The influence of the endothelial protein C receptor (EPCR) on the host response to Escherichia coli was studied. Animals were treated with 4 separate protocols for survival studies and analysis of physiologic and biochemical parameters: (1) monoclonal antibody (mAb) that blocks protein C/activated protein C binding to EPCR plus sublethal numbers of E coli (SLEC) (n = 4); (2) mAb to EPCR that does not block binding plus SLEC (n = 3); (3) SLEC alone (n = 4); and (4) blocking mAB alone (n = 1). Those animals receiving blocking mAb to EPCR plus sublethal E coli died 7 to 54 hours after challenge, whereas all animals treated with the other protocols were permanent survivors. Histopathologic studies of tissues from animals receiving blocking mAb plus SLEC removed at postmortem were compared with those animals receiving SLEC alone killed at T+24 hours. The animals receiving the blocking mAb exhibited consumption of fibrinogen, microvascular thrombosis with hemorrhage of both the adrenal and renal cortex, and an intense influx of neutrophils into the adrenal, renal, and hepatic microvasculature, whereas the tissues from animals receiving only sublethal E coli exhibited none of these abnormal histopathologic changes. Compared with the control animals, the animals receiving the blocking mAb exhibited significantly elevated serum glutamic pyruvic transaminase, anion gap, thrombin-antithrombin complex, IL-6, IL-8, and soluble thrombomodulin. The levels of circulating activated protein C varied too widely to allow a clear determination of whether the extent of protein C activation was altered in vivo by blocking protein C binding to EPCR. We conclude that protein C/activated protein C binding to EPCR contributes to the negative regulation of the coagulopathic and inflammatory response to E coli and that EPCR provides an additional critical step in the host defense against E coli.
The protein C anticoagulant pathway serves as an “on demand” anticoagulant system.1 Thrombin binding to thrombomodulin (TM) results in a complex that rapidly converts protein C to the active anticoagulant serine protease, activated protein C (APC). TM also increases the rate of thrombin activation of a procarboxypeptidase B, referred to as thrombin activatable fibrinolysis inhibitor.2 APC in complex with protein S functions as an anticoagulant by inactivating factors Va and VIIIa by limited proteolysis.
Defects in this pathway are the most common basis for hereditary thrombophilia3,4 and infants born with homozygous protein C deficiency usually develop microvascular thrombosis of the skin (purpura fulminans).5 Clinical studies have shown that protein C and protein S levels often decrease dramatically in septic shock patients6 and in meningococcemia, in particular. The extent of the decrease is correlated with risk of mortality and the development of purpura fulminans7 reminiscent of that seen in homozygous protein C–deficient infants. In addition to protein C consumption, several mechanisms impair the function of the pathway. These include inhibition of TM function by cytokine-mediated down-regulation8-12 and proteolysis of TM by neutrophil elastase13 or of protein S by thrombin and other proteases.6
Previous studies have shown that infusion of APC protects baboons from a lethal response to LD100Escherichia coli and that inhibition of protein C activation exacerbates the response to sublethal numbers of E coli resulting in a lethal coagulant and inflammatory response.14 Modulation of the pathway by inhibition of protein S function results in a similar exacerbation of the septic response that can be reversed by increasing the protein S levels to near normal.15 In rodent models of septic shock, infusion of TM has been shown to prevent disseminated intravascular coagulation (DIC) and organ failure16-20 and to suppress tumor necrosis factor elaboration.21,22 Initial clinical results have suggested that protein C infusion may be beneficial in meningococcemia and other forms of septic shock.23-25 Taken together, these results suggest that the pathway is both a target of inflammatory injury that occurs in sepsis and that this down-regulation of function contributes to the morbidity and mortality associated with at least some forms of sepsis.
Recently, an endothelial cell protein C receptor (EPCR) was identified.26 EPCR is a type 1 transmembrane protein that is expressed primarily by endothelial cells of the large blood vessels.26,27 It is homologous to the major histocompatibility class 1/CD1 family of proteins involved in the immune response.28 Human endothelium exposed to tumor necrosis factor α (TNFα) in culture exhibits a time-dependent decrease in EPCR expression and mRNA levels. In vitro studies demonstrated that cellular EPCR augments the activation of protein C on endothelium,29,30 whereas soluble EPCR inhibits APC anticoagulant activity.31 Soluble EPCR is released constitutively and the levels of soluble EPCR rise in patients with gram negative sepsis and lupus erythematosus.32 33 These observations raise 2 broad questions: does endogenous EPCR play a role in the host response to E coli, and if so, is this protection related solely to its role in protein C activation?
In this study, we challenged baboons with sublethal E coli and blocked protein C binding to EPCR with an anti-EPCR mAb to determine whether EPCR plays a critical role in the host response toE coli.
Methods
Reagents and monoclonal antibodies B 2 IgG1κ mAbs against human EPCR that cross react with baboon EPCR, 1494 (blocking) and 1510 (nonblocking), were prepared as described by Laszik et al.27 Fab fragments of these mAbs were prepared as described by Xu et al.29E coli organisms (33 985 Type B7-86a:K1; American Type Culture Collection, Rockville, MD) used in the infusion study were isolated from a stool specimen at Children's Memorial Hospital (Oklahoma City, OK). They were stored in the lyophilized state at 4°C after growth in tryptic soybean agar and reconstituted and characterized as described previously.34 To eliminate differences due to E coli strain variations, all animals were infused with E coli from this single isolate.
Preexperimentation and experimentation procedures
The study protocol received prior approval by the Institutional Animal Care and Use Committees of both the Oklahoma Medical Research Foundation and the University of Oklahoma Health Sciences Center (OUHSC). Papio cyanocephalus cynocephalus or Papio cyanocephalus anubis baboons were purchased from either a breeding colony maintained at OUHSC or the Biomedical Research Foundation, Inc (Houston, TX). Animals weighed 4.3 to 13.6 kg, had leukocyte counts of 5000 to 10 000/μL, and hematocrits exceeding 36%. They were free of tuberculosis. The animals were held for 30 days at the OUHSC animal facility, where the infusion studies were performed. All animals were observed continuously during the first 8 hours after infusion of the test materials. Only animals with a negative blood culture before experimentation were included in the study. This led to exclusion of 1 control female who subsequently was found to have a positive blood culture beforeE coli infusion.
Infusion procedures
Experiments were performed on 16 baboons. Animals were fasted overnight before each experiment, but were allowed water ad libitum. Each animal was sedated with ketamine hydrochloride (14 mg/kg, intramuscularly) on the morning of the study, and then, using a percutaneous catheter in the cephalic vein, anesthetized with sodium pentobarbital (2 mg/kg initially and with additional amounts approximately every 20 minutes for 8 hours to maintain a light level of surgical anesthesia).35 Animals were intubated orally and allowed to breathe spontaneously. The femoral artery and vein were cannulated aseptically and used for measuring arterial pressure and obtaining blood samples, respectively. A percutaneous catheter was placed in the saphenous vein and used to infuse the E coli.35 Each anesthetized baboon was positioned on its side in contact with controlled temperature heating pads.
Experimental groups
Table 1 shows the 4 groups of animals studied: group 1 was infused with an anti-EPCR mAb that blocked protein C/APC binding plus sublethal E coli; group 2 was infused with anti-EPCR mAb that binds EPCR but does not block protein C/APC binding plus sublethal E coli; group 3 was infused with sublethal E coli; group 4 was infused with blocking anti-EPCR mAb alone. One animal from group 4 was humanely killed at 24 hours for histology and the other was a permanent (7 day) survivor. Animals number 9 (nonblocking group 2) and number 10 (blocking group 1) received Fab fragments of their respective anti-EPCR mAbs, while the remaining animals in these 2 groups received intact anti EPCR mAbs.
Experimental groups
| Exp. No. . | Sex . | Wt. (kg) . | E coli Infused (CFU/kg × 109) . | E coli Col. Count at T + 2 hr (CFU/mL × 106) . | Anti-EPCR Blocking mAb (mg/kg) . | Anti-EPCR Nonblocking mAb (mg/kg) . | Survival (hs) . |
|---|---|---|---|---|---|---|---|
| Anti-EPCR blocking mAb plus sublethal E coli | |||||||
| 4 | M | 7.1 | 6.0 | 0.4 | 5 | — | 7 |
| 5 | M | 7.3 | 5.5 | 1.0 | 5 | — | 12 |
| 7 | M | 5.7 | 4.8 | 0.8 | 5 | — | 54 |
| 10 | F | 7.7 | 5.0 | 1.5 | 5 (fab) | — | 32 |
| Average | 7.0 | 5.3 | 0.9 | 5 | — | 26.3 | |
| SE | 0.4 | 0.3 | 0.2 | 0 | — | 10.7 | |
| Anti-EPCR nonblocking mAb plus sublethal E coli | |||||||
| 6 | M | 5.9 | 5.5 | 2.3 | — | 5 | 168 |
| 9 | F | 6.4 | 4.7 | 3.0 | — | 5 (fab) | 168 |
| 11 | M | 11.1 | 6.0 | 1.0 | — | 5 | 168 |
| Average | 7.8 | 5.4 | 2.1 | — | 5 | 168 | |
| SE | 1.7 | 0.4 | 0.6 | — | 0 | 0 | |
| Saline plus sublethal E coli | |||||||
| 11294 | F | 4.3 | 5.4 | 0.4 | — | — | 24* |
| 2994 | M | 5.1 | 5.4 | 0.8 | — | — | 24* |
| 21694 | M | 5.1 | 5.8 | 0.1 | — | — | 24* |
| 31694 | M | 5.1 | 5.8 | 0.8 | — | — | 168 |
| 32394 | M | 4.8 | 4.8 | 0.6 | — | — | 168 |
| 72795 | M | 13.6 | 5.8 | 1.4 | — | — | 168 |
| 81897 | F | 11.8 | 5.8 | 0.2 | — | — | 168 |
| Average | 7.1 | 5.5 | 0.6 | — | — | 168 | |
| SE | 1.5 | 0.1 | 0.2 | — | — | 0 | |
| EPCR blocking mAb | |||||||
| 3 | F | 5.7 | — | — | 5 | — | 24* |
| 12 | F | 8.2 | — | — | 5 | — | 168 |
| Exp. No. . | Sex . | Wt. (kg) . | E coli Infused (CFU/kg × 109) . | E coli Col. Count at T + 2 hr (CFU/mL × 106) . | Anti-EPCR Blocking mAb (mg/kg) . | Anti-EPCR Nonblocking mAb (mg/kg) . | Survival (hs) . |
|---|---|---|---|---|---|---|---|
| Anti-EPCR blocking mAb plus sublethal E coli | |||||||
| 4 | M | 7.1 | 6.0 | 0.4 | 5 | — | 7 |
| 5 | M | 7.3 | 5.5 | 1.0 | 5 | — | 12 |
| 7 | M | 5.7 | 4.8 | 0.8 | 5 | — | 54 |
| 10 | F | 7.7 | 5.0 | 1.5 | 5 (fab) | — | 32 |
| Average | 7.0 | 5.3 | 0.9 | 5 | — | 26.3 | |
| SE | 0.4 | 0.3 | 0.2 | 0 | — | 10.7 | |
| Anti-EPCR nonblocking mAb plus sublethal E coli | |||||||
| 6 | M | 5.9 | 5.5 | 2.3 | — | 5 | 168 |
| 9 | F | 6.4 | 4.7 | 3.0 | — | 5 (fab) | 168 |
| 11 | M | 11.1 | 6.0 | 1.0 | — | 5 | 168 |
| Average | 7.8 | 5.4 | 2.1 | — | 5 | 168 | |
| SE | 1.7 | 0.4 | 0.6 | — | 0 | 0 | |
| Saline plus sublethal E coli | |||||||
| 11294 | F | 4.3 | 5.4 | 0.4 | — | — | 24* |
| 2994 | M | 5.1 | 5.4 | 0.8 | — | — | 24* |
| 21694 | M | 5.1 | 5.8 | 0.1 | — | — | 24* |
| 31694 | M | 5.1 | 5.8 | 0.8 | — | — | 168 |
| 32394 | M | 4.8 | 4.8 | 0.6 | — | — | 168 |
| 72795 | M | 13.6 | 5.8 | 1.4 | — | — | 168 |
| 81897 | F | 11.8 | 5.8 | 0.2 | — | — | 168 |
| Average | 7.1 | 5.5 | 0.6 | — | — | 168 | |
| SE | 1.5 | 0.1 | 0.2 | — | — | 0 | |
| EPCR blocking mAb | |||||||
| 3 | F | 5.7 | — | — | 5 | — | 24* |
| 12 | F | 8.2 | — | — | 5 | — | 168 |
EPCR = Endothelial cell protein C receptor; E coli = Escherichia coli; CFU = colony-forming units.
One animal (#8) that was infused with nonblocking EPCR mAb plus sublethal E coli went into estrus and died after 78 hs. As this animal had “positive” blood culture before infusion of E coli, and had been found to have had been in early estrus, and because estrus increases sensitivity to E coli this animal was excluded from the summary 1 above studies.
After the initial 8 hours, those animals that were still alive were returned to their cages and observed until they either exhibited signs of irreversible organ failure at which time they were killed or until they survived for a full 168 hours of observation (permanent survivors). Those that survived were returned to the colony. Three animals infused with sublethal E coli alone were killed at T+24 hours to enable histopathologic comparison of tissues collected from animals infused with blocking mAb plus sublethal E coli (group 1).
The mAbs against EPCR were infused at a concentration of 5 mg/kg as a bolus at T-0.5 hours in all groups in which they were given. The peak mAb concentrations of groups 1, 2, and 4 were 39 ± 2.7, 45 ± 3.1, and 57 ± 1 μg/mL, respectively. The rate of decline of plasma concentrations were similar (T2 = 5.6 hours). The sublethal E coli infusions were begun at T-0 hours. These infusions were carried out over a 2-hour period at the end of which time colony counts were performed on blood taken at that time. Sublethal E coli doses administered ranged from 4.73 to 6.0 × 109 CFU/kg with mean values of 5.3, 5.4, and 5.5 in groups 1, 2, and 3, respectively. The mean blood concentrations of E coli at T+2 hours in 1, 2, and 3 were 0.9 ± 0.2, 2.1 ± 0.6, and 0.6 ± 0.2 × 106 CFU/mL, respectively.
Sampling
Mean systemic arterial pressure (MSAP) and heart rate were monitored with a Stathem pressure transducer and Hewlett Packard (Avondale, PA) recorder. Rectal temperature was measured with a telethermometer (Yellow Springs Instrument Co, Yellow Springs, OH). The above measurements were made, and blood samples were collected, at T = -0.5, 0, +1, +2, +3, +4, +6, +8, and +24 hours where T = 0 designates when the infusion of E coli was begun. Less than 10% of the animals' calculated blood volume (70 mL/kg) was withdrawn over the 8-hour monitoring period. At the time of death, tissue specimens were collected from the animal's lungs, kidneys, liver, adrenal glands, heart, spleen, and brain for light microscopic examination and the gross pathologic findings were recorded.
Assays
The levels of circulating tumor necrosis factor α (TNFα),36 interleukin-6 (IL-6),36interleukin-8 (IL-8),36 thrombin-antithrombin complex (TAT),37 monoclonal Ab to EPCR,37elastase-α1-antitrypsin,38 tPA,39 and soluble TM40,41 were determined by enzyme-linked immunosorbent assay (ELISA). Plasma APC levels were measured by a minor modification of the enzyme capture assay described by Gruber and Griffin.42 The modification involved using a different mAb (HPC 1241) to trap the APC in the baboon plasma. Fibrin degradation products (FDP) were measured by latex agglutination assay.43 Fibrinogen concentration was determined based on the thrombin clotting time.44 Platelet and white cell counts were determined in a Coulter counter. Serum creatinine (CR),45 blood urea nitrogen (BUN),46 anion gap,46 and glutamic pyruvic transaminase (SGPT)47 were measured by automated methods. A PTAH (phosphotungstic acid and hematoxylin) stain was used to estimate the extent of thrombosis.
Statistical analysis and scoring of tissue histopathology B
The clinical chemistry data were analyzed using analysis of variance with Duncan's multicomparison test to determine significant differences for a given variable between groups at given times. An analysis of variance was also used to determine significant differences (P < .05) between time 0 (T-0) and baseline and subsequent times for a given variable and a given group. The adrenal glands, kidneys and lungs were examined for polymorphonuclear (PMN) cell influx, congestion, hemorrhage, thrombosis, and necrosis. The tissues were rated according to the severity of the histopathologic lesions. The scale ranged from 0 to +4, with 4 being the most severe. All microscopic sections were read by Dr Kosanke, who was blinded as to which study was being analyzed. The Kruskal-Wallis test, a nonparametric test, was used to determine significant differences (P < .05) between groups for a given pathologic lesion.
Results
The survival time of animals receiving sublethal E coli plus blocking mAb to EPCR (group 1) ranged from 7 to 54 hours, whereas 4 of 4 receiving sublethal E coli plus saline (group3) and 3 of 3 receiving sublethal E coli plus nonblocking mAb to EPCR (group 2) were permanent (7 day) survivors (Table 1). The dosage of E coli infused ranged from 4.7 to 6.0 × 109CFU/kg. The animal infused with the blocking mAb alone showed no signs of organ injury, no increase in markers of coagulation or inflammation, and survived for the 7-day period of observation.
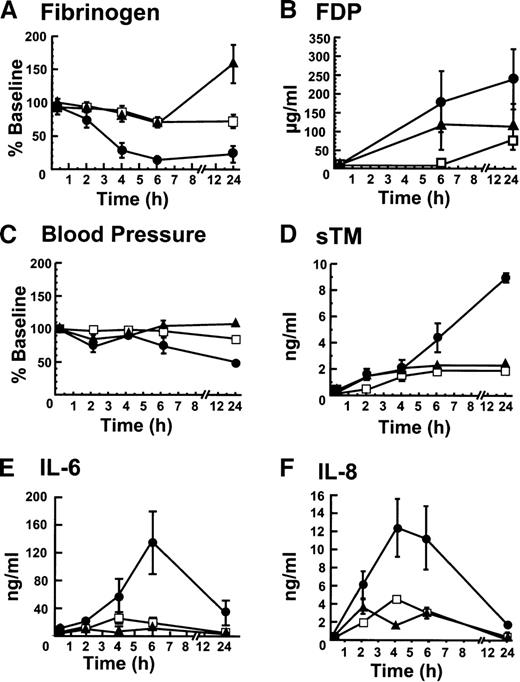
The responses among groups with respect to coagulation, hemodynamics, vascular injury, and inflammation were monitored by determining fibrinogen levels, fibrin degradation products, blood pressure, soluble TM, IL-6 and IL-8 (Figure 1). In the blocking mAb group, fibrinogen (Figure 1A) dropped significantly more than in either of the control groups. Conversely, FDPs were higher in the blocking mAb group than in either of the control groups (Figure1B). The blood pressure decreased more in the blocking group than in either control group (Figure 1C). Similarly, soluble TM levels, a marker of endothelial cell injury, were significantly higher in the blocking than in either of the control groups (Figure 1D). Unlike the control groups in which soluble TM concentrations remained essentially constant after 4 hours, the soluble TM levels continued to rise over the initial 24 hours in the group receiving the blocking mAb, suggesting ongoing vascular injury. IL-6 (Figure 1E) and IL-8 (Figure1F) reached significantly higher concentrations in the blocking mAb group than in the other 2 groups, indicating an increased inflammatory response.
The blocking mAB to EPCR induces changes in fibrinogen consumption, fibrin degradation products, blood pressure, soluble TM, IL-6, and IL-8 levels.
(A) Fibrinogen levels were determined as a function of time after infusion of sublethal E coli and (1) the blocking mAb (■) (2) the control mAb (□), and (3) saline (▴). The same symbols are used to identify the experimental groups throughout this figure; (B) Fibrin degradation products; (C) Blood pressure (MSAP); (D) Soluble TM; (E) IL-6; and (F) IL-8. All assays were performed at least in triplicate and the error bar represents the standard error of the mean from all animals in the group. When error bars are not shown in this and other figures, the error was smaller than the data point on the graph.
The blocking mAB to EPCR induces changes in fibrinogen consumption, fibrin degradation products, blood pressure, soluble TM, IL-6, and IL-8 levels.
(A) Fibrinogen levels were determined as a function of time after infusion of sublethal E coli and (1) the blocking mAb (■) (2) the control mAb (□), and (3) saline (▴). The same symbols are used to identify the experimental groups throughout this figure; (B) Fibrin degradation products; (C) Blood pressure (MSAP); (D) Soluble TM; (E) IL-6; and (F) IL-8. All assays were performed at least in triplicate and the error bar represents the standard error of the mean from all animals in the group. When error bars are not shown in this and other figures, the error was smaller than the data point on the graph.
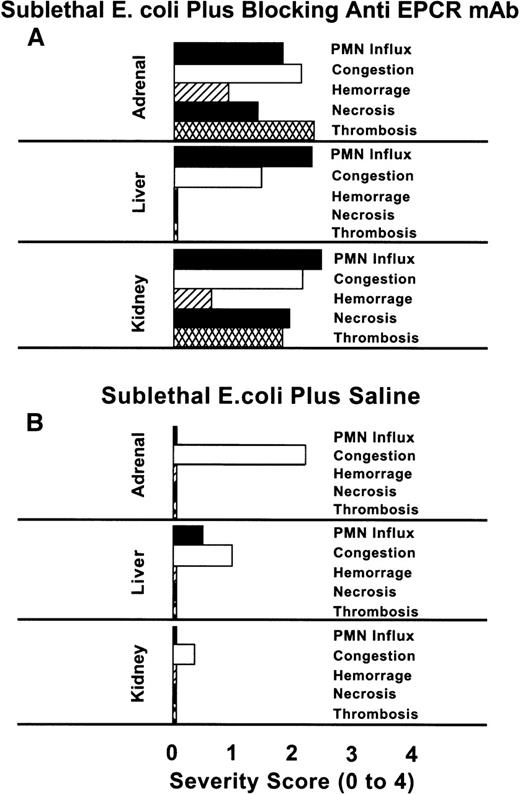
The scoring of the histopathologic lesions of the adrenal glands, liver, and kidneys is shown in Figure 2. The lesions of the blocking mAb group, with the exception of congestion of the adrenal sinusoids, were significantly more severe than those of animals in the saline group. The influx of neutrophils in the liver, and thrombosis of adrenal and renal parenchyma were severe in the blocking mAb group. In addition to these histopathologic changes, in the animals receiving the blocking mAb and sublethal E coli, petechial hemorrhagic lesions were observed in the stomach, all mucosal membranes, and skin. Such lesions were never seen in animals receiving sublethal E coli alone.
Comparison of the histochemical changes in organs from animals with or without the EPCR blocking mAb.
The histopathologic responses of the animals given the blocking mAb plus sublethal E coli are compared with those given sublethalE coli alone. The tissues from animals in the E colialone group were recovered after death at T + 24 hours, whereas those from the blocking mAb group were recovered at the time of impending death (7 to 54 hours). No histopathologic studies of tissues from animals in the nonblocking mAb group were performed because we wished to compare survival time and biochemical parameters between the blocking and nonblocking groups. Evaluations of the parameters were performed in a blinded fashion. PMN influx, congestion, hemorrhage, necrosis, and thrombosis were graded on a scale from 0 to 4, with 0 being normal and 4 being severe. The histopathologic changes of the tissues recovered from the blocking mAb group are significantly more severe than those of the sublethal E coli control groupP < .001, with the exception of the adrenal and hepatic congestion.
Comparison of the histochemical changes in organs from animals with or without the EPCR blocking mAb.
The histopathologic responses of the animals given the blocking mAb plus sublethal E coli are compared with those given sublethalE coli alone. The tissues from animals in the E colialone group were recovered after death at T + 24 hours, whereas those from the blocking mAb group were recovered at the time of impending death (7 to 54 hours). No histopathologic studies of tissues from animals in the nonblocking mAb group were performed because we wished to compare survival time and biochemical parameters between the blocking and nonblocking groups. Evaluations of the parameters were performed in a blinded fashion. PMN influx, congestion, hemorrhage, necrosis, and thrombosis were graded on a scale from 0 to 4, with 0 being normal and 4 being severe. The histopathologic changes of the tissues recovered from the blocking mAb group are significantly more severe than those of the sublethal E coli control groupP < .001, with the exception of the adrenal and hepatic congestion.
Because inhibition of protein C activation was one of the key potential mechanisms to explain the deleterious effects of EPCR-protein C interaction blockade, we examined the circulating plasma APC in the groups infused with the blocking or nonblocking mAbs. Unfortunately, these levels varied widely among individuals within each group. Because APC formation is dependent on thrombin generation, we examined the TAT complex levels as a surrogate marker for thrombin generation. These levels also varied widely between animals in each group. The levels of plasma APC in the control and blocking mAb groups were similar and varied too widely to allow a determination of the extent of inhibition of protein C activation in this model. It is clear, however, that protein C activation can occur relatively well even in the presence of the blocking mAB.
Time-dependent changes in markers of organ and cardiovascular function, as well as inflammatory responses, are compared among the groups in Table 2. The creatinine is significantly higher in blocking mAb group than in either of the control groups (P ≤ .05), indicating significantly greater renal damage in the group where EPCR-protein C binding is blocked. The anion gap and SGPT were also elevated, but did not reach statistical significance. In contrast, the white blood cells, tumor necrosis factor, tissue plasminogen activator, and platelet responses did not differ among any of the groups receiving E coli.
Summary of clinical laboratory and markers of hemostatic and inflammatory system responses to sublethal Escherichia coli plus blocking or nonblocking mAb to EPCR, or saline
| Measurements . | Blocking Anti-EPCR mAb Plus SLEC . | Nonblocking Anti-EPCR mAb Plus SLEC . | Saline Plus SLEC . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-0 . | 2 h . | 4 h . | 6 h . | 24 h . | T-0 . | 2 h . | 4 h . | 6 h . | 24 h . | T-0 . | 2 h . | 4 h . | 6 h . | 24 hr . | |
| Temperature (R) (°C) | 7.3 ± 0.2 | 8.4 ± 0.3 | 38.1 ± 0.5 | 38.1 ± 0.3 | 38.9 ± 0.1 | 6.8 ± 0.2 | 7.5 ± 0.4 | 38 ± 0.3 | 7.9 ± 0.2 | 9.2 ± 0.3 | 6.8 ± 0.2 | 37 ± 0.2 | 7.9 ± 0.2 | 37.9 ± 0.2 | 37.9 ± 0.2 |
| t-PA (ng/mL) | .7 ± 0.6 | 131.8 ± 13.2 | 17.5 ± 14.7 | 164 ± 35.7 | 19.5 ± 22.1 | 3.1 ± 1.6 | 118.8 ± 15.0 | 91.2 ± 12.3 | 103.1 ± 20.1 | 3.7 ± 8.2 | .3 ± 0.5 | 79.4 ± 13.9 | 4.6 ± 59.1 | 4.5 ± 12.9 | 33.9 ± 18.7 |
| Platelets ×106/nm3 | 206.2 ± 28.9 | 159.7 ± 26.2 | 135 ± 9.1 | 14.7 ± 16.5 | 80.5 ± 7.5 | 365.3 ± 44.6 | 277.3 ± 25.9 | 242 ± 10.6 | 91.33 ± 3.5 | 9.7 ± 2.3 | 306.2 ± 20.1 | 223.3 ± 14.7 | 215.2 ± 26.4 | 66.7 ± 13.1 | 84.4 ± 9.5 |
| WBC ×103/mm3 | .5 ± 1.7 | 1.3 ± 0.3 | 1.7 ± 0.2 | 2.7 ± 0.6 | 6.8 ± 0.5 | 3.8 ± 0.3 | 0.9 ± 0.2 | .6 ± 0.4 | 2 ± 0.5 | .8 ± 2.1 | .1 ± 0.8 | .7 ± 0.4 | 5 ± 2 | 4.7 ± 0.9 | 15.3 ± 2.3 |
| Elastase | 0.7 ± 0 | 3.4 ± 0.5 | 4.3 ± 0.3 | 5.6 ± 0.5 | 4.0 ± 0.6 | 0.8 ± 0.3 | 3.0 ± 0.5 | 4.1 ± 0.4 | 4.9 ± 0.5 | 3.4 ± 0.5 | — | — | — | — | — |
| TNF (ng/mL) | <5 ± 0 | 4.5 ± 4.4 | <5 ± 0 | <5 ± 0 | <5 ± 0 | <5 ± 0 | 4.4 ± 12.3 | 5 ± 0 | 5 ± 0 | 5 ± 0 | 7.9 ± 1 | 1.1 ± 9.6 | 8.3 ± 1.7 | 10 ± 2 | 8.2 ± 1.1 |
| Creatinine (mg/dL) | .6 ± 0.1 | — | 1.1 ± 0.3 | 1.9 | 4.3 ± 1 | 0.5 ± 0.1 | — | — | — | .6 ± 0.1 | .6 ± 0.1 | — | — | 0.7 ± 0.1 | 1.5 ± 0.6 |
| Anion Gap | 12.3 ± 1.8 | — | 14.5 ± 1.5 | 24 | 22.3 ± 2.6 | 11 | — | — | — | 12 | 12.8 ± 1.2 | — | — | 15.7 ± 1.1 | 15.3 ± 1.3 |
| SGPT/ALT (u/L) | 53.8 ± 9.3 | — | 52 ± 10 | 91 | 492.3 ± 129.5 | 72 ± 19 | — | — | — | 184.5 ± 93.8 | 59.4 ± 10.1 | — | — | 4.8 ± 19.3 | 97.3 ± 93.4 |
| Measurements . | Blocking Anti-EPCR mAb Plus SLEC . | Nonblocking Anti-EPCR mAb Plus SLEC . | Saline Plus SLEC . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-0 . | 2 h . | 4 h . | 6 h . | 24 h . | T-0 . | 2 h . | 4 h . | 6 h . | 24 h . | T-0 . | 2 h . | 4 h . | 6 h . | 24 hr . | |
| Temperature (R) (°C) | 7.3 ± 0.2 | 8.4 ± 0.3 | 38.1 ± 0.5 | 38.1 ± 0.3 | 38.9 ± 0.1 | 6.8 ± 0.2 | 7.5 ± 0.4 | 38 ± 0.3 | 7.9 ± 0.2 | 9.2 ± 0.3 | 6.8 ± 0.2 | 37 ± 0.2 | 7.9 ± 0.2 | 37.9 ± 0.2 | 37.9 ± 0.2 |
| t-PA (ng/mL) | .7 ± 0.6 | 131.8 ± 13.2 | 17.5 ± 14.7 | 164 ± 35.7 | 19.5 ± 22.1 | 3.1 ± 1.6 | 118.8 ± 15.0 | 91.2 ± 12.3 | 103.1 ± 20.1 | 3.7 ± 8.2 | .3 ± 0.5 | 79.4 ± 13.9 | 4.6 ± 59.1 | 4.5 ± 12.9 | 33.9 ± 18.7 |
| Platelets ×106/nm3 | 206.2 ± 28.9 | 159.7 ± 26.2 | 135 ± 9.1 | 14.7 ± 16.5 | 80.5 ± 7.5 | 365.3 ± 44.6 | 277.3 ± 25.9 | 242 ± 10.6 | 91.33 ± 3.5 | 9.7 ± 2.3 | 306.2 ± 20.1 | 223.3 ± 14.7 | 215.2 ± 26.4 | 66.7 ± 13.1 | 84.4 ± 9.5 |
| WBC ×103/mm3 | .5 ± 1.7 | 1.3 ± 0.3 | 1.7 ± 0.2 | 2.7 ± 0.6 | 6.8 ± 0.5 | 3.8 ± 0.3 | 0.9 ± 0.2 | .6 ± 0.4 | 2 ± 0.5 | .8 ± 2.1 | .1 ± 0.8 | .7 ± 0.4 | 5 ± 2 | 4.7 ± 0.9 | 15.3 ± 2.3 |
| Elastase | 0.7 ± 0 | 3.4 ± 0.5 | 4.3 ± 0.3 | 5.6 ± 0.5 | 4.0 ± 0.6 | 0.8 ± 0.3 | 3.0 ± 0.5 | 4.1 ± 0.4 | 4.9 ± 0.5 | 3.4 ± 0.5 | — | — | — | — | — |
| TNF (ng/mL) | <5 ± 0 | 4.5 ± 4.4 | <5 ± 0 | <5 ± 0 | <5 ± 0 | <5 ± 0 | 4.4 ± 12.3 | 5 ± 0 | 5 ± 0 | 5 ± 0 | 7.9 ± 1 | 1.1 ± 9.6 | 8.3 ± 1.7 | 10 ± 2 | 8.2 ± 1.1 |
| Creatinine (mg/dL) | .6 ± 0.1 | — | 1.1 ± 0.3 | 1.9 | 4.3 ± 1 | 0.5 ± 0.1 | — | — | — | .6 ± 0.1 | .6 ± 0.1 | — | — | 0.7 ± 0.1 | 1.5 ± 0.6 |
| Anion Gap | 12.3 ± 1.8 | — | 14.5 ± 1.5 | 24 | 22.3 ± 2.6 | 11 | — | — | — | 12 | 12.8 ± 1.2 | — | — | 15.7 ± 1.1 | 15.3 ± 1.3 |
| SGPT/ALT (u/L) | 53.8 ± 9.3 | — | 52 ± 10 | 91 | 492.3 ± 129.5 | 72 ± 19 | — | — | — | 184.5 ± 93.8 | 59.4 ± 10.1 | — | — | 4.8 ± 19.3 | 97.3 ± 93.4 |
EPCR = Endothelial cell protein C receptor; SLECs = SublethalE coli; WBC = white blood cell count.
The histologic appearance of adrenal, liver, kidney, and lung tissues collected at 24 hours after the infusion of sublethal E coliplus saline revealed no evidence of abnormal neutrophil influx, congestion, hemorrhage, necrosis, or thrombosis and, with the exception of the significant congestion of the adrenals and minor congestion of the liver, the tissues appeared normal (data not shown). This is in marked contrast to the histologic appearance of the adrenals (Figure 3A and B) and kidneys (Figure 3C and D) in baboons infused with sublethal E coli plus blocking anti-EPCR mAb. In the adrenal gland, 2 types of pathology were observed: one dominated by microthrombus formation (Figure 3A, arrow) and hemorrhage and the other dominated by leukocyte infiltration (Figure 3B) with little thrombosis or hemorrhage present. The thrombosis was observed in the 2 animals that died earliest and the leukocyte infiltration was seen in the other 2 animals. Thrombosis (arrow) involving 90% of the glomeruli of the kidney was also seen in the 2 animals that died the earliest (Figure 3C). This was accompanied in the kidney by karyopyknosis of 75% of the proximal tubular cells (arrow), indicating early ischemia. In contrast, severe acute necrosis of the proximal tubular epithelial cells of the kidney is seen in the 2 animals that died the latest (Figure 3D).
Photographs of sections of adrenal and kidneys from baboons injected with sublethal doses of E coli and blocking anti-EPCR mAbs.
The organs were harvested at the time of impending death. The “early” changes (ie, changes in animals with relatively short survival) consist of prominent microthrombus formation in the subcapsular vessels (arrows) of the adrenals (A) and also in the renal glomeruli (arrow) (C). Note the extensive cortical hemorrhage in the adrenal gland (A) and the early ischemic/necrobiotic changes (karyopyknosis) in the proximal tubular epithelial cells (triangle) of the kidney (C). In B and D, the animals that had relatively long survival, widespread necrotic foci were apparent with associated polymorphonuclear leukocyte infiltrates in the adrenal cortex (B) and severe acute tubular necrosis involving the proximal tubules (P) of the kidney (D). The distal tubules (D) were well-preserved. Occasional mitotic figures of the proximal tubular epithelial cells are also seen as features of regeneration (arrow) (D). Note the lack of thrombi and hemorrhage in the “late” group (B and D).
Photographs of sections of adrenal and kidneys from baboons injected with sublethal doses of E coli and blocking anti-EPCR mAbs.
The organs were harvested at the time of impending death. The “early” changes (ie, changes in animals with relatively short survival) consist of prominent microthrombus formation in the subcapsular vessels (arrows) of the adrenals (A) and also in the renal glomeruli (arrow) (C). Note the extensive cortical hemorrhage in the adrenal gland (A) and the early ischemic/necrobiotic changes (karyopyknosis) in the proximal tubular epithelial cells (triangle) of the kidney (C). In B and D, the animals that had relatively long survival, widespread necrotic foci were apparent with associated polymorphonuclear leukocyte infiltrates in the adrenal cortex (B) and severe acute tubular necrosis involving the proximal tubules (P) of the kidney (D). The distal tubules (D) were well-preserved. Occasional mitotic figures of the proximal tubular epithelial cells are also seen as features of regeneration (arrow) (D). Note the lack of thrombi and hemorrhage in the “late” group (B and D).
Treatment with the blocking mAb plus sublethal E coli resulted in significant pathologic changes in the liver (Figure4), compared with the animals treated with sublethal E coli alone. Both the portal and centrilobular zones of the animals that died early (Figure 4A and C) are characterized by well-preserved architecture. The centrilobular region, however, exhibits an intense polymorphonuclear leukocyte infiltrate in association with some necrobiotic/necrotic changes of scattered hepatocytes. In contrast, the histopathology of the portal and centrilobular zones of the animals that died later (Figure 4B and D) is dominated by “open” nuclei (pale chromatin pattern) with prominent nucleoli and vacuolar degeneration, which is most prominent in the centrilobular region, indicating hepatocellular degeneration.
Photographs of sections of the liver from baboons injected with sublethal doses of E coli and blocking anti-EPCR mAbs.
In the “early” animals (ie, animals with relatively short survival) the portal areas of the liver (A) are quite unremarkable with well-preserved architecture showing the bile duct (B), portal vein (P), hepatic artery, and numerous hepatocytes. The centrilobular regions (C) of the liver from the “early” animals exhibit prominent PMN infiltrate in association with necrobiotic/necrotic changes of scattered hepatocytes. The “late” changes (ie, changes in animals with relatively long survival) consist of “open” nuclei (ie, nuclei with pale chromatin pattern) and prominent nucleoli in the hepatocytes both in the portal (B) and centrilobular (D) regions and vacuolar degeneration of the hepatocytes more severe in the centrilobular area (D). Note (B) the well-preserved portal structures with bile duct (B), hepatic artery, and portal vein (P). Note also the lack of PMN infiltrate in the centrilobular area (D).
Photographs of sections of the liver from baboons injected with sublethal doses of E coli and blocking anti-EPCR mAbs.
In the “early” animals (ie, animals with relatively short survival) the portal areas of the liver (A) are quite unremarkable with well-preserved architecture showing the bile duct (B), portal vein (P), hepatic artery, and numerous hepatocytes. The centrilobular regions (C) of the liver from the “early” animals exhibit prominent PMN infiltrate in association with necrobiotic/necrotic changes of scattered hepatocytes. The “late” changes (ie, changes in animals with relatively long survival) consist of “open” nuclei (ie, nuclei with pale chromatin pattern) and prominent nucleoli in the hepatocytes both in the portal (B) and centrilobular (D) regions and vacuolar degeneration of the hepatocytes more severe in the centrilobular area (D). Note (B) the well-preserved portal structures with bile duct (B), hepatic artery, and portal vein (P). Note also the lack of PMN infiltrate in the centrilobular area (D).
Discussion
This study demonstrates that inhibition of protein C binding to EPCR converts the response to sublethal concentrations of E coliinto a lethal response. These animals exhibit DIC, intense neutrophil influx into the tissues, and elevation of some of the inflammatory cytokines. These responses are not due to nonspecific effects as the mAb because Fab fragments, which cannot fix complement, caused a similar response, and a class similar mAb to EPCR that does not block protein C-EPCR interaction was without significant effects on survival, inflammatory cytokine levels, or markers of organ damage. Furthermore, in the absence of a challenge to the system, the infusion of the blocking mAb elicited neither a change in organ morphology nor death. This study provides the first evidence that EPCR plays an important physiological role. It will be of considerable interest to compare the results of these studies with the murine EPCR gene deletion model currently in progress.
There are several possible mechanisms by which EPCR might protect against E coli sepsis. The first, and most obvious, mechanism is that animals receiving the blocking mAb might activate less protein C than the controls. It is clear, however, that protein C activation occurs relatively well even in the presence of the blocking mAb. This is consistent with the majority of the TM being in small vessels and the majority of the EPCR being in large vessels.27,28Because the coagulant response to E coli varies among animals whether or not EPCR mAbs are present48 and the blocking mAb elicits vascular damage in this model, the model is not well suited for investigations of the role of EPCR in protein C activation in vivo. This question is being approached using thrombin infusion in the presence and absence of blocking antibodies following the procedures described by Lentz's group.49
A second mechanism of protection could involve regulation of the inflammatory response. Consistent with this possibility, compared with controls, animals receiving the blocking mAb exhibited increased leukocyte infiltration into the tissues. The molecular basis for this response is not known. However, soluble EPCR has been shown to interact with leukocytes,50 a process mediated in part by proteinase 3, the autoantigen of Wegener's granulomatosis. The soluble TM levels in the blocking mAb group continued to rise for 24 hours or until death. This latter observation probably reflects progressive injury to the vascular endothelium,13,51 possibly mediated by the adherent leukocytes.51
IL-6 and IL-8 levels are increased in the blocking mAb group compared with the controls. Both cytokines appear early and remain elevated until death instead of returning to baseline as occurs in the controls. These results imply that inhibition of the protein C-EPCR interaction results in an increased inflammatory response that fails to be regulated normally. Whether this is a direct or indirect response is unclear.
Protein C and APC are currently in clinical trials for septic shock. The observation that EPCR plays an important role in the host defense against sepsis may be quite relevant in terms of evaluating patients capable of responding to either therapy. It is known that cytokines such as TNFα can down-regulate EPCR expression in cell culture26 suggesting that in some patients the levels of EPCR may be too low for the protein C/APC to be effective. Consistent with this possibility, histopathologic studies of skin biopsy specimens of patients with meningococcemia have shown markedly reduced levels of EPCR expression in the thrombosed vasculature compared with biopsy specimens from adjacent skin.52 Analysis of samples from these trials will not only test this hypothesis, but may provide insights into appropriate therapeutic regimens.50 52
Funded by: Grant # P01 HL54804 (C.T.E.) (C.T.E. is an investigator of the Howard Hughes Medical Institute); NIH Grant #2R01GMHL37704-12 (F.B.T.).
Reprints: Fletcher B. Taylor Jr, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: marie-brewer@omrf.ouhsc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal