A new bone marrow transplantation (BMT) method for treating severe autoimmune diseases in chimeric resistant MRL/lpr mice is presented. The method consists of fractionated irradiation (5.5 Gy × 2), followed by portal venous (PV) injection of whole bone marrow cells (BMCs) from allogeneic normal C57BL/6 (B6) mice and intravenous (IV) injection of whole B6 BMCs 5 days after the PV injection (abbreviated as 5.5 Gy × 2 + PV + IV). All recipients survived more than 1 year after this treatment (more than 64 weeks after birth). Abnormal T cells (Thy1.2+/B220+/CD3+/CD4−/CD8−) present in MRL/lpr mice before the treatment disappear, and hematolymphoid cells are reconstituted with donor-derived cells. The treated mice are free from autoimmune diseases. Levels of autoantibodies (IgG/IgM anti-ssDNA antibodies and IgG/IgM rheumatoid factors) decrease to normal levels. Successful cooperation is achieved among T cells, B cells, and antigen-presenting cells (APCs) of the treated MRL/lpr mice when evaluated by in vitro anti-SRBC responses. Newly developed T cells are tolerant to both donor (B6)-type and host (MRL/lpr)-type major histocompatibility complex (MHC) determinants. These findings clearly indicate that severe autoimmune diseases in MRL/lpr mice are completely ameliorated by the treatment without recourse to immunosuppressants, and that the treated MRL/lpr mice show normal immune functions, strongly suggesting that this strategy would be applicable to humans.
Various mouse strains that spontaneously develop autoimmune diseases have contributed not only to a better understanding of the fundamental nature of autoimmune diseases but also to the analysis of their etiopathogenesis.1 Using these mice, we have previously found that allogeneic bone marrow transplantation (BMT) (not autologous BMT) can be used to treat autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), immune thrombocytic purpura (ITP), insulin-dependent diabetes mellitus (IDDM), chronic glomerulonephritis, and a certain type of non-insulin–dependent diabetes mellitus (NIDDM).2-10 In contrast, we have found that the transplantation of T-cell–depleted bone marrow cells (BMCs) or partially purified hematopoietic stem cells (HSCs) from autoimmune-prone mice to normal mice leads to the induction of autoimmune diseases in the recipients.11,12 These findings have recently been confirmed even in humans; autoimmune diseases such as RA, SLE, multiple sclerosis (MS), and Crohn's disease were resolved after allogeneic BMT.13-20 Conversely, the adoptive transfer of autoimmune diseases such as myasthenia gravis, IDDM, and Graves' disease by allogeneic BMT from donors to recipients has been reported.21-29 On the basis of these findings, we have proposed that autoimmune disease is “a stem cell disorder.”9-11 However, in humans, autologous BMT or peripheral blood stem cell transplantation (PBSCT) is now carried out for the treatment of autoimmune diseases,30-32 because the success rate of BMT across major histocompatibility (MHC) barriers is not high as a result of (1) graft-versus-host reaction (GvHR), (2) graft rejection, and (3) incomplete T-cell recovery. There have, however, been reports on the rapid recurrence or persistence of autoimmune diseases after autologous BMT.33
In mice, we have previously shown that allogeneic BMT can be used to prevent and treat autoimmune diseases in B/WF1, BXSB, W/BF1, and NOD mice.2-4,8 In MRL/lpr mice that are radiosensitive (<8.5 Gy), we have found that conventional BMT (8.5 Gy irradiation plus allogeneic BMT) has a transient effect on autoimmune diseases,2 whereas the autoimmune diseases recur 3 months after BMT.8 However, we have found that BMT plus bone grafts (to recruit donor stromal cells) completely prevents the recurrence of autoimmune diseases in MRL/lpr mice.34Donor-derived stromal cells play a crucial role in successful allogeneic BMT,34,35 because MHC restriction exists between HSCs and stromal cells.36 However, we have found that the combination of BMT plus bone grafts has no effect on the treatment of autoimmune diseases in MRL/lpr mice37; MRL/lpr mice become more radiosensitive after the onset of lupus nephritis, due to uremic enterocolitis. To determine the optimal radiation dose to treat autoimmune diseases in MRL/lpr mice, we have irradiated MRL/lpr mice with 6 to 9.5 Gy in combination with BMT plus bone grafts. Almost all MRL/lpr mice (after the onset) died because of infection from the intestine if the doses were more than 8.5 Gy. Because irradiation doses less than 8.5 Gy do not kill abnormal HSCs in MRL/lpr mice (as we previously described8), we carried out fractionated irradiation and devised a new strategy. The strategy includes the injection of cyclophosphamide (CY), fractionated irradiation (5 Gy × 2), bone grafts (to recruit stromal cells), and 2 transplantations of whole BMCs from B6 mice.37 However, this strategy is not applicable to humans, because we have to carry out bone grafts to recruit donor stromal cells.
Recently, we have found that most donor HSCs are trapped and retained in the liver when they are injected either portal venously (PV) or even intravenously (IV),38 and that the HSCs induce anergy in host CD8+ T cells.39 In addition, we have found that a strategy (PV [on day 0] plus IV [on day 5] injections of donor whole BMCs) can induce persistent tolerance in the skin allograft system.40 On the basis of these findings, we attempted to establish a new strategy for allogeneic BMT applicable to humans: fractionated irradiation 5.5 Gy × 2 and the PV administration of 3 × 107 whole B6 BMCs, followed 5 days later by the IV administration of 3 × 107 whole B6 BMCs. Using this method, we show that severe autoimmune diseases in chimeric resistant MRL/lpr mice are completely resolved without recourse to any immunosuppressants.
Materials and methods
Mice
Female MRL/Mp-lpr/lpr (MRL/lpr), C57BL/6 (B6), BALB/c, and C3H/HeN mice were obtained from SLC (Shizuoka, Japan) and maintained until use in our animal facilities under specific pathogen-free conditions.
Preparation of allogeneic blood marrow cells and injection of blood marrow cells via portal vein
BMCs were collected from the femurs and tibias of B6 mice. In some experiments, a purified rat monoclonal antibody (mAb) against Thy 1.2 (PharMingen, San Diego, CA) was used to deplete T cells, in combination with magnetic beads conjugated with sheep antirat IgG Ab (Dynabeads M-450; Dynal AS, Oslo, Norway). Residual T cells after the treatment were less than 0.05% when stained with FITC- or PE-anti-CD4, CD8, or Thy 1.2Ab, and examined by a FACScan® (Becton Dickinson & Co, Mountain View, CA). The BMCs were injected via the portal vein, as described previously.38 In brief, the donor BMCs (3 × 107) in 0.3 mL of RPMI 1640 medium were injected via the superior mesenteric vein using a 27-gauge needle.
Experimental protocols
The onset of autoimmune diseases in MRL/lpr mice was monitored by proteinuria (more than 2.5+) and lymphadenopathy. The mice (4 to 5 months of age) with autoimmune diseases were irradiated in fractionated irradiations (5.5 Gy × 2 = 11 Gy; 4-hour interval). One day after the irradiation, the mice were transplanted with 3 × 107 whole BMCs via the portal vein (5.5 Gy × 2 + PV). MRL/lpr mice that had been irradiated 5.5 Gy × 2 and transplanted with 3 × 107 whole BMCs via the portal vein were further transplanted with whole BMCs intravenously 5 days after the PV administration (5.5 Gy × 2 + PV + IV). In addition, the following groups were prepared: (1) (5.5 Gy × 2 + IV), (2) (5.5 Gy × 2 + IV + IV), (3) (5 Gy × 2 + PV), (4) (5 Gy × 2 + PV + IV), (5) (6 Gy × 2 + PV), (6) (5.5 Gy × 2 + PV [−T] + IV [−T]): fractionated irradiation, (5.5 Gy × 2) PV administration of T-cell–depleted BMCs (3 × 107), and IV administration of T-cell–depleted BMCs (3 × 107) 5 days after PV administration; and (7) (8.5 Gy + IV): single dose irradiation (8.5 Gy), and IV administration of 3 × 107 whole BMCs.
Preparation of hepatic mononuclear cells (HMNCs)
Mice were systematically perfused with 4 mL of heparinized (10 units/ mL) phosphate-buffered saline (PBS) (0.01 mol/L pH 7.2) to eliminate blood. The liver was further perfused with 5 mL of PBS containing collagenase (Type IV, 400 units/mL, Sigma Chemical Co, St Louis, MO) via the portal vein. After perfusion, the liver was suspended in 5 mL of the collagenase-PBS and incubated at 37°C for 40 minutes. A single cell-suspension was then obtained by simply cutting and flushing. After washing twice with PBS, the cells were suspended in 10 mL of PBS containing 1% FCS and placed on 10 mL of Lympholyte-Mammal density solution (1.0860 g/mL, Cedarlane Laboratories Ltd, Hornby, Ontario, Canada). After centrifugation for 30 minutes at 2500 rpm at room temperature, the mononuclear cells (MNCs) were collected from the defined layer at the interface.
Immunological assays
Recipient mice were killed, and their spleens were removed. The immunological functions of the mice were examined as follows: (1) antibody production against sheep red blood cells (SRBCs) and (2) mixed leukocyte reaction (MLR). In the anti-SRBC antibody response, 4 × 106 spleen cells were cultured with the same number of SRBCs in 24-well flat-bottom culture plates (INC Biomedicals Inc, Ohio) for 5 days, and anti-SRBC antibody production was measured by the modified Jerne's plaque-forming cell (PFC) assay, as previously described.8 MLR was performed as follows: Spleen cells were treated with the mixture of mAbs against B cells (B220, RA3-6B2), macrophages (Mac-1, M1/70), granulocytes (Gr-1, RB6), and erythroid-lineage cells (TER119) (PharMingen), in combination with magnetic beads conjugated with sheep antirat IgG Ab (Dynabeads M-450). The resultant T-cell–enriched cells were used as responders. Triplicate cultures were set up in 96-well flat-bottom microwell trays (Corning Inc, Corning, NY). Each well contained 2 × 105 responder T cells and 2 × 105 irradiated (12 Gy) stimulator spleen cells in a total of 0.2 mL of RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, and 50 μmol/L 2-mercaptoethanol (2-ME: Wako, Osaka, Japan). The culture was incubated for 72 hours and pulsed with 0.5 μCi of 3H]-thymidine for the last 16 hours of the culturing period.
Surface marker analyses
FITC-coupled anti-H-2Db and PE-coupled anti-H-2Kk mAbs from PharMingen were used for H-2 typings. FITC- or PE-coupled mAbs against Thy 1.2, B220, CD4, and CD8 (PharMingen) were used to analyze the cell surface phenotypes. Lymph node and spleen cells were prepared from recipient mice, and the cells were stained with appropriate FITC- or PE-conjugated mAbs to detect abnormal T cells with the immunophenotypes of Thy1.2+/B220+/CD4−/CD8−or donor-derived cells. Cells were analyzed by a FACScan®.
Proteinuria
Proteinuria was measured using testing papers (Albustix: Miles-Sankyo Ltd Co, Tokyo, Japan).
Measurement of autoantibodies
RF (IgG and IgM) and anti-ssDNA Abs (IgG and IgM) in the sera of the recipient mice were determined by a standard enzyme-linked immunosorbent assay (ELISA). Autoantibodies were measured by absorbance at OD405 nm, which is the maximum absorbance using the phosphatase substrate tablet (Sigma 104; SIGMAD).
Pathologic findings
The kidneys of the recipient mice were removed and fixed in 10% formalin, and the sections were stained with hematoxylin and eosin (H-E). For the immunofluorescence study, the specimens were frozen in dry ice/acetone, as previously described.7 Briefly, 3-μm sections were stained with FITC-conjugated rabbit antimouse IgG or FITC-conjugated antimouse C3 (Medical and Biological Laboratories, Nagoya, Japan).
Statistical analyses
Statistical analyses of the survival rate of recipient mice were performed using a log rank test.
Results
Survival rates after various treatments
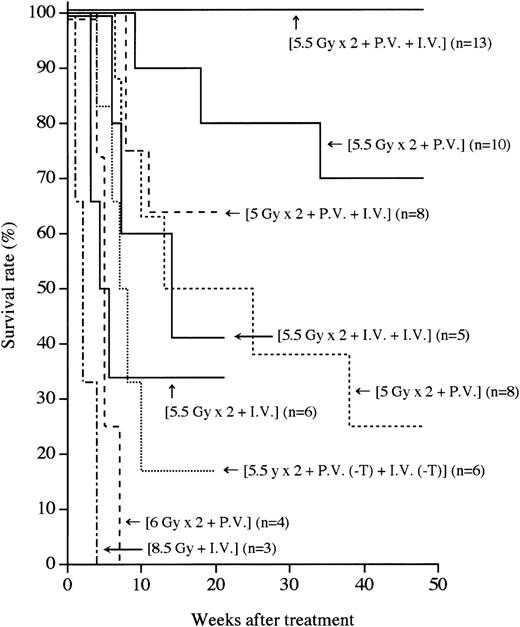
MRL/lpr mice (4 to 5 months of age) that had developed the symptoms of autoimmune diseases such as massive lymphadenopathy and proteinuria (more than 2.5+) were first treated with (5.5 Gy × 2 + PV), as described in the “Methods.” As shown in Figure1, more than 70% of the mice thus treated survived more than 1 year, indicating that this treatment has some effect on the treatment of autoimmune diseases. We next treated autoimmune diseases in MRL/lpr mice with (5.5 Gy × 2 + PV + IV), as described in the “Methods.” Thus treated MRL/lpr mice showed a 100% survival rate 1 year after the treatment, indicating that the supplemental IV injection is helpful for successful engraftment. In contrast, all the recipients treated with (8.5 Gy + IV) died within 4 weeks because of the side effects of radiation, as previously reported.37 The MRL/lpr mice treated with either (5.5 Gy × 2 + IV) or (5.5 Gy × 2 + IV + IV) showed survival rates of 33% and 40%, respectively, 21 weeks after the treatment. This appears to be due to graft rejection, because donor hematolymphoid cells cannot be detected in the recipients. These findings suggest that the PV injection of BMCs has much more effect on prolonging survival than the IV injection. To confirm that the PV injection is effective in successful engraftment of donor cells, the mice were transplanted with a small number (3 × 106) of whole BMCs via the portal vein. Sixty percent (6/10) of the recipients survived more than 32 weeks after the (5.5 Gy × 2 + PV [3 × 106]) treatment (data not shown), indicating an advantage to the PV administration of BMCs.
Survival rates after various treatments.
Survival rates of the recipients treated with single or fractionated irradiation, followed by PV and/or IV administrations of BMCs are shown. Numbers in parentheses represent the numbers of mice in each group. Statistical analyses were performed by a logrank test:P < .05, (5.5 Gy × 2 + PV + IV) vs (5.5 Gy × 2 + PV).
Survival rates after various treatments.
Survival rates of the recipients treated with single or fractionated irradiation, followed by PV and/or IV administrations of BMCs are shown. Numbers in parentheses represent the numbers of mice in each group. Statistical analyses were performed by a logrank test:P < .05, (5.5 Gy × 2 + PV + IV) vs (5.5 Gy × 2 + PV).
To investigate whether T cells in the BMCs are necessary for the engraftment, T-cell–depleted BMCs (3 × 107) were used (5.5 Gy × 2 + PV [−T] + IV [−T]). Eighty percent of the mice that had received such treatment died by 20 weeks (Figure 1), indicating that T cells (approximately 1%) in BMCs are essential for the engraftment and treatment, as previouslyreported.37
We next examined whether the radiation dose could be reduced when BMCs were PV injected. We treated MRL/lpr mice with (5 Gy × 2 + PV) or (5 Gy × 2 + PV + IV). However, more than 70% of the recipients treated with (5 Gy × 2 + PV) died within 35 weeks because of a recurrence of the autoimmune diseases (renal failure), and the mice treated with (5 Gy × 2 + PV + IV) showed a 65% survival rate 15 weeks after the treatment (Figure 1), indicating that the 5 Gy × 2 irradiation (without recourse to CY administration plus bone grafts, as previously reported37) was insufficient for depleting residual MRL/lpr HSCs. All the recipients that had been treated with a higher dose of irradiation (6 Gy × 2 + PV) died because of intestinal infection by 7 weeks (Figure 1). These findings suggest that (5.5 Gy × 2) is the most suitable irradiation dose. It should be noted that no immunosuppressants are necessary for this strategy.
Chimerism in MRL/lpr mice treated with (5.5 Gy × 2 + PV) or (5.5 Gy × 2 + IV)
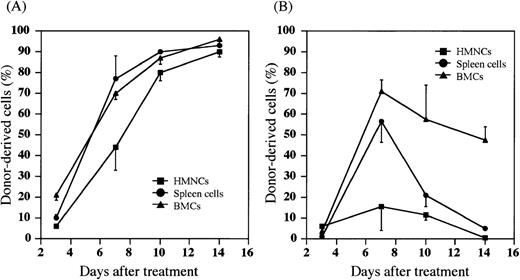
We have recently found that most donor HSCs are trapped and retained in the liver after either PV or IV injection of HSCs, and that the percentage of allogeneic donor HSCs in the liver is significantly higher after the PV injection than the IV injection.38Therefore, we next examined whether the donor cells were detected in the host hematolymphoid organs after the PV injection of BMCs. Hepatic mononuclear cells (HMNCs), spleen cells, and BMCs in the recipients were kinetically analyzed after the treatment with (5.5 Gy × 2 + PV) or (5.5 Gy × 2 + IV). When MRL/lpr mice were treated with (5.5 Gy × 2 + PV), percentages of donor-derived cells in HMNCs, spleen cells and BMCs gradually increased to almost 100% 14 days after the treatment (Figure 2A). The cells with mature lineage markers (B220+, CD4+, CD8+, Mac-1+, Gr-1+, or CD11c+) were contained in these donor-derived cells (data not shown). Therefore, hematolymphoid cells in the recipients were completely reconstituted with the cells of donor origin (6/6). In contrast, as shown in Figure 2B, donor cell-engraftment in the recipients treated with (5.5 Gy × 2 + IV) was transient in the spleen cells or HMNCs, although a high percentage of donor cells was found in the bone marrow (approximately 50% 14 days after treatment). These findings again indicate that the PV injection may facilitate the early engraftment of donor cells in the hematolymphoid organs.
Percentages of donor-derived cells in MRL/lpr mice treated with (5.5 Gy × 2 + PV) or (5.5 Gy × 2 + IV).
HMNCs (closed square), spleen cells (closed circle), and BMCs (closed triangle) in the recipient mice were collected and stained with FITC anti-H-2b mAb to detect donor-derived cells at the days indicated on the X-axis. (A) MRL/lpr mice treated with (5.5 Gy × 2 + PV), (B) MRL/lpr mice treated with (5.5 Gy × 2 + IV). The results are expressed as the mean ± SD of 6 mice.
Percentages of donor-derived cells in MRL/lpr mice treated with (5.5 Gy × 2 + PV) or (5.5 Gy × 2 + IV).
HMNCs (closed square), spleen cells (closed circle), and BMCs (closed triangle) in the recipient mice were collected and stained with FITC anti-H-2b mAb to detect donor-derived cells at the days indicated on the X-axis. (A) MRL/lpr mice treated with (5.5 Gy × 2 + PV), (B) MRL/lpr mice treated with (5.5 Gy × 2 + IV). The results are expressed as the mean ± SD of 6 mice.
Immunological findings in MRL/lpr mice treated with (5.5 Gy × 2 + PV + IV)
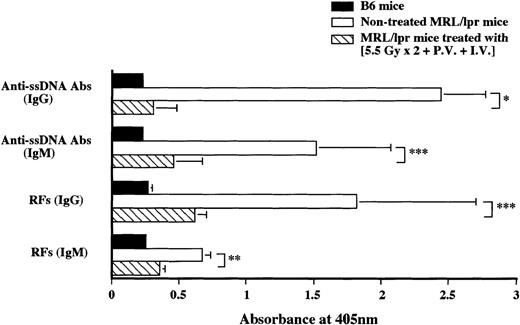
Nontreated MRL/lpr mice at the age of 20 weeks showed increased anti-ssDNA Abs (IgG and IgM) and RFs (IgG and IgM), whereas MRL/lpr mice treated with (5.5 Gy × 2 + PV + IV) showed low autoantibody levels (almost to the normal level) 48 weeks after the treatment (Figure 3).
Measurement of serum autoantibody levels in MRL/lpr mice treated with (5.5 Gy × 2 + PV + IV).
RFs and anti-ssDNA Abs were measured 48 weeks after the treatment (striped bars), B6 mice (black bars), and nontreated MRL/lpr mice (white bars). Autoantibodies in normal B6 and nontreated MRL/lpr mice were measured at 20 weeks of age. The results are expressed as the mean ± SD of 6 mice. Asterisks represent P values of treated versus nontreated MRL/lpr mice; *P < .001, **P < .01, and ***P < .05.
Measurement of serum autoantibody levels in MRL/lpr mice treated with (5.5 Gy × 2 + PV + IV).
RFs and anti-ssDNA Abs were measured 48 weeks after the treatment (striped bars), B6 mice (black bars), and nontreated MRL/lpr mice (white bars). Autoantibodies in normal B6 and nontreated MRL/lpr mice were measured at 20 weeks of age. The results are expressed as the mean ± SD of 6 mice. Asterisks represent P values of treated versus nontreated MRL/lpr mice; *P < .001, **P < .01, and ***P < .05.
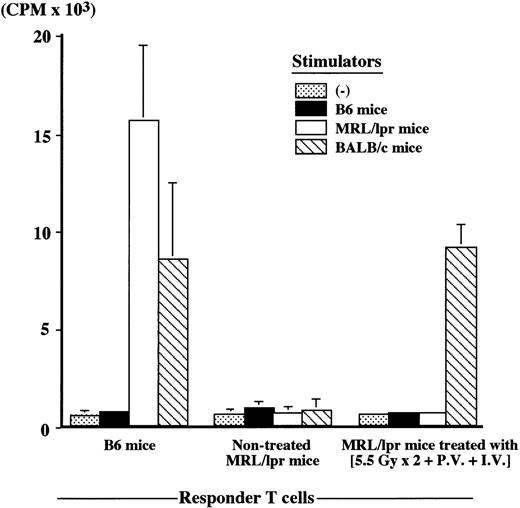
Nontreated MRL/lpr mice at the age of 20 weeks showed an extremely low anti-SRBC antibody response (number of PFC/culture, 2 ± 3), whereas MRL/lpr mice (64 weeks old) treated with (5.5 Gy × 2 + PV + IV) showed a high anti-SRBC response 48 weeks after the treatment (number of PFC/culture, 742 ± 28), the level being completely restored to the normal level (number of PFC/culture, 806 ± 82) seen in B6 mice (12 weeks old). Furthermore, newly developed T cells were tolerant to both host (MRL/lpr)-type and donor (B6)-type MHC determinants, whereas they showed a normal responsiveness to the third-party (BALB/c) cells, when examined in MLR (Figure4). These findings indicate that successful cooperation can be achieved among newly developed T cells, B cells, and APCs in the treated MRL/lpr mice.
Mixed lymphocyte reaction (MLR) in MRL/lpr mice treated with (5.5 Gy × 2 + PV + IV).
MLR was performed 48 weeks after the treatment, and compared with B6 (12 weeks old) and nontreated MRL/lpr mice at 20 weeks of age. Responder T cells (2 × 105) were mixed with 2 × 105 stimulator cells. The results are expressed as the mean ± SD of 6 mice.
Mixed lymphocyte reaction (MLR) in MRL/lpr mice treated with (5.5 Gy × 2 + PV + IV).
MLR was performed 48 weeks after the treatment, and compared with B6 (12 weeks old) and nontreated MRL/lpr mice at 20 weeks of age. Responder T cells (2 × 105) were mixed with 2 × 105 stimulator cells. The results are expressed as the mean ± SD of 6 mice.
The numbers of abnormal T cells (Thy1.2+/B220+) increased in the spleen and lymph nodes of nontreated MRL/lpr mice at the age of 20 weeks (Table 1). In contrast, abnormal T cells disappeared in MRL/lpr (64-week-old) mice treated with the (5.5 Gy × 2 + PV + IV) 48 weeks after the treatment; all the cells in the spleen and lymph nodes were of donor origin. This indicates that the hematolymphoid cells are completely reconstituted with donor-derived cells, although the treated MRL/lpr mice (64 weeks old) showed, due to aging, a lower percentage (26.6%) of T cells in the lymph node than young (12-week-old) B6 mice.
Analyses of cell surface antigens in MRL/lpr mice treated with (5.5 Gy × 2 + PV + LV) or nontreated MRL/lpr mice
| Mice (age) . | Organs . | Cell Surface Antigens (%) . | H-2 Typing (%) . | |||
|---|---|---|---|---|---|---|
| Thy 1.2+/ B220− . | Thy 1.2−/ B220+ . | Thy 1.2+/ B220+ . | H-2k . | H-2b . | ||
| Treated MRL/lpr (64 wk) | Spleen | 13.2 ± 8.8 | 60.0 ± 14.9 | 0.3 ± 0.2 | 0.0 ± 0.0 | 97.9 ± 2.3 |
| Lymph nodes | 26.6 ± 0.0 | 45.6 ± 5.2 | 0.8 ± 0.2 | 0.4 ± 0.5 | 93.7 ± 4.2 | |
| Nontreated MRL/lpr (20 wk) | Spleen | 30.2 ± 8.5 | 26.7 ± 11.5 | 24.7 ± 11.0 | 99.3 ± 0.6 | 0.0 ± 0.0 |
| Lymph nodes | 27.0 ± 4.6 | 7.5 ± 2.1 | 62.1 ± 23.6 | 99.9 ± 0.2 | 0.0 ± 0.0 | |
| B6 (12 wk) | Spleen | 19.2 ± 2.0 | 71.2 ± 3.6 | 0.8 ± 0.18 | 0.0 ± 0.0 | 99.7 ± 0.1 |
| Lymph nodes | 66.5 ± 9.7 | 29.2 ± 7.0 | 1.3 ± 0.4 | 0.0 ± 0.0 | 99.8 ± 0.1 | |
| Mice (age) . | Organs . | Cell Surface Antigens (%) . | H-2 Typing (%) . | |||
|---|---|---|---|---|---|---|
| Thy 1.2+/ B220− . | Thy 1.2−/ B220+ . | Thy 1.2+/ B220+ . | H-2k . | H-2b . | ||
| Treated MRL/lpr (64 wk) | Spleen | 13.2 ± 8.8 | 60.0 ± 14.9 | 0.3 ± 0.2 | 0.0 ± 0.0 | 97.9 ± 2.3 |
| Lymph nodes | 26.6 ± 0.0 | 45.6 ± 5.2 | 0.8 ± 0.2 | 0.4 ± 0.5 | 93.7 ± 4.2 | |
| Nontreated MRL/lpr (20 wk) | Spleen | 30.2 ± 8.5 | 26.7 ± 11.5 | 24.7 ± 11.0 | 99.3 ± 0.6 | 0.0 ± 0.0 |
| Lymph nodes | 27.0 ± 4.6 | 7.5 ± 2.1 | 62.1 ± 23.6 | 99.9 ± 0.2 | 0.0 ± 0.0 | |
| B6 (12 wk) | Spleen | 19.2 ± 2.0 | 71.2 ± 3.6 | 0.8 ± 0.18 | 0.0 ± 0.0 | 99.7 ± 0.1 |
| Lymph nodes | 66.5 ± 9.7 | 29.2 ± 7.0 | 1.3 ± 0.4 | 0.0 ± 0.0 | 99.8 ± 0.1 | |
The results are expressed as the mean ± SD of 6 mice. Cells from the spleen and lymph nodes (inguinal and axillary regions) were stained with FITC- or PE-conjugated mAbs, and analyzed using a FACScan.
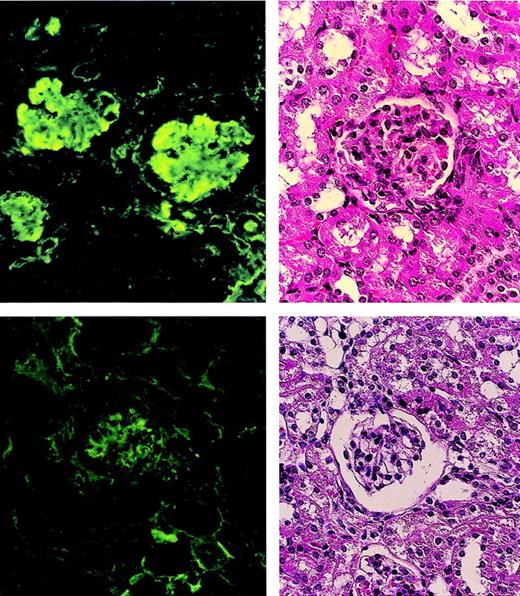
The glomeruli of the nontreated MRL/lpr mice showed the proliferation of mesangial cells and marked IgG deposits (Figure5 left), whereas the glomeruli of the MRL/lpr mice treated with the (5.5 Gy × 2 + PV + IV) showed normal appearance without IgG deposits (Figure 5 right).
Pathologic findings in glomeruli of MRL/lpr mouse kidneys.
A nontreated MRL/lpr mouse (20 weeks old) shows the proliferation of mesangial cells in the glomerulus (left, top) and marked IgG deposits (left, bottom). An MRL/lpr mouse (64 weeks old) treated with (5.5 Gy × 2 + PV + IV) shows normal appearance (right, top) without IgG deposits (right, bottom) 48 weeks after treatment.
Pathologic findings in glomeruli of MRL/lpr mouse kidneys.
A nontreated MRL/lpr mouse (20 weeks old) shows the proliferation of mesangial cells in the glomerulus (left, top) and marked IgG deposits (left, bottom). An MRL/lpr mouse (64 weeks old) treated with (5.5 Gy × 2 + PV + IV) shows normal appearance (right, top) without IgG deposits (right, bottom) 48 weeks after treatment.
Discussion
We have previously found that the IV injection of allogeneic BMCs (T-cell–depleted) plus bone grafts after a single dose of irradiation (8.5 Gy) has completely preventative effects on the autoimmune diseases in MRL/lpr mice,34 and that 2 IV injections of allogeneic whole BMCs plus bone grafts after the injection of CY and fractionated irradiations (5 Gy × 2) can resolve autoimmune diseases.37 However, these strategies are not applicable to humans, because we have to carry out bone grafts to recruit donor stromal cells. Considering this problem, we have devised a new method to treat severe autoimmune diseases in MRL/lpr mice. The method includes the PV injection of whole B6 BMCs after fractionated irradiations (5.5 Gy × 2) without recourse to any immunosuppressants or bone grafts.
We have previously reported that MHC-matched stromal cells are required for the proliferation and differentiation of HSCs,36 and that the donor-derived stromal cells play a crucial role in the success of allogeneic BMT.34,37 41 Therefore, the PV injection of donor whole BMCs (including stromal cells) should help create a suitable microenvironment in the liver and facilitate donor cell engraftment. Indeed, we have very recently found that the PV injection of BMCs after depleting stromal cells results in decreased CFU-S counts and a low survival rate (Fan T et al, manuscript in preparation). This finding strongly suggests that stromal cells contained in the donor whole BMCs are cotransplanted to the host liver by the PV injection, and that this facilitates the proliferation of donor HSCs in collaboration with MHC-matched donor stromal cells in the liver.
We have also previously shown that a small number (approximately 0.5%-1%) of T cells (particularly CD8+ T cells) contained in the BMCs are necessary for the engraftment of BMCs in the treatment of autoimmune diseases in MRL/lpr mice.37Martin42 has also found that CD8+ cells in the BMCs are essential for successful engraftment of donor-type hematolymphoid cells. This is the case in our study: The administration of T-cell–depleted BMCs was found to have no effect on the donor cell engraftment even via the portal vein route; 80% of the mice that had received such treatment died by 20 weeks after the treatment because of graft rejection. This likely reflects that the donor T cells are necessary for preventing the rejection of HSCs.
It has been shown that long-term injections of MRL/lpr mice with Abs against T cells (anti-Thy1.2 or anti-CD4 Ab) retard both lymphadenopathy and the progression of autoimmune diseases, as long as the agents are continued.43 44 However, these treatments are not applicable to humans. Actually, the long-term administration of agents such as anti-inflammatory agents, any immunosuppressants, and cytotoxic agents that have been used to treat SLE and RA results in cumulative side effects and a large burden on the patient. In contrast, the PV administration dispensed with the need for immunosuppressants, which are essential for conventional BMT via the IV route.
There are two types of animal models for autoimmune diseases: in one type, the disease is antigen-induced, whereas it develops spontaneously in the other. The animal models for RA and MS are well known as belonging to the former type. However, it seems likely that the abnormality in this model of autoimmune disease resides in immunocompetent cells, particularly T cells and B cells (but not HSCs). Therefore, autologous BMT or peripheral blood stem cell transplantation (PBSCT) can be used to treat antigen-induced autoimmune disease,45-49 because autoreactive clones are eliminated by irradiation when the BMT is carried out. In contrast, autologous BMT or PBSCT cannot be used to treat spontaneous autoimmune diseases, because spontaneous autoimmune diseases are “stem cell disorders,” as we have previously shown.11,12 To date, no one has been able to identify which patients have antigen-induced autoimmune diseases and which have spontaneous autoimmune diseases. It is therefore not possible to say which patients should, preferentially, receive autologous BMT or PBSCT. Because, during long-term observation (7 to 20 years), no autoimmune diseases recurred after allogeneic BMT in the 13 patients with autoimmune diseases plus leukemia or aplastic anemia,20 hopes have been pinned on the development of a new strategy for safer allogeneic BMT. In this study, we have shown an approach to allogeneic BMT: the administration of BMCs via the portal vein route facilitates the recovery of hemopoiesis, which reduces the physical burden on the patient. Therefore, we believe that this method will likely become useful for human application, because laparoscope-guided PV administration is a well-established and safe technique.
Acknowledgments
We thank Ms Y. Tokuyama and Ms M. Shinkawa for their expert technical assistance, and Mr Hilary Eastwick-Field and Ms K. Ando for their help in the preparation of the manuscript.
This work was supported by a grant for Experimental Models for Intractable Disease from the Ministry of Health and Welfare of Japan and a grant from the Japanese Private School Foundation, a grant from “Haiteku Research Center” of the Ministry of Education, grants-in-aid for scientific research (B) 11470062 and grant-in-aid for scientific research on priority areas (A)10181225,11162221.
Reprints:Susumu Ikehara, MD, PhD, First Department of Pathology, Kansai Medical University, 10-15 Fumizono-cho, Moriguchi City, Osaka 570-8506, Japan; e-mail: ikehara@takii.kmu.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal