Abstract
The yolk sac and aorto-gonad-mesonephros region are well recognized as the principal sites of hematopoiesis in the developing embryo, and the liver is the principal site of hematopoiesis in the fetus. However, little is known about circulating hematopoietic stem and progenitor cells in early fetal life. We investigated the number and characteristics of circulating progenitors in first trimester blood of 64 human fetuses (median gestational age, 10+4 weeks; range, 7+6-13+6 weeks). CD34+ cells accounted for 5.1 ± 1.0% of CD45+ cells in first trimester blood, which is significantly more than in term cord blood (0.4 ± 0.03%;P = .0015). However, the concentration of CD34+ cells (6.6 ± 2.4 × 104/mL) was similar to that in term cord blood (5.6 ± 3.9 × 104/mL). The total number of progenitors cultured from unsorted mononuclear cells (MNCs) in first trimester blood was 19.2 ± 2.1 × 103/mL, which is similar to that in term cord blood (26.4 ± 5.6 × 103/mL). All lineages were seen: colony-forming unit–GEMM (CFU-GEMM), CFU-GM, BFU-e, BFU-MK, and CFU-MK. Clonogenic assays of CD34+ cells purified from first trimester samples produced mainly two lineages: BFU-e (39.0 ± 9.6 × 103/mL CD34+ cells) and CFU-GEMM (22.6 ± 4.7 × 103/mL CD34+ cells). Short-term liquid culture of first trimester blood MNCs in SCF + IL-3 + Flt-3 (stem cell factor + interleukin-3 + Flt-3) increased, by 7-fold, the numbers of CFU-GEMM and induced a dramatic increase in BFU-e (65.6 ± 12.1–fold). These data show that significant numbers of committed and multipotent progenitors with capacity for expansion circulate in first trimester fetal blood and can be CD34 selected. These cells should be suitable targets for gene transfer and stem cell transplantation and, because fetal hematopoietic progenitors have been demonstrated in the maternal circulation from early gestation, may also be manipulated for noninvasive prenatal diagnosis of major genetic disorders.
Hematopoietic stem and progenitor cells reside in diverse anatomical locations in developing mammals including the yolk sac1 and para-aortic region within the embryo2,3,4 and the liver, spleen, and bone marrow of the fetus.5,6,7 The site of origin of definitive hematopoietic stem cells in the developing fetus remains controversial. Evidence from some studies indicates that hematopoietic stem cells from the yolk sac are responsible for transient primitive hematopoiesis, but they appear to lack the ability to reconstitute the hematopoietic system in adult animals.3,8 Instead, stem cells derived from an intraembryonic site, the aorta-gonad-mesonephros (AGM) region, have been shown, both in mice and man,4 to be responsible for definitive hematopoiesis2,3,9 by first colonizing the fetal liver and later the bone marrow.10 On the other hand, evidence from other studies suggests that hematopoietic stem cells with the capacity to contribute to definitive hematopoiesis are present both in the yolk sac and in the para-aortic splanchnopleura of murine embryos prior to fetal liver colonization.11 12
In vitro studies5,13 and in vivo retroviral-mediated transduction experiments in animal models14 indicate that these changes in the site of hematopoiesis occur through migration of multipotent hematopoietic stem and/or progenitor cells via the fetal circulation. In support of this, numerous studies have demonstrated that both early second trimester15-17 and preterm fetal blood18 19 have greater frequencies of hematopoietic progenitors than term cord blood and that this frequency declines with advancing gestational age. However, because of the difficulty in obtaining blood samples early in gestation, data are lacking on the frequency and characteristics of circulating progenitor cells in first trimester human fetal blood. These data should provide insights into developmental hematopoiesis, which may prove useful both for developing autologous in utero gene therapy protocols using fetal hematopoietic stem and/or progenitor cells and for early prenatal diagnosis of genetic abnormalities by expanding fetal hematopoietic stem and/or progenitor cells present in maternal blood.
The purpose of this study was to measure the frequency of hematopoietic stem and progenitor cells in first trimester fetal blood and to determine whether such cells could be CD34 selected and amplified in vitro.
Materials and methods
Fetal blood sample collection
Blood samples were obtained from 109 human fetuses between the gestational ages of 7+6 and 40+2 postmenstrual weeks. First trimester samples (n = 64) were collected by ultrasound-guided percutaneous intracardiac blood sampling at the time of clinically indicated surgical termination of pregnancy. In the second trimester an additional 1- to 2-mL aliquot was collected in ongoing pregnancies at the time of clinically indicated ultrasound-guided blood sampling from the umbilical vein, either at its intrahepatic portion or at the placental cord insertion. The indications were suspected chromosomal abnormalities for which rapid karyotyping was performed. All samples were negative for the conditions for which they were tested. Samples from gestational ages of 38+0-40+2 weeks (n = 30) were obtained at delivery from the cord umbilical vein in uncomplicated term pregnancies. All babies were healthy, with birth weights appropriate for gestational age, and there was no evidence of birth asphyxia (cord, pH less than 7.1) or congenital abnormalities. All samples were collected in heparinized tubes, and care was taken to exclude amniotic fluid contamination; maternal contamination was excluded by Kleihauer testing. Blood collection for research purposes was approved by the Research Ethics Committee of Hammersmith and Queen Charlotte's Hospitals, and we complied with national guidelines (Polkinghorne) regarding the use of fetal tissues for research purposes. All pregnant women gave written informed consent.
CD34+ cell enumeration by flow cytometry
First trimester fetal blood (median gestational age, 9+5weeks; range, 9+0-13+0 weeks; n = 8) and term cord blood samples (median gestational age, 39+6 weeks; range, 38+2-40+2 weeks; n = 6) were simultaneously stained with 20 μL/106 nucleated cells of fluorescein isothiocyanate–conjugated (FITC-conjugated) anti-CD45 monoclonal antibodies (mAbs) and 20 μL/106 nucleated cells of phycoerythrin–conjugated (PE-conjugated) anti-CD34 mAbs (Becton Dickinson, Oxford, England). Following red blood cell lysis with fluorescence-activated cell sorter (FACS) lysis buffer (Becton Dickinson), multiparameter flow cytometry was performed (FACScan, Coulter Electronics, Miami, FL). The analysis was completed using the cell gating guidelines recommended by the International Society of Hematotherapy and Graft Engineering (ISHAGE) as previously described.20 Briefly, the cells were first gated into region 1 (R1) so that the CD45+ cells could include all nucleated white blood cells and exclude red blood cells, nucleated red blood cells, platelets, and cellular debris. CD45+ events in R1 were then analyzed for CD34 staining, and positive events were gated into region 2 (R2). Events defined by R1 and R2 were analyzed on a dot-plot describing light scatter versus CD45 staining and on a second dot-plot describing the light-scattering characteristics of the cells. When the CD34+ fraction is analyzed for CD45 expression versus side scatter, true CD34+ blast cells form a discrete cluster that exhibits low-density CD45 expression and low side scatter characteristics. The number of CD34+ cells meeting all fluorescence and light scatter criteria was derived from the number of events representing specific staining, as determined by CD45-FITC/CD34-PE staining, less the number of events representing nonspecific staining, as determined by the CD45-FITC/isotype-PE control. For the calculation of absolute CD34+ cells, the corrected number of CD34+ cells was divided by the average total number of CD45+ events from the CD45 FITC/CD34-PE staining; this value was multiplied by the absolute leukocyte count. A minimum number of 100 CD34+ events and 75 000 CD45+ events (for term cord blood samples) was collected for the CD34+ cell quantitation by flow cytometry.
Flow cytometry analysis of CD34+ subpopulations
First trimester whole fetal blood samples (median gestational age, 10+0 weeks; range, 9+2-12+5 weeks; n = 5) were simultaneously stained with 20 μL/106nucleated cells of FITC-conjugated anti-CD34 and 20 μL/106 nucleated cells of either PE-conjugated anti-CD38 or anti–HLA-DR (an anti–human leukocyte antigen chain) mAbs (Becton Dickinson). Following red blood cell lysis with FACS lysis buffer (Becton Dickinson), multiparameter flow cytometric analysis was performed (FACScan, Coulter Electronics). For comparison, we also studied 3 second trimester fetal blood samples (median gestational age, 17+4 weeks) and 5 term cord blood samples (median gestational age, 38+6 weeks). To define the CD34+ subpopulations 150 000 events were acquired, and further analysis was performed after gating on the cells with both low side and forward scatter. Quadrants were defined using FITC- and PE-labeled isotype control mAbs. The CD34+ population was divided into (1) CD34+CD38bright or CD34+HLA-DRbrightsubpopulations containing those cells with high CD34 antigen expression and high anti-CD38 or anti–HLA-DR fluorescence and (2) CD34+CD38 or CD34+HLA-DR subpopulations containing those cells with high CD34 antigen and anti-CD38 or anti–HLA-DR fluorescence at less than one-half of the maximal PE-fluorescence of the isotype control.
CD34+ cell enrichment
First trimester (median gestational age, 12+0 weeks; range, 10+0-13+6 weeks; n = 7), second trimester (median gestational age, 22 + 0 weeks; range, 20+0-23+0 weeks; n = 3), and third trimester (median gestational age, 40+0 weeks; range, 38+0-40+3 weeks; n = 6) blood samples were enriched for CD34+ cells. Low-density MNCs were separated by density gradient centrifugation (d = 1077g/mL) (Ficoll-Hypaque, Sigma Chemical, St Louis, MO), and stained with hapten-conjugated anti-CD34 mAbs (QBEND/10 mouse immunoglobulin G1 [IgG1]). Antihapten antibodies attached to microbeads were passed twice through columns (MiniMACS columns, Miltenyi Biotech, Bisley, England) according to the manufacturer's instructions. The purity of CD34+ cells enriched from term cord blood samples was determined by flow cytometry after staining with FITC-conjugated anti-CD34 mAbs (HPCA-2, Becton Dickinson) and was found to be 91.6 ± 1.0%. Although the purity of the CD34+ population enriched from first and second trimester fetal blood could not be determined by flow cytometry due to the low number of CD34+ cells, 90% of the enriched cells had a blast-like appearance.
Colony assays
Colony assays were performed either on adherent cell-depleted nucleated cells or sorted CD34+ cells. First trimester fetal blood samples (median gestational age, 10+0 weeks; range, 7+6-13+1 weeks; n = 30) were first depleted of adherent cells by 1-hour incubation in Iscove's modified Dulbecco's medium (IMDM, Gibco Life Technologies, Grand Island, NY) with 10% fetal bovine serum (FBS) (StemCell Technologies, Vancouver, BC, Canada) at 37°C. Second trimester fetal blood samples (median gestational age, 15+0 weeks; range, 14+5-20+0 weeks; n = 5) were first incubated for red blood cell lysis using ammonium chloride (StemCell Technologies) on ice and then depleted of adherent cells. Cord blood from term neonates (median gestational age, 39+1 weeks; range, 38+0-40+0 weeks; n = 8) was enriched for low-density MNCs by density centrifugation and then depleted of adherent cells.
For CFU-GEMM, BFU-e, and CFU-GM assays, adherent cell–depleted nucleated cells were resuspended either at 3 × 105/mL (first trimester samples) or 3 × 104/mL (second trimester and term cord blood samples) in 0.9% methylcellulose with IMDM supplemented with 30% FBS, 1% bovine serum albumin (BSA), 10−42-mercaptoethanol, 50 ng/mL human recombinant SCF (rhSCF), 10 ng/mL human recombinant granulocyte-macrophage colony-stimulating factor (rhGM-CSF), 10 ng/mL rhIL-3 (StemCell Technologies), and 3 units/mL human recombinant erythropoietin (rhEPO) (R&D Systems, Abingdon, England). Using this suspension, 100-μL aliquots were placed into the wells of flat-bottomed 96-well tissue culture plates (Nunc, Naperville, IL). Plates were then incubated at 37°C in a humidified atmosphere of 5% carbon dioxide (CO2) in air. First, second, and third trimester blood-derived colonies were scored by morphological features after 10,12, and 14 days of culture, respectively. BFU-e colonies (200-1000 cells/colony) were identified on the basis of their orange-red color. Colonies containing only nonhemoglobinized cells were scored as CFU-GM, while large colonies (more than 2000 cells/colony) with both central hemoglobinized and peripheral nonhemoglobinized areas were classified as CFU-GEMM. When plucked, such colonies contained erythroid cells, macrophages, myelocytes, and some immature granulocytes.
Megakaryocyte progenitor assays (BFU-MK and CFU-MK) were established, where sufficient cells were available, from adherent cell–depleted MNCs plated in agar at 2 × 105/mL in the presence of 20% FCS, 1% BSA, 100 ng/mL thrombopoietin, penicillin/streptomycin, L-glutamine, modified Eagle's medium (MEM), nonessential amino acid and vitamin solution, 200 μg/mL iron-saturated transferrin, and IMDM in 96-well flat-bottomed microtitre plates as previously described.19 The number of progenitors was counted after 14 days in culture, and all megakaryocyte lineage colonies were identified by morphologic analysis and staining with CD61 (Becton Dickinson) using the alkaline phosphatase antialkaline phosphatase (APAAP) method.19
CD34+ cells enriched from first and second trimester fetal blood and term cord blood were cultured in colony-forming assays using the same techniques as described above, at starting concentrations of 2000 cells/mL for fetal blood and 1000 cells/mL for term cord blood.
Suspension cultures
Adherent cell–depleted nucleated cells (5 × 105/mL) from 9 first trimester fetal blood samples (median gestational age, 9+2 weeks; range, 8+0-11+2 weeks) were suspended in 1 mL IMDM supplemented with 30% FBS; 1% BSA (StemCell Technologies); 10−4 mol/L 2-mercaptoethanol (Sigma); 50 μg/mL streptomycin; 50 units/mL penicillin (Gibco); and 4 combinations of the following cytokines (R&D Systems): 50 ng/mL rhSCF, 10 ng/mL rhIL-6, 10 ng/mL rhIL-3, 50 ng/mL rhFlt-3 ligand, 50 ng/mL rhTPO, and 3 units/mL rhEPO. We plated 100 μL culture in individual wells of U-bottomed 96-well culture plates (Nunc) and incubated the culture in 5% CO2 in air for up to 7 days. At the end of the 7-day culture period, nonadherent cells from each well were pooled. Morphological analysis of the cultured cells was performed on Leishman-stained cytospins, while the BFU-e, CFU-GM, and CFU-GEMM progenitor content was determined by colony assays, as described above, and compared with that detected in nonexpanded blood samples.
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). Statistical comparisons were performed using the Studentt test.
Results
CD34+ cell enumeration by flow cytometry
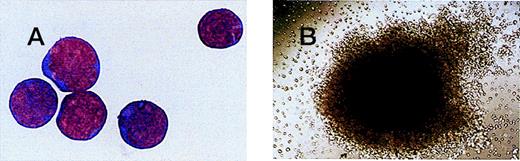
The frequency of CD34+ cells in first trimester fetal blood (median gestational age, 9+5 weeks) was 5.1 ± 1.0% of total CD45+ cells. This was significantly higher than that in term cord blood (0.4 ± 0.03% of total CD45+ cells; P = .0015). However, the absolute number of CD45+ cells, largely leukocytes, was significantly higher in term cord blood (11.9 ± 1.3 × 106/mL) than in first trimester blood (1.4 ± 0.4 × 106/mL;P < .0001). As a result, the concentration of CD34+ cells in whole blood samples was similar in first trimester (6.6 ± 2.4 × 104/mL) and term (5.6 ± 3.9 × 104/mL) fetal blood (P = .7). The percentage of CD34+ cells in first trimester samples, which was CD38−, was high (14.2 ± 6.4%; n = 5), and the first trimester samples were higher than the second trimester samples (5.5 ± 1.9%; n = 3) and term cord blood samples (3.9 ± 0.9%; n = 5). This suggests that there is a greater proportion of immature stem cells in first trimester fetal blood,21 22 although the correlation between the percentage of CD34+/CD38− cells and gestational age did not reach statistical significance. Similarly, the percentage of CD34+/HLA-DR cells was higher in first trimester fetal blood (23.0 ± 7.1%) than in term cord blood (18.6 ± 1.1%). The morphology of CD34+ cells in first trimester blood was similar to that of CD34+ cells from term cord blood (Figure 1A).
Magnetically sorted CD34+ cells and a CFU-GEMM colony from fetal blood.
(A) CD34+ cells magnetically sorted from a 12+0 week fetal blood sample and stained with Leishman's stain (original magnification × 100). (B) CFU-GEMM colony grown from a 7+6 week fetal blood sample (original magnification × 10).
Magnetically sorted CD34+ cells and a CFU-GEMM colony from fetal blood.
(A) CD34+ cells magnetically sorted from a 12+0 week fetal blood sample and stained with Leishman's stain (original magnification × 100). (B) CFU-GEMM colony grown from a 7+6 week fetal blood sample (original magnification × 10).
Hematopoietic progenitor cell assays
Both erythroid and nonerythroid progenitors that generated colonies of more than 1000 cells were detected at all gestations, from 7 weeks to term, in every fetal blood sample tested. When unsorted MNCs were plated in semisolid medium, the total number of progenitors per mL was found to peak in the second trimester (83.6 ± 31.3 × 103/mL), with similar numbers of progenitors in the first trimester (19.2 ± 2.1 × 103/mL) and third trimester (26.4 ± 5.6 × 103/mL) (Table1).
Hematopoietic progenitors circulating in fetal blood
| Median Gestational Age (Range) . | No. of Progenitors × 103/mL . | |||
|---|---|---|---|---|
| CFU-GEMM . | CFU-GM . | BFU-e . | Total . | |
| 10+0 wks (7+6-13+1) | 10.5 ± 1.3 | 3.3 ± 0.4 | 5.4 ± 0.8 | 19.2 ± 2.1 |
| 15+0 wks (14+5-20+0) | 32.5 ± 15.7* | 31.4 ± 12.0† | 19.7 ± 12.6* | 83.6 ± 31.3† |
| 39+0 wks (38+0-40+0) | 5.6 ± 0.7 | 9.7 ± 5.7 | 11.1 ± 2.1* | 26.4 ± 5.6 |
| Median Gestational Age (Range) . | No. of Progenitors × 103/mL . | |||
|---|---|---|---|---|
| CFU-GEMM . | CFU-GM . | BFU-e . | Total . | |
| 10+0 wks (7+6-13+1) | 10.5 ± 1.3 | 3.3 ± 0.4 | 5.4 ± 0.8 | 19.2 ± 2.1 |
| 15+0 wks (14+5-20+0) | 32.5 ± 15.7* | 31.4 ± 12.0† | 19.7 ± 12.6* | 83.6 ± 31.3† |
| 39+0 wks (38+0-40+0) | 5.6 ± 0.7 | 9.7 ± 5.7 | 11.1 ± 2.1* | 26.4 ± 5.6 |
Figures given in mean ± SEM.
Indicates that P < .01, comparing mean ± SEM with first trimester;
indicates P < .0001, comparing mean ± SEM with first trimester.
The nature of progenitors circulating in fetal blood changed with gestation. In first trimester blood the predominant progenitor type was CFU-GEMM (10.5 ± 1.3 × 103/mL), and there were fewer BFU-e cells (5.4 ± 0.8 × 103/mL) and CFU-GM cells (3.3 ± 0.4 × 103/mL) (Table1). In the second trimester, there were significantly more CFU-GEMM (P = .002), BFU-e (P = .01), and CFU-GM (P < .0001) progenitors compared with the first trimester. In third trimester fetal blood, the predominant progenitor type was BFU-e, although large numbers of CFU-GEMM and CFU-GM progenitors were also grown. First trimester CFU-GEMM progenitors appeared as large colonies with a tight center of hemoglobinized cells surrounded by dispersed cells; their average size (2.2 ± 1.3 × 104 cells/colony) was comparable to that of CFU-GEMM colonies in second trimester and term cord blood (Figure 1B). Cytological examination of first trimester CFU-GEMM progenitors revealed the presence of a majority of differentiated erythroblasts along with macrophages, myelocytes, immature granulocytes, and occasional megakaryocytes. In addition, first trimester CFU-GEMM progenitors appeared in culture earlier (at 8-10 days) than their second and third trimester counterparts (at 12 and 14 days, respectively). In some experiments sufficient cells were available to establish megakaryocyte progenitor assays. BFU-MK and CFU-MK progenitors were seen in all first trimester fetal blood samples (median gestational age, 11+0 weeks; range, 9+0-13+6 weeks; n = 6). The number of BFU-MK progenitors in first trimester blood was similar (0.57 ± 0.21 × 103/mL) to the number of BFU-MK progenitors in term cord blood (0.49 ± 0.22 × 103/mL;n = 10). By contrast, CFU-MK progenitors were present in large numbers in first trimester blood (5.05 ± 2.05 × 103/mL), and they were similar in morphology, although greater in number, than those in term cord blood (2.06 ± 0.52 × 103/mL;P = .07).
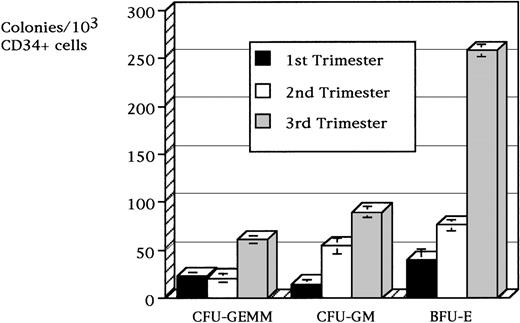
When a purified population of CD34+ cells was isolated from fetal blood and assessed for its content of hematopoietic progenitors, the progenitor population increased in frequency with advancing gestational age. In the first trimester, the progenitors totaled 76.2 ± 14.4 × 103/mL CD34+ cells, and this number increased to 150.6 ± 14.7 × 103/mL CD34+ cells in the second trimester and 410.6 ± 4.4 × 103/mL CD34+ cells at term. The relative distribution of progenitor types was similar throughout gestation, and BFU-e progenitors were prevalent at all gestations. The frequency of BFU-e progenitors increased from 39.0 ± 9.6 × 103/mL CD34+ cells in the first trimester to 76.1 ± 9.8 × 103/mL CD34+ cells in the second trimester and 258.3 ± 6.5 × 103/mL CD34+ cells in the third trimester (Figure 2). The frequency of CFU-GM colonies also increased from 14.5 ± 2.0 × 103/mL CD34+ cells in the first trimester to 54.1 ± 8.8 × 103/mL CD34+ cells in the second trimester and 89.5 ± 6.1 × 103/mL CD34+ cells in the third trimester. The frequency of CFU-GEMM colonies was similar in the first and second trimesters (22.6 ± 4.7 × 103/mL and 21.0 ± 2.9 × 103/mL CD34+ cells, respectively), but the colonies increased to 60.7 ± 2.0 × 103/mL CD34+ cells in the third trimester. No progenitors were cultured from the CD34− fraction of early fetal (n = 6), second trimester (n = 3), or term cord (n = 4) blood samples.
Frequency of CD34+ derived cell colonies per 103/mL CD34+ cells magnetically sorted from fetal blood samples.
Frequency of CD34+ derived cell colonies per 103/mL CD34+ cells magnetically sorted from fetal blood samples.
Suspension cultures
To determine whether it was possible to expand these cells in vitro, we cultured adherent cell–depleted MNCs from first trimester fetal blood (n = 9) for 7 days in liquid medium supplemented with each of the 5 different cytokines alone or with the cytokines in combination with SCF + IL-3, SCF + IL-3 + IL-6, SCF + IL-3 + TPO, and SCF + IL-3 + Flt-3. In the presence of all these cytokine combinations, there was a dramatic increase in the number of BFU-e progenitors, ranging from 47.7 ± 11.5–fold in the presence of SCF + [IL-3‖[plus] IL-6 to 65.6 ± 12.1–fold with SCF + IL-3 + Flt-3. The number of CFU-GEMM progenitors following liquid culture in the presence of these cytokines also significantly increased, although the increase was more modest, ranging from 3.6 ± 1.5–fold in the presence of SCF + IL-3 + IL-6 to 6.8 ± 1.8–fold with SCF + IL-3 + Flt-3 (Table2). In addition, although the light microscopy size and morphology of CFU-GEMM progenitors were the same following liquid culture as they were in preculture, CFU-GEMM–plucked post-liquid culture contained greater numbers of mature neutrophils and eosinophils as well as erythroblasts, immature myeloid cells, and megakaryocytes.
Liquid culture of first trimester fetal blood
| Growth Factor Combination . | Fold Expansion . | ||
|---|---|---|---|
| CFU-GEMM . | CFU-GM . | BFU-e . | |
| SCF + IL-3 | 6.1 ± 1.4 | 5.1 ± 1.7 | 51.0 ± 12.4 |
| SCF + IL-3 + IL-6 | 3.6 ± 1.5 | 2.4 ± 1.0 | 47.7 ± 11.5 |
| SCF + IL-3 + TPO | 5.0 ± 1.8 | 2.1 ± 0.5 | 61.1 ± 13.9 |
| SCF + IL-3 + Flt-3 | 6.8 ± 1.8 | 1.8 ± 0.6 | 65.6 ± 12.1 |
| Growth Factor Combination . | Fold Expansion . | ||
|---|---|---|---|
| CFU-GEMM . | CFU-GM . | BFU-e . | |
| SCF + IL-3 | 6.1 ± 1.4 | 5.1 ± 1.7 | 51.0 ± 12.4 |
| SCF + IL-3 + IL-6 | 3.6 ± 1.5 | 2.4 ± 1.0 | 47.7 ± 11.5 |
| SCF + IL-3 + TPO | 5.0 ± 1.8 | 2.1 ± 0.5 | 61.1 ± 13.9 |
| SCF + IL-3 + Flt-3 | 6.8 ± 1.8 | 1.8 ± 0.6 | 65.6 ± 12.1 |
Figures given in mean ± SEM.
Discussion
During ontogenesis of the human hematopoietic system, sequential changes in the sites of hematopoiesis are believed to occur through the migration of stem and/or progenitor cells in fetal blood. Evidence in support of this migratory process is provided by the detection of circulating hematopoietic multipotent stem and progenitor cells at all stages of fetal development. Although progenitor cells have been clearly detected in fetal blood samples from 12 weeks of gestation onward,15,16 their presence at earlier gestational ages has only been demonstrated in the extra-embryonic yolk sac and in different compartments of the embryo such as the fetal liver, bone marrow, and ventral wall of the aorta.1,4,5,23 To investigate the number and types of hematopoietic progenitor cells circulating in early fetal life, fetal blood samples were obtained during the first trimester. We used an ultrasound-guided needling technique, similar to the technique used for diagnostic and therapeutic purposes in the second and third trimesters, which has recently been adapted for research purposes in the late first trimester.24-26
Evidence to date shows that the majority of hematopoietic progenitors are contained within the CD34-expressing cell fraction in the human embryo and in fetal liver and bone marrow.4,27-29 As a result, we determined that the frequency of double-stained CD45+/CD34+ cells in first trimester blood (median gestational age, 9+5weeks) was 5.1 ± 1.0% of the total number of CD45+ cells. This was significantly higher than the frequency of double-stained CD45+/CD34+ cells in term cord blood (0.4 ± 0.03%). These data are in agreement with studies of later fetal blood (12-38 weeks) by Thilaganathan et al30 and suggest that circulating CD34+ cells are likely to contribute significantly to hematopoiesis in early fetal life.
In support of this, we found that CD34+ cells isolated from first trimester fetal blood gave rise to significant numbers of both multipotent progenitors (22.6 ± 4.7 × 103/mL CD34+ cells for CFU-GEMM) and committed progenitors (39.0 ± 9.6 × 103/mL CD34+ cells for BFU-e and 14.5 ± 2.0 × 103/mL CD34+ cells for CFU-GM). The number of BFU-e progenitors found in first trimester fetal blood was similar to the number of BFU-e progenitors found in unsorted cells from fetal liver at the same gestation5 and from CD34+ cells from the yolk sac, embryonic tissues, and fetal liver.1 Interestingly, circulating CFU-e progenitors were not cultured from early fetal CD34+ cells. This is in agreement with data from other researchers,1,5 which indicates that CFU-e progenitors are not found in fetal liver, although they are present in large numbers in the yolk sac. This is also consistent with the notion that during human gestation, circulating stem and progenitor cells are not derived from the yolk sac, but instead they are derived from, or en route to, the fetal liver from an intraembryonic site, specifically the AGM.4,31 The number of CFU-GEMM progenitors is similar to or slightly higher than that previously reported for CD34+ cells isolated from the yolk sac, embryo, and fetal liver. This may reflect differences in the classification of colonies such as BFU-e versus CFU-GEMM. In our study the majority of colonies derived from early fetal blood contained a prevalence of erythroid cells along with some macrophages, myelocytes, and immature granulocytes, as described by Huyhn et al1 in the yolk sac and embryonic tissues.
Interestingly, we also demonstrated for the first time that there are large numbers of circulating megakaryocyte progenitors in first trimester fetal blood. The majority of progenitors were CFU-MK, as found in second and third trimester samples from live born babies.19,32 This is in contrast to the findings of Zauli et al33 who demonstrated predominantly BFU-MK progenitors in umbilical vein samples from fetuses with gestational ages ranging from 18-22 weeks. The presence of large numbers of megakaryocyte progenitors in first trimester blood raises the possibility that they are the principal source of platelets early in gestation because megakaryocytopoiesis in fetal liver or marrow does not appear to be significant before 10-11 weeks gestation.34 35
The number of circulating progenitors in fetal blood showed quite a striking peak in the second trimester. The fall in the number of progenitors from the second to the third trimester is consistent with observations by ourselves and others in preterm babies, which show an inverse relationship between progenitor cell numbers and gestational age from 24-40 weeks.17-19,32 This is in line with the establishment of marrow hematopoiesis and the smaller role of the fetal liver as a site of hematopoiesis later in gestation. The increase in the number of circulating progenitors from the first to the second trimester may be due to a number of reasons. First, the increase may reflect an increase in the number of cells migrating from the fetal liver to establish hematopoiesis in the fetal bone marrow, which is still at a very low level in the first trimester.7 Second, the increase may also reflect greater hematopoietic activity in the second trimester, when both liver and marrow hematopoiesis are established; indeed the increase in circulating progenitors parallels the increase in fetal bone marrow between 14 and 24 weeks.36 Third, our data indicate that the clonogenicity of circulating CD34+ cells is relatively low in the first trimester and increases with advancing gestational age.
The increase in clonogenicity of progenitors with advancing gestational age in fetal blood, as shown by others15-17,36 using fetal liver and bone marrow, suggests that there may be significant differences in the characteristics of CD34+ cells in the first, second, and third trimesters. Differences have been noted in cell cycle status, as reported for fetal CFU-GM37 and BFU-e progenitors,38,39 or in the sensitivity to cytokines.1,32,40 Indeed, as reported for first trimester fetal liver,41 we found that a high percentage of CD34+ cells in the first trimester blood was CD38−, which suggests that CD34+ cells in the first trimester are more primitive than those circulating later in ontogeny.42 Consequently, it is possible that circulating CD34+ cells in the first trimester are less able to form colonies in vitro, at least under conditions optimal for cord blood or adult bone marrow,22 as has been reported for CD34+ cells from the AGM.43 However, these hypotheses remain to be clarified in future studies with purified CD34+ cells and in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice.
The concentration of CD34+ cells in first trimester blood was 6.6 ± 2.4 × 104/mL, which is similar to that in term cord blood. Studies of fetal weight and blood volume in the first and early second trimester44 indicate that for ex vivo manipulation (including gene transfer), it would be possible to collect an estimated 103/mL CD34+ cells. However, our data showed that circulating progenitors from first trimester blood proliferated extensively in vitro in response to various cytokine combinations (multipotent progenitors increasing 7-fold and BFU-e increasing 66-fold after 7 days of liquid culture in SCF+IL-3+Flt-3). This suggests that sufficient numbers of progenitors and possibly stem cells could be collected even from very immature fetuses. Clearly more work will be required both before and after in vitro “amplification” (1) to identify the optimal cytokine combination for stem cell expansion and the long-term repopulating ability of such candidate stem cells by using appropriate animal models, such as NOD/SCID mice,42,45 and (2) to determine whether CD34− repopulating cells circulate in early fetal blood.41
This study demonstrates the ability to isolate multipotent progenitor cells from the fetal circulation during the first trimester. This is relevant to the development of noninvasive prenatal diagnosis by expansion of fetal hematopoietic progenitors present in maternal blood and the development of intrauterine gene therapy for inherited hematopoietic and metabolic disorders. First, fetal hematopoietic progenitor and stem cells have been demonstrated in the maternal circulation from early gestation onwards,46-50 and it may be possible to select the cells in vitro and amplify them for noninvasive prenatal diagnosis. Although to date some success has been reported,48 most groups have found the degree of expansion insufficient against the higher number (approximately a million-fold) of background maternal cells.49 50 We speculate that further development of this approach will involve detection of differences in optimal growth factor requirements between maternal and fetal hematopoietic progenitor cells in order to exploit them for selective fetal cell expansion in maternal blood.
Second, development of in utero gene therapy is likely to require access to early circulating fetal hematopoietic stem cells because fetal bone marrow cannot be harvested in ongoing pregnancies, and fetal liver has not been harvested in ongoing pregnancies before 17 weeks. It seems likely that such samples will need to be acquired before gestation reaches 13-14 weeks. Although first trimester samples in our study were obtained from noncontinuing pregnancies, fetal blood sampling has been reported from as early as 12 weeks of gestation in ongoing pregnancies.51 52 We acknowledge that eventual development, such as high resolution ultrasound or endoscopically guided fine needling of the umbilical cord, will require safe techniques for harvesting fetal blood in the late first trimester.
Supported in part by a project grant from Wellbeing, London, England.
Reprints:Cesare Campagnoli, Institute of Obstetrics and Gynaecology, Imperial College School of Medicine, Queen Charlotte's and Chelsea Hospitals, Goldhawk Road, London W6 OXG, England.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal