Abstract
Regulated lymphocyte trafficking is essential for the control and integration of systemic immune responses. This homing process disperses the immunologic repertoire, guides lymphocyte subsets to the specialized microenvironments that control their differentiation and survival, and targets immune effector cells to sites of antigenic insult. This review discusses data indicating that the adhesion receptors regulating the trafficking of normal lymphocytes are also expressed and functionally active in their malignant counterparts, the non-Hodgkin lymphomas. These “homing receptors” appear to mediate the highly tissue-specific dissemination of specific lymphoma subtypes, such as lymphomas of the mucosa-associated lymphoid tissues and lymphomas of the skin. Furthermore, as a result of their capability to enhance lymphoma dissemination and to transduce signals into the cell, promoting cell growth and survival, adhesion receptors may contribute to lymphoma aggressiveness. Taken together, the data offer a framework for understanding the dissemination routes of non-Hodgkin lymphomas and suggest that adhesion receptors, specifically those of the CD44 family, may present useful tools to predict prognosis in patients with lymphomas.
Successful defense of the body against microbial invasion is critically dependent on the presence of lymphocyte clones with the right antigen specificity at the right position at the right time. To accomplish this task, evolution has created great antigen-receptor diversity and has equipped lymphocytes with exquisite motility. Most mature lymphocytes recirculate continuously, going from blood to tissue and back to the bloodstream again.1 This recirculation is not random, but rather is guided by lymphocyte-endothelial recognition and subsequent diapedesis, directing lymphocyte homing.2-5 Thus, lymphocyte-endothelial interaction is a central regulatory point in the immune system, controlling the access of specialized lymphocyte subsets to particular tissues and influencing the nature of local immune and inflammatory responses. At the molecular level, this process is regulated by adhesion receptors on the cell membrane of lymphocytes and their counterreceptors on endothelial cells in concert with chemokines. Specialization of both lymphocyte subsets and endothelial cells in their expression of adhesion receptors allows lymphocytes to move selectively to specific functional compartments of the immune system, such as the mucosa- and skin-associated lymphoid tissues.2-5
Adhesion receptors play a key role in many biologic processes such as embryogenesis, hemostasis, and inflammation.3-16 Their function is to orchestrate cell-cell and cell-extracellular matrix interactions and is complex and dynamic. As has also been emphasized in a recent review by Shattil et al,10 engagement of adhesion receptors with their ligands does not represent a simple Velcro-type of interaction, but instead transduces signals into the cell-regulating cytoskeletal organization,7,8,10 cell cycle progression,9 and cell survival17,18 through a process known as outside-in signaling. On the other hand, cytoplasmic signals, for example, generated via antigen or chemokine receptors, regulate the cell surface expression and functional activity of adhesion receptors, a process termed inside-out signaling.7,10,13,14,16,19 The adhesion molecules involved in lymphocyte homing constitute a structurally diverse group of cell membrane receptors belonging to distinct adhesion receptor families, that is, the selectin,11 the integrin,7 the immunoglobulin,6 and the CD44 families.13 Each of these receptors exhibits specific ligand-binding properties that are needed for specific tasks in the trafficking process.2-5For example, when blood flows through postcapillary venules in lymphoid tissues, lymphocytes slow down and roll on the endothelial surface. In most instances, this rolling is mediated by interactions of adhesion receptors of the selectin family with their carbohydrate ligands. Major selectins involved in lymphocyte homing are L-selectin and E-selectin, which are expressed on the lymphocyte and endothelial cell surface, respectively. Once the rolling process has slowed down the cells, they are arrested at the right site by activated integrins on the lymphocyte cell surface interacting with their immunoglobulin family counterreceptors on the endothelium, resulting in extravasation.2-5 Integrins with an important function in lymphocyte homing are αLβ2, α4β1, and α4β7. Whereas CD44 presumably is of minor importance in the physiology of lymphocyte homing, recent studies indicate that this class of adhesion receptors is of great significance for lymphocyte migration to sites of inflammation.20-26 At these sites, CD44 may interact with its major ligand, hyaluronate, which is expressed on the luminal surface of activated endothelium.
Apart from their physiologic functions, adhesion receptors are also involved in tumor invasion and metastasis.12 The non-Hodgkin lymphomas (NHLs) represent the malignant counterparts of normal lymphocytes, arrested at specific stages of maturation. This review describes the paradigm of lymphocyte homing and discusses data indicating that the adhesion receptor programs directing the homing of normal lymphocytes are, at least partly, preserved in lymphomas. These adhesion receptors contribute to lymphoma aggressiveness and appear to determine the highly tissue-specific dissemination patterns of certain lymphoma subtypes.
Lymphocyte adhesion receptors in the regulation of lymphocyte homing
An overview of the most important lymphocyte adhesion receptors involved in homing, their distribution on various lymphocyte subsets, their ligands, sites of interaction, and predominant role(s) in homing is given in Table 1. Before we focus on the specific roles of these molecules, we will discuss the general principles of lymphocyte-endothelial interaction.
Lymphocyte adhesion molecules and their role in homing
| Receptor . | Expression on Lymphocytes . | Ligands . | Interacting Cells/Substrates . | Predominant Role(s) in Homing . |
|---|---|---|---|---|
| L-selectin | Resting T and B cells | PNAd GlyCAM CD34 (MAdCAM-1) | High endothelial venules | Peripheral lymph node-homing |
| CLA | Memory T-cell subset | E-selectin | Endothelium (skin) | Skin-homing |
| αLβ2 (LFA-1) | Broad expression on T and B cells | ICAM-1 ICAM-2 ICAM-3 | Endothelium High endothelial venules Dendritic cells FDC Activated epithelium | Broad function in lymphocyte homing |
| α4β1 (VLA-4) | Broad expression on T and B cells | VCAM-1 Fibronectin (CS-1) | Activated endothelium Dendritic cells FDC | Homing to inflammatory sites |
| α4β7 | Naive T and B cells (low) Memory T-cell subset (high) Peripheral B-cell subset (low) | MAdCAM-1 (VCAM-1) (Fibronectin) | Endothelium of intestinal mucosa Peyer patch and mesenteric lymph node high endothelial venules | Gut-homing |
| αEβ7 | Intraepithelial T cells | E-cadherin | Epithelium | Epitheliotropism |
| CD44 (multiple isoforms) | Broad expression on T and B cells Splice variants on activated T and B cells | Hyaluronate Collagen IV Fibronectin Serglycin Osteopontin Growth factors (FGF-2, HGF, MIP-1β) | Extracellular matrix Endothelium | Homing to sites of inflammation Modulation of cell growth and motility |
| Receptor . | Expression on Lymphocytes . | Ligands . | Interacting Cells/Substrates . | Predominant Role(s) in Homing . |
|---|---|---|---|---|
| L-selectin | Resting T and B cells | PNAd GlyCAM CD34 (MAdCAM-1) | High endothelial venules | Peripheral lymph node-homing |
| CLA | Memory T-cell subset | E-selectin | Endothelium (skin) | Skin-homing |
| αLβ2 (LFA-1) | Broad expression on T and B cells | ICAM-1 ICAM-2 ICAM-3 | Endothelium High endothelial venules Dendritic cells FDC Activated epithelium | Broad function in lymphocyte homing |
| α4β1 (VLA-4) | Broad expression on T and B cells | VCAM-1 Fibronectin (CS-1) | Activated endothelium Dendritic cells FDC | Homing to inflammatory sites |
| α4β7 | Naive T and B cells (low) Memory T-cell subset (high) Peripheral B-cell subset (low) | MAdCAM-1 (VCAM-1) (Fibronectin) | Endothelium of intestinal mucosa Peyer patch and mesenteric lymph node high endothelial venules | Gut-homing |
| αEβ7 | Intraepithelial T cells | E-cadherin | Epithelium | Epitheliotropism |
| CD44 (multiple isoforms) | Broad expression on T and B cells Splice variants on activated T and B cells | Hyaluronate Collagen IV Fibronectin Serglycin Osteopontin Growth factors (FGF-2, HGF, MIP-1β) | Extracellular matrix Endothelium | Homing to sites of inflammation Modulation of cell growth and motility |
Only molecules discussed in the present paper are listed. The αβ heterodimers belong to the integrin family. CLA indicates cutaneous lymphocyte antigen; FDC, follicular dendritic cells; FGF, fibroblast growth factor; GlyCAM, glycosylation-dependent cell adhesion molecule; HGF, hepatocyte growth factor; ICAM, intercellular adhesion molecule; LFA, lymphocyte function-associated; MAdCAM, mucosal addressin cell adhesion molecule; MIP, macrophage inflammatory protein; PNAd, peripheral lymph node addressin; VCAM, vascular cell adhesion molecule; VLA, very late antigens.
Engagement of lymphocytes with endothelium directs lymphocyte homing and is a multistep process 2-5,27-30 (Figure1). The initial step consists of a loose “tethering” engagement between the lymphocyte and the endothelium, leading to a rolling movement of the lymphocyte over the vascular endothelium of the postcapillary venule (Figure 1). This step generally is mediated by molecules of the selectin family, which are strategically localized on the tips of the cell membrane's microvilli,31-33 thus allowing for effective interaction of the selectin with its sialomucin ligand. However, other molecules such as the integrins, α4β1 and α4β7, and CD44 also can mediate rolling.21,30,34-36 Lymphocyte rolling is transient and reversible, unless followed by a signal leading to activation of adhesion molecules of the integrin family. These molecules, that is, αLβ2, also known as lymphocyte function-associated molecule (LFA)-1, α4β1, and α4β7, mediate stable adhesion and promote migration of lymphocytes across the vessel wall (Figure 1). The extremely rapid integrin activation that must occur in the bloodstream is mediated by cytokines of the chemokine family, interacting with their G protein-coupled receptors.37 Recently, several chemokines like SDF-1, SLC (6-C-kine), BLC/BCA-1, MIP-3β, MIP-3α, IP10, Mig, and TARC have been identified that are capable of mediating rapid (milliseconds) integrin-dependent lymphocyte arrest under flow conditions.38-40 Consistent with their important regulatory role in homing, these chemokines display site-specific production as well as specificity for distinct lymphocyte subsets 39,40-45 (Table 2). For example, the chemokine SLC is specifically expressed by high endothelial venules and selectively recruits naive T cells, expressing the chemokine receptor CCR7, into the secondary lymphoid organs,43 whereas TARC recruits CCR4-positive memory T cells to the skin.40a BLC/BCA, by contrast, is involved in the recruitment of B cells into B-cell areas. This molecule is specifically expressed in B-cell follicles, presumably by follicular dendritic cells (FDC). Disruption of CXCR5, the receptor for BLC, leads to a disturbed development of primary follicles and germinal centers in the spleen and Peyer patches.44,46 In addition to chemokines, heparan sulfate (HS) proteoglycans expressed on endothelium or extracellular matrix contribute to integrin activation and promote diapedesis by concentrating and presenting chemokines to their receptors (Figure 1). Because specific modifications of HS chains appear to determine cytokine-binding specificity, chemokine interaction with HS proteoglycans may add an additional level of specificity to the lymphocyte-endothelial cell interaction cascade.47-49
Lymphocyte interaction with endothelium.
In the postcapillary venules selectin-sialomucin interactions mediate “rolling” of lymphocytes on endothelium. Chemokines presented by heparan sulfate proteoglycans on the endothelium can bind to chemokine receptors, which are G protein-coupled 7-membrane-spanning molecules. This leads to activation of members of the integrin family on the surface of lymphocytes. Interaction of integrins with their ligands results in stable adhesion of lymphocytes to endothelium and in diapedesis.
Lymphocyte interaction with endothelium.
In the postcapillary venules selectin-sialomucin interactions mediate “rolling” of lymphocytes on endothelium. Chemokines presented by heparan sulfate proteoglycans on the endothelium can bind to chemokine receptors, which are G protein-coupled 7-membrane-spanning molecules. This leads to activation of members of the integrin family on the surface of lymphocytes. Interaction of integrins with their ligands results in stable adhesion of lymphocytes to endothelium and in diapedesis.
Chemokines involved in lymphocyte migration
| . | Source of Production . | Receptor . | Target Cell(s) . | Reference . |
|---|---|---|---|---|
| CXC-Chemokine | ||||
| IP10 | Monocytes, T cells, keratinocytes, fibroblasts, endothelial cells | CXCR3 | Activated T cells | 40, 172, 173 |
| Mig | Monocytes/macrophages | CXCR3 | Activated T-cells | 40, 172, 174 |
| SDF-1 | Several tissues | CXCR4 | Pro- and pre-B cells, T cells, bone marrow progenitor cells, bone marrow-derived dendritic cells | 175, 176 |
| BLC/BCA-1 | B-cell follicles (secondary lymphoid tissues) | CXCR5 | B cells | 44, 45 |
| CC-Chemokine | ||||
| MIP-3α | Activated monocytes, activated dendritic cells, lymphoid tissues | CCR6 | CD34+ dendritic cells, monocytes, subset of memory T cells | 177, 178 |
| MIP-3β | Dendritic cells, lymphoid tissues | CCR7 | memory T cells, mature dendritic cells | 177, 178 |
| SLC | High endothelial venules (secondary lymphoid tissues) | CCR7 | naive T cells, mature dendritic cells | 43, 179 |
| TARC | Activated endothelium | CCR4 | skin-homing memory T cells | 40a |
| . | Source of Production . | Receptor . | Target Cell(s) . | Reference . |
|---|---|---|---|---|
| CXC-Chemokine | ||||
| IP10 | Monocytes, T cells, keratinocytes, fibroblasts, endothelial cells | CXCR3 | Activated T cells | 40, 172, 173 |
| Mig | Monocytes/macrophages | CXCR3 | Activated T-cells | 40, 172, 174 |
| SDF-1 | Several tissues | CXCR4 | Pro- and pre-B cells, T cells, bone marrow progenitor cells, bone marrow-derived dendritic cells | 175, 176 |
| BLC/BCA-1 | B-cell follicles (secondary lymphoid tissues) | CXCR5 | B cells | 44, 45 |
| CC-Chemokine | ||||
| MIP-3α | Activated monocytes, activated dendritic cells, lymphoid tissues | CCR6 | CD34+ dendritic cells, monocytes, subset of memory T cells | 177, 178 |
| MIP-3β | Dendritic cells, lymphoid tissues | CCR7 | memory T cells, mature dendritic cells | 177, 178 |
| SLC | High endothelial venules (secondary lymphoid tissues) | CCR7 | naive T cells, mature dendritic cells | 43, 179 |
| TARC | Activated endothelium | CCR4 | skin-homing memory T cells | 40a |
Only chemokines discussed in the present paper are listed.
In the multistep lymphocyte-endothelial cell interaction model described above (Figure 1), specificity is determined by unique combinations of primary and secondary adhesion receptor pairs, as well as by chemokine-mediated activation events.4,27-30,33,43For example, whereas lymphocyte homing to peripheral lymph nodes is directed by the adhesion receptors (1) L-selectin and (2) LFA-1, and by the chemokine (3) SLC, homing to the skin is mediated by the adhesion receptors (1) CLA and (2) LFA-1 and by the chemokine (3) TARC.33,40a,43 Important factors regulating the adhesion receptor profile of lymphocytes are prior antigenic stimulation and state of activation.2-5 50 Imprinting of the lymphocyte at the site of antigenic experience leads to expression of a specific set of adhesion receptors on the lymphocyte. These “homing” receptors permit interaction with endothelial area codes “vascular addressins,” crucial in tissue-specific recirculation of lymphocytes to the sites of primary antigenic stimulation. Combinations of lymphocyte adhesion molecules and vascular addressins involved in tissue-specific homing are α4β7/MAdCAM-1 for homing to mucosa-associated lymphoid tissues (MALT), cutaneous lymphocyte antigen (CLA)/E-selectin for homing to skin, and L-selectin/peripheral lymph node addressins (PNAds) for peripheral lymph node homing. Binding of αEβ7 to the adhesion molecule E-cadherin expressed on epithelial cells is involved in positioning lymphocytes in epithelium (Figure2).
Lymphocyte migration.
Lymphocyte migration is strictly regulated by cell adhesion receptors on lymphocytes (lymphocyte homing receptors) and endothelium (vascular addressins). Naive lymphocytes migrate randomly through the body because they express both α4β7 (for mucosal homing) and L-selectin (for homing to peripheral lymph nodes). Migration of activated lymphocytes to sites of inflammation involves several receptor-ligand pairs, including selectin-sialomucin, α4β1-VCAM-1, α4β1-CS-1, and CD44-hyaluronate interactions. Homing of memory lymphocytes is largely restricted to organs of primary antigenic stimulation. By binding to their vascular addressins, lymphocyte homing receptors α4β7, L-selectin, and CLA mediate tissue-specific homing to the mucosa, peripheral lymph node, and skin, respectively. Interaction of αEβ7 with E-cadherin A metastasisexpressed on epithelial cells is involved in positioning of lymphocytes in the epithelium of skin and mucosa. The NHLs related to lymphocyte populations with tissue-specific homing properties are shown in the boxes. These tumors usually display tissue-specific dissemination patterns and express homing receptors corresponding to the tissue of origin.
Lymphocyte migration.
Lymphocyte migration is strictly regulated by cell adhesion receptors on lymphocytes (lymphocyte homing receptors) and endothelium (vascular addressins). Naive lymphocytes migrate randomly through the body because they express both α4β7 (for mucosal homing) and L-selectin (for homing to peripheral lymph nodes). Migration of activated lymphocytes to sites of inflammation involves several receptor-ligand pairs, including selectin-sialomucin, α4β1-VCAM-1, α4β1-CS-1, and CD44-hyaluronate interactions. Homing of memory lymphocytes is largely restricted to organs of primary antigenic stimulation. By binding to their vascular addressins, lymphocyte homing receptors α4β7, L-selectin, and CLA mediate tissue-specific homing to the mucosa, peripheral lymph node, and skin, respectively. Interaction of αEβ7 with E-cadherin A metastasisexpressed on epithelial cells is involved in positioning of lymphocytes in the epithelium of skin and mucosa. The NHLs related to lymphocyte populations with tissue-specific homing properties are shown in the boxes. These tumors usually display tissue-specific dissemination patterns and express homing receptors corresponding to the tissue of origin.
Adhesion receptors in lymphoma dissemination
At least 3 sets of clinical observations suggest that conserved homing programs might play an important role in the dissemination of NHLs.51 First, NHLs related to small resting lymphocytes, such as lymphocytic and mantle-cell lymphoma, usually are disseminated to multiple sites at presentation, whereas NHLs related to activated lymphocytes, such as diffuse large B-cell lymphomas, often are initially localized.52 These differences in dissemination propensity presumably reflect the recirculating versus sessile character of the normal counterparts of these NHLs. Second, extranodal NHLs arising in the MALT or the skin preferentially disseminate to mucosal sites and skin, respectively.52-59 This strongly suggests that they make use of specific area codes, similar to those used during normal lymphocyte homing. Third, specific lymphoma dissemination to sites of (micro)trauma and inflammation has been reported.51 59 This phenomenon might be explained by interaction of tumor cells with activated endothelium at the site of injury. In the following paragraphs, studies focusing on the role of specific adhesion receptors in lymphoma dissemination are discussed.
Selectinsand their carbohydrate ligands
L-selectin; homing to peripheral lymph nodes.
L-selectin, like the other members of the selectin family, consists of lectin, epidermal growth factor, and short consensus repeat domains.11 L-selectin is expressed by lymphocytes, monocytes, and neutrophils and is rapidly down-regulated on cell activation.60 In vitro evidence for a selective role of L-selectin in lymphocyte homing to peripheral lymph nodes came from experiments demonstrating that monoclonal antibodies (mAbs) against L-selectin interfere with binding of lymphocytes to high endothelial venules of peripheral lymph nodes, but not to high endothelial venules of Peyer patches.61 In vivo inactivation of L-selectin by mAbs or gene knockout results in a nearly complete inhibition of lymphocyte homing to peripheral lymph nodes, whereas homing to Peyer patches remains largely intact.33,62-64 The PNAds, the main ligands for L-selectin, are selectively but not exclusively expressed on high endothelial venules in peripheral nodes.65Anti-PNAd (mAb MECA-79) decreases lymphocyte adherence to peripheral lymph node high endothelial venules by 60% to 90%.65 The MECA-79 epitope is a carbohydrate moiety that decorates a number of different protein backbones including CD34 and GlyCAM-1.5,66,67 Lymphocyte homing to peripheral lymph nodes also is markedly reduced in α (1,3) fucosyltransferase Fuc-TVII knockout mice, which are deficient in selectin ligands.68Hence, both L-selectin and its ligands are needed for lymphocyte homing to peripheral lymph nodes (Figure 2).
Studies of L-selectin expression on NHLs have shown that the vast majority of nodal lymphomas express L-selectin (Table3). This holds for low-grade and aggressive B-cell lymphomas, as well as for T-cell lymphomas with a primary nodal localization.69 By contrast, expression of L-selectin in extranodal lymphomas is variable. Aggressive mucosal B-cell and T-cell lymphomas are generally L-selectin negative.69,70Similarly, cutaneous T-cell lymphomas express low levels of L-selectin71 (Table 3). However, 2 types of gastrointestinal tract lymphomas express L-selectin, low-grade B-cell lymphoma of MALT-type and malignant lymphomatous polyposis (MLP).69,72 Whereas MLP, a variant of mantle-cell lymphoma, typically shows widespread dissemination to both mucosal sites and peripheral lymph nodes, MALT-type lymphoma generally disseminates to mucosal sites only. Possibly, in the dissemination of the latter lymphoma type to peripheral lymph nodes, other steps in the lymphocyte-endothelial interaction cascade are rate limiting.4,27 Alternatively, because Helicobacter pylori antigens promote the growth of gastric lymphoma cells,73 the absence of antigenic stimulation within the peripheral lymph node microenvironment might explain the low incidence of MALT-type lymphoma at these sites.
Expression of adhesion molecules in non-Hodgkin lymphomas
| REAL Classification . | L-selectin . | α4β7 . | CLA . | αEβ7 . | CD44s . | References . |
|---|---|---|---|---|---|---|
| B-CLL | 69, 82, 136, 148, 162, 165, 167, 180-184 | |||||
| nodal | + | − | − | − | + | |
| Mantle cell lymphoma | 70, 72, 82, 123, 148, 181 | |||||
| nodal | + | − | − | − | + | |
| GI-tract (MLP) | + | + | − | − | + | |
| Marginal zone B-cell lymphoma | 70, 82, 124, 148, 181, 186 | |||||
| GI-tract (low-grade MALT) | + | + | − | −/+ | + | |
| Follicle center lymphoma | 69, 82, 136, 148, 162, 165, 167, 180, 181, 183 | |||||
| nodal | +/− | − | − | − | +/− | |
| Diffuse large B-cell lymphoma | 69, 70, 82, 102, 136, 148, 162, 164, 165, 167, 169, 180, 181, 183 | |||||
| nodal | +/− | − | − | − | +/− | |
| GI tract | − | − | − | − | + | |
| Peripheral T-cell lymphoma | 69, 71, 75, 80-82, 125, 127, 130, 148, 183, 185 | |||||
| nodal | + | − | − | − | + | |
| GI tract | − | +/− | − | +/− | + | |
| skin (MF) | −/+ | − | + | −/+ | + | |
| Anaplastic large cell lymphoma | 80, 81, 128 | |||||
| nodal | − | −/+ | −/+ | − | + | |
| skin | −/+ | − | + | −/+ | nt |
| REAL Classification . | L-selectin . | α4β7 . | CLA . | αEβ7 . | CD44s . | References . |
|---|---|---|---|---|---|---|
| B-CLL | 69, 82, 136, 148, 162, 165, 167, 180-184 | |||||
| nodal | + | − | − | − | + | |
| Mantle cell lymphoma | 70, 72, 82, 123, 148, 181 | |||||
| nodal | + | − | − | − | + | |
| GI-tract (MLP) | + | + | − | − | + | |
| Marginal zone B-cell lymphoma | 70, 82, 124, 148, 181, 186 | |||||
| GI-tract (low-grade MALT) | + | + | − | −/+ | + | |
| Follicle center lymphoma | 69, 82, 136, 148, 162, 165, 167, 180, 181, 183 | |||||
| nodal | +/− | − | − | − | +/− | |
| Diffuse large B-cell lymphoma | 69, 70, 82, 102, 136, 148, 162, 164, 165, 167, 169, 180, 181, 183 | |||||
| nodal | +/− | − | − | − | +/− | |
| GI tract | − | − | − | − | + | |
| Peripheral T-cell lymphoma | 69, 71, 75, 80-82, 125, 127, 130, 148, 183, 185 | |||||
| nodal | + | − | − | − | + | |
| GI tract | − | +/− | − | +/− | + | |
| skin (MF) | −/+ | − | + | −/+ | + | |
| Anaplastic large cell lymphoma | 80, 81, 128 | |||||
| nodal | − | −/+ | −/+ | − | + | |
| skin | −/+ | − | + | −/+ | nt |
+ Reported cases all or almost all positive; +/− variable reports, most cases positive; −/+ variable reports, most cases negative; − reported cases negative or sporadically positive. CLL indicates chronic lymphocytic leukemia; GI, gastrointestinal tract; MALT, mucosa-associated lymphoid tissue; MF, mycosis fungoides; MLP, malignant lymphomatous polyposis; nt, not tested.
Cutaneous lymphocyte antigen; homing to the skin.
CLA is a carbohydrate antigen that is closely related to the sialyl Lewis x antigen (sLex).2,74,75 CLA is present on a minor subset (10%–15%) of memory T lymphocytes in the peripheral blood, tonsils, and lymph nodes.75,76 However, 40% to 90% of the T cells in the normal and inflamed skin, respectively, are CLA positive.75,77,78 CLA mediates skin homing via interaction with E-selectin, which is constitutively expressed on skin endothelium2 79 (Figure 2).
Interestingly, mycosis fungoides and other subtypes of cutaneous lymphoma express CLA75,80,81 but not the mucosal homing receptor α4β7 (Drillenburg and Pals, unpublished observation). On the other hand, gastrointestinal T-cell lymphomas express the mucosal homing receptor α4β7,82 but not CLA (Table 3). Hence, the malignant counterparts of 2 normal lymphoid tissues express mutually exclusive adhesion molecules concordant with their tissue of origin. The highly selective expression of CLA on cutaneous T-cell lymphomas suggests that CLA mediates the skin-specific dissemination of these lymphomas in vivo (Figures 2 and 3).
Integrins
Integrins form a large family of heterodimeric transmembrane glycoproteins consisting of noncovalently associated α- and β-subunits.7 Only limited numbers of integrins are expressed on lymphocytes. They serve as receptors and mediate interactions of lymphocytes with a variety of cells as well as with components of the extracellular matrix. Integrins expressed on lymphocytes are not constitutively active but their function is activation dependent.2-5,7 19
Lβ2 (LFA-1).
The leukocyte integrin αLβ2, which is generally designated by its original name LFA-1, is one of the most important integrins of the immune system. It mediates lymphocyte interactions with other cells, including adhesion to endothelium,3-5,88-93 dendritic cells,85,86follicular dendritic cells (FDC),87 and epithelium.88 The ligands for LFA-1 on endothelium are the intercellular adhesion molecules (ICAM)-1, -2, and -3.29,89-91 LFA-1 interaction with ICAM-1 plays a major role in lymphocyte adhesion and transmigration through high endothelial venules at various anatomic sites.3-5,29,92,93 Lymphocytes share LFA-1 with other leukocytes. Defective expression of the β2-subunit leads to impaired emigration of leukocytes from the blood to sites of inflammation, resulting in severe immunodeficiency.94
This integrin supports in vitro invasiveness of T-cell hybridomas and lymphoma cell lines in hepatocyte and fibroblast monolayers and promotes experimental lymphoma metastasis in nude mice.95-98 In human lymphomas, expression of LFA-1 is closely related to lineage derivation and stage of differentiation.99 Although an initial study by Clayburger et al100 suggested an association between the absence of LFA-1 expression on lymphoma cells and unfavorable prognosis, subsequent studies by these authors101 as well as by our own laboratory99,102 did not confirm this finding. Whether or not expression of ICAM-1, a major ligand of LFA-1, plays a role in lymphoma dissemination and disease outcome is controversial. Our own studies in large-cell NHLs did not reveal a relation of ICAM-1 expression with either dissemination or prognosis102; however, Terol et al103 recently reported that ICAM-1 correlates inversely with dissemination and overall survival in aggressive NHLs.
Integrin 4β1.
The leukocyte integrin α4β1 is unique among β1-integrins because it is not only involved in cell-matrix but also in cell-cell interaction. It can bind the CS-1 domain of the extracellular matrix component fibronectin as well as the vascular cell adhesion molecule (VCAM)-1, a transmembrane glycoprotein. The latter molecule is constitutively expressed by FDC and is induced on endothelium at sites of inflammation.
Integrin α4β1 plays an important role in lymphocyte migration to sites of inflammation, including inflamed joints in rheumatoid arthritis and inflammatory brain lesions in experimental allergic encephalitis, an animal model for multiple sclerosis 104-107 (Figure 2). During antigen-specific B-cell differentiation, α4β1 in concert with LFA-1 mediates the binding of germinal center B lymphocytes to FDC.83,108 This interaction is crucial for B-cell selection and for affinity maturation.109,110 It has been demonstrated that α4β1–VCAM-1 as well as LFA-1–ICAM interaction inhibits apoptosis of germinal center B cells in vitro.17 This suggests that α4β1 (and LFA-1) may have a dual role in the germinal center. Apart from mediating physical contact between B cells and FDC, these integrins may contribute to the B-cell selection process via outside-in signaling.17 111
In human NHLs, α4β1 is widely expressed in a pattern that matches the expression on related stages of normal lymphocyte differentiation.70,112 Like binding of normal germinal center B cells, the binding of the tumor cells of follicle-center cell lymphomas to FDC cells is mediated by α4β1.113 This interaction may play a role in the ongoing somatic hypermutation process in these lymphomas, which is believed to be antigen driven.114Transformation of follicular lymphoma to a diffuse phenotype is associated with loss of FDC from the tumor, rather than with down-regulation of α4β1 on the tumor cells.113 As with LFA-1, no consistent association between α4β1 expression and disease parameters has been found in NHLs.70 112 Conceivably, functional overlap between LFA-1 and α4β1 causes redundancy in interactions relevant for lymphoma dissemination, including those with endothelium and FDC (see below). Furthermore, because the function of integrins is activation dependent, their expression per se does not reflect their function.
Integrin 4β7; homing to the mucosa.
The integrin α4β7 mediates lymphocyte homing to the intestinal mucosa by binding to MAdCAM-1, a vascular addressin selectively expressed on mucosal endothelium (Figure2).63,115,116 MAdCAM-1 is an immunoglobulin family member with domains that display homology to the adhesion receptors ICAM-1 and VCAM-1, as well as to another mucosa-associated immunoglobulin family member, IgA1.115 In β7-knockout mice, the formation of MALT is severely impaired. This abnormality is caused by a defect in lymphocyte-endothelial interaction at the stage of tight adhesion and extravasation; β7-deficient lymphocytes show normal rolling on the endothelial lining of the high endothelial venules of Peyer patches, but they fail to stably arrest and to transmigrate.117 Using α4-null chimeric mice, Arroyo et al118 showed that lymphocytes lacking α4 also are unable to enter Peyer patches, whereas their homing to lymph nodes and spleen is not disturbed. Together, these findings demonstrate the key role of α4β7in mucosal homing in the mouse. In man, α4β7 appears to have a similar function. It is expressed on mucosal lymphocytes,82,119,120 and moreover, it is present on a subset of peripheral blood memory T cells with gut homing properties.76 Recently, the human MAdCAM-1 has been cloned.121 Like its murine homologue, it is preferentially expressed in the MALT.122
We have studied the expression of the α4β7mucosal homing receptor in NHLs.72,82 In MLP, an unusual presentation of NHL of mantle cell type characterized by multifocal involvement of the gastrointestinal tract, expression of α4β7 was present in 7 of 8 cases.72 By contrast, all cases of nodal mantle cell lymphoma in our study were α4β7 negative. The association between α4β7 expression and gastrointestinal tract dissemination of mantle cell lymphoma was recently confirmed by Geismann et al.123 These authors reported that α4β7, when present on nodal mantle cell lymphoma, predicts multifocal digestive tract involvement.
In most cases of low-grade B-cell lymphoma of MALT-type (Figure3) as well as in intestinal T-cell lymphoma, we also observed α4β7 expression. By contrast, expression of α4β7 on lymphomas with a nonmucosal primary localization was uncommon.82 Taken together, these findings suggest that α4β7 plays an important role in determining gastrointestinal tract involvement by lymphoma as well as in the characteristic mucosal dissemination often found in lymphomas of the MALT (Figure 2; Table 3). Interestingly, Dogan et al124recently reported that low-grade B-cell lymphomas of MALT type up-regulate α4β7 after H. pylori-induced T–cell-dependent proliferation of neoplastic cells.
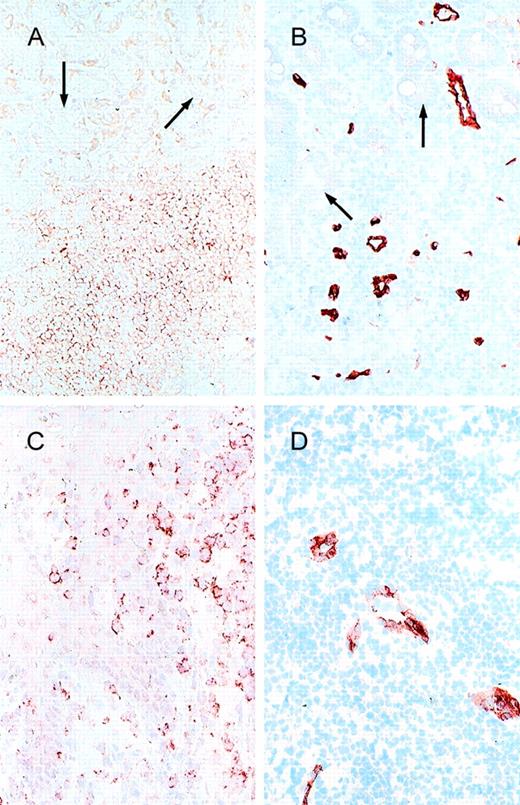
Cell adhesion receptors and vascular addressins.
(A) α4β7 and (B) MAdCAM-1 expression in a low-grade B-cell lymphoma of MALT type (stomach; arrow, epithelial structures); (C) CLA and (D) E-selectin expression in a cutaneous T-cell lymphoma.
Cell adhesion receptors and vascular addressins.
(A) α4β7 and (B) MAdCAM-1 expression in a low-grade B-cell lymphoma of MALT type (stomach; arrow, epithelial structures); (C) CLA and (D) E-selectin expression in a cutaneous T-cell lymphoma.
Eβ7; interaction with epithelium.
In the gut, the integrin αEβ7 is found on nearly all intestinal intraepithelial lymphocytes and on approximately 50% of the T cells in the lamina propria. αEβ7 can bind E-cadherin on epithelial cells and in this way mediates the positioning of lymphocytes in the epithelium125 (Figure 2).
Expression of αEβ7 is present on celiac disease-associated intestinal T-cell lymphomas.126Furthermore, intraepidermal malignant T lymphocytes in mycosis fungoides express αEβ7127 (Figure 2; Table3). In advanced stages of this disease with loss of epitheliotropism, expression of αEβ7 decreases, suggesting a direct involvement of αEβ7 in epitheliotropism. Consistent with this notion, we recently demonstrated a strong expression of αEβ7 in pagetoid reticulosis, a rare form of cutaneous T-cell lymphoma characterized by an extreme epitheliotropism and a pagetoid pattern of lymphocyte infiltration between keratinocytes.128
TheCD44 family
CD44 represents a family of glycoproteins encoded by a single gene on the human chromosome 11 (Figure4).13,129-134 The members of this family show a broad tissue distribution and have been implicated in a number of important biologic processes, including lymphocyte homing and activation, hematopoiesis, and tumor progression and metastasis.12,13,15,130,135-142 The CD44 gene consists of 19 exons.143 Structural and functional diversity of CD44 is generated by alternative splicing messenger RNA (mRNA) involving 10 exons encoding domains of the extracellular portion of the CD44 molecule. In addition to variable exon usage, variations in glycosylation contribute to the diversity of CD44.13
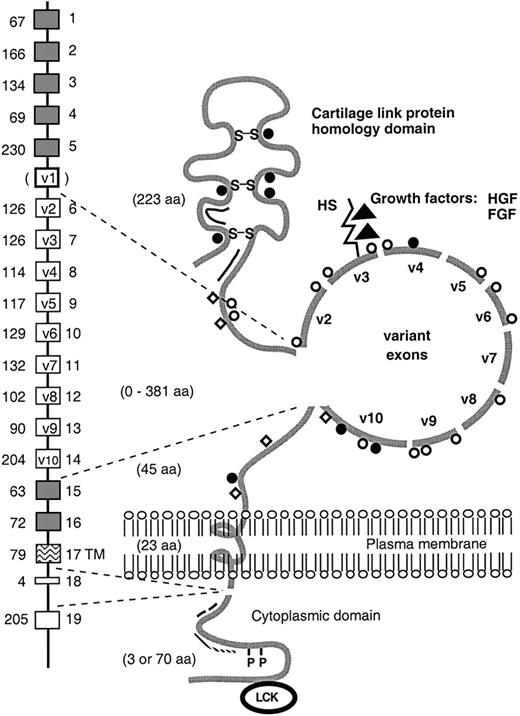
Schematic representation of the CD44 gene and its encoded proteins.
The extracellular domain and cytoplasmic tail of CD44 isoforms vary in size as the result of alternative splicing. The alternatively spliced exons are indicated by open boxes. The human v1 exon contains a stop codon. In the model of the protein, all putative glycosylation sites are indicated: O-glycosylation (open circles); N-glycosylation (closed circles); chondroitin sulfate (open squares); heparan sulfate (HS) (rod). As indicated, the HS-binding site in exon v3 has the ability to bind growth factors, including hepatocyte growth factor/scatter factor (HGF/SF) and fibroblast growth factor (FGF). In addition, the hyaluronate-binding sites (double line), the disulfide bonds (S-S), the ankyrin binding site (. . . . .), the Ezrin-binding site (– – –), the phosphorylation sites (P), and the putative interaction site for the src-family kinase p56lckare indicated. aa, aminoacid; TM, transmembrane.
Schematic representation of the CD44 gene and its encoded proteins.
The extracellular domain and cytoplasmic tail of CD44 isoforms vary in size as the result of alternative splicing. The alternatively spliced exons are indicated by open boxes. The human v1 exon contains a stop codon. In the model of the protein, all putative glycosylation sites are indicated: O-glycosylation (open circles); N-glycosylation (closed circles); chondroitin sulfate (open squares); heparan sulfate (HS) (rod). As indicated, the HS-binding site in exon v3 has the ability to bind growth factors, including hepatocyte growth factor/scatter factor (HGF/SF) and fibroblast growth factor (FGF). In addition, the hyaluronate-binding sites (double line), the disulfide bonds (S-S), the ankyrin binding site (. . . . .), the Ezrin-binding site (– – –), the phosphorylation sites (P), and the putative interaction site for the src-family kinase p56lckare indicated. aa, aminoacid; TM, transmembrane.
Most CD44-expressing cells express the “standard” CD44 (CD44s) isoform translated from an mRNA species containing none of the “variant” (v) exons.13,132,141 On hematopoietic cells and lymphocytes this 85- to 95-kd molecule is the principle CD44 isoform.132,141,144-146 Larger CD44 isoforms containing various combinations of variant exons are preferentially expressed on epithelial cells,144-146 but they can also be expressed on activated lymphocytes141,147 and aggressive malignant lymphomas.141 During lymphocyte ontogeny and activation, CD44 is strictly regulated, suggesting an important function in these processess.13,135,148 Indeed, CD44 has been reported to be involved in lymphopoiesis137 and lymphocyte activation,149-153 as well as in lymphocyte migration and homing.130 In the homing process, CD44 mediates lymphocyte binding to high endothelial venules,13,130,133,154lymphocyte rolling,21,36 and migration to inflammatory sites.20-26 Cross-linking of CD44 on lymphocytes costimulates antigen cell receptor-mediated proliferation, cytokine release, and integrin activation through outside-in signaling14,149-153 and leads to protein tyrosine kinase activation. This lymphocyte activation presumably involves src-family tyrosine kinases, because CD44 was shown to be physically and functionally associated with the p56lck in T cells.155 The CD44 cytoplasmic tail associates with the actin cytoskeleton via ankyrin and molecules of the ERM family.156-158 These cytoskeletal interactions may regulate hyaluronate binding and CD44-dependent motility as well as inside-out signaling events.
Several experimental and clinical studies suggest an important role of CD44 in the biologic behavior of NHLs and indicate that CD44 represents a clinically useful marker predicting disease outcome. In a nude mouse model, CD44s (but not CD44v8-10) enhances the growth and metastatic capacity of Burkitt lymphoma cell lines.140 Engagement of CD44 on the tumor cells with hyaluronate on the luminal surface of endothelial cells presumably plays an important role in these tumor-promoting effects. Furthermore, CD44 interaction with hyaluronate in the extracellular matrix may enhance tumor cell growth by providing a molecular bridge facilitating tumor cell attachment. The matrix might serve as a scaffold for tumor cell growth and as a reservoir for growth and motility factors with biologic effects on the tumor cells.159 In this context, it is of interest that CD44 variants containing exon v3 can be decorated with HS side chains, and by this virtue, can serve as receptors for heparin-binding growth factors, including MIP-1β, fibroblast growth factor (FGF)-2, and hepatocyte growth factor (HGF).47,160,161 Presentation of HGF to its receptor tyrosine kinase c-Met by HS forms of CD44 was recently shown to promote Met activation as well as activation of the RAS/MAP kinase and PI3 kinase pathways downstream of Met, and may enhance tumor growth and motility.161
Clinical studies support the importance of CD44 in the biology of human NHLs (Table 4). In diffuse large-cell lymphomas (DLCL), we observed a strong correlation between CD44 expression and lymphoma dissemination.102,136 Furthermore, CD44 expression is an unfavorable prognosticator in these tumors.102 Similar findings were reported by the group of Jalkanen139,162 for high-grade B-cell lymphomas. In a recent study, we explored the prognostic value of CD44 in a group of 276 patients diagnosed as having DLCL of the B lineage according to the criteria of the REAL classification.52 In this multicenter population-based study group, expression of CD44 was a powerful prognosticator for both overall and disease-free survival in patients with localized disease.163 In a multivariate comparison with the clinical parameters of the International Prognostic Index, currently used to predict prognosis in malignant lymphoma, CD44 expression was the major prognosticator for the subgroup of patients with localized nodal disease.
CD44 expression, relation to lymphoma dissemination, and prognosis
| Author . | Diagnosis . | No. Patients . | Exon . | Correlation . | Independent Prognostic Factor . | |
|---|---|---|---|---|---|---|
| Stage . | Prognosis . | |||||
| Pals136 | DLCL | 36 | CD44s | + | nt | nt |
| Horst102 | DLCL | 78 | CD44s | + | + | − |
| Jalkanen162 | Low-, intermediate-, and high-grade lymphomas | 104 | CD44s | + | + | + |
| Jalkanen139 | Low-, intermediate-, and high-grade lymphomas | 245 | CD44s | + | + | + |
| Joensuu186 | Low- and high-grade MALT | 27 | CD44s | − | + | nt |
| Stauder167 | High-grade B-cell lymphomas | 62 | CD44s | + | + | − |
| CD44v3 | + | − | − | |||
| CD44v6 | − | + | + | |||
| Drillenburg163 | DLCL | 276 | CD44s | + | +4-150 | +4-150 |
| CD44v6 | + | − | − | |||
| Author . | Diagnosis . | No. Patients . | Exon . | Correlation . | Independent Prognostic Factor . | |
|---|---|---|---|---|---|---|
| Stage . | Prognosis . | |||||
| Pals136 | DLCL | 36 | CD44s | + | nt | nt |
| Horst102 | DLCL | 78 | CD44s | + | + | − |
| Jalkanen162 | Low-, intermediate-, and high-grade lymphomas | 104 | CD44s | + | + | + |
| Jalkanen139 | Low-, intermediate-, and high-grade lymphomas | 245 | CD44s | + | + | + |
| Joensuu186 | Low- and high-grade MALT | 27 | CD44s | − | + | nt |
| Stauder167 | High-grade B-cell lymphomas | 62 | CD44s | + | + | − |
| CD44v3 | + | − | − | |||
| CD44v6 | − | + | + | |||
| Drillenburg163 | DLCL | 276 | CD44s | + | +4-150 | +4-150 |
| CD44v6 | + | − | − | |||
Only in patients with localized disease. DLCL indicates large B-cell lymphoma; MALT, mucosa-associated lymphoid tissue; nt, not tested.
In all the above-mentioned clinical studies, mAbs against epitopes on the constant part of CD44 (CD44s) were used to assess CD44 expression (Table 4). However, in addition to CD44s, NHLs may express CD44 isoforms containing variant exons.141,164-168 These larger splice variants appear to be predominantly expressed on a subgroup of aggressive lymphomas.141,165,167,169 They often contain exon v6/7 encoded sequences, which have been reported to confer metastatic behavior in rat carcinoma cell lines.138 The function of CD44 splice variants on lymphocytes is as yet incompletely understood, but they presumably have a role in lymphocyte activation. Antibodies against v6 are immunosuppressive, whereas mice carrying a CD44v4-v7 transgene in their T cells show an accelerated immune response against T–cell-dependent antigens.170,171 Stauder and colleagues167 studied CD44 variant expression in a mixed group of high-grade NHLs, including precursor B-cell lymphomas, Burkitt lymphomas, and DLCL. In this group, CD44v6 was an independent prognosticator predicting tumor-related death. However, in our own study focusing on a single diagnostic group (ie, DLCL), CD44v6 had no prognostic value, whereas CD44s expression was an important prognosticator.163 Thus, although there is general agreement that CD44 is a potentially useful biologic prognosticator (co)determining lymphoma dissemination, the question whether epitopes on the constant or variant part of the molecule are the most suitable for assessing prognosis needs further exploration. To answer this question, studies comparing different anti-CD44 mAbs by standardized techniques in patients belonging to defined clinicopathologic subgroups and receiving uniform treatment are needed.
Supported by grants from the Dutch Cancer Society and the Association for International Cancer Research.
Reprints:Steven T. Pals, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: s.t.pals@amc.uva.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal