold in homozygous mutant VEGF cultures. Homozygous mutant cultures could be partially rescued by the addition of exogenous VEGF. RNA levels for the endothelial adhesion receptors ICAM-2 and PECAM were reduced in homozygous mutant cultures, but ICAM-2 RNA levels decreased substantially, whereas PECAM RNA levels remained at hemizygous levels. The quantitative data correlated with the antibody staining patterns because cells that were not organized into vessels expressed PECAM but not ICAM-2. These PECAM+ cell clumps accumulated in mutant cultures as vessel density decreased, suggesting that they were endothelial cell precursors blocked from maturation. A subset of PECAM+ cells in clumps expressed stage-specific embryonic antigen-1 (SSEA-1), and all were ICAM-2(−) and CD34(−), whereas vascular endothelial cells incorporated into vessels were PECAM(+), ICAM-2(+), CD34(+), and SSEA-1(−). Analysis of flk-1 expression indicated that a subset of vascular precursor cells coexpressed PECAM and flk-1. These data suggest that VEGF signaling acts in a dose-dependent manner to affect both a specific differentiation step and the subsequent expansion of endothelial cells.
Our understanding of mammalian vascular development is far from complete. Mesoderm-derived cells differentiate into endothelial cells that coalesce into blood vessels in a process called vasculogenesis, and the sprouting of endothelial cells from vessels produces new vessels in a process called angiogenesis.1-3 A relationship between the vascular and hematopoietic lineages through a common precursor cell, the hemangioblast, has long been hypothesized.1,4 Immature vascular precursor cells are called angioblasts, but in most cases the angioblast is operationally defined as a cell that participates in vasculogenesis. Nevertheless, several markers are consistently associated with embryonic stem (ES) cell-derived precursor cells having vascular potential, including the vascular endothelial growth factor (VEGF) receptor flk-1, the cell adhesion receptor platelet endothelial cell adhesion molecule (PECAM), and the adhesion molecule VE-cadherin.5-7 These molecules also have in vivo expression patterns that are consistent with their being early markers of vascular lineage.8-12 However, the paucity of early precursor cells available from mouse embryos makes functional analysis of these putative precursor cells difficult.
VEGF signaling is critical for blood vessel formation during development (reviews13-15). VEGF is a 45-kd homodimer produced at sites of vasculogenesis and angiogenesis, and alternative splicing results in 3 different isoforms in the mouse. The homodimer of VEGF-164 is the most active form, and its properties include mitogenesis, chemotaxis, and permeability for endothelial cells. VEGF binds to 2 high-affinity receptors, flk-1 (VEGFR-2) and flt-1 (VEGFR-1), that are expressed in endothelium. Recently, a third molecule that binds VEGF with high affinity was identified as neuropilin-1, a receptor that also signals in the nervous system through a different ligand.16 Available data suggest that neuropilin-1 acts as a coreceptor with flk-1 in vascular tissues.
Expression patterns of receptors and ligands suggest that VEGF may be a highly specific mediator of blood vessel formation in vivo,8,17,18 and analysis of targeted mutations in the mouse supports this hypothesis. Both flk-1 and flt-1 receptor mutations are recessive embryonic lethals at days 8.5 to 9.5 of gestation.19,20 The flk-1 mutation severely impairs vasculogenesis and hematopoiesis, whereas the flt-1 mutation affects vascular organization. A targeted mutation in VEGF confirmed that this ligand is necessary for vascular development and showed a surprising dosage effect because embryos that were hemizygous for the VEGF-targeted mutation died in utero with vascular defects.21,22 The importance of flk-1 in developmental blood vessel formation and hematopoiesis was further shown in a chimera analysis with flk-1−/−ES cells.23 Although these data suggest that VEGF signaling through the flk-1 receptor is responsible for vasculogenesis and hematopoiesis, the recent identification of VEGF-related molecules that can bind flk-1, flt-1,24-27 , or both, makes it important to analyze the effects of the lack of VEGF on developmental processes.
ES cell differentiation in vitro has provided a tool to study specific aspects of mammalian development (review28). ES cell differentiation in serum without added factors recapitulates many aspects of yolk sac development, including vascular and hematopoietic differentiation in the context of other yolk sac cell types.5,29-32,33 This system was exploited to identify early hematopoietic precursor cells and putative hemangioblasts with vascular and hematopoietic potential.34,35 Differentiation of ES cells with specific mutations indicates that defects in hematopoiesis and vascular development are recapitulated in vitro. Differentiation of flk-1−/−ES cells revealed the expected defects in vasculogenesis, but primitive erythropoiesis was not compromised as it is in vivo.23 36 The authors suggested that VEGF signaling may be necessary in vivo for the migration of precursors to the yolk sac environment, whereas in vitro the signals are produced in the vicinity of the precursor cells.
To further elucidate the role of VEGF signaling in vascular development in a system with the potential for functional analysis, we analyzed VEGF mutant ES cells differentiated in vitro. Vascular development was compromised in a dose-dependent manner and could be partially rescued by the exogenous addition of VEGF. A population of cells that expressed flk-1 but formed blood islands inefficiently accumulated in the VEGF mutants, and this population partially overlapped with a second population of cells that accumulated and expressed the cell adhesion receptor PECAM. The PECAM+ and flk-1+ cells were negative for several other vascular markers, including intercellular adhesion molecule-2 (ICAM-2) and CD34. These findings suggest that VEGF signaling is required for the transition of flk-1+ and PECAM+ cells to vascular endothelial cells that express ICAM-2 and CD34.
Materials and methods
Cell culture and in vitro differentiation
ES cells wild-type (wt, +/+), hemizygous mutants for the targeted VEGF mutation (het, +/−), and homozygous mutants for the targeted VEGF mutation (null, −/−)21 were cultured with 5637 cell-conditioned medium as a source of leukemia inhibitory factor. They were differentiated by a modification of a protocol for embryoid body differentiation that allows for reattachment of the embryoid bodies to tissue culture plastic during the differentiation.32 In vitro differentiation in attached cultures was commenced by treatment with Dispase, 3 days in suspension culture in differentiation media (DMEM-H, 20% fetal bovine serum (FBS; lot tested), 150 μm α-thioglycerol, and 50 μg/mL gentamicin), and transfer to 24-well tissue culture plates. Differentiation cultures were fed every 48 hours, and in some cases the medium was supplemented with human rVEGF-165 (R&D Systems, Minneapolis, MN) at 15 ng/mL at each feeding from day 3 to day 8.
Antibody staining
Antibody staining was performed using a modified protocol.32 Cultures were rinsed in PBS and fixed in the appropriate fixative for 6 minutes—ice-cold methanol:acetone (50:50) for PECAM, ICAM-2, SSEA-1, and flk-1 antibodies, and fresh 4% paraformaldehyde for CD34 antibody. PECAM/CD34 double stains were fixed in paraformaldehyde, and all others were fixed in methanol:acetone. Fixed cultures were blocked in staining media (3% FBS, 0.1% NaN3 in PBS), incubated in primary antibody for 1 to 2 hours at 37°C, and rinsed in staining media. Secondary antibody was incubated for 1 hour at 37°C. For double-labeling experiments, the first reaction was followed by incubation with a rabbit anti-PECAM antibody (gift of Beat Imhof) followed by the appropriate secondary antibody. PECAM and flk-1 were double labeled by staining first with rat antimouse PECAM, followed by staining with rabbit antimouse flk-1. Cultures were rinsed in PBS and viewed with an Olympus IX-50 inverted microscope using epifluorescence.
Primary antibodies and dilutions used were: rat antimouse PECAM at 1:1000 (Mec 13.3; Pharmingen, San Diego, CA); rat antimouse ICAM-2 at 1:500 (3C4; Pharmingen); rat antimouse CD34 at 1:500 (RAM34; Pharmingen); mouse antimouse SSEA-1 at 1:50 (MC-480; Developmental Studies Hybridoma Bank); purified rabbit polyclonal antimouse PECAM at 1:500; and rabbit polyclonal antimouse flk-1 at 1:50 (sc-504; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies used were donkey antirat B-phycoerythrin (BPE) cross-absorbed at 1:300 (712-106-150; Jackson Immunoresearch, West Grove, PA); goat antimouse IgM Fab fragment-BPE cross-absorbed at 1:500 (115-106-075; Jackson Immunoresearch) for MC-480; goat antirabbit IgG (H + L)-fluorescein isothiocyanate (FITC) cross-absorbed at 1:500 (4050-02; Southern Biotechnology Associates, Birmingham, AL) for polyclonal PECAM; and donkey antirabbit IgG (H + L) TRITC cross-absorbed at 1:100 (711-025-152; Jackson Immunoresearch) for polyclonal flk-1. Py-4-1 endothelial cells37 and NIH 3T3 fibroblast cell lines were used as positive and negative controls, respectively, for PECAM, ICAM-2, CD34, and flk-1. For SSEA-1 reactivity, ES cells were the positive control and NIH 3T3 cells were the negative control. In all cases controls with no primary antibody or an irrelevant isotype-matched control did not produce signal over background (data not shown).
Imaging and quantitation
Embryonic stem cells were plated onto a 48-well tissue culture dish, maintained as described above, and fixed on day 8 of differentiation. After staining with the appropriate primary and secondary antibodies, good-quality wells were photographed using Tmax 400 film at a fixed exposure time at 10× magnification. Sequential frames were set up to provide 7 nonoverlapping frames with 100% cell coverage from each well. This strategy allowed for the analysis of more than 60% of each well area. Each frame was scanned using a SprintScan 35 (Polaroid, Cambridge, MA), and the resultant digital image was manually modified in Adobe Photoshop 3.0 to remove the background. The modified image was analyzed using an Image Processing Tool Kit (Rev. 2.1; Reindeer Games, Asheville, NC) that allowed quantitation of the stained area. The average for each well was calculated, the numbers for 2 to 4 wells of each condition were averaged, and the SD was calculated. The relative abundance of PECAM+ aggregates was determined by visual examination of multiple PECAM-stained wells of each genotype in several independent differentiations.
RNA analysis
Total RNA was isolated by centrifugation through a CsCl cushion38 and analyzed using a modified RNase protection assay.3932P-labeled antisense RNA probes were generated by in vitro transcription of the following cloned gene fragments: p4-21L1 (flk-1, nt 2399-2687) (gift of D. Dumont); clone 13 (flt-1, nt 2321-2598), Te2 (tie-2, nt 2057-2435), VEGFCon (VEGF, nt 1-400, a subclone of VEGFAllie) (all gifts of K. Peters); I-2700 (ICAM-2, nt 556-817), and PECAM-dCPa (PECAM, nt 1425-1904). After overnight hybridization at 45°C with a specific probe and β-actin as an internal control, the reactions were digested with RNase A and RNase T1. The protected fragments were electrophoresed through a 5% acrylamide/8 mol/L urea gel, then visualized and quantitated using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
In situ hybridization
In situ hybridization was performed by modification of a standard protocol used for whole-mount hybridization.40 RNA probes were synthesized using p421L1 as a template for flk-1 and PECAM-dCP as a template for PECAM. PECAM-dCP was a reverse transcription–polymerase chain reaction amplification product of 660 bp from nt 1244 to nt 1904 generated from Py-4-1 endothelial cell RNA using a TA cloning kit (Invitrogen, Carlsbad, CA) (M. Inamdar and V. L. Bautch, unpublished results). Cultures from days 8 or 9 were fixed in fresh 4% paraformaldehyde for 1 hour and dehydrated through a methanol:PBS series for storage at −20°C. Wells were rehydrated, incubated first in 6% H2O2 in PBT (PBS + 0.1% Tween-20) for 30 minutes, incubated in proteinase K (3 μg/mL in PBT) for 10 minutes, and then incubated in fresh glycine (2 mg/mL in PBT) for 4 minutes. All incubations were followed by three 5-minute washes in PBT. Wells were refixed in 0.2% glutaraldehyde and 4% paraformaldehyde in PBT for 8 minutes, washed, and prehybridized for 2 to 3 hours at 70°C as described. Wells were hybridized in hybridization buffer with denatured probe (2 μL of a 50-μL probe reaction/0.25 mL hybridization buffer/well). An initial 3-hour incubation at 80°C was followed by a 36-hour incubation at 69°C. Posthybridization washes were as described at 65°C. RNase treatment, blocking incubation, antibody reaction, washes, and substrate development were all as described. Cultures were developed for 25 to 36 hours, then postfixed and stored in PBT at 4°C.
Statistical analysis
The average area stained (imaging) or protected counts (RNA) were calculated from multiple wells, multiple experiments, or both. Averages for each genotype were compared to wild-type values using the Studentt test. For the VEGF rescue experiments, the rescue average was compared to the average of unrescued wells of the same genotype using the Student t test. Significant differences are denoted by asterisks in the appropriate figures.
Results
Vascular development is compromised in the absence of VEGF
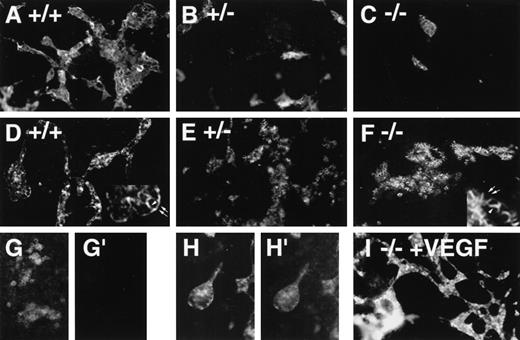
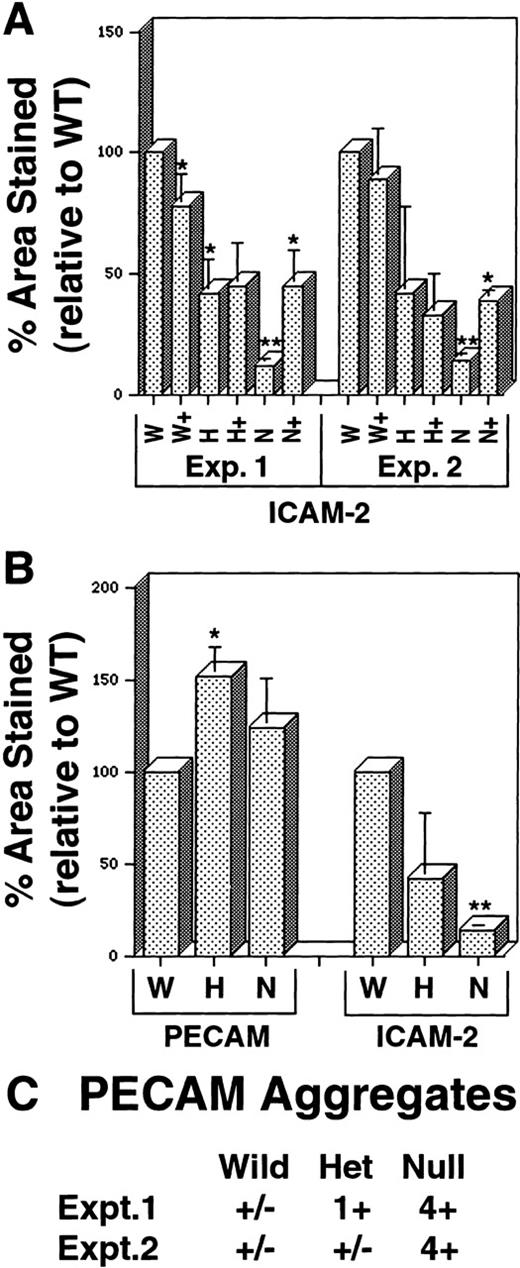
In vitro differentiation of wild-type (+/+, wt), hemizygous (+/−, het), and homozygous (−/−, null) VEGF mutant ES cells21 to day 8 revealed defective vascular development in both hemizygous and null VEGF mutant cultures (Figures1 and2). Cultures were analyzed by immunofluorescent antibody staining for expression of the vascular adhesion molecule ICAM-2 (Figures 1A-1C). ICAM-2 is a member of the immunoglobulin superfamily expressed on endothelium and on certain leukocyte subpopulations.41 In our hands ICAM-2 expression was detected only in patent vasculature (Figures 1G-1H′). Quantitative imaging of the ICAM-2 staining showed that hemizygous and null cultures had 50% and 10% of the vascular structures of wild-type cultures, respectively (Figure 2A). The vasculature that formed in the null mutant cultures was predominantly small and rounded, with a paucity of branch points (Figure 1C).
Immunofluorescent antibody staining with vascular markers in VEGF mutant ES cell cultures.
ES cells were differentiated, fixed on day 6 (G, G′), day 8 (A-F, I), or day 9 (H-H′), and stained with antibodies to ICAM-2 (A-C, G′, H′) or PECAM (D-G, H, I), followed by a PE-conjugated secondary antibody. (A, D, G, G′, H, H′),VEGF +/+ cultures; (B, E) VEGF +/− cultures; (C, F) VEGF −/−cultures; (I) −/−VEGF culture differentiated in the presence of 15 ng/mL recombinant hVEGF-165 from day 3 to day 8. The insets in D and F illustrate the differences in PECAM staining patterns, with arrowheads showing staining of borders between PECAM-expressing cells and arrows showing staining of borders between PECAM-expressing and nonexpressing cells. (G, G′) +/+ day 6 culture stained with PECAM (G) and ICAM-2 (G′) to show the lack of ICAM-2 staining before the development of patent vasculature. (H, H′) +/+ day 9 culture stained with PECAM (H) and ICAM-2 (H′) to show ICAM-2 staining of patent vasculature. Magnification, 50×.
Immunofluorescent antibody staining with vascular markers in VEGF mutant ES cell cultures.
ES cells were differentiated, fixed on day 6 (G, G′), day 8 (A-F, I), or day 9 (H-H′), and stained with antibodies to ICAM-2 (A-C, G′, H′) or PECAM (D-G, H, I), followed by a PE-conjugated secondary antibody. (A, D, G, G′, H, H′),VEGF +/+ cultures; (B, E) VEGF +/− cultures; (C, F) VEGF −/−cultures; (I) −/−VEGF culture differentiated in the presence of 15 ng/mL recombinant hVEGF-165 from day 3 to day 8. The insets in D and F illustrate the differences in PECAM staining patterns, with arrowheads showing staining of borders between PECAM-expressing cells and arrows showing staining of borders between PECAM-expressing and nonexpressing cells. (G, G′) +/+ day 6 culture stained with PECAM (G) and ICAM-2 (G′) to show the lack of ICAM-2 staining before the development of patent vasculature. (H, H′) +/+ day 9 culture stained with PECAM (H) and ICAM-2 (H′) to show ICAM-2 staining of patent vasculature. Magnification, 50×.
Quantitative imaging analysis of VEGF mutant ES cell cultures.
Day 8 ES cells were processed as shown in Figure 1, and imaging analysis was performed as described in “Materials and methods.” (A) Percentage area stained with antibody to ICAM-2 in 2 separate experiments. W, wild-type (+/+) VEGF cultures; H, hemizygous (+/−) VEGF cultures; N, null (−/−) VEGF cultures. In all cases, (+) after the letter denotes the same genotype ES cells incubated with 15 ng/mL recombinant hVEGF-165 from days 3 to 8. (B) Percentage area stained with antibody to PECAM or ICAM-2 in duplicate wells of the same experiment. Abbreviations are as for A. In all cases, statistical analysis was performed comparing H and N figures to the W figure in the same experiment, and each (+) figure was compared to the nonrescued figure for the same genotype. *P < .01; **P < .001. (C) Semiquantitative assessment of PECAM+ aggregates (examples in Figures 1F, 1G) was conducted by visual inspection of 2 to 4 wells of each genotype in separate experiments. The relative abundance of the aggregate staining was estimated as follows: +/−, trace; 1+, 2% to 5%; 2+, 5% to 10%; 3+, 10% to 30%, 4+, more than 30%.
Quantitative imaging analysis of VEGF mutant ES cell cultures.
Day 8 ES cells were processed as shown in Figure 1, and imaging analysis was performed as described in “Materials and methods.” (A) Percentage area stained with antibody to ICAM-2 in 2 separate experiments. W, wild-type (+/+) VEGF cultures; H, hemizygous (+/−) VEGF cultures; N, null (−/−) VEGF cultures. In all cases, (+) after the letter denotes the same genotype ES cells incubated with 15 ng/mL recombinant hVEGF-165 from days 3 to 8. (B) Percentage area stained with antibody to PECAM or ICAM-2 in duplicate wells of the same experiment. Abbreviations are as for A. In all cases, statistical analysis was performed comparing H and N figures to the W figure in the same experiment, and each (+) figure was compared to the nonrescued figure for the same genotype. *P < .01; **P < .001. (C) Semiquantitative assessment of PECAM+ aggregates (examples in Figures 1F, 1G) was conducted by visual inspection of 2 to 4 wells of each genotype in separate experiments. The relative abundance of the aggregate staining was estimated as follows: +/−, trace; 1+, 2% to 5%; 2+, 5% to 10%; 3+, 10% to 30%, 4+, more than 30%.
The staining pattern of another vascular cell adhesion molecule, PECAM, indicated a similar reduction in the amount of patent vasculature in the mutant cultures (Figures 1D-1F). However, a second population of PECAM+ cells was identified that formed small clumps that were not patent vasculature (Figure 1F). These clumps of PECAM+ cells were rare in wild-type cultures, more prevalent in hemizygous cultures, and fairly abundant in null cultures (Figure 2C). In the cell clumps at least part of each cell border was free of PECAM antibody staining, whereas in the patent vasculature the PECAM staining pattern was continuous for each endothelial cell (Figure 1, insets to D and F). Moreover, the PECAM+ cells in the clumps appeared more rounded than the endothelial cells incorporated into vascular structures. The staining pattern seen in the clumps closely resembled the pattern of PECAM staining seen at early times during ES cell differentiation before the development of vascular structures (Figure 1G and 12). Quantitative imaging of wells stained with PECAM showed that the PECAM-stained area was increased in hemizygous and null cultures compared with wild-type cultures, though only the increase in hemizygous cultures was statistically significant (Figure 2B). This contrasted with the levels of ICAM-2 staining and was consistent with the observation that increased numbers of PECAM+ cells were found in clumps as the number of PECAM+ and ICAM-2+ vascular structures decreased.
The addition of exogenous recombinant VEGF (human 165 isoform) from day 3 to day 8 of differentiation enabled the rescue of vascular development in the null mutant cultures (Figures 1I and 2A). ICAM-2 staining of rescued null mutant cultures hemizygous showed amounts of patent vasculature similar to those of the hemizygous cultures. However, in no case were mutant cultures rescued to wild-type levels of vasculature (Figure 2A). Moreover, the exogenous VEGF had little effect on the hemizygous cultures and no significant effect on the wild-type cultures. Optimal rescue was seen at 15 ng/mL, with lower concentrations giving less rescue and higher concentrations showing no significant differences from 15 ng/mL effects (data not shown). These findings showed that the soluble human VEGF 165 isoform could rescue vascular development in the cultures, but they suggested that additional components were required to bring vascular development to wild-type levels.
RNA analysis shows effects of the VEGF mutation
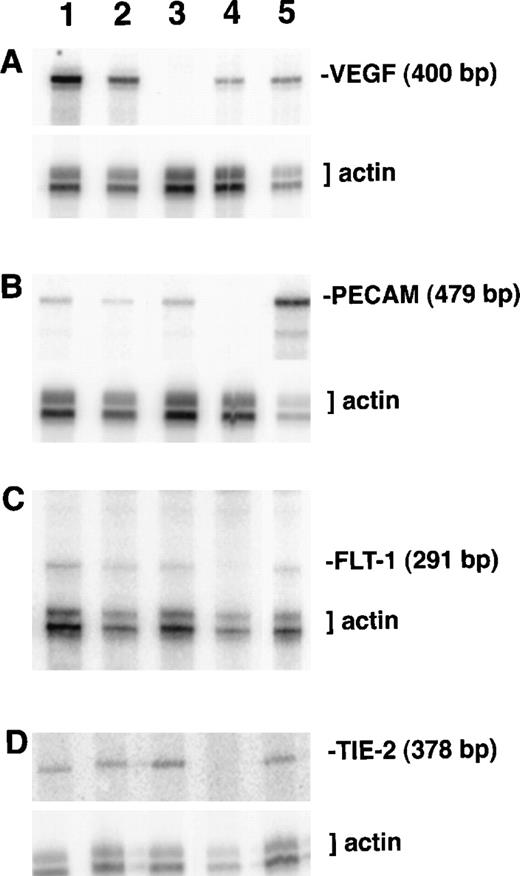
To further characterize the effects of the lack of VEGF signaling during ES cell differentiation, RNase protection analysis was performed on day 8 cultures (Figures 3,4). For each reaction an antisense probe to β-actin was included and was used to normalize the radioactivity in the protected bands. The levels of VEGF RNA showed the expected relationships among the cultures (Figures 3A, 4),21 and they mirrored the relative amounts of vasculature defined by ICAM-2 staining (Figure 2). The targeted VEGF locus produces a small amount of truncated transcript that does not encode a functional protein,21 and Northern blot analysis showed only the truncated transcript in RNA from the null VEGF mutant cultures (data not shown). The absence of functional VEGF in the null mutant cultures indicated that the residual vascular development must have resulted from some other mechanism.
RNA protection analysis of VEGF mutant ES cell cultures.
Total RNA from cultures on day 8 of differentiation was hybridized with the indicated 32P-labeled probes and a β-actin probe as described in “Materials and methods.” After separation on a polyacrylamide–urea gel, protected fragments were visualized and quantitated using a PhosphorImager. Lane 1, VEGF (+/+) ES culture RNA; lane 2, VEGF (+/−) ES culture RNA; lane 3, VEGF (−/−) ES culture RNA; lane 4, NIH 3T3 fibroblast cell line RNA; lane 5, Py-4-1 endothelial cell line RNA. (A) Protection with antisense VEGF probe. (B) Protection with antisense PECAM probe. (C) Protection with antisense flt-1 probe. (D) Protection with antisense tie-2/tek probe.
RNA protection analysis of VEGF mutant ES cell cultures.
Total RNA from cultures on day 8 of differentiation was hybridized with the indicated 32P-labeled probes and a β-actin probe as described in “Materials and methods.” After separation on a polyacrylamide–urea gel, protected fragments were visualized and quantitated using a PhosphorImager. Lane 1, VEGF (+/+) ES culture RNA; lane 2, VEGF (+/−) ES culture RNA; lane 3, VEGF (−/−) ES culture RNA; lane 4, NIH 3T3 fibroblast cell line RNA; lane 5, Py-4-1 endothelial cell line RNA. (A) Protection with antisense VEGF probe. (B) Protection with antisense PECAM probe. (C) Protection with antisense flt-1 probe. (D) Protection with antisense tie-2/tek probe.
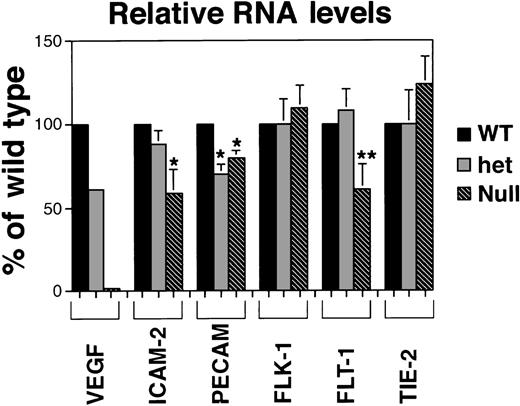
Quantitative RNA analysis of VEGF mutant ES cell cultures.
PhosphorImager data from the gels in Figure 3 were manipulated to subtract background and to normalize to the β-actin protected signal in the same lane. Relative band densities for each probe in het (+/−) and null (−/−) lanes were then compared to (+/+) wild-type levels for that probe. Each result, except for the VEGF analysis, is a compilation of at least 3 experiments on at least 2 (and sometimes 3) different sets of RNA prepared on different days and at different passage numbers. In all cases, statistical analyses compared VEGF het and null RNA levels to the VEGF wild-type RNA levels for that probe. *P < .05; **P < .000 0001.
Quantitative RNA analysis of VEGF mutant ES cell cultures.
PhosphorImager data from the gels in Figure 3 were manipulated to subtract background and to normalize to the β-actin protected signal in the same lane. Relative band densities for each probe in het (+/−) and null (−/−) lanes were then compared to (+/+) wild-type levels for that probe. Each result, except for the VEGF analysis, is a compilation of at least 3 experiments on at least 2 (and sometimes 3) different sets of RNA prepared on different days and at different passage numbers. In all cases, statistical analyses compared VEGF het and null RNA levels to the VEGF wild-type RNA levels for that probe. *P < .05; **P < .000 0001.
RNA levels of the endothelial adhesion receptors ICAM-2 and PECAM also showed the expected trends, though several differences between relative RNA levels and quantitative imaging comparisons were noted (Figures 2B, 4). ICAM-2 RNA levels were not significantly lower in hemizygous than in wild-type cultures, but ICAM-2 protein imaging analysis showed a 50% reduction in stained areas of the hemizygous cultures compared with wild-type cultures (Figure 2B). Similarly, the null cultures showed a significant decrease in ICAM-2 RNA levels but only to 50% of the wild-type and hemizygous levels, suggesting that ICAM-2 RNA may accumulate in the absence of translation to protein. Levels of PECAM RNA decreased to 75% of wild-type levels in hemizygous cultures but were not further decreased in null mutant cultures. Overall the trends for each adhesion receptor were similar between imaging and RNA analyses, and they were consistent with the presence of nonvessel PECAM+ cells in the mutant cultures.
RNA levels for the high-affinity VEGF receptors showed different trends in response to the lack of VEGF signaling (Figures 3, 4). Levels of flk-1 RNA did not vary significantly among the different cultures, but the amount of flt-1 RNA in the null mutant VEGF cultures decreased to half the levels seen in the wild-type and hemizygous cultures. These data show that RNA for the 2 receptors accumulated differently in the absence of VEGF signaling. A third endothelial tyrosine kinase receptor not involved in VEGF signaling, tie-2/tek, did not show significant changes in RNA levels among the different cultures.
Characterization of PECAM+ cells not incorporated into vasculature
Double-label immunofluorescent antibody staining was performed on day 8 VEGF mutant cultures to further characterize the PECAM+ cells found in clumps. As expected from the initial staining, double labeling with ICAM-2 and PECAM showed that the PECAM+ cell clumps did not express detectable ICAM-2 (Figure 5). Moreover, the PECAM+, ICAM-2− clumps were more prevalent in hemizygous cultures than in wild-type cultures and more prevalent still in null VEGF mutant cultures. This staining pattern confirmed that only vascular endothelial cells incorporated into patent vasculature expressed detectable ICAM-2. A second marker of early vasculature, CD34, was also not detectable in the PECAM+ clumps (Figure6). CD34 staining, in fact, mirrored ICAM-2 staining, and only vascular endothelial cells that were incorporated into patent vasculature expressed both CD34 and ICAM-2. However, some CD34+ cells were found outside the vasculature and did not express either PECAM or ICAM-2 (Figure 6). In contrast, ICAM-2 staining was confined to the patent vasculature and possibly to hematopoietic cells within the blood islands.
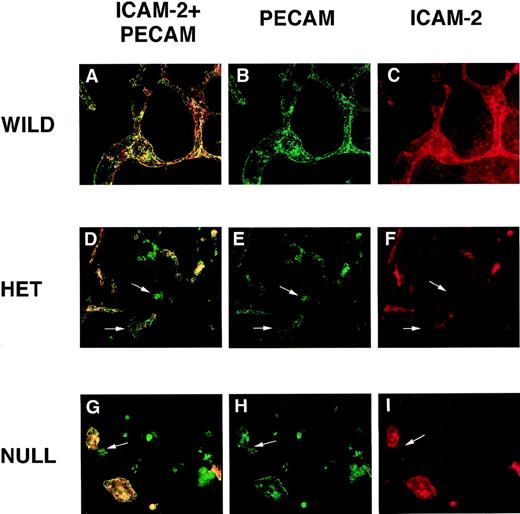
PECAM/ICAM-2 double-label immunofluorescent antibody staining of VEGF mutant ES cultures.
Day 8 cultures were fixed and stained with rat antimouse ICAM-2 (red) and rabbit polyclonal antimouse PECAM (green) antibodies, along with the appropriate secondary antibodies. Images were photographed with the appropriate FITC (B, E, H) or rhodamine (C, F, I) filters or with a double exposure using both filters sequentially (A, D, G). (A-C) Wild-type (+/+) VEGF ES cultures. (D-F) Hemizygous (+/−) VEGF mutant ES cell cultures. (G-I) Null (−/−) VEGF mutant ES cultures. The arrows in (D-I) point to clumps of cells that stain with the PECAM antibody but not with the ICAM-2 antibody. Magnification, 50×.
PECAM/ICAM-2 double-label immunofluorescent antibody staining of VEGF mutant ES cultures.
Day 8 cultures were fixed and stained with rat antimouse ICAM-2 (red) and rabbit polyclonal antimouse PECAM (green) antibodies, along with the appropriate secondary antibodies. Images were photographed with the appropriate FITC (B, E, H) or rhodamine (C, F, I) filters or with a double exposure using both filters sequentially (A, D, G). (A-C) Wild-type (+/+) VEGF ES cultures. (D-F) Hemizygous (+/−) VEGF mutant ES cell cultures. (G-I) Null (−/−) VEGF mutant ES cultures. The arrows in (D-I) point to clumps of cells that stain with the PECAM antibody but not with the ICAM-2 antibody. Magnification, 50×.
PECAM/CD34 double-label immunofluorescent antibody staining of hemizygous VEGF mutant ES cultures.
Day 8 VEGF hemizygous (+/−) ES cultures were fixed and stained with rat antimouse CD34 (red) and rabbit polyclonal antimouse PECAM (green) antibodies, along with the appropriate secondary antibodies. Images were photographed with the appropriate FITC (B) or rhodamine (C) filters or with a double exposure using both filters sequentially (A). The arrow in each frame points to a clump of cells that stained with the PECAM antibody but not with the CD34 antibody. The arrowheads in each frame point to several individual cells that stained with the CD34 antibody but not with the PECAM antibody. Magnification, 50×.
PECAM/CD34 double-label immunofluorescent antibody staining of hemizygous VEGF mutant ES cultures.
Day 8 VEGF hemizygous (+/−) ES cultures were fixed and stained with rat antimouse CD34 (red) and rabbit polyclonal antimouse PECAM (green) antibodies, along with the appropriate secondary antibodies. Images were photographed with the appropriate FITC (B) or rhodamine (C) filters or with a double exposure using both filters sequentially (A). The arrow in each frame points to a clump of cells that stained with the PECAM antibody but not with the CD34 antibody. The arrowheads in each frame point to several individual cells that stained with the CD34 antibody but not with the PECAM antibody. Magnification, 50×.
Double-label immunofluorescent antibody staining with PECAM and SSEA-1 (Figure 7) showed that some of the PECAM+ cells in clumps also stained for the SSEA-1 marker. In contrast, none of the patent vasculature stained with SSEA-1. SSEA-1 reacts with a carbohydrate epitope that is expressed on undifferentiated and partially differentiated embryonic cells.42 43 Although many of the small PECAM+ clumps found in the wild-type cultures appeared to contain only double-positive cells (Figure 7A), the larger clumps in hemizygous and null cultures seemed to have both double-positive cells and cells that stained only for PECAM (Figures 7D, 7G).
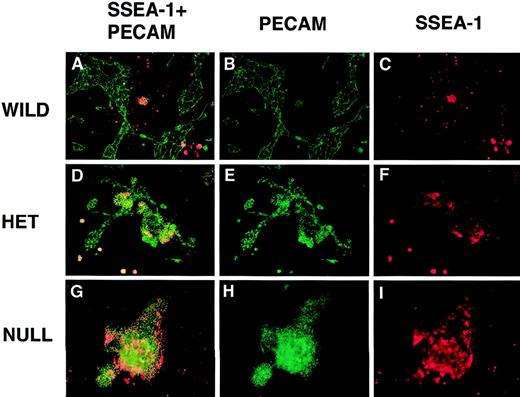
PECAM/SSEA-1 double-label immunofluorescent antibody staining of VEGF mutant ES cultures.
Day 8 cultures were fixed and stained with mouse antimouse SSEA-1 (red) and rabbit polyclonal antimouse PECAM (green) antibodies, along with the appropriate secondary antibodies. Images were photographed with the appropriate FITC (B, E, H) or rhodamine (C, F, I) filters or with a double exposure using both filters sequentially (A, D, G). (A-C) Wild-type (+/+) VEGF ES cultures. (D-F) Hemizygous (+/−) VEGF mutant ES cultures. (G-I) Null (−/−) VEGF mutant ES cultures. Magnification, 50×.
PECAM/SSEA-1 double-label immunofluorescent antibody staining of VEGF mutant ES cultures.
Day 8 cultures were fixed and stained with mouse antimouse SSEA-1 (red) and rabbit polyclonal antimouse PECAM (green) antibodies, along with the appropriate secondary antibodies. Images were photographed with the appropriate FITC (B, E, H) or rhodamine (C, F, I) filters or with a double exposure using both filters sequentially (A, D, G). (A-C) Wild-type (+/+) VEGF ES cultures. (D-F) Hemizygous (+/−) VEGF mutant ES cultures. (G-I) Null (−/−) VEGF mutant ES cultures. Magnification, 50×.
Expression of flk-1 in vascular endothelial growth factor mutant cultures
Because the amount of flk-1 RNA did not decrease in the mutant cultures, though the amount of vasculature was dramatically reduced, we examined the localization of flk-1 RNA-expressing cells by in situ hybridization analysis and compared the flk-1 pattern to the PECAM pattern (Figure 8). As expected, in wild-type cultures both probes were localized to patent vasculature (Figures 8A, 8B). In hemizygous cultures hybridized with the PECAM probe, patent vasculature and clumps of PECAM+ cells were seen (Figure 8C). The hemizygous cultures hybridized with the flk-1 probe showed patent vasculature, small clumps of cells, and isolated single cells that stained (Figure 8D). The null cultures showed different but partially overlapping patterns of expression for the 2 probes. The PECAM probe hybridized to numerous clumps of cells in the null cultures that resembled in size and morphology the PECAM+ cell clumps stained with the antibody (Figure 8E, 8G). The PECAM probe also hybridized to the small amount of patent vasculature present (data not shown). The flk-1 probe hybridized to areas of loosely aggregated mesenchymal-looking cells (Figure 8F and to the small amount of patent vasculature and clumps of cells that resembled the PECAM+ cells (Figure8H).
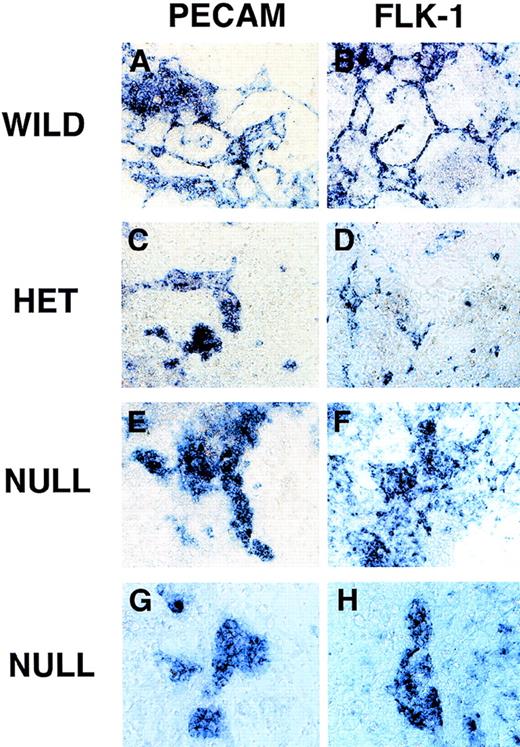
RNA in situ hybridization of VEGF mutant ES cultures.
Day 8 cultures were fixed and processed for in situ hybridization as described in “Materials and methods.” Cultures were hybridized with an antisense PECAM probe (A, C, E, G) or an antisense flk-1 probe (B, D, F, H). (A, B) Wild-type (+/+) VEGF ES cultures. (C, D) Hemizygous (+/−) VEGF mutant ES cultures. (E-H) Null (−/−) VEGF mutant ES cultures. Magnification, (A-F) 50×, (G-H) 100×.
RNA in situ hybridization of VEGF mutant ES cultures.
Day 8 cultures were fixed and processed for in situ hybridization as described in “Materials and methods.” Cultures were hybridized with an antisense PECAM probe (A, C, E, G) or an antisense flk-1 probe (B, D, F, H). (A, B) Wild-type (+/+) VEGF ES cultures. (C, D) Hemizygous (+/−) VEGF mutant ES cultures. (E-H) Null (−/−) VEGF mutant ES cultures. Magnification, (A-F) 50×, (G-H) 100×.
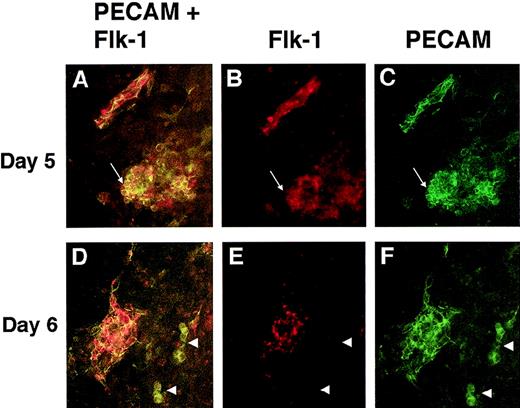
To determine whether mutant cells expressing both PECAM and flk-1 were present in the VEGF null cultures, double-label immunofluorescence with PECAM and flk-1 antibodies was performed (Figure 9). VEGF null cultures at day 5 and day 6 of differentiation had cells that stained for both PECAM and flk-1 and cells that stained only for PECAM. Some of the double-positive cells were incorporated into patent vasculature, and some of the double-positive cells were found in the PECAM+ clumps of cells (Figure 9A-9C). However, not all cells of the PECAM+ clumps had detectable flk-1 staining, and some small clumps of PECAM+ cells had no detectable flk-1 staining (Figures 9D-9F). Similar staining patterns were seen on days 7 and 8, but the intensity of the flk-1 antibody signal was significantly reduced (data not shown). Interestingly, few cells had detectable flk-1 antibody staining but no detectable PECAM antibody staining (data not shown), suggesting that flk-1 RNA may be expressed in the mutant cells in the absence of detectable levels of flk-1 protein.
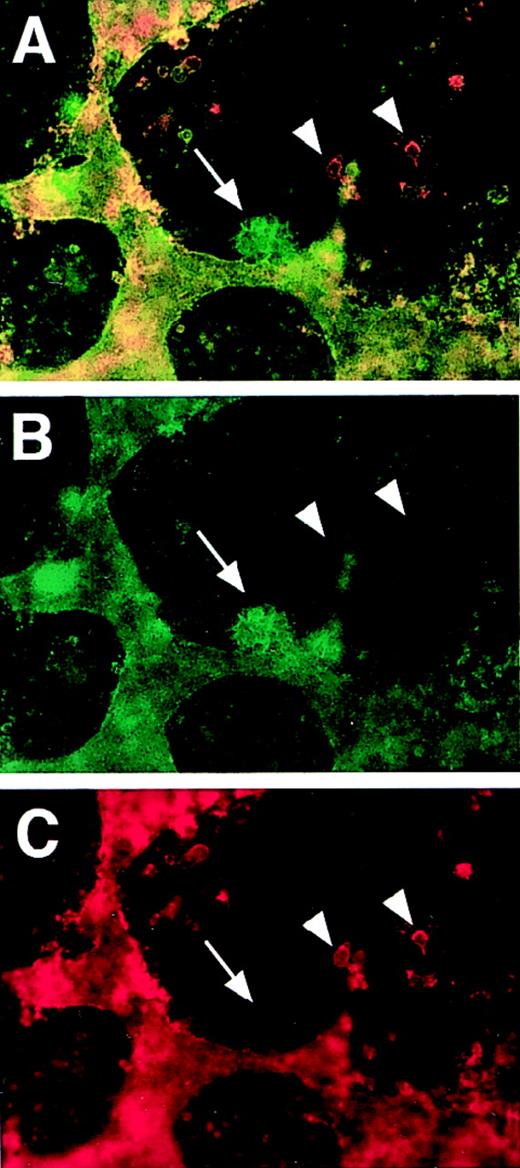
PECAM/flk-1 double-label immunofluorescent antibody staining of VEGF mutant ES cultures.
Day 5 (A-C) or day 6 (D-F) null (−/−) VEGF mutant cultures were fixed and stained with rat antimouse PECAM (green) and rabbit antimouse flk-1 (red) antibodies, along with the appropriate secondary antibodies. Images were photographed with the appropriate FITC (C, F) or rhodamine (B, E) filters or with a double exposure using both filters sequentially (A, D). The arrows in A to C point to a clump of PECAM+ cells that partially stained for flk-1. The structure in the upper left is patent vasculature. Arrowheads in D to F point to clumps of PECAM+ cells that had no detectable staining for flk-1. The structure to the left is patent vasculature. Magnification, 50×.
PECAM/flk-1 double-label immunofluorescent antibody staining of VEGF mutant ES cultures.
Day 5 (A-C) or day 6 (D-F) null (−/−) VEGF mutant cultures were fixed and stained with rat antimouse PECAM (green) and rabbit antimouse flk-1 (red) antibodies, along with the appropriate secondary antibodies. Images were photographed with the appropriate FITC (C, F) or rhodamine (B, E) filters or with a double exposure using both filters sequentially (A, D). The arrows in A to C point to a clump of PECAM+ cells that partially stained for flk-1. The structure in the upper left is patent vasculature. Arrowheads in D to F point to clumps of PECAM+ cells that had no detectable staining for flk-1. The structure to the left is patent vasculature. Magnification, 50×.
Discussion
The role of the VEGF signaling pathway in vascular development was examined by differentiating and analyzing ES cells with a targeted mutation at the VEGF locus. Our results clarify that VEGF signaling is crucial to vascular development, and they provide quantitative information about the effects of both the hemizygous and homozygous VEGF mutation on vasculogenesis. Most important, a population of precursor cells that exhibit a unique marker pattern accumulates in the mutant cultures. The reciprocal relationship between the amount of patent vasculature and the precursor cells suggests that they are blocked from further development by the absence of VEGF signaling during ES cell differentiation. This is the most specific understanding to date of the first point at which VEGF signaling is critical to embryonic blood vessel formation.
Our quantitation of the effects of the VEGF mutation showed that vascular development was reduced by approximately 50% in the hemizygous VEGF mutant cultures and that VEGF RNA levels were also reduced by approximately 50% in these cultures. Previous analysis of the requirement for VEGF in vivo revealed that the loss of 1 copy of VEGF produced embryonic lethality, suggesting a tight dose requirement for VEGF signaling during vascular development.21,22Although the null mutant VEGF cultures did not produce functional VEGF protein, a low but reproducible level of vascular development did occur, as defined by the presence of groups of cells with contiguous cell border expression of PECAM and ICAM-2. This primitive blood vessel formation occurred at levels reduced by approximately 10 times those in wild-type cultures. This finding suggests that blood vessel formation is not completely dependent on signaling mediated by VEGF (VEGF-A), and it is consistent with the presence of some vasculature in the VEGF null mutant embryos.21 The recent identification of several VEGF-related molecules indicates that some redundancy may exist in the VEGF signaling required for vascular development. For example, both VEGF-C and VEGF-D can signal through the flk-1 (VEGFR-2) receptor, whereas PIGF and VEGF-B can signal through the flt-1 (VEGFR-1) receptor. Moreover, a third receptor, called flt-4 (VEGFR-3), can signal through VEGF-C and VEGF-D,25 and a targeted mutation in flt-4 is a homozygous embryonic lethal with vascular defects.44 In fact, in light of the plethora of VEGF-related molecules and receptors, it seems surprising that the VEGF mutation has such a profound effect on embryonic vascular development because our study clearly showed that 90% of vascular development during ES cell differentiation required VEGF-dependent signaling.
VEGF signaling is also implicated in the expansion and branching of endothelial cells that occur after primary differentiation events, and our results indicate that VEGF is important for these events during ES cell differentiation. The vessels that formed in the VEGF mutant cultures were small and rounded, and they had fewer branch points than wild-type cultures when normalized to the amount of vasculature (Scalia A, Bautch VL, unpublished results). Vascular defects were partially rescued by addition of exogenous VEGF-165, which produced more vasculature with more branch points. Thus this isoform is likely to make major contributions to VEGF signaling in vivo, consistent with in vitro studies showing that VEGF-165 has strong mitogenic and chemotactic activities. Our inability to rescue vascular development completely with exogenous VEGF-165 indicates that either the local concentration or presentation of exogenous VEGF did not reach levels sufficient for complete rescue or that other isoforms also contribute significantly to VEGF signaling in vivo.
Quantitative RNA analysis showed that the 2 high-affinity VEGF receptors, flk-1 and flt-1, responded differently to the absence of ligand. The flk-1 levels did not differ significantly among the genotypes, and the lack of patent vasculature in the mutant cultures suggested that flk-1 RNA expressing precursor cells accumulated in the absence of VEGF signaling. This was confirmed by in situ hybridization analysis that showed strong flk-1 RNA expression from dispersed cells and clumps of cells. Thus flk-1 RNA expression is activated in the absence of VEGF, but the flk-1 expressing cells do not efficiently mature to endothelial cells that form patent vasculature. Interestingly, the number of cells expressing detectable flk-1 protein were reduced in the VEGF null mutant cultures compared with the flk-1 RNA expression pattern, suggesting that in the absence of VEGF the receptor protein is either not translated at high levels or is not stable. In contrast, flt-1 RNA levels were maintained in hemizygous VEGF mutant cultures but decreased significantly in null VEGF mutant cultures. The VEGF mutant cultures may express flt-1 RNA at reduced levels because VEGF signaling is required to regulate expression of the flt-1 receptor.45
The trends seen in the relative RNA levels of the 2 vascular adhesion receptors, ICAM-2 and PECAM, in general followed the patterns seen with the quantitative imaging of the staining of each protein. In our hands the ICAM-2 antibody reacted only with endothelial cells incorporated into patent vasculature, and ICAM-2 RNA levels and antibody-stained areas decreased with reduced VEGF production. In contrast, PECAM antibody stained clumps of unincorporated cells and patent vasculature, and both the quantitative imaging and the relative RNA levels reflect this expanded PECAM expression profile relative to ICAM-2. These results show that the quantitative change in PECAM expression levels was minimal, yet the corresponding immunofluorescent antibody staining patterns show a dramatic difference in the type of cells stained in the mutant series.
The PECAM+ cells that accumulated in the VEGF mutant cultures may be precursor cells blocked from further differentiation by the lack of VEGF signaling. Several lines of evidence support this hypothesis. First, there is a rough reciprocal relationship between the amount of patent vasculature and the amount of PECAM staining outside vessels. Wild-type cultures have only rare clumps of PECAM+ cells, hemizygous cultures have fewer patent vessels and more PECAM+ clumps, and null cultures have only rare small vessels and numerous clumps of PECAM+ cells. Second, similar clumps of PECAM+ cells are prevalent at early times of in vitro differentiation, before the development of patent vasculature.12 Third, clumps of mesodermal cells that aggregate in the yolk sac just before blood island formation express PECAM and resemble in morphology the PECAM+ cell clumps seen during ES cell differentiation.10 12 Finally, rescue of the VEGF null cultures by exogenous VEGF produces more patent vasculature and correspondingly fewer clumps of PECAM+ cells, suggesting that VEGF can promote the maturation of the PECAM+ cell clumps into patent vasculature.
Several markers distinguish the PECAM+ cells in clumps from those in vessels. ICAM-2 and CD34 are both expressed by endothelial cells incorporated into patent vasculature but not by PECAM+ cell clumps. This marker profile also distinguishes the PECAM+ clumps in normal ES cell cultures that form early in differentiation from the patent vasculature that forms later.12 CD34 is expressed on early vessels and hematopoietic cells in mouse development,46 but it has not been associated with immature endothelial cells; in a recent study, it was also associated with more mature ES cell-derived endothelial cells.7 This study also identified VE-cadherin as an early marker of the endothelial lineage, so it will be informative to determine the expression of VE-cadherin relative to PECAM in the VEGF mutant cultures. The developmental expression of ICAM-2 is less well documented. ICAM-2 RNA is detected in a vascular and endocardial pattern during mouse embryogenesis, but ICAM-2 RNA expression is not associated with early yolk sac mesodermal condensations (Bautch VL, unpublished results).
In contrast, SSEA-1 is expressed by a subset of the PECAM+ cells in clumps but not by endothelial cells incorporated into vessels. SSEA-1 recognizes a carbohydrate epitope that is expressed by several embryonic lineages, so it is less useful as a lineage marker than as a way to distinguish PECAM+ cell clumps from PECAM+ vasculature. Flk-1 protein is also detectable on a subset of PECAM+ cells in clumps, which is consistent with a model in which PECAM+ cells in clumps respond to VEGF signaling through the flk-1 receptor to mature into endothelial cells. Flk-1 is also expressed on patent vasculature early during ES cell differentiation. Our results are consistent with dual roles for VEGF/flk-1 receptor signaling in endothelial cell differentiation and in expansion and branching of endothelial cells. A recent study analyzed flk-1 null mutant ES cell differentiation and suggested that flk-1 signaling was not required for endothelial cell differentiation.36 However, in our hands, ES cells carrying a homozygous null mutation for flk-1 differentiated similarly to the VEGF null mutant ES cells, with an accumulation of PECAM+ cell clumps and residual patent vasculature that was positive for PECAM and ICAM-2 (Lau DC, Bautch VL, unpublished results). Thus our data support a model that has a primary requirement for VEGF/flk-1 receptor signaling in endothelial cell differentiation that is not absolute and a requirement for the same signaling pathway in later stages of vascular development, such as endothelial cell expansion and branching.
Acknowledgments
We thank Dan Dumont and Kevin Peters for the probes and Beat Imhof for the polyclonal PECAM antiserum. We thank Susan Whitfield and David Miller for artwork. We thank fellow laboratory members for fruitful discussions. The MC-480 hybridoma was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine (Baltimore, MD) and the Department of Biological Sciences, University of Iowa, (Iowa City, IA) under contract N01-HD-6-2915 from the NICHD.
Supported by National Institutes of Health grant HL43174 to V.L.B. and by grants from the European Community (Biomed BMH4-CT98-3380) and Actie Levenslijn (#7.0019.98) to P.C. V.L.B. was supported by a National Institutes of Health RCDA Award, and S.D.R. was supported by a National Institutes of Health NSRA Award.
Reprints:Victoria L. Bautch, Department of Biology, University of North Carolina at Chapel Hill, CB#3280, Chapel Hill, NC 27599; e-mail: bautch@med.unc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal