When granulocytes are stimulated under certain clinical conditions, elastase is released therefrom and digests fibrin(ogen) independently of the plasmin system, which may also be mobilized simultaneously. Thus, discrimination of these 2 systems becomes urgent for the diagnosis and treatment of the underlying diseases. Using as immunogen a 97-kd granulocyte-elastase digest of human fibrinogen, we raised an antibody IF-123 that specifically recognizes elastase digests of human fibrin(ogen). The 97-kd elastase fragment resembles plasmic fragment D1, and the epitope of this antibody is located on the A (196-204) residue segment. This segment appears to be masked in fibrin(ogen) but exposed when the A Leu 204-Ile 205 peptide bond is cleaved by elastase. Cathepsin G concomitantly released from granulocytes failed to expose the epitope. By an enzyme immunoassay using IF-123 as the capture antibody, the elastase digests of fibrin(ogen) can be measured in plasma samples without interference by abundantly coexisting fibrinogen. Indeed, we found that the elastase digests were mostly elevated in patients with inflammation or malignant tumors, but remained in a normal range in patients with a benign gastrointestinal tract disease such as duodenal ulcer and polyps in the gallbladder or the colon. Like the plasmic D-dimer, the elastase digests predominantly consisted of the DD/E complex and DD/E-containing high-molecular weight derivatives apparently corresponding to the phase-3 plasmic digests of cross-linked fibrin.
Granulocyte-derived elastase (GE) is localized in azurophilic and specific granules of granulocytes and is released extracellularly in response to various stimuli such as endotoxin and cytokines.1 Released GE may degrade the components of the extracellular matrix, such as elastin and a variety of proteoglycans, and also plasma proteins including fibrin(ogen).2 3 GE has thus been implicated in the pathogenesis of a wide variety of diseases.
Granulocyte-derived elastase is regulated predominantly by α1-proteinase inhibitor (α1-PI) in vivo, although it is inhibited by several other inhibitors including α2-macroglobulin in vitro.4,5 The granulocyte-associated elastase, however, has been shown to be more resistant against α1-PI than free elastase.4-6 Like plasmin produced in blood of patients with certain diseases such as disseminated intravascular coagulation, GE may be released into the blood circulation and may digest fibrinogen and fibrin clots before being neutralized by α1-PI. Several lines of evidence have indicated that besides the major fibrinolytic protease plasmin, GE plays a role in fibrin(ogen) degradation in vitro7-10 as well as in vivo.11-13 Of particular interest is that both plasmin and GE systems are often mobilized simultaneously, and discrimination of their fibrin(ogen) degradation products becomes necessary to grasp the clinical conditions and to appropriately monitor their treatment. Based on these pieces of information, we have attempted to raise monoclonal antibodies specific to the GE digests of fibrin(ogen) and to characterize and measure the GE digests in plasma from patients with a variety of diseases.
Materials and methods
Chemicals and reagents
All chemicals and reagents were purchased from commercial sources and used without purification unless otherwise specified.
Preparation of GE digests of human fibrinogen corresponding to plasmic fragment D1
A commercial human fibrinogen product (FIB-I: Enzyme Research Laboratories, South Bend, IN, about 2.6% [w/v] in 20 mmol/L citric acid-HCl/glycine, pH 7.4) was diluted to 1.0%(w/v) with 50 mmol/L Tris-HCl, pH 7.5, containing 0.15 mol/L NaCl (TBS) and 1.0 mmol/L disodium EDTA and applied onto a lysine-Sepharose 4B column (Pharmacia Biotech, Tokyo, Japan) to remove plasminogen and plasmin, if any. The pass-through fractions were pooled and treated with 2.0 mol/L urea to denature factor XIII, a trace contaminant.14 The fibrinogen fraction was then dialyzed against TBS and brought to 10 mg/mL with the same buffer. The fibrinogen fraction (20 mL) was digested with 2.0 mg human GE (purified from the human purulent sputum,15 and free of cathepsin G, myeloperoxidase, and lysozyme, Elastin Products, Owensville, MO) in the presence of 5.0 mmol/L CaCl2, 1.0 mmol/L benzamidine HCl, and 1.0 mmol/L trans-4-aminomethyl-1-cyclohexanecarboxylic acid (t-AMCHA) for 2 hours at 37°C. This enzyme was also found to be free of trypsin-type and chymotrypsin-type enzymes (data not shown). The ratio of enzyme/substrate (wt/wt) was 1:100. To terminate the digestion, diisopropylfluorophosphate (DFP) was added to the reaction mixture at 1.0 mmol/L. The digests were applied onto a Sephacryl S-300 HR column (5.0 × 90 cm; Pharmacia Biotech), and the fractions containing a 97-kd fragment corresponding to plasmic fragment D1 were collected and pooled. We tentatively designated this 97-kd fragment as GE-D.
Preparation of GE digests of cross-linked fibrin
The human fibrinogen fraction was brought to 10 mg/mL in TBS containing 5.0 mmol/L CaCl2 (TBS-CaCl2). The fibrinogen fraction (10 mL) enriched with 1.0 μg/mL factor XIII was clotted with 0.6 NIH-u/mL human thrombin (Sigma, St. Louis, MO). The fibrin clots formed were squeezed with filter paper, washed extensively with 0.15 mol/L NaCl, lyophilized, and ground with a glass rod. A 50-mg portion of the ground fibrin was suspended in 8.0 mL TBS-CaCl2 containing 1.0 mmol/L benzamidine HCl and 1.0 mmol/L t-AMCHA, and digested with 37 μg of GE for 3 hours at 37°C. The enzyme/substrate ratio (wt/wt) was 1:1333. The reaction was terminated with 1.0 mmol/L DFP, and the digests were centrifuged for 20 minutes at 10,000g. The supernatant was applied to a Sephacryl S-300 HR column (5.0 × 90 cm), and the first peak fractions were collected and pooled. The GE digests in this pooled fraction were found to correspond to the phase-3 plasmic digests of cross-linked fibrin (plasmic XDP)10,16 as examined by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE).17 This fraction was named GE-XDP.
Preparation of plasmic degradation products
Plasmic degradation products of human fibrinogen were prepared essentially as described.18 The degradation products were found to contain fragments X, Y, D1A, D1, and E3. When necessary, fragment D119and phase-3 digests of cross-linked fibrin16 were prepared as described. Absorbance coefficients (A1%, 1 cm at 280 nm) for calculating the protein concentration were 15.1 for fibrinogen,20 20.8 for fragment D1,20 18.1 for plasmic XDP, which was assessed on the basis of 20.0 and 12.0 for plasmic fragments DD and E,20 respectively. The same values were used for the corresponding GE digests.
Preparation of monoclonal antibodies specific to the GE digests of fibrinogen
We inoculated Balb/c mice with 50 μg of GE-D in complete Freund's adjuvant followed by 50 μg of GE-D without the adjuvant as immunogen essentially by a hybridoma technique of Köhler and Milstein21 with minor modifications as described elsewhere.22 Selection of clones secreting monoclonal antibodies (mAbs) specific to the GE digests of fibrinogen, but not to the parent molecule fibrinogen or it plasmic digests was carried out by a direct-binding enzyme-linked immunosorbent assay (ELISA) using GE-D, plasmic fragment D1, and fibrinogen as antigens.
Binding of mAbs to antigens determined by a direct-binding ELISA
Binding between the antigens and mAbs was studied by a direct-binding ELISA as described previously.22 Briefly, wells of polystyrene microtiter plates (Immulon-II, Dynatech, Chantilly, VA) were coated overnight at 4°C with 50 μL of respective antigens at 5 μg/mL in 50 mmol/L Tris-HCl, pH 8.5. The antigen-coated wells were washed with 0.15 mol/L NaCl containing 0.05% (w/v) Tween-20 (NaCl-Tween) and incubated with 50 μL of the culture supernatant for 1 hour at 25°C. After decantation of the reaction mixture, the wells were washed with NaCl-Tween. Horseradish peroxidase (HRPO)-conjugated antimouse IgG rabbit antibody (DAKO, Glostrup, Denmark) diluted 200-fold with 50 mmol/L Tris-HCl, pH 8.0, containing 0.15 mol/L NaCl and 0.05% (w/v) Tween-20 was added to each well as the second antibody. The bound antibodies were determined using 50 mmol/L Tris-HCl, pH 7.5, containing 0.5 mmol/L 4-aminoantipyrine, 10 mmol/L phenol, and 0.005% hydrogen peroxide as substrate, and the color produced was read at 492 nm on an MPR-A4i Microplate reader (Tosoh, Tokyo, Japan).
Determination of the dissociation constant of IF-123
Binding between antigens and IF-123 was studied by a solid-phase ELISA using microtiter plates coated with either GE-D or GE-XDP as described previously.22 The amounts of bound antibodies were calculated from calibration curves constructed with known amounts of the bound respective antibodies. The dissociation constant was calculated as described previously.23
Immunoblotting
Separation of the -chain remnants of GE-D bound to IF-123 and their digestion with lysyl endopeptidase
Purified IF-123 (50 mg) was coupled to an NHS-activated HiTrap column (5 mL, Pharmacia Biotech) according to the manufacturer's instruction, and 10 mg GE-D was applied to the column. After washing the column with 20 mmol/L Tris-HCl, pH 8.0, containing 1 mol/L NaCl, 1.0 mmol/L benzamidine HCl, and 1.0 mmol/L t-AMCHA, proteins were eluted with 0.2 mol/L glycine-HCl, pH 2.5, and immediately neutralized with 1.0 mol/L Tris-HCl, pH 8.0. The affinity-purified GE-D was estimated to be 4.9 mg. After reduction and S-pyridylethylation of 4.5 mg of the affinity-purified GE-D, the 3 subunit polypeptides of this fraction were separated by reverse-phase high-performance liquid chromatography (HPLC) using a POROS R1/M column (4.6 × 100 mm, PerSeptive Biosystems, Framingham, MA) on BioCAD Workstation (PerSeptive Biosystems). They were eluted in the order the α-, β- and γ-chain remnants with a linear gradient from 30% to 60% acetonitrile in 5 minutes. The pyridylethylated α-remnant (Pe-α/GE-D) was further purified by rechromatography on the same column, and the collected fraction was dried using a Speed Vac (Savant Instruments, Farmingdale, NY). The dried Pe-α/GE-D (510 μg) was dissolved in 235 μL of 50 mmol/L Tris-HCl, pH 9.0, containing 3 mol/L urea, and digested with lysyl endopeptidase (Wako Chemical, Osaka, Japan) at an enzyme/substrate molar ratio of 1:50 for 18 hours at 37°C. The digests were analyzed by reverse-phase HPLC using a Cosmosil 5C18P column (4.6 × 150 mm, Nacalai Tesque, Kyoto, Japan). A 0.1% trifluoroacetic acid/water (solvent A) and 0.1% trifluoroacetic acid/acetonitrile (solvent B) gradient system was used, and a linear gradient from 0% to 40% solvent B in 100 minutes was used. The flow rate was 0.5 mL/min, and the column effluent was monitored by absorbance at 215 nm (A215). Individual peak fractions were dried with a Speed Vac, dissolved in 0.2 mL of 10 mmol/L bicarbonate buffer, pH 8.6, and coated to wells of polystyrene microtiter plates (Immulon-II) after 500-fold dilution. The peptide-coated wells were washed 3 times with NaCl-Tween and incubated with IF-123 at 5 μg/mL in TBS-Tween for 1 hour at 25°C. The bound antibodies were detected by antimouse IgG conjugated with HRPO and visualized by incubation with 4-aminoantipyrine-phenol-hydrogen peroxide as substrate.
Inhibition by synthetic peptides of the binding of IF-123 to immobilized affinity-purified GE-D or a synthetic 13-residue peptide corresponding to A (192-204)
When the separated peptides were tested for binding with IF-123, only a single peptide, named peptide #19, was found to react with the antibody in a direct-binding ELISA. Its full sequence was determined by a protein sequencer, model 476A (Applied Biosystems, Foster City, CA), and assigned to the Aα (192-204) residue segment.
Wells of polystyrene microtiter plates were coated with 50 μL of 4 nmol/L affinity-purified GE-D or 40 nmol/L synthetic peptide with a sequence of Aα (192-204), Asp-Leu-Leu-Phe-Ser-Arg-Asp-Arg-Gln-His-Leu-Pro-Leu, tentatively named s-peptide 19, in 50 mmol/L Tris-HCl, pH 8.5, for 16 hours at 4°C. The antigen-coated wells were washed 3 times with NaCl-Tween and incubated with IF-123, which had been mixed with various concentrations of either one of the synthetic peptides (TANA Laboratories, Houston, TX) in TBS-Tween for 1 hour at 25°C. The bound antibody was detected as described above.
Preparation of protease(s) released from activated granulocytes
To release the enzymes stored in granules, the granulocytes (5.7 × 107 cells) isolated from 30 mL of heparinized venous blood obtained from healthy volunteers described elsewhere,25 were preincubated for 3 minutes at 37°C with 5 μg/mL of cytochalasin B (Sigma) in the presence of 1.3 mmol/L CaCl2 and 1.0 mmol/L MgCl2, and activated with 500 nmol/L N-formyl-Met-Leu-Phe (fMLP, Sigma) for 5 minutes at 37°C. After centrifugation, the supernatant was collected and passed through a cellulose acetate membrane filter (0.2 μm pore, Advantec, Tokyo, Japan). The concentrations of GE and cathepsin G in the supernatant were measured by spectrophotometric assays using MeO-Suc-Ala-Ala-Pro-Val-NA and Suc-Ala-Ala-Pro-Phe-NA (Sigma) as substrates, respectively.26 Concentrations of GE and cathepsin G were calculated from calibration curves constructed with known amounts of the commercially available purified GE and cathepsin G (purified from human purulent sputum; elastase, myeloperoxidase, lysozyme free; Elastin Products).
Characterization of GE digests by immunoprecipitation with IF-123–conjugated Sepharose 4B
Purified IgG fractions of IF-123 and JIF-23,27 an mAb that specifically recognizes the amino-terminal disulfide-linked conformation of the plasmic fragment D species (D1, D2, and D3), were individually coupled to CNBr-activated Sepharose 4B (Pharmacia Biotech) according to the manufacturer's instruction, and the IgG-conjugated Sepharose 4B was suspended in an equal volume of 50 mmol/L Tris-HCl, pH 8.0, containing 0.15 mol/L NaCl and 0.05% sodium azide. One hundred microliters of either purified GE digests (10 μg of GE digests of fibrinogen and GE-XDP) or patient-derived plasma samples were incubated with 200 μL of the gel suspension and 1 mL of 20 mmol/L Tris-HCl, pH 7.6, containing 0.5 mol/L NaCl and 0.05%(w/v) Tween-20 (suspension buffer) for 16 hours at 4°C, and the mixture was centrifuged at 10 000g for 3 minutes at 25°C. The gels precipitated were suspended and washed 5 times with the same buffer, each time by rotating the tubes for 20 minutes at 25°C, and then centrifuged at 10 000g for 3 minutes at 25°C. The precipitate was resuspended in 1.0 mL of 20 mmol/L Tris-HCl, pH 7.6, containing 0.15 mol/L NaCl and 2% SDS to solubilize the proteins bound to the antibody-coupled gels. Proteins in 0.2 mL of the supernatant after centrifugation at 10 000g for 5 minutes at 25°C were isolated by acetone precipitation and subjected to SDS-PAGE using 3.5% to 9.0% gradient gels under nonreducing conditions as described elsewhere.28 The proteins were visualized by silver staining.
Measurement of GE digests of fibrinogen and fibrin in clinical samples by a sandwich ELISA
Wells of polystyrene microtiter plates were coated overnight at 4°C with 20 μg/mL F(ab′)2 fragment of IF-123 in 50 mmol/L Tris-HCl, pH 8.5, and 50 fold- diluted plasma samples in 50 mmol/L Tris-HCl, pH 8.0, containing 0.05%(w/v) Tween-20 and 1 mol/L urea were allowed to react with the immobilized F(ab′)2 fragment of IF-123 for 1 hour at 25°C. After decantation of the reaction mixture, the wells were washed with NaCl-Tween, and HRPO-conjugated antihuman fibrinogen rabbit antibody (DAKO) was added to each well as the second antibody. The amounts of bound antibodies were determined by reading A492 on an MPR-A4i Microplate reader using 4-aminoantipyrine-phenol-hydrogen peroxide as substrate. Proteins specifically bound to IF-123 were determined on a calibration curve constructed with pooled normal plasma spiked with known amounts of GE-XDP.
Statistical analysis
To compare the levels of GE digests in plasma between patients and the control, Welch's t test was used. A P value of less than 0.05 was considered significant.
Results
Characterization of an mAb specific to GE digests of human fibrinogen and cross-linked fibrin
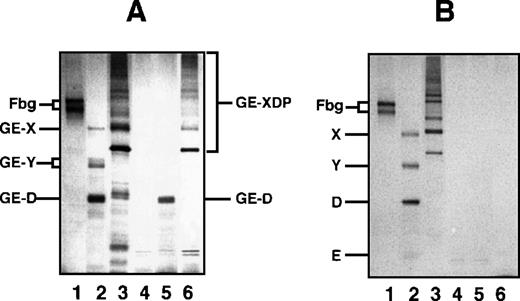
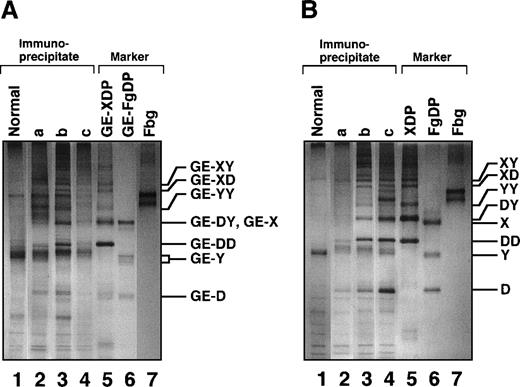
Among the mAbs thus prepared, there was an antibody that specifically reacted with GE-D but not with fibrinogen or its plasmic D1, when analyzed by a direct-binding ELISA. The reactivity of this antibody IF-123 was independent of calcium ions, although the fibrinogen D domain contains a high-affinity calcium binding site29 30 (data not shown). This antibody was classified into IgG1 with κ-type light chains. When analyzed by immunoprecipitation followed by SDS-PAGE, GE-D and GE-XDP were adsorbed to IF-123 (Figure 1A, GE-D and GE-XDP in lanes 5 and 6, respectively). Fibrinogen, its plasmic digests, or plasmic XDP were not adsorbed (Figure 1B, lanes 4-6). The dissociation constants of IF-123 with GE-D and GE-XDP were 1.20 × 10−9 mol/L and 1.23 × 10−9 mol/L, respectively.
SDS-PAGE analysis of immunoprecipitate.
SDS-PAGE analysis was performed under nonreducing conditions for fibrinogen and GE and plasmic digests of fibrinogen and cross-linked fibrin immunoprecipitated with IF-123–conjugated Sepharose 4B. (A) GE digests. (B) Plasmic digests. In both panels, lanes 1 to 3 represent the applied samples and lanes 4 to 6 the bound proteins to the gels. Lanes 1 and 4, fibrinogen; lanes 2 and 5, fibrinogen digests; and lanes 3 and 6, cross-linked fibrin digests.
SDS-PAGE analysis of immunoprecipitate.
SDS-PAGE analysis was performed under nonreducing conditions for fibrinogen and GE and plasmic digests of fibrinogen and cross-linked fibrin immunoprecipitated with IF-123–conjugated Sepharose 4B. (A) GE digests. (B) Plasmic digests. In both panels, lanes 1 to 3 represent the applied samples and lanes 4 to 6 the bound proteins to the gels. Lanes 1 and 4, fibrinogen; lanes 2 and 5, fibrinogen digests; and lanes 3 and 6, cross-linked fibrin digests.
Epitope mapping for IF-123
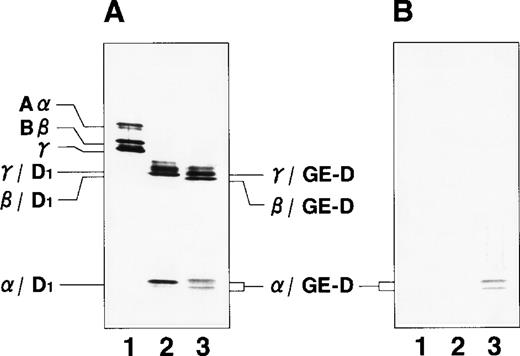
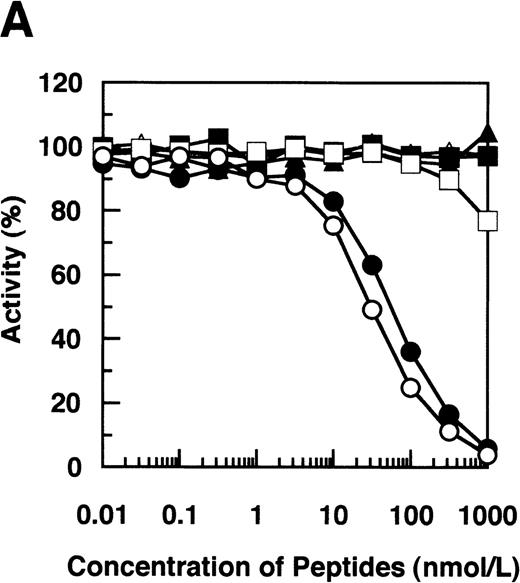
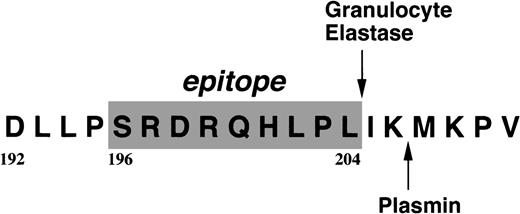
By immunoblotting run under reducing conditions, neither fibrinogen nor plasmic D1 was stained (Figure2B, lanes 1 and 2), whereas 2 α-remnant species of GE-D were stained with this antibody (Figure 2B, lane 3). The epitope was thus localized to an approximately 12-kd Aα-chain–derived segment specifically cleaved by GE. The 12-kd fragment purified (Figure3A, Pe-α/GE-D) and their lysyl endopeptidase digests were fractionated by reverse-phase HPLC on a Cosmosil 5C18P column (Figure 3B). Separated peak fractions were coated onto immunoplates and their reactivities with IF-123 were examined. Among them, a fraction denoted by peak #19 (Figure 3B) reacted with IF-123. By sequence analysis, we assigned this peptide to the Aα (192-204) residues, Asp-Leu-Leu-Phe-Ser-Arg-Asp-Arg-Gln-His-Leu-Pro-Leu. Because lysyl endopeptidase hydrolyzes specifically the carboxyl side bond of lysyl residues,31 it is very likely that peptide #19 lacking the carboxyl-terminal Lys residue constitutes the carboxyl-terminal segment of the α-remnant of GE-D. To confirm that the epitope resides in peptide #19, we conducted inhibition assays by using ELISAs, where the affinity-purified GE-D and a synthetic peptide with the same sequence as peptide #19, s-peptide 19, were individually immobilized, and binding of IF-123 was tested in the presence of various synthetic analogs of peptide #19. Two synthetic peptides, s-peptide 19 and a peptide analog, Ser 196-Leu 204, lacking the first 4 amino acids of s-peptide 19 were able to inhibit the binding of IF-123 to the affinity-purified GE-D (Figure 4A) as well as to s-peptide 19 (Figure 4B) in the same manner. When the first 3 Ser-Arg-Asp residues of the (Ser 196-Leu 204) peptide had been removed, binding with IF-123 decreased nearly two magnitudes (Figure 4A and B). On the other hand, deletion of Leu 204 from or addition of Ile 205 or Ile 205-Lys 206 to the (Ser 196-Leu 204) peptide resulted in complete loss of binding with IF-123 (Figure 4A and B). The Aα Lys 206 has been reported to be a potential plasmic cleavage P1 site.32The results together suggested that the Aα (Ser 196-Leu 204) residue segment functioned as the epitope for IF-123, and that its carboxyl-side residues were critical for full expression of the epitope, as schematically shown in Figure 5.
Immunoblot analyses.
Immunoblot analyses were run under reducing conditions for fibrinogen and its plasmic and GE-digests with an antihuman fibrinogen rabbit antibody (A) and IF-123 (B). Lane 1, fibrinogen; lane 2, plasmic fragment D1; and lane 3, GE-D.
Immunoblot analyses.
Immunoblot analyses were run under reducing conditions for fibrinogen and its plasmic and GE-digests with an antihuman fibrinogen rabbit antibody (A) and IF-123 (B). Lane 1, fibrinogen; lane 2, plasmic fragment D1; and lane 3, GE-D.
Reverse-phase HPLC.
Separation of the α-remnants of GE-D (A) and its lysyl endopeptidase-digests (B) by reverse-phase HPLC. Only peptide #19 was reactive to IF-123.
Reverse-phase HPLC.
Separation of the α-remnants of GE-D (A) and its lysyl endopeptidase-digests (B) by reverse-phase HPLC. Only peptide #19 was reactive to IF-123.
Synthetic peptide inhibition.
Inhibition by synthetic peptides Aα (192-204), Aα (196-204), and their analogs of binding of IF-123 to immunoaffinity-purified GE-D (A) and the Aα (192-204) peptide corresponding to peptide #19 (B). Synthetic peptides correspond to Aα (192-204) (○), Aα (196-204) (•), Aα (199-204) (□), Aα (196-203) lacking Leu 204 (▪), Aα (196-205) linked with Ile 205 (Δ) and Aα (196-206) linked with Ile 205-Lys 206 (▴).
Synthetic peptide inhibition.
Inhibition by synthetic peptides Aα (192-204), Aα (196-204), and their analogs of binding of IF-123 to immunoaffinity-purified GE-D (A) and the Aα (192-204) peptide corresponding to peptide #19 (B). Synthetic peptides correspond to Aα (192-204) (○), Aα (196-204) (•), Aα (199-204) (□), Aα (196-203) lacking Leu 204 (▪), Aα (196-205) linked with Ile 205 (Δ) and Aα (196-206) linked with Ile 205-Lys 206 (▴).
Localization of the epitope for IF-123.
The residues constituting the epitope are shaded, and a plasmic cleavage site is shown in the carboxyl terminal 6 residue extension.
Localization of the epitope for IF-123.
The residues constituting the epitope are shaded, and a plasmic cleavage site is shown in the carboxyl terminal 6 residue extension.
Expression of the epitope by proteases released from activated granulocytes
When granulocytes (107 cells/mL) had been stimulated with 500 nmol/L fMLP, they were estimated to release GE and cathepsin G at the concentration of 610 nmol/L and 56 nmol/L, respectively. These proteases were tested for digestion of fibrinogen with or without prior treatment with various protease inhibitors. When the supernatant of activated granulocytes had been treated with a mixture of Z-Gly-Leu-Phe-CH2Cl, Nα-tosyl-l-lysine chloromethylketone (TLCK), and Nα-tosyl-l-phenylalanine chloromethylketone (TPCK), inhibitors of cathepsin G, trypsin-type enzymes, and chymotrypsin-type enzymes, respectively, profiles of fibrinogen degradation and appearance of the reactivity with IF-123 (Figure 6B) were nearly identical with those for the nontreated supernatant (Figure 6A) as examined by SDS-PAGE and a sandwich ELISA. When the supernatant had been treated with a GE-specific inhibitor, MeO-Suc-Ala-Ala-Pro-Val-CH2Cl, fibrinogen was degraded gradually, but none of the degradation products reacted with IF-123 (Figure 6C).
GE-induced epitope expression.
Digestion of fibrinogen was performed by proteases released from the activated granulocytes as analyzed by SDS-PAGE (upper panels) and concomitant appearance of the epitope for IF-123 by a sandwich ELISA (lower panels). (A) Control without prior treatment of the proteases with any inhibitors. (B) Prior treatment with Z-Gly-Leu-Phe-CH2Cl, a specific inhibitor to cathepsin G, TPCK, and TLCK. (C) Prior treatment with MeO-Suc-Ala-Ala-Pro-Val-CH2Cl, a specific inhibitor to GE.
GE-induced epitope expression.
Digestion of fibrinogen was performed by proteases released from the activated granulocytes as analyzed by SDS-PAGE (upper panels) and concomitant appearance of the epitope for IF-123 by a sandwich ELISA (lower panels). (A) Control without prior treatment of the proteases with any inhibitors. (B) Prior treatment with Z-Gly-Leu-Phe-CH2Cl, a specific inhibitor to cathepsin G, TPCK, and TLCK. (C) Prior treatment with MeO-Suc-Ala-Ala-Pro-Val-CH2Cl, a specific inhibitor to GE.
Effects on the epitope expression of plasmic digestion of GE-XDP and GE digestion of plasmic XDP
To see whether plasmic digestion of GE-XDP and GE digestion of plasmic XDP affect the structure required for the epitope expression, we digested GE-XDP with plasmin and plasmic XDP with GE, both at 1:1000 of the enzyme/substrate ratio (wt/wt), and examined the reactivity of the digests to IF-123 by the immunoprecipitation method. Although GE-XDP was converted by plasmin to the phase-4 digests (Figure7A, lanes 1-4), the epitope for IF-123 was retained in the digests containing the fragment D components (Figure 7A, lanes 5-8). Digestion of plasmic XDP with GE also yielded the phase-4 digests (Figure 7B, lanes 1-4), and the new epitope was expressed at later stages, where plasmic DD and DY had been further degraded to smaller fragments DD and DY (Figure 7B, lanes 7 and 8, indicated by arrowheads).
Epitope expression by a combination of GE and plasmin.
Effects on the epitope expression for IF-123 are noted by further digestion of GE-XDP by plasmin (A) and plasmic XDP by GE (B). The 2 XDP fractions were digested by respective enzymes at 37°C, and samples removed at various timed intervals were analyzed by SDS-PAGE under nonreducing conditions before and after immunoprecipitation with IF-123–conjugated Sepharose. Lanes 1 and 5, 0 minutes; lanes 2 and 6, 15 minutes; lanes 3 and 7, 60 minutes; and lanes 4 and 8, 120 minutes.
Epitope expression by a combination of GE and plasmin.
Effects on the epitope expression for IF-123 are noted by further digestion of GE-XDP by plasmin (A) and plasmic XDP by GE (B). The 2 XDP fractions were digested by respective enzymes at 37°C, and samples removed at various timed intervals were analyzed by SDS-PAGE under nonreducing conditions before and after immunoprecipitation with IF-123–conjugated Sepharose. Lanes 1 and 5, 0 minutes; lanes 2 and 6, 15 minutes; lanes 3 and 7, 60 minutes; and lanes 4 and 8, 120 minutes.
Measurement of GE digests in plasma by a sandwich ELISA
By a sandwich ELISA, we were able to measure GE-D and GE-XDP spiked in plasma up to 80 μg/mL without any interference by fibrinogen (Figure 8, open and closed circles, respectively). When plasma samples, 282 in total, derived from patients with a variety of diseases were subjected to measurement of plasmic fragment D species and GE-digests by ELISAs using JIF-23 and IF-123, respectively, there was a moderate correlation, r = 0.652, between these 2 fibrin(ogen) digests (Figure 9). However, they were found to be independent entities, as shown by 3 representative examples: (a) GE digests were markedly elevated but plasmic digests were low; (b) both GE and plasmic digests were elevated; and (c) GE digests were low but plasmic digests were elevated. By immunoprecipitation analysis, multiple protein fractions were abundantly precipitated with IF-123 from examples a and b (Figure 10A, lanes 2 and 3), but only a little from example c (Figure 10A, lane 4). On the contrary, only small amounts of proteins were precipitated with JIF-23 from example a (Figure 10B, lane 2), whereas considerable amounts of multiple proteins were precipitated from examples b and c (Figure 10B, lanes 3 and 4).
Measurement of GE-D and GE-XDP added to pooled normal plasma by a sandwich ELISA using IF-123.
Plasma was spiked with GE-D (○); GE-XDP (•); plasmic D1(□) or plasmic XDP (▪).
Measurement of GE-D and GE-XDP added to pooled normal plasma by a sandwich ELISA using IF-123.
Plasma was spiked with GE-D (○); GE-XDP (•); plasmic D1(□) or plasmic XDP (▪).
Comparison of levels of GE digests and plasmic digests in plasmas derived from patients with various diseases.
They include APL; acute myelocytic leukemia; acute lymphocytic leukemia; malignant lymphoma; solid cancers in the stomach, liver, colon, and lungs; sepsis; gestation toxicosis; SLE; and benign gastrointestinal tract diseases such as duodenal ulcer and polyp(s) in the gallbladder and the colon.
Comparison of levels of GE digests and plasmic digests in plasmas derived from patients with various diseases.
They include APL; acute myelocytic leukemia; acute lymphocytic leukemia; malignant lymphoma; solid cancers in the stomach, liver, colon, and lungs; sepsis; gestation toxicosis; SLE; and benign gastrointestinal tract diseases such as duodenal ulcer and polyp(s) in the gallbladder and the colon.
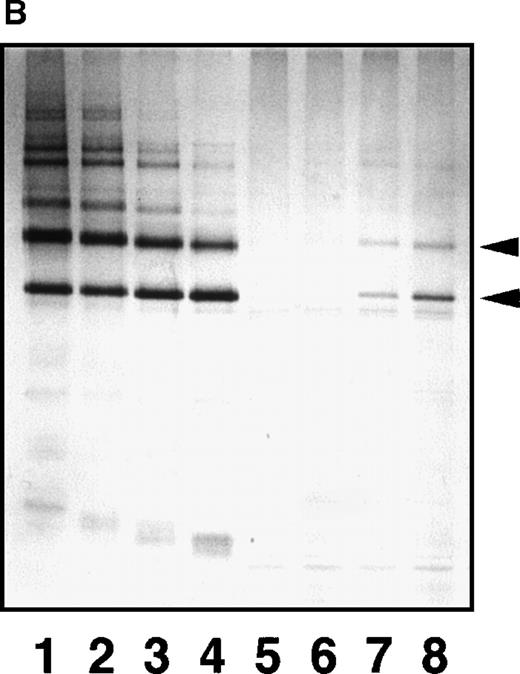
SDS-PAGE analyses of immunoprecipitated GE digests and plasmic digests in 3 representative plasma samples.
(A) In sample a, GE digests are elevated but plasmic digests are low. In sample b, both GE and plasmic digests are elevated. In sample c, GE digests are low but plasmic digests are elevated. Individual fragments in the GE digests are termed according to their corresponding fragments assigned for plasmic digests.16 20 (B) Purified samples are shown as reference.
SDS-PAGE analyses of immunoprecipitated GE digests and plasmic digests in 3 representative plasma samples.
(A) In sample a, GE digests are elevated but plasmic digests are low. In sample b, both GE and plasmic digests are elevated. In sample c, GE digests are low but plasmic digests are elevated. Individual fragments in the GE digests are termed according to their corresponding fragments assigned for plasmic digests.16 20 (B) Purified samples are shown as reference.
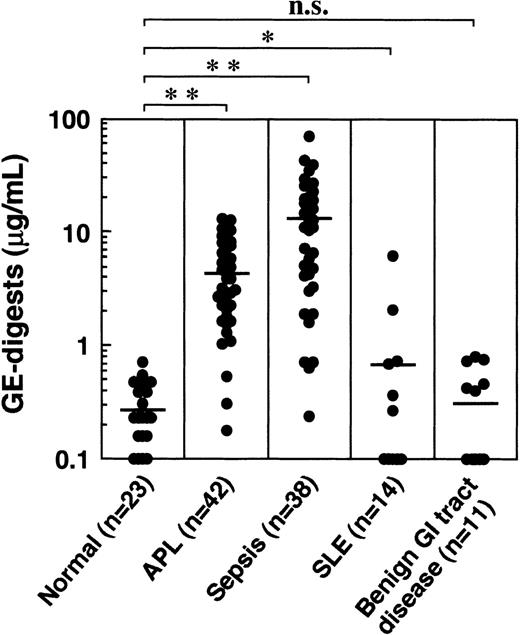
When the GE digests were measured in plasmas derived from various diseases, including acute promyelocytic leukemia (APL), sepsis, systemic lupus erythematosus (SLE), and benign gastrointestinal tract diseases, they were highly elevated in APL and sepsis, and moderately elevated in SLE (Figure 11). No significant increase was observed in benign gastrointestinal tract diseases. The GE digests in 23 normal healthy men and women between 22 and 48 years of age ranged from 0 to 0.70 μg/mL (mean ± SD: 0.30 ± 0.17 μg/mL).
GE digests in plasmas derived from patients with various diseases.
Horizontal bars represent the means. *P < 0.05; **P < 0.001 versus normal control. n.s., not significant.
GE digests in plasmas derived from patients with various diseases.
Horizontal bars represent the means. *P < 0.05; **P < 0.001 versus normal control. n.s., not significant.
Discussion
Granulocytes are known to release intrinsic proteolytic enzymes including elastase and cathepsin G in a variety of pathologic conditions.1-3 In fact, they may degrade the tissue-constituent proteins such as elastin and a variety of proteoglycans, and also plasma proteins including fibrinogen and fibrin.4,7-13 Although a variety of proteases may be released, cathepsin G, the other major granulocyte-derived protease, or trypsin-type and chymotrypsin-type proteases if any, were not able to affect the epitope expression in the GE digests of fibrinogen and fibrin (see Figure 6). Degradation of fibrinogen and fibrin by GE was distinct from degradation of those by plasmin,7-13 and discrimination of their fibrin(ogen) degradation products have been attempted by SDS-PAGE10 and immunochemical techniques.9,13 In this study, we raised an mAb IF-123 that recognized the α-remnants of GE-D, but not fibrinogen nor its plasmic fragment D. By HPLC analysis of GE-D, and inhibition studies by ELISAs using a variety of peptide analogs, the epitope was localized within the Aα (196-204) residue segment (Figures 4 and 5). Although removal of the amino-terminal 3 residues of this peptide decreased the reactivity to IF-123 significantly by 2 magnitudes, deletion of the carboxyl-terminal Leu 204, and addition of Ile 205 and Ile 205-Lys 206 resulted in complete loss of the reactivity. Thus, for eliciting the epitope, a full sequence of the Aα (196-204) residues and its local conformation seems to be needed, and its carboxyl-terminal portion appears to be critical. In a crystal structure study on tryptic fragment D of human fibrinogen,33 the Aα 196-197 residues, exactly corresponding to the amino-terminal dipeptide of the epitope (Figure 5), were not appropriately positioned, however. Furthermore, the hydrophobicity at the carboxyl-terminal residue of this segment seems to contribute to the local conformation needed for the epitope as observed by partial inhibition of the reaction to IF-123 by peptide analogs with either a Val or a Phe residue at their carboxyl-terminus (profiles not shown). The Aα Lys 206 is a potential P1-site for plasmic cleavage of the Aα-chain to form the fragment D species,32 and the carboxyl-terminal 404 residues, Aα (Met 207-Val 610), are proposed to compose the flexible polar protuberance.34 Therefore, the epitope for IF-123 seems to be situated very close to the carboxyl-terminal globular D domain, but is still masked even though the carboxyl-terminal flexible protuberance is cleaved by plasmin at the Aα Lys 206-Met 207 peptide bond. Only when the Aα 204-205 peptide linkage of the fibrinogen Aα-chain is cleaved directly by GE, or when the carboxyl-terminal 2 Ile 205-Lys 206 residues of plasmic fragment D are removed by GE, the epitopic segment becomes accessible to IF-123 (Figure 4A and B and Figure 5). As shown in an experiment, in which the plasmic phase-3 digests were further degraded by GE to smaller phase-4 digests, the reactivity to IF-123 was clearly demonstrated in at least 2 bands (Figure 7B, lanes 7 and 8, indicated by arrowheads). Although not shown in results, sequence analysis of the first 3 cycles of Pe-α/GE-D revealed the presence of 4 amino-termini to be assigned to Aα Ser 100, Asn 102, Tyr 108, and Ser 112. Thus, the 2 bands that reacted with IF-123 on immunoblotting are likely to have these amino acids at their amino-termini. Conversely, when the GE-XDP was further digested with plasmin, the epitope was retained in all the D- and DD-containing segments (see Figure 7A). These data altogether indicate that the epitope is buried in fibrinogen and plasmic fragments D and XDP, and is exposed when the Aα Leu 204-Ile 205 peptide bond is cleaved by GE either directly from fibrinogen and fibrin, or from plasmic fragments D and XDP, which may have been produced prior to digestion by GE. Therefore, GE digests and plasmic digests were certainly distinct entities, even though they may be produced simultaneously under many disease conditions (Figure 9). Furthermore, GE digests of cross-linked fibrin resembled the plasmic phase-3 digests consisting of DD/E and much higher molecular weight fragments (Figure 10). Therefore, discrimination of these 2 species of fibrin(ogen) degradation products will allow us to grasp the patient's condition more accurately and to respond more quickly and appropriately than monitoring by plasmic fragments alone in terms of fibrinogen/fibrin degradation products.
Although the diseases tested are still limited at this stage of this investigation, we found that the level of GE-D species, that is, GE-D and GE-XDP, were increased significantly in APL and sepsis, and moderately in SLE. On the other hand, the level of GE-D species was in the normal range in patients with a benign gastrointestinal tract disease. To comprehend clinical implications of the plasma level of GE-D species in relation to diseases, and also to evaluate the proposed assay system using IF-123, we must await further investigation.
Acknowledgments
The authors are indebted to Chizuko Nakamikawa for skillful assistance and to Michiko Takano for clerical work for construction of this manuscript.
Supported in part by Grants-in-Aid for Scientific Research 08407034 and 11470250, by the International Scientific Research Program, and Joint Research Grant 09044329 and 11694308 from the Ministry of Education, Science and Culture of the Government of Japan.
Reprints:Michio Matsuda, Division of Hemostasis and Thrombosis Research, Institute of Hematology, Jichi Medical School, Yakushiji 3311-1, Minamikawachi, Kawachi-Gun, Tochigi-ken 329-0498, Japan; e-mail: thmichi@jichi.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal