Stimulation of the platelet nonintegrin collagen receptor, glycoprotein VI, evokes a signaling response similar to that induced by antigen receptor activation in B and T lymphocytes. A key transducer of the lymphocyte signaling pathways is the Bruton's tyrosine kinase (Btk)/Tec kinase family, which connects receptors to the elevation of intracellular-free calcium levels. An important signaling function for Btk in collagen-induced platelet activation in vitro was recently demonstrated by other researchers using Btk-deficient platelets from patients with X-linked agammaglobulinemia (XLA). Since Btk-deficiency does not induce an overt platelet-based bleeding disorder in vivo, collagen receptor responses may include other Btk/Tec kinase family members in normal platelets. Both Btk and Tec had increased tyrosine following stimulation of collagen receptors or CD32 cross-linking. Data from kinetic analyses and inhibitor studies and the use of phosphopeptide-specific antibodies recognizing 2 Btk regulatory phosphorylated tyrosine residues suggest a mechanism for coordinate recruitment of Btk and Tec through the immunoreceptor tyrosine-based activation motif, Src family kinases, and phosphatidylinositol 3-kinase. In XLA platelets, collagen treatment increased tyrosine phosphorylation of Tec and several other signaling proteins, including Lyn, Fyb, Slp-76, and the Wiskott-Aldrich syndrome protein. This indicates that important elements of the collagen signaling pathway proximal and distal to Btk and Tec are preserved despite the lack of functional Btk. The results are consistent with the conclusion that activation of Tec may sustain XLA platelet function in vivo, while some in vitro assays of nonintegrin collagen receptor signaling through the Btk/Tec kinase family reflect the additive dosage of the transducers.
Disruption of vessel walls through injury or rupture of atherosclerotic plaques exposes subendothelial collagen to blood platelets and results in formation of a hemostatic thrombus.1-3 Impairment of the platelet-collagen binding interaction due to absence or blocking of platelet receptors is associated with bleeding diathesis, thus illustrating the essential role for this interaction in hemostasis.1-3 Platelets bind to collagen through several specific membrane receptor types. Collagen engagement of these receptors induces a series of biochemical and morphological events as the platelets are incorporated into a forming thrombus. The platelet membrane protein integrin α2β1 binds collagen directly, whereas platelet glycoprotein Ib binds indirectly to collagen through the von Willebrand factor. After initial adhesion to collagen, other nonintegrin receptors, such as glycoprotein VI (GPVI), are engaged, and the platelet proceeds through an irreversible activation sequence.
Collagen-induced platelet intracellular signaling through GPVI resembles the functional response of B- and T-cell antigen receptors (BCR and TCR, respectively) to cross-linking.3-8 Activated GPVI signals the platelets by physical association with a critical immunoreceptor tyrosine-based activation motif (ITAM)-containing Fcγ receptor (FcγR) chain, which is a docking site for proximal transducers of receptor signaling pathways.3Dimerization and aggregation of receptor chains after ligand binding or antibody cross-linking activates the proximal transducers initiating an intracellular signal. In platelets, loss of the FcγR chain function abrogates collagen-induced platelet activation and aggregation through a decrease in GPVI-mediated intracellular signal transduction.7 Collagen receptor signaling through the FcγR chain results in activation of multiple transducers including the Src family kinases, syk tyrosine kinase, phosphatidylinositol 3-kinase (PI 3-kinase), Btk/Tec kinase family, and phospholipase C-γ2 (PLC-γ2).3-9 These proximal mediators of signal transduction function in a variety of hematopoietic cells to link lineage-specific surface receptors to common downstream responses such as release of intracellular calcium, redistribution of cytoskeletal proteins, and secretion of granules.3
The Btk/Tec tyrosine kinase family comprises structurally homologous, functionally interchangeable, dosage-sensitive signal transducing proteins.10 Multiple intracellular signaling pathways, including the Src family kinases, PI 3-kinase, protein kinase C, and G proteins,11-21 appear to converge in the modulation of Btk/Tec kinase activity. By influencing PLC-γ2 activity and other targets, Btk/Tec kinases are important components of receptor pathways that lead to increased intracellular calcium levels.9,13,22,23 Btk/Tec kinases may also function in platelets and lymphoid cells by binding to and tyrosine phosphorylating the Wiskott-Aldrich syndrome protein (WASP).24-30 Genetic deficiency of WASP causes abnormal platelet morphology, number, and functional responses (reviewed in Ochs31 and in Snapper and Rosen32). Tyrosine phosphorylation of WASP by the Btk/Tec kinases may be an important mechanism to regulate WASP function in platelet signaling pathways.
Btk deficiency dramatically influences BCR signaling pathways in human and murine B lineage cells, and more subtle defects have been identified in murine mast cell FcεR function.10,29 A role for Btk/Tec kinases in platelets was recently demonstrated by the observation of decreased in vitro collagen-induced aggregation of platelets from XLA patients.9 This influence on platelet function correlated with decreased collagen-induced tyrosine phosphorylation of PLC-γ2, delayed intracellular calcium release, and decreased secretion from dense granules. In contrast to the profound influence of Btk deficiency on the B-cell lineage, XLA patients do not exhibit a platelet-dependent bleeding disorder. The presence of a functional defect of XLA platelets in vitro, without a corresponding clinical phenotype of bleeding diathesis, suggests that expression of alternative members of the Btk/Tec kinase family may permit normal collagen receptor signaling in vivo. To test the hypothesis that multiple Btk/Tec kinases contribute to collagen receptor signaling, we tested platelets for expression of these kinases and their tyrosine phosphorylation in response to receptor stimulation. The data were consistent with the coordinated activation of Btk and Tec in receptor pathways signaling through the ITAM, PI 3-kinase, and Src kinases. The parallel activation of Btk and Tec suggests that both contribute to functional responses in platelets.
Materials and methods
The following products were purchased for this study: prostaglandin E1 (PGE1), Arg-Gly-Asp-Ser (arginine, glycine, aspartic acid, and serine; RGDS) peptide, dimethylsulfoxide (DMSO), aspirin, apyrase (type VIII), N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes), sodium dodecyl sulfate (SDS), 2-mercaptoethanol, sodium orthovanadate, bovine serum albumin (BSA), protein A-Sepharose, Triton X-100, and tris(hydroxymethyl) aminomethane (Tris) (all from Sigma, St. Louis, MO); nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA); SDS-PAGE (polyacrylamide gel electrophoresis) molecular standards, [γ-32P]ATP (adenosine triphosphate), and enhanced chemiluminesence (ECL) reagents including secondary antibodies (Amersham, Arlington Heights, IL); PI 3-kinase inhibitors LY294002 and wortmannin (Calbiochem, La Jolla, CA); anti-CD32 monoclonal antibody (mAb) IV.3 (MEDAREX, Annandale, NJ); F(ab′)2 fragment of goat antimouse immunoglobulin G (IgG) (ICN Pharmaceutical, Aurora, OH); anti-Btk goat serum (Santa Cruz Biotechnology, Santa Cruz, CA); anti-Fyb/Slp-130 mAb (Transduction Laboratories, Lexington, KY); anti-Tec and anti-Shc rabbit polyclonal antibody (pAb) (Upstate Biotechnology, Lake Placid, NY); and horse tendon collagen (Hormon Chemie, Munich, Germany).
Antisera against the N-terminal of Btk, phosphotyrosine 551, and phosphotyrosine 223 have been described.13 An antiphosphotyrosine antibody, 4G10, was used as described. We also received the following gifts: purified human thrombin (Green Cross, Osaka, Japan) and recombinant thrombopoietin (TPO) (Kirin Brewery, Tokyo, Japan).
Platelet preparation
Human blood from XLA patients and healthy volunteers was drawn by venipuncture into 1/10 volume of 3.8% (wt/vol) trisodium citrate and gently mixed. Before drawing blood, informed consent was obtained. Genetic studies were performed in different laboratories for XLA patients A and B (Dr S. Tuskada, Osaka University School of Medicine, Osaka, Japan) and XLA patients C-F (H.D.O., University of Washington School of Medicine, Seattle, WA). Patients A and B are brothers, and the genetic study revealed that the Btk allele in both patients has a T558→G(TAT→TAG) mutation resulting in the appearance of a premature stop codon. Patients C and D are brothers, and both have a Btk allele containing an A1898→G(Glu589→Gly) missense mutation. The Btk allele in patient E has a C403→ T(Glu91→STOP) mutation. The Btk alleles in the unrelated patients F and G have missense mutations: A1223→C(His364→Pro) mutation for patient F and G1970→A(Gly613→Asp) mutation for patient G. Western blot analysis and/or flow cytometry for detection of intracellular Btk revealed that platelets from patients A, B, and E do not express even truncated Btk, while platelets from the other patients may express a trace amount of mutated Btk.
Platelet rich plasma (PRP) was prepared by centrifugation of whole blood at 200g for 20 minutes. PRP was aspirated and incubated with 2 μmol/L aspirin for 30 minutes at room temperature. Following addition of 1 μmol/L PGE1 from a stock solution in 1 mmol/L absolute ethanol, the PRP was spun at 800g to form a soft platelet pellet. The pellet was resuspended in 1 mL modified Hepes-Tyrode buffer containing 129 mmol/L sodium chloride (NaCl), 8.9 mmol/L sodium hydrogen carbonate (NaHCO3), 0.8 mmol/L monopotassium phosphate (KH2PO4), 0.8 mmol/L magnesium dichloride (MgCl2), 5.6 mmol/L dextrose, and 10 mmol/L Hepes (pH 7.4); supplemented with 0.6 units/mL apyrase; and washed twice. Platelets (3 × 108 cells/mL) were resuspended for experiments in the modified Hepes-Tyrode buffer supplemented with 0.6 units/mL apyrase and 200 μg/mL RGDS peptide.
Platelet stimulation and protein analysis
Aliquots of platelets (0.5 mL at a concentration of 3 × 108 cells/mL) were incubated in modified Hepes-Tyrode buffer supplemented with 0.6 units/mL apyrase and 200 μg/mL RGDS peptide. Platelet stimulation by agonists was terminated by the addition of an equal volume of lysis buffer containing 15 mmol/L Hepes (pH 7.4), 150 mmol/L NaCl, 1 mmol/L phenyl methyl sulfonyl fluoride (PMSF), 10 mmol/L EGTA (ethylenegylcotetraacetic acid), 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, and 2% Triton X-100 (wt/vol). In some experiments, 2 mmol/L MgCl2 was added to ensure the presence of Mg2+ during precipitation. After 20 minutes on ice, the lysates were centrifuged at 10 000g at 4°C for 20 minutes. The lysate soluble supernatant was recovered, incubated with protein A-Sepharose (40 μL of 50% slurry) for 1 hour, and centrifuged to obtain the cleared supernatant. Immunoprecipitating antibody (1-5 μg/sample) was added to the cleared supernatants for 2-3 hours on ice, then immune complexes were bound by the addition of protein A-Sepharose (40 μL of 50% slurry/mL supernatant). The immunoprecipitates were washed 3 times with 1 mL cold buffer (the same as the lysis buffer except using 1% Triton X-100 [wt/vol]). Proteins were denatured by heating at 95°C for 5 minutes in modified Laemmli's sample buffer (10% glycerol, 1% SDS, 5% 2-mercaptoethanol, 50 mmol/L Tris-HCl [pH 6.8], 0.002% bromophenol blue, 10 mmol/L EGTA, and 1 mmol/L sodium orthovanadate), then separated by 1-dimensional SDS-electrophoresis with 10% or 7.5%-15% polyacrylamide gels.
Proteins were transferred from the gel onto nitrocellulose membranes in transfer buffer (25 mmol/L Tris, 192 mmol/L glycine, and 20% methanol). To block residual protein binding sites, membranes were incubated in Tris-buffered saline-Tween (TBST; 10 mmol/L Tris [pH 7.6], 150 mmol/L NaCl, and 0.1% Tween 20) with 10% BSA. The membranes were washed with TBST, then incubated overnight with primary antibodies diluted in TBST as indicated: 1.0 μg/mL each of 4G10 antibody, anti-Btk goat antibody, and 1.0 μg/mL anti-Tec pAb; 1:1000 dilution anti-Btk rabbit antibody; and 0.5 μg/mL each of antibodies 223PY and 551PY. The membranes were washed with TBST then incubated with horseradish peroxidase–conjugated second antibody diluted in TBST at a concentration of 1:3000. The antibody binding to proteins was visualized with ECL according to the manufacturer's instructions.
In vitro kinase assays
Immunoprecipitated Btk was washed twice with kinase buffer (150 mmol/L NaCl, 5 mmol/L MgCl2, 5 mmol/L manganese dichloride (MnCl2), 1 mmol/L sodium orthovanadate (Na3VO4), and 10 mmol/L Hepes [pH 7.4]), then incubated for 10 minutes at room temperature in 30 μL kinase buffer containing 10 μmol/L ATP and 9.25 MBq (0.25 mCi/mL) [γ-32P]ATP. Reactions were terminated with an equal volume of modified Laemmli sample buffer, the proteins were separated with SDS-PAGE, and the incorporation of 32P into Btk was visualized by autoradiography.
Results
Btk and Tec in human platelets
The Btk/Tec kinase family plays important roles during development and function of hematopoietic lineages. Each family member displays a distinct pattern of expression in these lineages. To evaluate the pattern of expression of Btk/Tec kinases in platelets, immunoblot analysis was performed after separation of platelet extract proteins by gel electrophoresis. Approximately 77-kd Btk proteins and approximately 65-kd Tec proteins were detected, but few Itk or Bmx proteins were detected (data not shown).
Tyrosine phosphorylation of Btk/Tec kinases specifically induced by nonintegrin collagen receptors
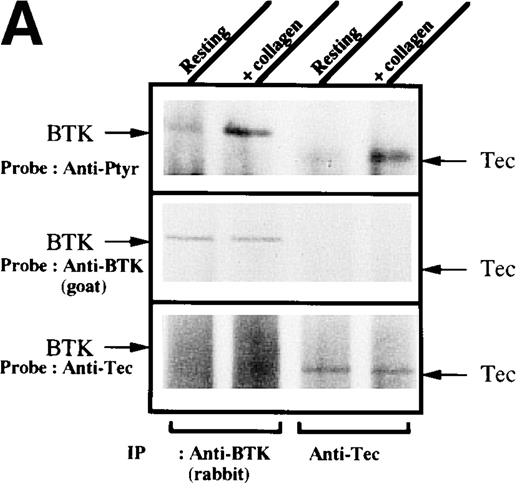
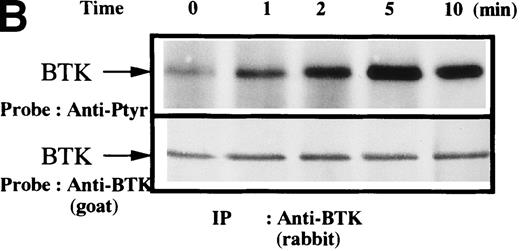
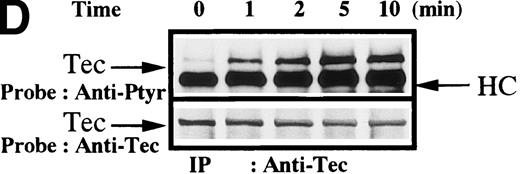
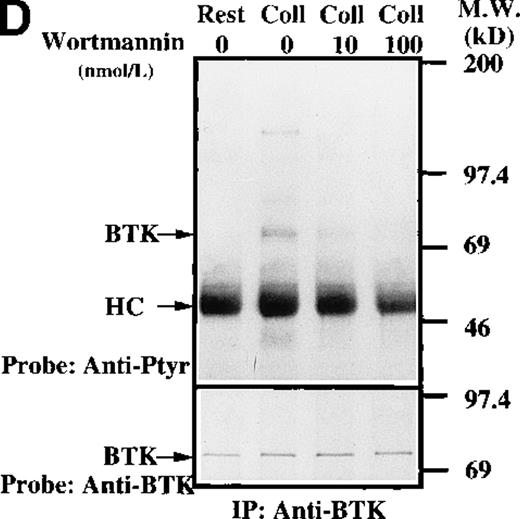
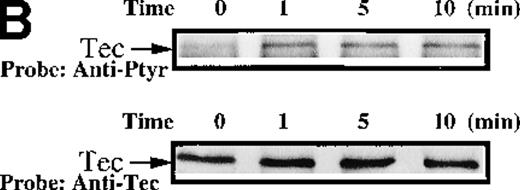
To test for the tyrosine phosphorylation of Btk and Tec, these proteins were immunopurified from extracts of control and collagen-treated platelets. Increases in the phosphorylation of Btk and Tec were detected using antiphosphotyrosine immunoblot in samples with similar amounts of either protein (Figure1A). Treatment of platelets with collagen for increasing lengths of time induced a detectable increase in Btk phosphorylation within 1 minute, with a peak at 5 minutes after collagen stimulation (Figure 1B). Phosphorylation at 10 minutes remained similar to the elevated levels at 5 minutes. To evaluate the role of integrin and nonintegrin collagen receptors in Btk activation, platelets were stimulated with collagen in the presence or absence of extracellular magnesium ion with 10 mmol/L EDTA added. The absence of magnesium ion blocks collagen binding to and stimulation of platelet α2β1.1-3 With or without magnesium, Btk tyrosine phosphorylation was retained in collagen-treated platelets, which indicates that nonintegrin receptors are sufficient for activation (Figure 1C). A kinetic evaluation of Tec phosphorylation after collagen stimulation of nonintegrin receptors was performed to compare it with the Btk response. Magnesium-independent increased Tec phosphorylation was detectable at 1 minute and was maximal at 5 minutes (Figure 1D). The similar kinetics of activation support the conclusion that both kinases participate in this pathway.
Increased tyrosine phosphorylation of Btk and Tec in collagen-stimulated platelets.
(A) Aliquots of platelets were treated with 50 μg/mL of either collagen or buffer for 5 minutes, then were lysed in detergent buffer as described in “Materials and methods.” Btk or Tec was purified from the soluble extracts by immunoprecipitation, and the denatured samples were divided into 3 equal aliquots. Replicate pairs of the samples (without or with collagen treatment) were separated by 7.5%-15% SDS-PAGE, transferred to a nitrocellulose membrane, then immunoblotted with either 4G10 total phosphotyrosine antibody (top panel), anti-Btk antibody (middle panel), or anti-Tec antibody (bottom panel). (B) Platelets were treated with 50 μg/mL of either collagen or buffer for 10 minutes or less. Btk was purified from the soluble extracts by immunoprecipitation, as described above, then immunoblotted with either 4G10 total phosphotyrosine antibody (top panel) or anti-Btk antibody (bottom panel). (C) Platelets were incubated in nominally Ca++– and Mg2+–free modified Hepes-Tyrode buffer and treated with 50 μg/mL of either collagen or buffer for 5 minutes, followed by the addition of 10 mmol/L EDTA. Btk was purified from the soluble extracts by immunoprecipitation as described above, then immunoblotted with either 4G10 total phosphotyrosine antibody (top panel) or anti-Btk antibody (bottom panel). (D) Aliquots of platelets, as described in (C), were treated with 50 μg/mL of either collagen or buffer for 0 to 10 minutes following the addition of 10 mmol/L EDTA. Tec was purified from the soluble extracts by immunoprecipitation as described above and immunoblotted with either 4G10 total phosphotyrosine antibody (top panel) or anti-Tec antibody (bottom panel).
Increased tyrosine phosphorylation of Btk and Tec in collagen-stimulated platelets.
(A) Aliquots of platelets were treated with 50 μg/mL of either collagen or buffer for 5 minutes, then were lysed in detergent buffer as described in “Materials and methods.” Btk or Tec was purified from the soluble extracts by immunoprecipitation, and the denatured samples were divided into 3 equal aliquots. Replicate pairs of the samples (without or with collagen treatment) were separated by 7.5%-15% SDS-PAGE, transferred to a nitrocellulose membrane, then immunoblotted with either 4G10 total phosphotyrosine antibody (top panel), anti-Btk antibody (middle panel), or anti-Tec antibody (bottom panel). (B) Platelets were treated with 50 μg/mL of either collagen or buffer for 10 minutes or less. Btk was purified from the soluble extracts by immunoprecipitation, as described above, then immunoblotted with either 4G10 total phosphotyrosine antibody (top panel) or anti-Btk antibody (bottom panel). (C) Platelets were incubated in nominally Ca++– and Mg2+–free modified Hepes-Tyrode buffer and treated with 50 μg/mL of either collagen or buffer for 5 minutes, followed by the addition of 10 mmol/L EDTA. Btk was purified from the soluble extracts by immunoprecipitation as described above, then immunoblotted with either 4G10 total phosphotyrosine antibody (top panel) or anti-Btk antibody (bottom panel). (D) Aliquots of platelets, as described in (C), were treated with 50 μg/mL of either collagen or buffer for 0 to 10 minutes following the addition of 10 mmol/L EDTA. Tec was purified from the soluble extracts by immunoprecipitation as described above and immunoblotted with either 4G10 total phosphotyrosine antibody (top panel) or anti-Tec antibody (bottom panel).
Collagen-induced Btk activation through the Src family kinases
An important mechanism for activation of the Btk/Tec kinases in receptor signaling pathways occurs through phosphorylation of 2 regulatory tyrosine residues.11-13 Receptor stimulation activates the Src family kinases, which then transphosphorylates the Btk Y551 residue. This site is within the kinase domain activation loop, and phosphorylation increases the catalytic activity of the enzyme. An early substrate for the activated kinase is the Btk Y223 residue. This phosphorylation site, within the SH3 domain, probably alters intramolecular and intermolecular binding interactions.
To test for evidence of an Src family kinase–mediated activation of Btk in the collagen signaling pathway, the phosphorylation of the 2 regulatory Btk tyrosine residues was measured for increasing lengths of time after platelet stimulation. The immunopurified Btk from each sample was immunoblotted with phosphopeptide site-specific antibodies that recognize either phosphorylated Y551 or Y223 residue, and the kinetics of phosphorylation of each site were compared (Figure2A). A maximal increase in phosphorylation of the activating residue Y551 was observed within 1 minute. In contrast to other B-cell and mast-cell receptor responses, in which the Y551 phosphorylation rapidly decreases, Btk in platelets retained a high level of phosphorylation for at least 10 minutes.13The Y223 residue was detectably phosphorylated in unstimulated platelets, similar to its phosphorylation in other systems. Phosphorylation of the Y223 residue increased slowly relative to the Y551 residue, with a maximal increase noted at approximately 5 minutes. To confirm that collagen-induced phosphorylation enhanced kinase activity, Btk was immunopurified from control and stimulated platelets, then incubated in the presence of radiolabeled [γ-32P]ATP. Autoradiography of the Btk protein (Figure 2B) demonstrated significantly higher in vitro autophosphorylation in the platelets treated with collagen for 1 minute. This strongly suggests that collagen treatment of platelets activates Btk through an Src family kinase–dependent mechanism similar to that described in other cell types.
Src-mediated activation of Btk in collagen-treated platelets.
(A) Platelets were treated with collagen as in Figure 1A for the time indicated. Btk was purified from the soluble extracts by immunoprecipitation as described above, then immunoblotted with Btk phosphopeptide site-specific antibodies 551PY (top panel) and 223PY (middle panel) or anti-Btk antibody (bottom panel). (B) Platelets were treated with either collagen or buffer for 1 minute. Btk was purified from the soluble extracts by immunoprecipitation as described above. The immune complexes were incubated in a kinase buffer containing [γ-32P]ATP for 5 minutes as described in “Materials and methods.” Incorporation of 32P into Btk was visualized by autoradiography.
Src-mediated activation of Btk in collagen-treated platelets.
(A) Platelets were treated with collagen as in Figure 1A for the time indicated. Btk was purified from the soluble extracts by immunoprecipitation as described above, then immunoblotted with Btk phosphopeptide site-specific antibodies 551PY (top panel) and 223PY (middle panel) or anti-Btk antibody (bottom panel). (B) Platelets were treated with either collagen or buffer for 1 minute. Btk was purified from the soluble extracts by immunoprecipitation as described above. The immune complexes were incubated in a kinase buffer containing [γ-32P]ATP for 5 minutes as described in “Materials and methods.” Incorporation of 32P into Btk was visualized by autoradiography.
PI 3-kinase in the platelet Btk activation pathway
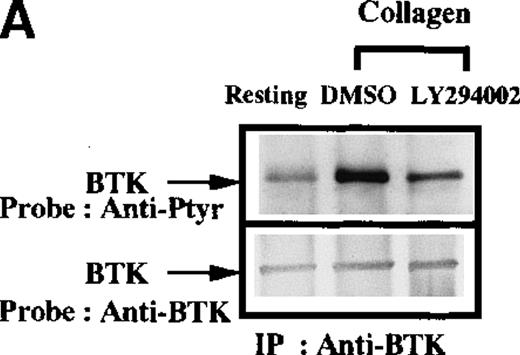
In B cells, Btk activation and function are critically influenced by the PI 3-kinase signaling pathway. A variety of structural and genetic data indicate that the Btk pleckstrin homology domain directly binds to membrane phospholipid products of PI 3-kinase.14 Modulation of this interaction regulates the capacity of receptor-associated Src family kinases to activate Btk. In platelets, PI 3-kinase is an important mediator of platelet aggregation,33-37 and inhibitors can prevent collagen-induced phosphorylation of the downstream target protein WASP.24 To test the hypothesis that the PI 3-kinase signaling pathway is required for Btk and Tec activation in platelets, collagen stimulation of platelets was performed in the absence or presence of a specific inhibitor of PI 3-kinase activity (LY294002). After immunopurification from platelet extracts, the phosphorylation of Btk (Figure 3A) and Tec (Figure 3B) was measured by antiphosphotyrosine immunoblot. The presence of the LY294002 inhibitor strongly attenuated the collagen-induced phosphorylation of Btk and Tec. The role of PI 3-kinase in collagen-stimulated Btk phosphorylation was further tested by using either a lower dose of inhibitor LY294002 (5 μmol/L) or using inhibitor wortmannin (Figures 3C and 3D, respectively).
Influence of PI 3-kinase activity on Btk and Tec phosphorylation.
Aliquots of platelets were treated with 0.1% DMSO (vehicle for LY294002) or 50 μmol/L LY294002 for 10 minutes. Then 50 μg/mL collagen was added for 5 minutes, as indicated. (A) Btk or (B) Tec were purified from the soluble extracts by immunoprecipitation as described above. The proteins were analyzed by immunoblot with 4G10 total antiphosphotyrosine (top panels) and anti-Btk or anti-Tec antibody (bottom panels). Collagen-stimulated Btk phosphorylation and coprecipitation of phosphoproteins was inhibited by low-dose (5 μmol/L) LY294 002, (C) 10 nmol/L wortmannin, and (D) 100 nmol/L wortmannin. MgCl2 was added to the platelet extracts in (C) and (D).
Influence of PI 3-kinase activity on Btk and Tec phosphorylation.
Aliquots of platelets were treated with 0.1% DMSO (vehicle for LY294002) or 50 μmol/L LY294002 for 10 minutes. Then 50 μg/mL collagen was added for 5 minutes, as indicated. (A) Btk or (B) Tec were purified from the soluble extracts by immunoprecipitation as described above. The proteins were analyzed by immunoblot with 4G10 total antiphosphotyrosine (top panels) and anti-Btk or anti-Tec antibody (bottom panels). Collagen-stimulated Btk phosphorylation and coprecipitation of phosphoproteins was inhibited by low-dose (5 μmol/L) LY294 002, (C) 10 nmol/L wortmannin, and (D) 100 nmol/L wortmannin. MgCl2 was added to the platelet extracts in (C) and (D).
As divalent cations can be essential to maintaining protein conformation and interactions, Mg2+ was added to the platelet extracts during immunoprecipitation to enhance the association of Btk with possible ligands or substrates. Interestingly, the presence of Mg2+ enhanced coprecipitation of several tyrosine phosphorylated proteins (apparent masses in approximate units of 150, 80, 70, and 40 kd) with Btk from extracts of collagen-stimulated platelets (Figure 3C). PI 3-kinase inhibition not only decreased Btk tyrosine phosphorylation, but it also blocked the apparent coprecipitation of the other phosphoproteins. Consistent with the influence of the PI-3 kinase inhibitor LY294002, treatment of platelets with inhibitor wortmannin resulted in a dose-dependent block of collagen-induced Btk phosphorylation and coprecipitation of phosphoproteins (Figure 3D). These results are consistent with the recent observations of Laffargue et al,37 who found that Btk tyrosine phosphorylation is enhanced by platelet aggregation and that this response is inhibited in a similar dosage range by wortmannin. These results confirm the importance of PI 3-kinase as an upstream regulator of Btk activation and association with other signaling proteins during collagen receptor signaling platelets. They also strengthen the similarity of the platelet mechanism to receptor pathways previously described in other hematopoietic lineages.
Capacity of alternative platelet receptors to activate Btk and Tec
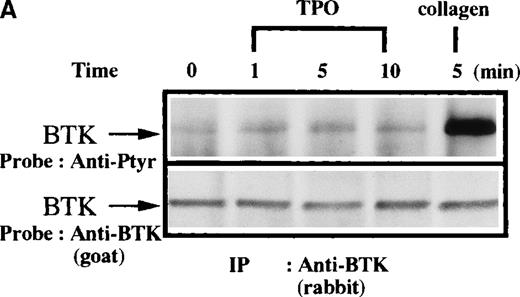
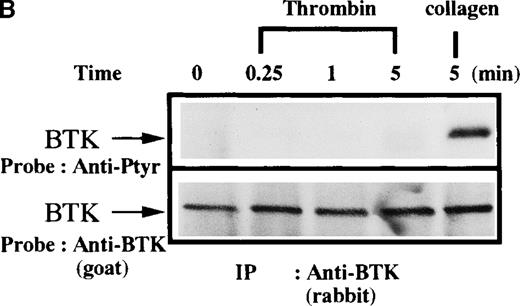
To further define the specificity of the activation of the Btk/Tec kinase family in platelet signaling responses, important regulators of platelet function were assessed for their capacity to induce tyrosine phosphorylation of Btk or Tec. TPO and thrombin bind to specific platelet membrane receptors that elicit tyrosine phosphorylation of multiple platelet proteins by mechanisms different from collagen. Thrombin is known to activate PLC in platelets through receptors coupled to trimeric G-proteins like Gq and Gi.38-40Interestingly, a role for Gq in activation of Btk has been postulated in other cells.20 On the other hand, TPO is known to induce activation of Jak2 and Tyk2 without activation of PLC.41-48To test whether Btk is recruited into these signaling pathways, platelets were treated with either TPO (Figure4A) or thrombin (Figure 4B) for increasing lengths of time, then Btk was immunopurified and analyzed for phosphotyrosine content. In comparison with collagen treatment, there was little or no increase of Btk phosphorylation induced by these agonists. This indicates that a robust Btk activation is not a general response to platelet activation, which is in agreement with the above cited report by Laffargue et al.37
Influence of TPO and thrombin on Btk phosphorylation.
Platelets were treated with 50 μg/mL collagen, (A) 100 ng/mL TPO, (B) 1 unit/mL thrombin for 0 to 10 minutes, or (C) 1 unit/mL thrombin for 0 to 5 minutes, as indicated. (A,B) Btk or (C) Tec was purified from soluble extracts by immunoprecipitation as described above and immunoblotted with either 4G10 antibody (top panels) or anti-Btk or anti-Tec antibody (bottom panels).
Influence of TPO and thrombin on Btk phosphorylation.
Platelets were treated with 50 μg/mL collagen, (A) 100 ng/mL TPO, (B) 1 unit/mL thrombin for 0 to 10 minutes, or (C) 1 unit/mL thrombin for 0 to 5 minutes, as indicated. (A,B) Btk or (C) Tec was purified from soluble extracts by immunoprecipitation as described above and immunoblotted with either 4G10 antibody (top panels) or anti-Btk or anti-Tec antibody (bottom panels).
Since XLA platelets have normal responses to thrombin treatment,9it is formally possible that thrombin may selectively signal through Tec, independently of Btk. To test the influence of thrombin directly, Tec was immunopurified from control and thrombin-treated platelets and immunoblotted with antiphosphotyrosine. A small increase in Tec tyrosine phosphorylation was observed (Figure 4C), which is consistent with a previous report that maximal thrombin-induced Tec phosphorylation is highly dependent on platelet aggregation.49 Hamazaki et al50 also found that Tec phosphorylation is significantly dependent on platelet aggregation, although to a lesser degree. The differences may be due to technical nuances. For example, Laffargue et al39 observed an inhibitory influence of cytochalasins D, whereas Hamazaki et al50 did not. Thus, collagen induces stronger Btk and Tec tyrosine phosphorylation than that provoked through other platelet receptor signaling pathways in our experimental conditions.
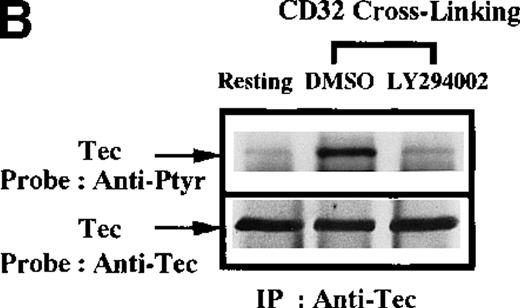
Recent observations indicate that the platelet surface FcγRIIA (CD32) is similar to the collagen nonintegrin receptor in its use of the ITAM-mediated signaling mechanisms.3 To test whether platelet receptor signaling through the ITAM-mediated signaling is sufficient for Btk and Tec activation, the CD32 protein was stimulated by cross-linking with the mAb IV.3. Btk (Figure5A) and Tec (Figure 5B) were immunopurified from platelet extracts and analyzed by antiphosphotyrosine immunoblot. Cross-linking of CD32 is sufficient to increase the tyrosine phosphorylation of both proteins. The similarity of CD32 signaling to the collagen receptor pathway was further tested by treating platelets with the inhibitor LY294002 prior to CD32 cross-linking. The capacity of CD32 cross-linking to increase the tyrosine phosphorylation of both Btk and Tec was blocked by inhibition of PI 3-kinase activity. This suggests that following ligation of CD32, PI 3-kinase activity is also necessary for tyrosine phosphorylation of Btk and Tec. These results demonstrate that the capacity to recruit the Btk/Tec kinase family in platelet signaling pathways is critically dependent upon the proximal mediators of the receptor activation.
Influence of CD32 cross-linking on Btk and Tec phosphorylation modulated by PI 3-kinase.
Platelets were incubated with 0.1% DMSO (vehicle for LY294002) or 50 μmol/L LY294002 for 10 minutes. We added 3 μg/mL anti-CD32 for 10 minutes, followed by 30 μg/mL (Fab′)2 of goat antimouse IgG for 5 minutes as indicated. (A) Btk or (B) Tec was purified from the soluble extracts by immunoprecipitation as described above, then immunoblotted with 4G10 antibody (top panels) and anti-Btk or anti-Tec antibody (bottom panels).
Influence of CD32 cross-linking on Btk and Tec phosphorylation modulated by PI 3-kinase.
Platelets were incubated with 0.1% DMSO (vehicle for LY294002) or 50 μmol/L LY294002 for 10 minutes. We added 3 μg/mL anti-CD32 for 10 minutes, followed by 30 μg/mL (Fab′)2 of goat antimouse IgG for 5 minutes as indicated. (A) Btk or (B) Tec was purified from the soluble extracts by immunoprecipitation as described above, then immunoblotted with 4G10 antibody (top panels) and anti-Btk or anti-Tec antibody (bottom panels).
Collagen-induced Tec tyrosine phosphorylation in XLA platelets
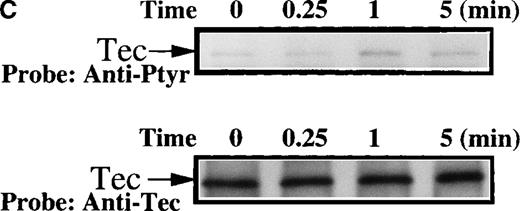
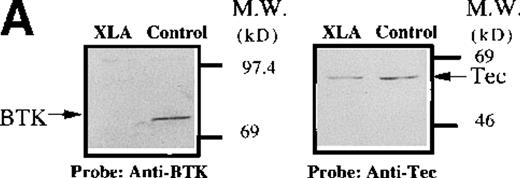
The evaluation of Btk and Tec tyrosine phosphorylation in platelets from normal subjects indicates that these signaling proteins are coordinately activated in response to collagen binding to nonintegrin receptors. Platelets from 7 classical XLA patients (5 families) were evaluated to determine whether the level of Tec expression or the activation of Tec in response to collagen is dependent on the presence of functional Btk protein. Immunoblot analysis of platelet extracts from XLA patients (A, B, and E) demonstrated an absence of the full-length Btk protein compared with normal subjects, as detailed in “Materials and methods” (Figure 6A; data not shown). In contrast, Tec protein, at levels similar to normal subjects, was present in platelet extracts from all 7 XLA patients, thereby indicating that the expression of Tec protein is not regulated by functional Btk (Figure 6A; data not shown).
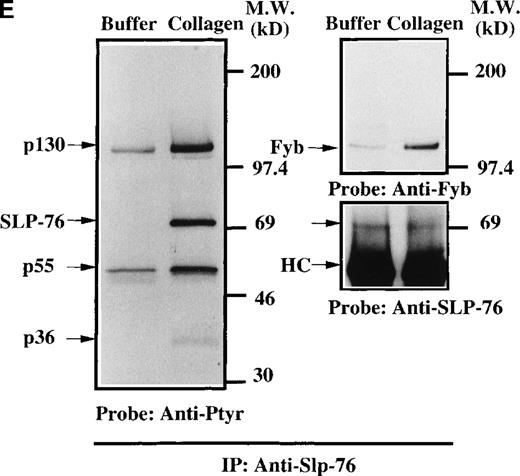
Analysis of platelets from XLA patients.
Soluble extracts were prepared from patient A or a normal control (3 × 106 platelets/sample) and were then subjected to immunoblot analysis with (A) Btk or Tec antiserum. Collagen-stimulated tyrosine phosphorylation of Tec was analyzed as described in the legend to Figure 1D, and analyses are given for (B) patient A and (C) patient B. Tyrosine phosphorylation of (D) WASP or (E) Slp-76 in XLA platelets prior to or after 50 μg/mL collagen treatment for 5 minutes was examined by immunoblot analysis with 4G10 antibody following immunoprecipitation (anti-WASP or anti–Slp-76, patient B). The upper and lower portions of the membrane from the (E) anti–Slp76 immunoprecipitate was immunoblotted with anti-Fyb/Slp-130 or anti–Slp-76 as indicated. Tec and WASP phosphorylation studies were also performed using platelets from patients C-G, and similar results were obtained.
Analysis of platelets from XLA patients.
Soluble extracts were prepared from patient A or a normal control (3 × 106 platelets/sample) and were then subjected to immunoblot analysis with (A) Btk or Tec antiserum. Collagen-stimulated tyrosine phosphorylation of Tec was analyzed as described in the legend to Figure 1D, and analyses are given for (B) patient A and (C) patient B. Tyrosine phosphorylation of (D) WASP or (E) Slp-76 in XLA platelets prior to or after 50 μg/mL collagen treatment for 5 minutes was examined by immunoblot analysis with 4G10 antibody following immunoprecipitation (anti-WASP or anti–Slp-76, patient B). The upper and lower portions of the membrane from the (E) anti–Slp76 immunoprecipitate was immunoblotted with anti-Fyb/Slp-130 or anti–Slp-76 as indicated. Tec and WASP phosphorylation studies were also performed using platelets from patients C-G, and similar results were obtained.
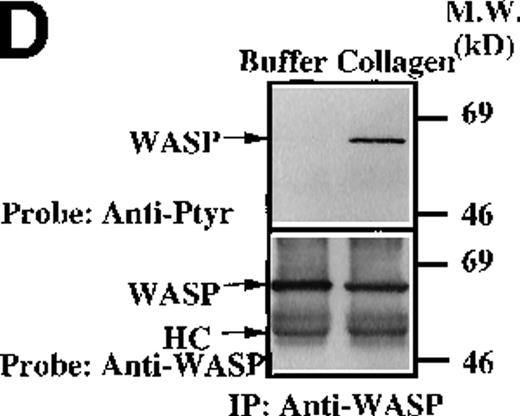
Collagen treatment of XLA platelets increased the level of Tec tyrosine phosphorylation maximally within 1 minute after stimulation (Figures 6B and 6C; data not shown). Increased Tec phosphorylation was observed in platelets from all XLA patients (7/7) tested. Thus, Tec recruitment into the collagen receptor signaling pathway does not require functional Btk. Increased tyrosine phosphorylation of Tec in XLA platelets is consistent with the hypothesis that Tec function can rescue platelet responses in the absence of Btk function, and by this mechanism, Tec function can prevent overt clinical platelet-dependent bleeding diathesis in XLA patients. We examined the platelet extracts from XLA patients for evidence of the recruitment of other signaling proteins in collagen receptor pathways. WASP is essential for normal platelet morphology and function, and it is tyrosine phosphorylated in normal collagen-stimulated platelets in a PI 3-kinase dependent manner.24 Recent reports indicate that WASP is a direct substrate of Btk in heterologous expression systems.28 30This suggests that the Btk/Tec kinase family may be important upstream regulators of WASP function in platelet receptor pathways. We tested the hypothesis that WASP recruitment into the platelet collagen nonintegrin receptor pathway occurs in the absence of Btk. XLA platelet extracts were prepared prior to and after collagen treatment, then WASP protein was immunopurified and immunoblotted with either antiphosphotyrosine or anti-WASP (Figure 6D). XLA platelet extracts were prepared prior to and after collagen treatment, then WASP protein was immunopurified and immunoblotted with either antiphosphotyrosine or anti-WASP (Figure 6D; data not shown). Collagen induced a clear increase in WASP tyrosine phosphorylation in the XLA platelets from 7/7 patients tested, suggesting that Tec activation is sufficient to preserve the WASP recruitment in this signaling pathway.
We investigated whether other collagen-dependent signaling events appear to be preserved in the XLA platelets. Previous studies have shown that Slp-76 is essential for activation of PLC-γ2 in collagen or collagen-related peptide-induced platelet activation.51,52 In XLA platelets, Slp-76 becomes tyrosine phosphorylated following collagen treatment and is immunoprecipitated in association with 130-, 55-, and 38-kd phosphoproteins (Figure 6E). The 130-kd protein in the anti–Slp-76 immunoprecipitate reacted with an anti-Fyb mAb (Figure 6E, upper panel). The 130-kd and 55-kd proteins were previously identified as Fyb/Slp-130 and Lyn, respectively.51 52 These observations indicate that the platelet collagen receptor signaling pathways are largely intact despite the absence of Btk and that phosphorylation of a downstream substrate requires only the presence of Tec.
Discussion
Platelet function in hemostasis can be promoted or inhibited by multiple extracellular factors binding to surface receptors.38 Alternative receptors use specific, sometimes overlapping, intracellular signaling pathways to regulate platelet incorporation into a forming thrombus. One of the strongest promoters of thrombogenesis, subendothelial collagen, plays a critical role both in normal hemostasis and in pathophysiological events such as vascular infarction subsequent to rupture of atherosclerotic plaques.1-3
Collagen receptor regulation of intracellular calcium levels in platelet functional responses is an important element of the irreversible engagement into a thrombus.1-3,38 The Btk/Tec kinase family provides critical elements for the regulation of receptor-dependent intracellular calcium responses in a variety of hematopoietic cell lineages. The important role of the Btk/Tec kinase family in the receptor signaling pathways has been confirmed by the phenotypic analysis of cells harboring either naturally occurring mutations, such as XLA and xid, or targeted-deletion of other Btk/Tec family members. In the B-cell antigen receptor signaling pathway, PI 3-kinase and Src kinases function upstream of Btk, while Btk, in conjunction with Syk, modulates phosphorylation of PLC-γ2 and intracellular calcium levels.15 22
Several observations suggest a similar functional role for the Btk/Tec kinase family in the platelet nonintegrin collagen receptor signaling pathway. Importantly, collagen-induced responses, such as PLC-γ2 phosphorylation, calcium mobilization, and granule release, are decreased in Btk-deficient platelets compared with those from normal donors.9 In our experiments, phosphorylation of Btk and Tec was more strongly activated by receptors that signal through an ITAM-containing subunit (collagen nonintegrin receptors and CD32) than in response to platelet agonists that signal by other mechanisms (thrombin or thrombopoietin). The role of Src family kinases in Btk activation was demonstrated by detection of enhanced phosphorylation of Btk tyrosine 551 (Tyr551), the Src family kinase substrate site within the Btk kinase domain activation loop, using phosphopeptide-specific antibodies.11-13 The increased phosphorylation of Btk coincided with increased Btk enzymatic activity in an in vitro kinase assay. We also observed that PI 3-kinase activity was required for collagen- and CD32-induced tyrosine phosphorylation of both Btk and Tec in platelets. Taken together, these results confirm that 2 of the major regulatory mechanisms controlling recruitment of the Btk/Tec kinase family into antigen receptor pathways are conserved in platelet receptor responses.
The evaluation of collagen regulation of platelet function is complicated by heterogeneity in responses among healthy volunteers, due in part to variation in the expression of the integrin α2β1, a major collagen receptor.53 Whereas collagen and CD32 were strong activators of Btk and Tec tyrosine phosphorylation, thrombin treatment of platelets in our experiments had only a weak influence. Thrombin-stimulated activation of Tec and Btk in platelets is largely dependent on platelet integrins and aggregation.37,49,50 In agreement with this, PI 3-kinase activity is essential for platelet integrin αIIbβ3 function and cytoskeletal reorganization.33 Thus, the small influence of thrombin on Btk and Tec phosphorylation in our experiments probably reflects RGDS inhibition of the integrin-mediated platelet aggregation.
Several important collagen-dependent platelet responses are diminished in Btk-deficient platelets derived from XLA patients.9Despite abnormalities in XLA platelet responses to collagen in vitro, XLA patients do not demonstrate a platelet-dependent abnormal bleeding tendency. These results suggest either that the Btk/Tec family kinase activity is dispensable to platelet function or that there is sufficient Btk/Tec kinase functional redundancy to rescue signaling in specific receptor pathways. Our comparison of the kinetics and mechanisms of Btk and Tec activation in a normal agonist-stimulated environment indicates that these signaling proteins are coordinately recruited into specific platelet receptor pathways. Furthermore, Tec protein is expressed in Btk-deficient platelets at levels similar to normal platelets, and Tec is rapidly phosphorylated in response to collagen stimulation of XLA platelets. These results favor the interpretation that functional redundancy of the Btk/Tec kinase family rescues XLA platelet responses to collagen in vivo.
In platelets, one important signaling function of the Btk/Tec kinase family may be to link specific receptors to the WASP signaling protein, a critical regulator of platelet morphology and function.31,32 This relationship is suggested by similarities in the recruitment of the Btk/Tec kinase family and the WASP signaling protein into platelet receptor signaling pathways. Both CD32 and collagen signal to WASP via a PI 3-kinase dependent mechanism.24-27,54 WASP becomes strongly tyrosine phosphorylated in collagen-stimulated platelets, and this response is blocked by wortmannin, whereas thrombin induces a much weaker increase in WASP phosphorylation.24 WASP binds directly to Btk and appears to be a direct substrate of Btk.28,30 However, in WASP-null mice generated by gene targeting, functional deficits were noted in T cells but not in B cells.55 Because T cells express Btk/Tec kinases but not Btk, and B cells express predominantly Btk, the physiologic significance of the Btk-induced WASP phosphorylation in B-cell receptor pathways is unclear. The robust increase in WASP phosphorylation observed in collagen-treated XLA platelets indicates the role of another kinase in the phosphorylation of WASP in this platelet receptor pathway. The current study reveals that Tec is recruited by mechanisms similar to those used to recruit WASP and Btk and that Tec expression is preserved in XLA platelets. Thus, Tec is a prime candidate as the WASP kinase in XLA platelets, and this role would support the hypothesis of functional redundancy in signaling through Btk/Tec kinases.
The capacity of Tec to partially rescue XLA platelet function would concur with the previous demonstration of functional similarity in signal transduction using Itk and Tec to reconstitute BCR-induced calcium responses in Btk-deficient B cells.15 As Tec is not overexpressed in the platelets from XLA patients, Tec may only partially compensate for the loss of Btk. Decreased XLA platelet responses detectable in vitro may indicate that Btk/Tec signaling function in the collagen receptor pathway is dosage dependent, which is similar to the graded reconstitution of B-cell function in a Btk transgenic murine model system.56 In contrast to the data regarding the collagen receptor pathway, Tec expression does not readily account for the normal response of XLA platelets to thrombin9 because thrombin has only a small influence on Tec tyrosine phosphorylation in our experimental conditions. This pathway may require Btk/Tec kinase function only distal to platelet aggregation.37,49 50
The emerging role of the Btk/Tec kinase family in the collagen signaling pathway helps to clarify the molecular mechanism of action of a major physiological regulator of platelet function. The observation that certain aspects of the nonintegrin collagen receptor signaling pathway are impaired in XLA platelets,9 coupled with an extensive catalog of Btk mutations resulting in XLA, suggests that platelets may provide an interesting system for the detailed genotypic/phenotypic evaluation of Btk signaling function.
Supported in part by grants in aid from The Ministry of Education, Science and Technology of Japan, Japan (Y.I. and A.O.), and The Ryoichi Naito Foundation for Medical Research, Japan (A.O.); Research Grants for Life Sciences and Medicine, Keio University Medical Science Fund, Keio, Japan (A.O.); grant HD17427 from the National Institutes of Health, Bethesda, MD (H.D.O.); grant 6-F496-0330 from the March of Dimes Birth Defects Foundation, (H.D.O.); the Jeffery Module Foundation; and the DeJoria Wiskott-Aldrich Research Fund (H.D.O.) O.N.W. is an Investigator of the Howard Hughes Medical Institute, and M.I.W. is supported by fellowship DRG-086 from the Cancer Research Fund of the Damon Runyon Walter Winchell Foundation Fellowship.
Reprints:Atsushi Oda, Hokkaido Red Cross Blood Center, Yamanote 2-2, Nishi-ku, Sapporo 063-0002, Japan; e-mail:aoda@hokkaido.bc.jrc.or.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 2. Src-mediated activation of Btk in collagen-treated platelets. / (A) Platelets were treated with collagen as in Figure 1A for the time indicated. Btk was purified from the soluble extracts by immunoprecipitation as described above, then immunoblotted with Btk phosphopeptide site-specific antibodies 551PY (top panel) and 223PY (middle panel) or anti-Btk antibody (bottom panel). (B) Platelets were treated with either collagen or buffer for 1 minute. Btk was purified from the soluble extracts by immunoprecipitation as described above. The immune complexes were incubated in a kinase buffer containing [γ-32P]ATP for 5 minutes as described in “Materials and methods.” Incorporation of 32P into Btk was visualized by autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1663.005k44_1663_1670/6/m_bloo00544002aw.jpeg?Expires=1765946326&Signature=RsxRWSgamai2RUCs07aDcVpcbPdo9fAzVz3HgVLI-Uj-~Bw8huNACfrtkl-~ic2pw5otmYxzfB2QDgtlyu0h7QkC0K6uQmoBY~jizbxY5bAKURZULDIiW2AHyKbii8affxwTS-iNvw-e2YsFjc0HxDoHLIkTPZH09IyMRU6y6gRobMETbFjELvAtOIGu1gK-jJmaXgrfcZ9HqgeBw1VcdF-V~El8N0PNlSdSL9CS2MwePjAPpbHzzIz~wgobHKbauRyrXPJYae7JfiiQjFfKlYDj0bcH3WgczIIwoFZCpZGiNXzf-lVcLuz6z6lZJSxpvZyoU3YLq7A5B7iBryY1OQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Src-mediated activation of Btk in collagen-treated platelets. / (A) Platelets were treated with collagen as in Figure 1A for the time indicated. Btk was purified from the soluble extracts by immunoprecipitation as described above, then immunoblotted with Btk phosphopeptide site-specific antibodies 551PY (top panel) and 223PY (middle panel) or anti-Btk antibody (bottom panel). (B) Platelets were treated with either collagen or buffer for 1 minute. Btk was purified from the soluble extracts by immunoprecipitation as described above. The immune complexes were incubated in a kinase buffer containing [γ-32P]ATP for 5 minutes as described in “Materials and methods.” Incorporation of 32P into Btk was visualized by autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1663.005k44_1663_1670/6/m_bloo00544002bw.jpeg?Expires=1765946326&Signature=u7GiL7eQqwJi3Y5oQGAHxnHw13~C8~17HvaPEZrV05d5ZdzzMp3-v~PJepsgJpsulxrOHZ22epVQIqY7ic~0Ngh2uQmhE5aaN9eCWKgaBYvt10orVeqxyAozbSHgNJF0sf341eSw171D53g7G9aexJlNZy-QQw8BB-RVgOBcqma32hCzrfu7ZWmj2yc2eOLJClqvxmfCiiB9kB4DHvaKNdcH65fcUOnY2WS-OCBkeF3JiTLiWtySUf9Zfzjiq1b010VnGLMDED8Ijt-em3yftoFkFZRKBPG3H36pXbZyrYtoA6hT~Bpj~h1JOaaTBN6zpq1FoxZ9ywnQVsrHL6Xjzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal