Germline mutations of the CDKN2A(p16INK4A) tumor suppressor gene predispose patients to melanoma and pancreatic carcinoma. In contrast, mutations of the murine CDKN2A gene predispose BALB/c mice to pristane-induced plasmacytoma. We describe here a family in which a germline mutation of CDKN2A is present in 4 individuals who developed melanoma as well as in a fifth family member who is suffering from multiple myeloma. To determine whether the CDKN2A mutation predisposed the myeloma patient to her disease, we carried out loss of heterozygosity studies on sorted bone marrow from this individual and observed loss of the wild type CDKN2A allele in the malignant plasma cells. We suggest that germline mutations of CDKN2A may predispose individuals to a wider variety of malignancy than has been hitherto reported, but that the expression of these cancers may depend heavily on the genetic background of the patient.
The CDKN2A tumor suppressor gene encodes a cell cycle inhibitor (designated protein 16 [p16], p16INK4A, or CDKN2A) that binds to and sequesters the CDK4 and CDK6 kinases from their regulatory cyclin D subunit. Deprived of its regulatory partner, cdk4 cannot phosphorylate the retinoblastoma protein (Rb), which is considered the “gatekeeper” of cell proliferation. As a result, the cells do not progress past the restriction point into the S phase.1 Deletion, transcriptional inactivation, or mutation of the CDKN2A tumor suppressor gene occurs in more than 50% of sporadic tumors arising from a large number of different tissues.2 This promiscuous and widespread alteration of CDKN2A in malignancy rivals or surpasses that of p53. Curiously, families bearing a germline mutation of CDKN2A typically develop a restricted spectrum of cancers including melanoma and occasionally pancreatic carcinoma.3 Some investigators have reported that a few kindreds display a broader range of tumor types including lung4 and oropharyngeal cancers, cholangiocarcinoma5, and glial tumors.6-8
Animal models of disease have the potential to shed considerable light on their human counterparts. It has been known for decades that an intraperitoneal injection of mineral oil, plastics, or pristane will induce plasmacytomas in BALB/c mice.9 As murine plasmacytoma corresponds to multiple myeloma in humans, there has been considerable interest in the genetic basis for susceptibility to this disease. To address this question, Mock et al11 mapped 4 plasmacytoma susceptibility/resistance loci (Pctrl) in BALB/c mice.10 One of these loci is allelic to CDKN2A, and BALB/c mice bearing the susceptibility allele possess a germline mutation of CDKN2A that encodes a protein deficient in binding to CDK4. The identities of the genes corresponding to the remainingPctrl are not known at this time. However, they likely represent additional modifiers that in conjunction with a germlineCDKN2A mutation, account for the predisposition to plasmacytoma in BALB/c mice.12
Previous studies have demonstrated that there is a significant familial risk of multiple myeloma.13 In light of the murine model described above, we hypothesized that germline CDKN2A mutations in humans might influence myeloma predisposition in some individuals. We describe here a melanoma-prone family in which a germlineCDKN2A mutation cosegregated with both cases of melanoma and a single case of myeloma (patient No. II:1). In the patient with myeloma, we further demonstrated loss of heterozygosity (LOH) of the wild type allele in the malignant plasma cells. This suggests that the germlineCDKN2A mutation played a role in the development of this patient's malignancy.
Study design
Sorting of bone marrow plasma cells
Following informed consent, cells from a bone marrow (BM) aspiration were acquired from patient No. II:1. Plasma cells were purified from the unsorted BM using the B-B4 monoclonal antibody (mAb) (α-syndecan/CD138; Serotec, Oxford, England) and immunomagnetic beads (DYNABEADS M450 rat antimouse immunoglobulin G1[IgG1]; Dynal, Oslo, Norway).14
Amplification and LOH analysis of CDKN2A exon 1
We prepared DNA from peripheral blood following standard protocols. We amplified CDKN2A exon 1 sequences from all available members of the family as well as the presort and postsort BM DNA obtained from patient No. II:1 by using the forward primer (p16E1-FF) 5′GAAAGAGGAGGGGCTGGCTGGTC and the reverse primer (p16E1-RR) 5′GCGCTACCTGATTCCAATTCCCCTGC. The reaction products were separated by polyacrylamide gel electrophoresis (PAGE) on an 8% ethidium-stained polyacrylamide gel and photographed under ultraviolet light.
Results and discussion
A melanoma-prone family containing a member with multiple myeloma
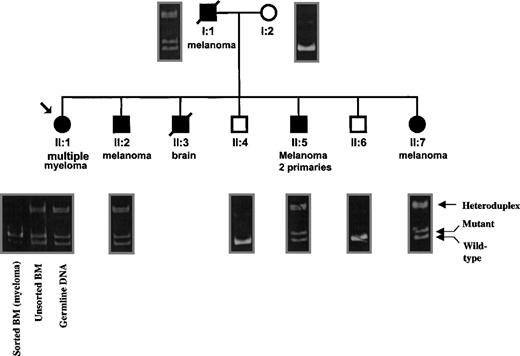
Family A is of northern European descent and comprises 9 members, 4 of whom developed melanoma (including one case of multiple primary disease; Figure 1). In addition to melanoma, one sibling (patient No. II:1) developed multiple myeloma at the age of 53, and a second developed a “brain tumor” (patient No. II:2–it is unknown whether this was a primary tumor or metastatic lesion). We sequenced the genomic DNA of the index case, patient No. II:1, and found that she harbors a germline CDKN2A mutation in exon 1, designated +24 (not shown). This mutation results from a duplication of the first 24 nucleotides of the CDKN2A coding sequence and has been previously associated with melanoma predisposition by multiple investigators worldwide.15
The +24 base pair mutation cosegregates with melanoma and myeloma in family A, and the wild type allele is deleted in the malignant plasma cells of patient No. II:1.
Shaded members denote cancer development with the cancer type given below. The arrow indicates the proband with multiple myeloma. Four individuals in family A have developed melanoma (patients No. I:1, II:2, II:5, and II:7). The positions of the wild type, mutant, and heteroduplex bands are indicated on the right-hand side of the figure. Note that all 5 individuals harboring the +24 mutation have developed cancer, while patients No. I:2, II:4, and II:6 are wild type for both alleles and have not developed any malignancy. (DNA was not available for patient No. II:3.) On the left side of the figure, the PCR reactions from germline, unsorted BM, and sorted BM cells are shown under patient No. II:1. Note that the LOH of the wild typeCDKN2A allele is seen only in the sorted (greater than 90% malignant plasma cells) obtained from this individual. Importantly, there is a large decrease in the amount of heteroduplex DNA present in the sorted BM sample, which was again due to lack of the wild type PCR product.
The +24 base pair mutation cosegregates with melanoma and myeloma in family A, and the wild type allele is deleted in the malignant plasma cells of patient No. II:1.
Shaded members denote cancer development with the cancer type given below. The arrow indicates the proband with multiple myeloma. Four individuals in family A have developed melanoma (patients No. I:1, II:2, II:5, and II:7). The positions of the wild type, mutant, and heteroduplex bands are indicated on the right-hand side of the figure. Note that all 5 individuals harboring the +24 mutation have developed cancer, while patients No. I:2, II:4, and II:6 are wild type for both alleles and have not developed any malignancy. (DNA was not available for patient No. II:3.) On the left side of the figure, the PCR reactions from germline, unsorted BM, and sorted BM cells are shown under patient No. II:1. Note that the LOH of the wild typeCDKN2A allele is seen only in the sorted (greater than 90% malignant plasma cells) obtained from this individual. Importantly, there is a large decrease in the amount of heteroduplex DNA present in the sorted BM sample, which was again due to lack of the wild type PCR product.
The +24 mutant and wild type CDKN2A exon 1 polymerase chain reaction (PCR) products can be resolved easily by PAGE. We obtained peripheral blood lymphocyte (PBL) genomic DNA from all available members of family A and used PAGE to analyze each member of the family (Figure 1). Interestingly, every member of this family possessing the +24 mutation has developed cancer; in contrast, the 3 noncarrier family members are cancer free. While the association of the germlineCDKN2A mutation and melanoma in this family was unsurprising, its role in the development of myeloma in patient No. II:1 was unclear. We hypothesized that the +24 mutation had predisposed patient No. II:1 to this disease.
LOH analysis of wild type CDKN2A in malignant plasma cells
If loss of CDKN2A expression played a role in the development of myeloma in patient No. II:1, the malignant cells should demonstrate loss or dysfunction of the wild type but not the mutantCDKN2A allele. To confirm this prediction, we performed LOH analysis on this patient's plasma cells. Following informed consent, we acquired an aliquot of a BM aspiration (performed as part of the routine clinical care of this patient). Examination of this BM sample revealed that it comprised both normal hematopoietic elements and 30% or less malignant plasma cells. To ensure that nonmalignant constituents of the BM did not interfere with the LOH analysis, we enriched the sample for the malignant clone using magnetic bead selection with a plasma cell-specific B-B4 mAb (α-syndecan/CD138, Serotec), which resulted in a final sample containing more than 90% plasma cells. We isolated genomic DNA from both the sorted and unsorted BM samples as well as from the PBLs from patient No. II:1, amplified exon 1 of CDKN2A from each sample, and resolved the PCR products on a PAGE gel.
Germline DNA amplified from the myeloma patient (No. II:1) demonstrated equal ratios of the wild type and mutant exon 1 alleles ofCDKN2A (Figure 1). Similarly, DNA from unsorted BM cells from this individual appears heterozygous as well because the minority of cells (30% or less) is malignant. However, DNA isolated from sorted BM, which is highly enriched with malignant plasma cells (greater than 90%), comprises mainly the mutant allele, as indicated by excess of the corresponding PCR product. The small amount of wild type PCR product present in this lane arises from residual nonmalignant cells in the sorted BM sample. We conclude that the wild type CDKN2Aallele is lost in the malignant cells and propose that this loss played a role in the development of myeloma in patient No. II:1 of family A.
Our hypothesis that abrogation of CDKN2A function plays a role in myeloma initiation or progression in patient No. II:1 is reinforced by 2 previous observations. First, germline CDKN2A mutations predispose mice to pristane-induced plasmacytoma. Second, loss ofCDKN2A function occurs commonly in human myeloma, albeit at a later stage of the disease.16 17 It is possible that the loss of CDKN2A in this patient may be a nonspecific event that occurred in the malignant cells due to an unstable genome. However, this explanation seems unlikely due to the important role played byCDKN2A in cell cycle control and the almost universal loss of control of the restriction point in the malignancy.
In light of the genetic complexity of both melanoma and myeloma susceptibility, it is not likely that most CDKN2Amutation-bearing individuals are equally predisposed to both diseases. Comparing an analogy between humans and BALB/c mice, which carry at least 4 Pctrl, germline CDKN2A mutations in humans may not be predisposed to myeloma unless other modifier genes that accelerate tumor development are present.
Acknowledgments
We thank the families and individuals who attended the Toronto Familial Melanoma Clinic and participated in the study. We thank Rakesh Nayer for technical assistance.
Supported by grant #7430 from The National Cancer Institute of Canada, Toronto, Ontario, Canada.
Reprints:David Hogg, University of Toronto, Medical Sciences Building, Room 7368, Toronto, Ontario, M5S 1A8 Canada; e-mail:david.hogg@utoronto.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal