Two Notch ligand families, Delta and Serrate/Jagged, have been identified in vertebrates. Members of the Jagged family have been shown to affect in vitro hematopoiesis. To determine whether members of the Delta family might play a similar role in hematopoiesis, we examined the expression of mouse Delta-like-1 (mDll1). mDll1 protein was detected in whole marrow and in a marrow stromal cell line MS-5. At the RNA level, both mDll1 and Notch1 were seen in marrow precursor, differentiated hematopoietic, marrow stromal, and MS-5 cells. We isolated a cDNA encoding the human homologue of mDll1, designated human Delta-like-1 (hDll1). A soluble form of hDll1, hDll1NDSL, containing the DSL domain and the N-terminal sequences, was expressed and purified from bacteria as a glutathione S-transferase (GST) fusion protein. We observed that hDll1NDSL delayed the acquisition of differentiation markers by murine hematopoietic progenitor cells (Lin−) cultured in vitro with cytokines. In addition, it promoted greater expansion (more than 3 times) of the primitive hematopoietic precursor cell population, measured in high-proliferative potential colony assay and day 12 colony-forming unit spleen (CFU-S) assay, than GST controls. We also observed that the percentage of apoptotic cells decreased and that the number of cells in the S-phase of the cell cycle increased in the cultures of Lin−cells with hDll1NDSL. The effects of hDll1NDSL were blocked by antibody against the mouse counterpart of hDll1NDSL, mDll1NDSL. These observations demonstrate that hDll1 plays a role in mediating cell fate decisions during hematopoiesis.
The development of multiple differentiated blood cell populations from a single stem cell involves an intricate interplay of cell proliferation, migration, growth, differentiation, and death.1-3 Recently, the Notch pathway has been implicated in the regulation of hematopoiesis.4 Notch signaling is an evolutionarily conserved mechanism that is used by metazoans to control cell fate. Notch, first cloned in Drosophila, encodes a receptor protein with 36 epidermal growth factor (EGF)-like repeats in the extracellular domain, a single transmembrane domain, and 6 cdc10/ankyrin repeats in the intracellular domain.5,6 FourNotch homologues have been identified in vertebrates: Notch 1, 2, 3, and 4.7-11 Studies in vertebrates and invertebrates indicate that signals transmitted through the Notch receptor, in combination with other cellular factors, influence differentiation, proliferation, and apoptotic events at all stages of development.12
Notch ligands have been identified, including Delta and Serrate inDrosophila13,14 and Lag-2 and Apx-1 inCaenorhabditis elegans.15-17 Two Notch ligand families, Delta and Serrate/Jagged, have been identified in mammals, including Delta-like-1, 3 in mouse for the Delta family18,19 and Jagged-1, 2 in rat and human for the Serrate/Jagged family.20-23 Like Notch, the ligands are single-transmembrane proteins characterized by a conserved region in Delta, Serrate, and Lag-2, referred to as the DSL domain.16The DSL domain is a 45-amino acid sequence containing a unique spacing of 6 cysteines and 3 glycines. It is required for DSL ligand binding to the Notch receptors and activation of the Notch pathways.24,25 DSL ligands are thought to act as transmembrane proteins that interact through their extracellular domains with Notch receptors that are expressed on adjacent cells.24,26 However, recent genetic and biochemical evidence demonstrates that proteolytic processing of Delta produces a secreted extracellular domain that is biologically active as a Notch agonist.27 This secreted form of Delta was detected in the culture medium from Delta-transfected cells and inDrosophila embryos.27,28 These observations raise the possibility of diffusible DSL ligands that could have physiological roles in activating ectopic Notch receptors. Functional analysis inC. elegans has revealed that the secreted forms of Lag-2 and Apx-1, containing only the N-terminal region and the adjacent DSL domain, are sufficient for ectopic receptor activation.25 29
Members of the Serrate/Jagged family have recently been shown to influence the development of hematopoietic progenitor cells.23 30-34 However, the roles of the Delta family in the regulation of hematopoiesis is unknown. In this article, we describe the identification and isolation of a human homologue of the Delta family, human Delta-like-1 (hDll1), and the expression pattern of the mouse counterpart, mDll1, in bone marrow. Using a soluble form of hDll1 (hDll1NDSL) that includes the DSL domain and the N-terminal sequences, we have demonstrated a function of hDll1 in the regulation of hematopoiesis. At multiple stages of their development, hDll1NDSL inhibited the differentiation of hematopoietic progenitor cells. It also promoted ex vivo expansion of progenitor cells and suppressed apoptosis of hematopoietic cells in cultures supplemented with cytokines. This result may be attributed to Notch activation blocking progenitor differentiation and promoting expansion of primitive progenitor population.
Materials and methods
Isolation of the human Delta-like-1 (hDll1) cDNA and in vitro transcription/translation of the gene
A human heart λ uni-Zap XR cDNA library (Stratagene, La Jolla, CA) was used to screen hDll1 cDNA using32P-labeled mDll1 cDNA18 as a probe by standard methods.35 DNA sequencing was performed at Cornell University DNA Sequencing Center (Ithaca, NY) using custom oligonucleotide primers from Gibco-BRL Life Science Technology (Grand Island, NY). DNA and protein sequence analysis was performed using DNA Strider software, and database searches were accomplished by using the BLAST network service at the National Center for Biotechnology Information.36 The full-length hDll1cDNA in pBluescript was in vitro translated in a coupled transcription/translation reaction using the TNT-coupled reticulocyte lysate system (Promega, Madison, WI).
Production of thioredoxin-mDll1NDSL fusion protein and generation of anti-mDll1NDSL antibody
The pTrx/mDll1NDSL plasmid was constructed by subcloning the polymerase chain reaction (PCR)-amplified mDll1NDSLregion of the mDll1 cDNA into an Escherichia coliexpression vector pTrxFus (Invitrogen, San Diego, CA). The PCR was carried out with 5' primer (5'- GATCTCTAGACCTCCATACAGACTCT), 3' primer (5'-GATCGTCGACAGATTGGGTCAGTGCA), and mDll1cDNA template.18 The mDll1NDSLincludes the DSL domain and its adjacent N-terminal 50 amino acids. The fusion protein recovered in the soluble fraction of a liter of bacteria culture was purified by gel filtration chromatography and separated further by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The fusion protein was used to immunize chickens. The IgY antibodies were purified from the egg yolk and by the thioredoxin-mDll1NDSL affinity column.
Western blots analysis and immunohistochemical staining
Western blot analysis was performed using the ECL luminescence detection method (Amersham Life Science, Arlington Heights, IL). Fifteen micrograms protein was analyzed for each sample. The affinity-purified anti-mDll1NDSL antibody was used at 1:100 dilution. Horseradish peroxidase-labeled rabbit antichicken IgY was then used at 1:5000 dilution. The MS-5 cell line was fixed with cold acetone and incubated with avidin D and biotin blocking solutions (Vector Laboratories, Burlingame, CA), which all contained 2% chicken serum, 1:50 dilution of IgY, and 1:50 dilution of anti-thioredoxin-mNotch1 antibody raised in chicken. Biotinylated anti-mDll1NDSL antibody was used at 50 μg/mL. Biotinylated IgY was used as negative control. The detection system was carried out using a Vectastain ABC-AP kit (Vector Laboratories).
Production of recombinant GST-hDll1NDSL and hDll1NDSL proteins
The hDll1NDSL region includes the DSL domain and its adjacent N-terminal 50 amino acids (aa 127-225). The cDNA fragment encoding this region was amplified by PCR and was subcloned into the expression vector pGEX-2T to create pGEX-hDll1NDSL. The PCR was carried out with 5'-primer (5'-CGGGATCCCTCCACACAGATTCTCCTG), 3'-primer (5'-CGGAA TTCTTAGATCGGCTCTGTGCAGTAG), andhDll1 cDNA template. pGEX-hDll1NDSL and pGEX-2T were induced to express the GST-hDll1NDSL fusion protein and GST on IPTG induction, respectively.
Bacterial pellet fractions containing GST-hDll1NDSL were resuspended in 8 mol/L urea buffer A (50 mmol/L Tris/HCl, pH 8, 1 mmol/L EDTA, 50 mmol/L NaCl, and 0.1 mmol/L phenylmethylsulfonyl fluoride) and were renatured by dilution to alkaline buffer without urea. Both GST and the renatured fusion protein were purified through a DEAE Sepharose CL-6B column (Pharmacia Biotech, Piscataway, NJ) with a bed volume of 90 mL. The column was washed by buffer A and eluted with NaCl gradient buffer A (50-500 mmol/L). GST or GST-hDll1NDSL in the positive DEAE fractions was further purified with affinity chromatography using a glutathione Sepharose 4B column (Pharmacia Biotech). To release hDll1NDSLfrom the fusion protein, GST-hDll1NDSL in the DEAE fractions was bound to the glutathione Sepharose 4B column. The column was washed with 4-column volumes of buffer B (50 mmol/L Tris-HCl, pH 8.3, 150 mmol/L NaCl) and then with 2.5 mmol/L CaCl2in buffer B. Thrombin protease (Pharmacia Biotech) was added at 1 U/100 μg fusion proteins. After digestion overnight, the hDll1NDSL was recovered from the glutathione column.
The protein preparations of GST, GST-hDll1NDSL, and hDll1NDSL were dialyzed in phosphate-buffered saline (PBS) and filtered through 0.2-μmol/L filters. Protein concentrations were assayed with the Bio-Rad protein assay solution (Bio-Rad, Hercules, CA). Endotoxin levels in the final protein preparations were measured using an endotoxin kit (Sigma, St Louis, MO).
Isolation and culture of mouse progenitor cells
A StemSep system (Stem Cell Technologies, Vancouver, Canada) was used to isolate bone marrow progenitor cells from the Balb/C mice. Monoclonal antibodies to the following murine cell-surface antigens were included: T lymphocytes (CD5), B lymphocytes (CD45R), macrophages (Mac-1), granulocytes (Gr-1), and erythroid (TER 119). The purity of the cells was verified by fluorescence-activated cell sorting (FACS) of Gr-1 and Mac-1 expression, which was less than 5%.
Lin− cells were cultured in serum-free medium (Ex-vivo 15; Biowhittaker, San Diego, CA) supplemented with cytokine cocktails. The cytokines used in this study included interleukin-3 (IL-3; 10 ng/mL), c-kit ligand (KL; 20 ng/mL), GM-CSF (1000 U/mL), IL-11 (100 ng/mL), Flt3 ligand (FL; 100 ng/mL), G-CSF (1000 U/mL), IL-1β (100 U/mL), IL-6 (20 ng/mL), and erythropoietin (Epo; 10 U/mL). Cytokine cocktails, GST, and GST-hDll1NDSL proteins were added to the cultures according to individual experiments (see “Results”). Cultures were incubated for 7 to 12 days at 37°C in a fully humidified 5% CO2atmosphere.
Flow cytometry analysis
Hematopoietic cells were washed with 2% fetal calf serum (FCS) in PBS at 4°C. The cells were resuspended in 0.1 mL 2% FCS in PBS with 1 μL Fc blocker (Pharmingen, San Diego, CA). Antibodies used were fluorescein isothiocyanate (FITC)-conjugated rat antimouse Mac-1 and antimouse Gr-1 (Pharmingen). One microliter antibody was added to the cells. After 30 minutes of incubation, the cells were washed twice in 2% FCS/PBS and fixed in 1% formalin/PBS.
Cell-cycle analysis was performed using DNA binding dye propidium iodide (PI). Hematopoietic cells were fixed in 50% ethanol and resuspended to 0.2 mL of 10 mg/mL RNAaseA and 50 μg/mL PI. Hematopoietic cells stained with PI in the sub-G1 phase of the cell cycle represented the apoptotic bodies and were measured as apoptosis events by the method of Fraker et al..37 Analysis of Mac-1/Gr-1 expression and cell-cycle kinetics was performed by our Institute Core Facility using the FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Progenitor assay
Cells were plated in 1 mL IMDM medium containing 1.2% (wt/vol) methylcellulose, 30% FCS, 2 mmol/L glutamine, 0.1 mmol/L 2-mercaptoethanol, and 4 mmol/L hemin plus cytokines (IL-3, GM-CSF, KL, and Epo). Triplicate cultures were incubated for 7 days at 37°C in a fully humidified 5% CO2 atmosphere, and colonies were counted under a microscope. CFU-S was measured by the injection of progenitor cells into irradiated Balb/C mice at 5 mice/group. Spleen colonies were counted after 12 days as CFU-Sday12.
Statistical analysis
Results are presented as the mean ± standard error (SE). Significance was determined using the two-tailed paired Studentt-test.
Results
Isolation, sequencing, and analysis of hDll1 cDNA
To clone a human Delta homologue, full-length mouseDelta-like-1 (mDll1) cDNA, generously provided by A. Gossler,18 was used as a probe to screen a human heart cDNA library (Stratagene). Six positive clones were isolated after the tertiary screening. The sequences of these clones showed the highest similarity to mDll1. We concluded that these clones contained partial cDNA fragments of the human homologue of mDll1(hDll1). A 2.1-kb clone was found to contain the 3' end ofhDll1 with 848 bp of untranslated region and 1252 bp of coding region by sequence analysis. The other 1.2-kb clone contained 511 bp of 5' untranslated region and 689 bp of 5' coding region. There was a gap of 228 bp between the two clones. To obtain a complete cDNA clone of hDll1, primers were designed according to the sequences from the two clones, and RT-PCR was performed using total mRNA isolated from human bone marrow endothelial cells, generously provided by S. Raffi (Weil Medical College, Cornell University). A cDNA fragment of 1080 bp was obtained and cloned into plasmid pBluescript. A full-length cDNA ofhDll1 (3039 bp) was assembled by combining the PCR fragment and the 2.1-kb clone.
This cDNA had one open reading frame of 2169 bp and was predicted to encode a protein product having 723 amino acids (Fig.1). The full-length cDNA was successfully translated in vitro to produce a 79-kd protein at the predicted molecular weight (Fig. 2D). Analysis of the amino acid sequence indicated that hDll1 is a transmembrane protein with a large extracellular domain (aa 1-543) and a short intracellular domain (aa 573-723). The hDll1 protein shares more structural features with the Delta family than with the Serrate/Jagged family, including a DSL motif and 8 EGF-like repeats within the extracellular domain.
Predicted amino acid sequence of human Delta-like-1.
Alignment of amino-acid sequences between human Delta-like-1 (hDll1) and mouse Delta-like-1 (mDll1). The secretion signal peptide (aa 1-25), the EGF-like repeats (aa 225-516), and the transmembrane domain (aa 543-572) are shown in bold type. The DSL domain (aa 177-221) is underlined. The Genbank accession number for hDll1 is AF196571.
Predicted amino acid sequence of human Delta-like-1.
Alignment of amino-acid sequences between human Delta-like-1 (hDll1) and mouse Delta-like-1 (mDll1). The secretion signal peptide (aa 1-25), the EGF-like repeats (aa 225-516), and the transmembrane domain (aa 543-572) are shown in bold type. The DSL domain (aa 177-221) is underlined. The Genbank accession number for hDll1 is AF196571.
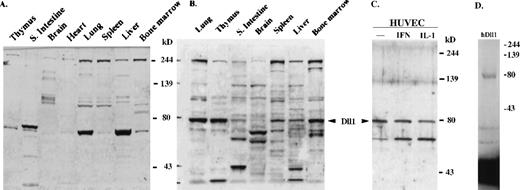
Delta-like-1 protein expression.
(A) Western blot with chicken isotype IgY control. No signal was observed at the position of 79 kd. (B,C) Western blot with affinity-purified anti-mDll1NDSL antibody. mDll1 protein (79 kd was detected in adult mouse tissues, including lung, thymus, small intestine, brain, spleen, liver, and bone marrow. hDll1 protein (79 kd was also detected with the same antibody in human umbilical vein endothelial cells (HUVEC), HUVEC treated with IFN-αb and IL-1β (IL-1). (D) In vitro transcription and translation of thehDll1 cDNA. The full-length hDll1 cDNA was in vitro translated into a 79-kd protein. Autoradiograph of the35S-labeled protein product separated by 10% SDS-PAGE was shown.
Delta-like-1 protein expression.
(A) Western blot with chicken isotype IgY control. No signal was observed at the position of 79 kd. (B,C) Western blot with affinity-purified anti-mDll1NDSL antibody. mDll1 protein (79 kd was detected in adult mouse tissues, including lung, thymus, small intestine, brain, spleen, liver, and bone marrow. hDll1 protein (79 kd was also detected with the same antibody in human umbilical vein endothelial cells (HUVEC), HUVEC treated with IFN-αb and IL-1β (IL-1). (D) In vitro transcription and translation of thehDll1 cDNA. The full-length hDll1 cDNA was in vitro translated into a 79-kd protein. Autoradiograph of the35S-labeled protein product separated by 10% SDS-PAGE was shown.
Figure 1 also shows the alignment of the amino acid sequences of hDll1 and mDll1. The hDll1 protein has 90% overall amino acid identity with mDll1, and it is longer than mDll1 by 1 amino acid. The extracellular domains have higher degrees of conservation (89%) than the intracellular domain (82%), and the N-terminal plus DSL sequences show the highest conservation (95%). In addition, comparison of hDll1 withDrosophila Delta shows that the best-matched sequences are in the extracellular domain, with 48% amino acid identity. However, there was only 41% identity when hDll1 was compared with human Jagged1, a member of Serrate/Jagged family, even in the most conserved extracellular domain. It is clear that members of the same Notch ligand family, ie, Delta or Serrate/Jagged family, in different species, share higher sequence identity than the members of the ligands in different families in the same species.
mDll1 is expressed in bone marrow hematopoietic cells and in stromal cells
To assess the expression of Dll1 proteins, cell extracts prepared from multiple tissues were analyzed by Western blot using a chicken polyclonal antibody that was raised against mDll1NDSL. A negative control was obtained using the preimmune antibody (Fig. 2A). No signals were seen in this blot in the predicted position of mDll1. A major band of molecular weight 79 kd, the correct molecular weight for mDll1 and hDll1, was detected in the blot with anti-mDll1 antibody (Figs. 2B, 2C). It is expressed at high levels in the mouse lung, thymus, and bone marrow and at lower levels in small intestine, brain, spleen, and liver (Fig. 2B). Because hDll1 shares high homology with mDll1 (95% identity in the region used to raise the antibody; Fig.4A), the antibody also detected the protein in human umbilical vein endothelial cells (HUVEC) and in HUVEC treated with interferon-α and IL-1 for 24 hours (Fig. 2C). No major difference was observed in the expression of hDll1 in untreated HUVEC versus cytokine-activated HUVEC. The sizes of mDll1 and hDll1, detected by the Western blot, matched the size of the in vitro translated hDll1 protein (Fig. 2D).
We examined the expression of mDll1 in MS-5 stromal cell line and primary stromal cells using immunohistochemical staining with our biotinylated anti-mDll1NDSL antibody. MS-5 is a mouse bone marrow stromal derived fibroblast cell line that supports both mouse and human long-term hematopoiesis in vitro.38 39 mDll1 was detected in the MS-5 cells (Fig. 3B), whereas no staining was observed with the biotinylated isotype IgY control (Fig. 3A). To confirm the specificity of the staining, the anti-mDll1NDSL antibody raised against thioredoxin-mDll1NDSL was preabsorbed with GST-hDll1NDSL-coupled agarose and also with the control GST agarose. We found that the anti-mDll1NDSL antibody staining in MS-5 cells was completely abolished with the GST-hDll1NDSL preabsorbed antibodies (Fig. 3C). We also detected mDll1 expression using a similar approach in primary bone marrow stromal cells cultured for 2 weeks in 20% FCS in RPMI medium (Figs. 3E, 3F), whereas no staining was observed in the negative control (Fig. 3D). The mDll1 staining appeared mostly in the cytoplasm, with stronger staining in the perinuclear areas of the cells but not in the nuclei (Fig. 3E). We concluded that mDll1 was expressed in a stromal-derived fibroblast cell line and in primary bone marrow stromal cells.
Immunohistochemical analysis of mDll1 protein expression.
The expression of mDll1 was detected using the affinity-purified anti-mDll1NDSL antibody conjugated with biotin. Avidin mediates the detection system through alkaline phosphatase (AP)-conjugated biotin. New fuscin substrate of AP-stained the cells expressing mDll1 red. Hematoxylin-stained the nucleus brown. (A) MS-5 cells stained with isotype IgY-biotin control and hematoxylin. The cells show no staining in the cytoplasm and brown staining in the nucleus. (B) MS-5 cells stained with anti-mDll1NDSL-biotin and hematoxylin. The cells show reddish cytoplasmic and brownish nuclear staining. (C) MS-5 cells stained with preabsorbed anti-mDll1NDSL-biotin and hematoxylin. The cells show no staining in the cytoplasm and brownish staining in the nucleus. (D) Bone marrow stromal cells stained with isotype IgY-biotin control. The cells show no cytoplasmic or nuclear staining. (E) Bone marrow stromal cells stained with anti-mDll1NDSL-biotin. The cells show reddish cytoplasmic and no nuclear staining. (F) Bone marrow cells stained with anti-mDll1NDSL-biotin and hematoxylin. The cells show reddish cytoplasmic and brownish nuclear staining.
Immunohistochemical analysis of mDll1 protein expression.
The expression of mDll1 was detected using the affinity-purified anti-mDll1NDSL antibody conjugated with biotin. Avidin mediates the detection system through alkaline phosphatase (AP)-conjugated biotin. New fuscin substrate of AP-stained the cells expressing mDll1 red. Hematoxylin-stained the nucleus brown. (A) MS-5 cells stained with isotype IgY-biotin control and hematoxylin. The cells show no staining in the cytoplasm and brown staining in the nucleus. (B) MS-5 cells stained with anti-mDll1NDSL-biotin and hematoxylin. The cells show reddish cytoplasmic and brownish nuclear staining. (C) MS-5 cells stained with preabsorbed anti-mDll1NDSL-biotin and hematoxylin. The cells show no staining in the cytoplasm and brownish staining in the nucleus. (D) Bone marrow stromal cells stained with isotype IgY-biotin control. The cells show no cytoplasmic or nuclear staining. (E) Bone marrow stromal cells stained with anti-mDll1NDSL-biotin. The cells show reddish cytoplasmic and no nuclear staining. (F) Bone marrow cells stained with anti-mDll1NDSL-biotin and hematoxylin. The cells show reddish cytoplasmic and brownish nuclear staining.
Generation of recombinant soluble hDll1NDSL and GST-hDll1NDSL proteins
We initially created a thioredoxin-mDll1NDSL protein containing the DSL domain of mDll1 and its adjacent N-terminal 50 amino acids using a pTrxFusion vector-mediated expression system. We observed that the fusion protein enhanced the expansion of primitive hematopoietic precursors, inhibited differentiation of myeloid progenitor cells, and suppressed apoptosis of hematopoietic cells (data not shown). However, the control thioredoxin protein showed inhibitory effects in the colony formation by progenitor cells, which might have compromised the function of the fusion protein. We, therefore, made a GST-hDll1NDSL fusion protein containing the DSL domain of hDll1 and its adjacent N-terminal 50 amino acids (Fig.4A). GST-hDll1NDSL was expressed and purified from the E. coli lysate. The fusion protein was estimated to be more than 95% pure by Coomassie-blue staining after purification by DEAE- and glutathione-affinity columns.
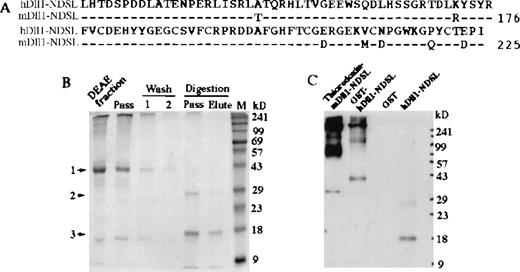
Production of soluble hDll1NDSL and GST-hDll1NDSL recombinant proteins.
(A) Amino acid sequences of hDll1 and mDll1 containing the N-terminal and DSL regions are termed hDll1NDSL and mDll1NDSL. The two sequences are highly conserved with 92% identity and 97% similarity in amino acid sequences. The cDNA sequences encoding hDll1NDSL was subcloned into pGEX-2T vector to create GST-hDll1NDSL fusion protein. (B) Production of hDll1NDSL by digestion of the fusion protein with thrombin protease. Coomassie-blue staining of the protein gel is shown. Five microliters DEAE fraction, DEAE column pass and wash (fractions 1 and 2) before digestion, and column pass and elute after digestion were analyzed by SDS-PAGE. 1, indicates the position of GST-hDll1NDSL at 43-kd marker; 2, indicates GST at 26 kd; 3, indicates hDll1NDSL at 17 kd. (C). Western blot analysis of the recombinant proteins. Monomeric forms of thioredoxin-mDll1NDSL, GST-hDll1NDSL, and hDll1NDSL were detected at positions 34 kd, 43 kd, and 17 kd, respectively by Western blot with anti-mDll1NDSLantibody. Polymers of the two fusion proteins were also revealed. No staining for GST was seen. A weak band in the hDll1-NDSL lane at approximately 27 kd was detected. It is highly possible that the band represented a degraded or partially digested product of GST-hDll1-NDSL during overnight incubation with thrombin protease.
Production of soluble hDll1NDSL and GST-hDll1NDSL recombinant proteins.
(A) Amino acid sequences of hDll1 and mDll1 containing the N-terminal and DSL regions are termed hDll1NDSL and mDll1NDSL. The two sequences are highly conserved with 92% identity and 97% similarity in amino acid sequences. The cDNA sequences encoding hDll1NDSL was subcloned into pGEX-2T vector to create GST-hDll1NDSL fusion protein. (B) Production of hDll1NDSL by digestion of the fusion protein with thrombin protease. Coomassie-blue staining of the protein gel is shown. Five microliters DEAE fraction, DEAE column pass and wash (fractions 1 and 2) before digestion, and column pass and elute after digestion were analyzed by SDS-PAGE. 1, indicates the position of GST-hDll1NDSL at 43-kd marker; 2, indicates GST at 26 kd; 3, indicates hDll1NDSL at 17 kd. (C). Western blot analysis of the recombinant proteins. Monomeric forms of thioredoxin-mDll1NDSL, GST-hDll1NDSL, and hDll1NDSL were detected at positions 34 kd, 43 kd, and 17 kd, respectively by Western blot with anti-mDll1NDSLantibody. Polymers of the two fusion proteins were also revealed. No staining for GST was seen. A weak band in the hDll1-NDSL lane at approximately 27 kd was detected. It is highly possible that the band represented a degraded or partially digested product of GST-hDll1-NDSL during overnight incubation with thrombin protease.
To remove GST from the fusion protein, the fusion protein bound to the glutathione beads was digested with thrombin protease. The released hDll1NDSL protein was recovered from the glutathione beads with limited GST contamination (Fig. 4B). It was difficult to remove the contaminated GST from hDll1NDSL even when the digested protein was passed through the glutathione-affinity column twice, suggesting that GST is probably in close association with hDll1NDSL and that protein–protein interactions may help to stabilize hDll1NDSL in solution.
The sequence of GST-hDll1NDSL in the pGEX-2T vector was verified by DNA sequencing, and the nature of the recombinant protein was examined in Western blot with anti-mDll1NDSL antibody (Fig. 4C). Thioredoxin-mDll1NDSL, GST-hDll1NDSL, GST, and hDll1NDSL were separated by SDS-PAGE in a 12% nonreducing polyacrylamide gel. Thioredoxin-mDll1 was detected as a 34-kd band, GST-hDll1NDSL as a 43-kd band, and hDll1NDSL as a 17-kd band. No band was detected in the GST lane. We noticed that both thioredoxin-mDll1NDSL and GST-hDll1NDSLformed polymers in solution, but not hDll1NDSL.
Effects of GST-hDll1NDSL in hematopoiesis
To test the function of the GST fusion protein, we first examined the possible hematopoietic effects of GST. Hematopoietic progenitor cells were isolated from adult mouse bone marrow by immunomagnetic negative selection. Lineage-negative (Lin−) progenitor cells that had not bound the antibodies against the lineage-associated cell surface markers were isolated. More than 95% of the isolated Lin− cells were Mac-1 negative, the marker for murine monocytes and granulocytes, as analyzed by flow cytometry; 104 Lin− cells/mL was cultured in 24-well plates in serum-free medium supplemented with IL-3, IL-1, and GM-CSF. Bovine serum albumin bovine serum albumin and GST were added to the above cultures, as indicated in Table1. After 7 days, the cultures were analyzed for their cellularity, total CFU, Mac-1 expression, and cell cycles. Data collected from three independent experiments were analyzed using overall F test. The differences in the four parameters among 6 tested protein groups were not statistically significant (Table 1). We concluded that GST used at concentrations of up to 0.4 μmol/L in the liquid culture of Lin− cells with serum-free medium was without effect on the hematopoietic cells.
GST has no effects in the culture of Lin−cells
| N = 3 . | Cytokines . | Cytokines + BSA (0.4 μmol/L) . | Cytokines + GST . | P Value . | |||
|---|---|---|---|---|---|---|---|
| 0.1 μmol/L . | 0.2 μmol/L . | 0.3 μmol/L . | 0.4 μmol/L . | ||||
| Live cells | 122 (±20) | 133 (±7) | 111 (±16) | 118 (±3) | 121 (±10) | 125 (±4) | >.05 |
| Total CFU | 918 (±122) | 732 (±9.1) | 808 (±88) | 767 (±59) | 908 (±57) | 846 (±42) | >.05 |
| Mac-1 cells (%) | 82 (±4.5) | 83 (±3.5) | 80 (±4) | 78 (±2.8) | 81 (±3.3) | 75 (±2.9) | >.05 |
| S-phase cells (%) | 26.8 (±2) | 29.3 (±3.1) | 25.1 (±2) | 25.5 (±4) | 23 (±3) | 23.3 (±1.7) | >.05 |
| N = 3 . | Cytokines . | Cytokines + BSA (0.4 μmol/L) . | Cytokines + GST . | P Value . | |||
|---|---|---|---|---|---|---|---|
| 0.1 μmol/L . | 0.2 μmol/L . | 0.3 μmol/L . | 0.4 μmol/L . | ||||
| Live cells | 122 (±20) | 133 (±7) | 111 (±16) | 118 (±3) | 121 (±10) | 125 (±4) | >.05 |
| Total CFU | 918 (±122) | 732 (±9.1) | 808 (±88) | 767 (±59) | 908 (±57) | 846 (±42) | >.05 |
| Mac-1 cells (%) | 82 (±4.5) | 83 (±3.5) | 80 (±4) | 78 (±2.8) | 81 (±3.3) | 75 (±2.9) | >.05 |
| S-phase cells (%) | 26.8 (±2) | 29.3 (±3.1) | 25.1 (±2) | 25.5 (±4) | 23 (±3) | 23.3 (±1.7) | >.05 |
Mouse Lin− bone marrow cells were cultured for 7 days in the serum-free medium supplemented with cytokines (IL-3, IL-1, and GM-CSF). The effects of 20 μg/mL bovine serum albumin and GST at different concentrations were tested under these culture conditions. Cultures were evaluated for their cellularity, total CFU, expression of Mac-1, and S-phase cells. Data are expressed as mean percentage of 3 independent experiments with SD in parentheses. Differences of the observed parameters among 6 tested groups are all not statistically significant as determined by overall F test for one-way analysis of variance (P > .05).
To determine the effective dosage of GST-hDll1NDSLin the regulation of hematopoietic differentiation, we titrated the fusion protein in the hematopoietic assays. GST and the fusion protein were added to the cytokine-supported cultures of Lin− cells at different concentrations, as indicated in Figure 5. Cultures were analyzed for the expression of Mac-1. Data from three independent experiments showed that Mac-1 expression was inhibited by the addition of the fusion proteins but not by the GST controls (Fig. 5). The inhibitory effect was protein concentration dependent, with the most effective dosage at 0.3 μmol/L.
A dose-response of GST-hDll1NDSL in inhibition of myeloid differentiation.
Mouse Lin− bone marrow cells were cultured for 6 days in the serum-free medium with IL-3, GM-CSF, and IL-1. GST-hDll1NDSL or control GST was added to the cultures at indicated concentrations at day 0. The cultures were initiated with 1 × 104 Lin− cells in 1 mL serum-free medium. All values represent mean ± SE from three independent experiments. Mac-1+ cells were analyzed by FACS after the cells were stained with anti-Mac-1-FITC antibodies.
A dose-response of GST-hDll1NDSL in inhibition of myeloid differentiation.
Mouse Lin− bone marrow cells were cultured for 6 days in the serum-free medium with IL-3, GM-CSF, and IL-1. GST-hDll1NDSL or control GST was added to the cultures at indicated concentrations at day 0. The cultures were initiated with 1 × 104 Lin− cells in 1 mL serum-free medium. All values represent mean ± SE from three independent experiments. Mac-1+ cells were analyzed by FACS after the cells were stained with anti-Mac-1-FITC antibodies.
To examine the hematopoietic effects of GST-hDll1NDSL, 3 × 104 Lin− cells were added to a single well of a 6-well plate containing 3 mL serum-free medium plus cytokines (IL-3, GM-CSF, and IL-1). GST-hDll1NDSL and control protein GST were added in 0.1 μmol/L every 3 days. After culture for 7 days, the cells were evaluated for their Mac-1 and Gr-1 expression, progenitor cell expansion, apoptosis, and cell-cycle kinetics. Data from five independent experiments are summarized in Table 1 and Figure 6.
Effects of GST-hDll1NDSL in myelopoiesis.
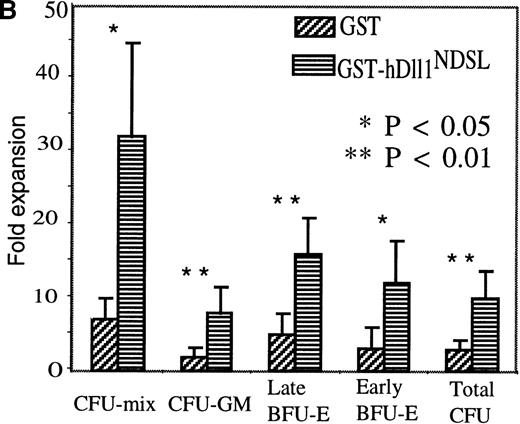
Mouse Lin− bone marrow cells were cultured for 7 days in the serum-free medium with IL-3, GM-CSF, and IL-1. GST-hDll1NDSL or control GST was added to the cultures at 0.1 μmol/L every 3 days. The cultures were initiated with 3 × 104 Lin−cells in 3 mL serum-free medium. All values represent mean ± SE from five independent experiments. (A) Differentiation analysis. Lin− cells cultured for 7 days with GST or GST-hDll1NDSL were washed and labeled with FITC- and phycoerythrin (PE)-conjugated mAbs directed to Mac-1 or Gr-1. The top panel of fluorescence histograms shows the cells stained with control isotype antibodies (IgG2b-FITC and IgG2b-PE). The middle panel shows the cells cultured with GST and stained with anti-Mac-1 or anti-Gr-1 antibodies. The bottom panel shows the cells cultured with GST-hDll1NDSL and stained with the above two antibodies. The x-axis represents log fluorescence intensity, and the y-axis represents cell numbers. Percentages of Mac-1- or Gr-1-positive cells are indicated in parentheses, and the MIF is shown for the corresponding M1 population of cells. (B) Progenitor cell assay. Lin− cells before and after culture for 7 days with GST and GST-hDll1NDSL were plated in triplicate in methylcellulose supplemented with IL-3, GM-CSF, KL, and Epo. Colonies were scored under microscope, including the CFU mix consisting of multiple lineages of at least granulocyte/macrophage and erythroid clusters, CFU-GM, burst-forming unit–erythrocyte (early BFU-E consisting of multiple erythroid clusters and late BFU-E as a single compact erythroid colony at day 7 of culture), and high-proliferation potential–CFU colonies (larger than 0.5 mm in diameter). Columns represent fold expansion of colonies formed by the progenitors. The fold expansion was calculated as the number of colonies from the day 7 cultured cells divided by the colonies from the cells before culture. Differences between the GST and GST-hDll1NDSL groups are statistically significant for CFU mix and early BFU-E (P = .02) and very significant for CFU-GM (P = .02), late BFU-E (P = .01), and total CFU (P = .001).
Effects of GST-hDll1NDSL in myelopoiesis.
Mouse Lin− bone marrow cells were cultured for 7 days in the serum-free medium with IL-3, GM-CSF, and IL-1. GST-hDll1NDSL or control GST was added to the cultures at 0.1 μmol/L every 3 days. The cultures were initiated with 3 × 104 Lin−cells in 3 mL serum-free medium. All values represent mean ± SE from five independent experiments. (A) Differentiation analysis. Lin− cells cultured for 7 days with GST or GST-hDll1NDSL were washed and labeled with FITC- and phycoerythrin (PE)-conjugated mAbs directed to Mac-1 or Gr-1. The top panel of fluorescence histograms shows the cells stained with control isotype antibodies (IgG2b-FITC and IgG2b-PE). The middle panel shows the cells cultured with GST and stained with anti-Mac-1 or anti-Gr-1 antibodies. The bottom panel shows the cells cultured with GST-hDll1NDSL and stained with the above two antibodies. The x-axis represents log fluorescence intensity, and the y-axis represents cell numbers. Percentages of Mac-1- or Gr-1-positive cells are indicated in parentheses, and the MIF is shown for the corresponding M1 population of cells. (B) Progenitor cell assay. Lin− cells before and after culture for 7 days with GST and GST-hDll1NDSL were plated in triplicate in methylcellulose supplemented with IL-3, GM-CSF, KL, and Epo. Colonies were scored under microscope, including the CFU mix consisting of multiple lineages of at least granulocyte/macrophage and erythroid clusters, CFU-GM, burst-forming unit–erythrocyte (early BFU-E consisting of multiple erythroid clusters and late BFU-E as a single compact erythroid colony at day 7 of culture), and high-proliferation potential–CFU colonies (larger than 0.5 mm in diameter). Columns represent fold expansion of colonies formed by the progenitors. The fold expansion was calculated as the number of colonies from the day 7 cultured cells divided by the colonies from the cells before culture. Differences between the GST and GST-hDll1NDSL groups are statistically significant for CFU mix and early BFU-E (P = .02) and very significant for CFU-GM (P = .02), late BFU-E (P = .01), and total CFU (P = .001).
GST-hDll1NDSL increased the cell viability of hematopoietic cells
Cells were counted using the trypan blue exclusion method. Live and dead cells were counted separately after the Lin−cells were cultured for 7 days. Cellularity analysis from five independent experiments are summarized (Table2). Progenitors cultured with GST-hDll1NDSL gave rise to a similar number of total cells compared with controls. The viable cells in the GST-hDll1NDSL culture represented 82% of the total cells compared with 67% in the controls (Table 2). Thus, GST-hDll1NDSL appeared to inhibit cell death.
Ligand stimulation of Notch affects differentiation, cell cycle, and apoptosis of the Lin− cells
| N = 5 . | Total Cells . | Live Cells . | Differentiation . | Cell Cycle . | Apoptosis . | ||
|---|---|---|---|---|---|---|---|
| Mac-1 . | Gr-1 . | G0/G1 . | S . | Sub-G1 . | |||
| GST | 426 (±19) | 284 (±31) | 84 (±2.5) | 76 (±6) | 86 (±3) | 7 (±1) | 46 (±6) |
| GSThDll1NDSL | 428 (±77) | 352 (±74) | 73 (±3.5) | 50 (±7) | 73 (±4) | 21 (±5) | 14 (±4) |
| P value | 0.9 | 0.04 | 0.007 | 0.002 | 0.01 | 0.01 | 0.005 |
| N = 5 . | Total Cells . | Live Cells . | Differentiation . | Cell Cycle . | Apoptosis . | ||
|---|---|---|---|---|---|---|---|
| Mac-1 . | Gr-1 . | G0/G1 . | S . | Sub-G1 . | |||
| GST | 426 (±19) | 284 (±31) | 84 (±2.5) | 76 (±6) | 86 (±3) | 7 (±1) | 46 (±6) |
| GSThDll1NDSL | 428 (±77) | 352 (±74) | 73 (±3.5) | 50 (±7) | 73 (±4) | 21 (±5) | 14 (±4) |
| P value | 0.9 | 0.04 | 0.007 | 0.002 | 0.01 | 0.01 | 0.005 |
Mouse Lin− bone marrow cells cultured for 7 days with GST or GST-hDll1NDSL were evaluated for their cellularity, expression of Mac-1 and Gr-1 antigens, cell-cycle kinetics, and apoptosis. Data are expressed as mean percentage of 5 independent experiments with SD in parentheses.
GST-hDll1NDSL inhibited the differentiation of myeloid progenitors
Cell differentiation was examined by flow cytometry using anti-Mac-1 (detecting monocyte and granulocyte) and anti-Gr-1 (detecting granulocyte) antibodies. In the five independent experiments, Lin− cells cultured with GST-hDll1NDSLshowed consistently lower percentages of cells expressing Mac-1 and Gr-1. As shown in Table 2, the mean percentage of cells expressing Mac-1 at day 7 was 73% in the population cultured with GST-hDll1NDSL versus 84% in the control population. Similarly, the mean percentage of cells expressing Gr-1 was 50% versus 76%. These differences in expression of Mac-1 and Gr-1 are statistically significant (P < .01). In addition, the median intensity of fluorescence (MIF) of cells expressing Mac-1 and Gr-1 was significantly decreased when the cells were treated with GST-hDll1NDSL. A representative experiment is shown in Figure 6A in which at day 7, 78% (MIF, 157) and 67% (MIF, 71) of the cells exposed to GST-hDll1NDSL ligand expressed Mac-1 and Gr-1 (bottom panel), respectively, compared with 92% (MIF, 253) and 88% (MIF, 1186) of control cells (middle panel).
GST-hDll1NDSL affects the cell cycle and apoptosis of hematopoietic cells
Cell-cycle profiles were analyzed by cell sorting of the PI-stained hematopoietic cells derived from the Lin− cells cultured for 7 days. In the five independent observations (Table 2), Lin− cells cultured with GST-hDll1NDSL showed a significantly lower fraction of cells in G0/G1 phase (73%) than control GST-treated cells (86%, and a higher fraction of cells in S phase (21%) than control cells (7%). The differences in cell cycles between the two groups were statistically significant (P = .01).
Because GST-hDll1NDSL appeared to inhibit cell death (Table2), the apoptotic cell population in the sub-G0/G1 phase was analyzed during the process of cell-cycle data collection. Cells treated with GST-hDll1NDSL had a significantly reduced apoptotic cell population (14%) compared with the control cells cultured with GST (46%) (Table 2). The difference between the two groups was statistically significant (P = .005).
GST-hDll1NDSL promotes expansion of primitive hematopoietic progenitors
The effect of GST-hDll1NDSL on the expansion of progenitor cells was analyzed by clonogenic assays. The number of colonies generated after the Lin− cells were cultured for 7 days (day 7 CFU) was divided by the input colonies, giving the fold expansion of the colony-forming progenitors during the culture. Data from the five independent experiments are presented (Fig. 6B). Lin− cells cultured with GST-hDll1NDSLshowed significantly greater (3.3 fold) expansion of progenitors compared with the GST controls (Total CFU). In addition, GST-hDll1NDSL promoted progenitor cell expansion in each category of progenitors including CFU-mix (4.6 fold), CFU-GM (4.2 fold), early BFU-E (3.9 fold), and late BFU-E (3.2 fold) over the control cultures with GST.
In summary, we investigated the phenotypic and functional changes of Lin− progenitor cells cultured with GST-hDll1NDSL compared with the cells treated with GST in conditions promoting myelopoiesis. Data from five independent experiments demonstrated that GST-hDll1NDSL partially suppressed differentiation and promoted expansion of myeloid progenitor cells. We also observed that the fraction of cells in the S phase of the cell cycle was increased and that the percentage of apoptotic cells was decreased.
hDll1NDSL functions similarly to the GST-hDll1NDSL fusion protein
To control the possibility that the effects of GST-hDll1NDSL fusion protein were caused by the novel fusion polypeptide rather than by the hDll1NDSL sequences, we examined the function of hDll1NDSL released from the fusion protein. hDll1NDSL was released by digestion of the fusion protein bound to the GST-affinity beads with thrombin protease. Because the free GST and thrombin were not totally removed from the hDll1NDSL fraction (Fig. 4), 4 protein groups were compared: (1) 0.1 μmol/L GST; (2) 0.1 μmol/L GST-hDll1NDSL; (3) 0.1 μmol/L GST and 30 ng/mL thrombin (the same amount used to digest the fusion protein); (4) 0.1 μmol/L hDll1NDSL, 0.1 μmol/L GST, and 30 ng/mL thrombin. The Lin− cells were cultured for 7 days at 104cells/mL (total of 3 ml per well in 6-well plates) in the serum-free medium with a cytokine cocktail of IL-3, GM-CSF, and IL-1. Under these culture conditions, the 4 protein groups were added every 3 days. Cell proliferation, differentiation, and apoptosis of the cultured cells were evaluated. Data from three independent experiments are summarized in Table 3. Essentially, the hDll1NDSL effects were similar to those seen with the GST-hDll1NDSL fusion protein. The numbers of live cells were increased in the GST-hDll1NDSL and hDll1NDSL group over the two control groups. The numbers of progenitor cells detected as CFU were all significantly higher in the GST-hDll1NDSL and hDll1NDSL groups than in the controls. The hDll1NDSL group also showed consistently lower percentages of cells expressing Mac-1 and Gr-1 than the control GST/thrombin group. Like the fusion protein, the hDll1NDSLgroup also showed a significantly lower fraction of cells in the G0/G1 phase than the control GST/thrombin-treated cells and a higher fraction of cells in the S phase than the control cells. The differences between the GST-hDll1NDSL and the GST groups and between the hDll1NDSL and the GST/thrombin groups were statistically significant. Those between the GST-hDll1NDSL and the hDll1NDSL groups and between the GST and the GST/thrombin groups were not statistically significant. In conclusion, the results demonstrated that the GST-hDll1NDSL fusion protein retained the function of hDll1NDSL in regulation of hematopoiesis. The observed hematopoietic effects of the fusion protein reflected the function of hDll1NDSL.
hDll1NDSL functions similarly to GST-hDll1NDSL fusion protein
| Protein Groups . | Live Cells* . | Total CFU3-151 . | Differentiation . | Cell Cycle . | Apoptosis . | ||
|---|---|---|---|---|---|---|---|
| Mac-13-151 . | Gr-13-151 . | G0/G13-151 . | S3-151 . | Sub-G13-151 . | |||
| 1. GST | 263 (±40) | 3518 (±456) | 82 (±1) | 70 (±6) | 88 (±1) | 6 (±1) | 46 (±4) |
| 2. GST-hDll1NDSL | 300 (±76) | 15135 (±272) | 75 (±1) | 56 (±3) | 65 (±2) | 30 (±2) | 12 (±4) |
| 3. GST, Thrombin | 288 (±64) | 3240 (±216) | 87 (±4) | 81 (±3) | 89 (±1) | 6 (±1) | 46 (±10) |
| 4. hDll1NDSL, GST, Thrombin | 356 (±72) | 15766 (±992) | 75 (±4) | 61 (±4) | 70 (±3) | 25 (±4) | 16 (±3) |
| Protein Groups . | Live Cells* . | Total CFU3-151 . | Differentiation . | Cell Cycle . | Apoptosis . | ||
|---|---|---|---|---|---|---|---|
| Mac-13-151 . | Gr-13-151 . | G0/G13-151 . | S3-151 . | Sub-G13-151 . | |||
| 1. GST | 263 (±40) | 3518 (±456) | 82 (±1) | 70 (±6) | 88 (±1) | 6 (±1) | 46 (±4) |
| 2. GST-hDll1NDSL | 300 (±76) | 15135 (±272) | 75 (±1) | 56 (±3) | 65 (±2) | 30 (±2) | 12 (±4) |
| 3. GST, Thrombin | 288 (±64) | 3240 (±216) | 87 (±4) | 81 (±3) | 89 (±1) | 6 (±1) | 46 (±10) |
| 4. hDll1NDSL, GST, Thrombin | 356 (±72) | 15766 (±992) | 75 (±4) | 61 (±4) | 70 (±3) | 25 (±4) | 16 (±3) |
Mouse Lin− bone marrow cells were cultured for 7 days in serum-free medium with IL-3, GM-CSF, and IL-1. GST, GST-hDll1NDSL, GST plus thrombin, or hDll1NDSLplus GST plus thrombin were added to the cultures every 3 days. Cultured cells were evaluated for their cellularity, CFU, Mac-1/Gr-1 expression, and cell-cycle kinetics. Data are expressed as mean number of live cells and CFU from 3 separate experiments ± SE. Data for differentiation and cell-cycle analyses are expressed as mean percentage of 3 independent experiments with SD in parentheses. Statistical significance is determined by the two-tailed paired Studentt-test. Differences between protein groups 1 and 3 and groups 2 and 4 are not statistically significant (P > .05 for all the observed parameters). Differences between protein groups 1 and 2 and protein groups 3 and 4 are statistically significant for the parameters of total CFU, differentiation, cell cycle, and apoptosis (
P < .01), and significant for live cells (*P < .05).
GST-hDll1NDSL promoted expansion of primitive hematopoietic progenitor cells
After defining the role of GST-hDll1NDSL in the regulation of Lin− cells, a population that included late-stage progenitors (44% of which are in the S phase of the cell cycle) and stem cells, we extended the functional analysis of GST-hDll1NDSL to the early-stage hematopoietic progenitors and stem cells. These cells were enriched in Lin−cells isolated from 5-fluorouracil-treated mouse bone marrow. The specificity of the GST-hDll1NDSL effects was also tested by the use of the neutralizing anti-thioredoxin-mDll1NDSLantibody (anti-mDll1). Five thousand cells were added to a single well of 24-well plate containing 1 mL serum-free medium plus cytokines (IL-11, KL, and FL). Four protein groups were tested: (1) 0.1 μmol/L GST; (2) 0.1 μmol/L GST-hDll1NDSL; (3) 0.1 μmol/L GST-hDll1NDSL plus 5 μg/mL anti-mDll1; and (4) 0.1 μmol/L GST-hDll1NDSL plus 5 μg/mL preimmune serum. Proteins were added to the cultures every 3 days. Cells were counted after culture for 12 days, and CFU cells including HPP-CFC (colony greater than 0.5 mm) and CFU-GM were measured in methylcellulose assay. Early-stage progenitors in the suspension population of cells were read out in standard CFU-Sday12 assay by injecting the cultured cells into the irradiated mice. Twelve days later, the spleen colonies were counted. Data from 3 independent experiments are summarized in Table 4. Cells cultured with GST-hDll1NDSL showed a 2.5-fold increase in HPP-CFC, a 1.6-fold increase in CFU-GM, and a 4-fold increase in CFU-Sday12 compared with the control cells cultured with GST. Furthermore, the GST-hDll1NDSLeffects were significantly blocked by the presence of anti-mDll1 antibody but not affected by the preimmune serum. The anti-mDll1 was raised against thioredoxin-mDll1NDSL, which showed 92% identity with the corresponding region of hDll1 (Fig. 4A). Thus, the data in Table 4 demonstrate that hDll1NDSL promoted the expansion of noncycling primitive progenitors. The hDll1NDSL effect was blocked by the anti-mDll1 antibody, which further confirmed that the function of the fusion protein was hDll1NDSL sequence dependent.
GST-hDll1NDSL promotes the expansion of primitive hematopoietic progenitor cells neutralized by anti-mDll1NDSL antibodies
| Protein Groups . | Live Cells . | HPP-CFU* . | CFU-GM* . | CFU-Sday12* . |
|---|---|---|---|---|
| 1. GST | 6 (±2) | 1220 (±200) | 4503 (±491) | 22 (±13) |
| 2. GST-hDll1NDSL | 5 (±2) | 3011 (±547) | 7187 (±840) | 88 (±22) |
| 3. GST-hDll1NDSL Anti-mDll1 Ab | 5 (±2) | 1787 (±110 ) | 4277 (±327) | 34 (±25 ) |
| 4. GST-hDll1NDSL Preimmune serum | 6 (±2 ) | 3107 (±639) | 9657 (±770) | 72 (±19) |
| Protein Groups . | Live Cells . | HPP-CFU* . | CFU-GM* . | CFU-Sday12* . |
|---|---|---|---|---|
| 1. GST | 6 (±2) | 1220 (±200) | 4503 (±491) | 22 (±13) |
| 2. GST-hDll1NDSL | 5 (±2) | 3011 (±547) | 7187 (±840) | 88 (±22) |
| 3. GST-hDll1NDSL Anti-mDll1 Ab | 5 (±2) | 1787 (±110 ) | 4277 (±327) | 34 (±25 ) |
| 4. GST-hDll1NDSL Preimmune serum | 6 (±2 ) | 3107 (±639) | 9657 (±770) | 72 (±19) |
Lin− cells were isolated from 5-Fu-treated mouse bone marrow and cultured for 12 days in serum-free medium with IL-11, KL, and FL. GST, GST-hDll1NDSL, GST-hDll1NDSLplus anti-mDll1NDSL antibody, or GST-hDll1NDSLplus preimmune serum was added to the cultures every 3 days. Cells were evaluated for their cellularity, HPP-CFU (colonies larger than 0.5 mm in diameter), CFU-GM, and CFU-Sday12. Data are expressed as mean number of 3 independent experiments with SE in parentheses. Statistical significance is determined by the two-tailed paired Studentt-test. Differences between protein groups 1 and 3 and groups 2 and 4 are not statistically significant (P > .05 for all the observed parameters). Differences between protein groups 1 and 2 and protein groups 3 and 4 are statistically very significant for the parameters of HPP-CFU, CFU-GM, CFU-Sday12(*P < .01) but not significant for the live cells (P > .05).
Discussion
In this article, we describe the isolation of a cDNA clone encoding a human Delta ligand (hDll1) for Notch and the creation of a soluble form of the ligand. A role for this soluble hDll1 in the regulation of hematopoiesis in vitro was revealed. Although hDll1 shares all the conserved features with other DSL proteins, it is most closely related to mDll1. Expression of mDll1 was detected in a variety of adult mouse tissues, including hematopoietic tissues of spleen and bone marrow. Together with Jagged1 and Jagged223,30-32 then, 3 DSL ligands are expressed by bone marrow stromal cells. Because Notch1 and Notch2 were detected in hematopoietic cells,30,40 it is likely that multiple DSL ligands and Notch receptors are involved in the regulation of bone marrow hematopoiesis. Recently, Radtke et al41 reported a conditional knock-out of Notch1 in mouse. In a competitive repopulation experiment, Notch-1-deficient bone marrow contributed normally to all hematopoietic lineages, but not to T cells. These observations indicate the redundancy of Notch signaling, probably caused by the expression of multiple members of Notch and its ligand in the hematopoietic system.
We have taken a protein biochemical approach to address hDll1 function by creating a soluble form of the newly identified Notch ligand. We made a soluble hDll1 as a GST fusion protein, containing the DSL domain and the adjacent N-terminal region, in a bacterial expression system. Compared to the soluble form of Jagged1 produced in COS cells,23,30 our production strategy achieved higher yield and purity. Our method of ligand production may prove to be a useful general strategy for the production of soluble receptor agonists using other members of the Notch ligand family. In the culture of murine Lin− cells with GST-hDll1NDSL and cytokines, we found decreased numbers of mature monocytes/macrophages and granulocytes and increased numbers of progenitor cells. We also observed decreased numbers of apoptotic cells and increased numbers of cells in the S-phase of the cell cycle. The function of hDll1 is thus consistent with reports on the action of Jagged1 and Jagged2 in hematopoiesis. In culture of primary human and mouse hematopoietic progenitor cells, both Jagged1 and Jagged2 were shown to increase the number of progenitors.30,31 Jagged2 also decreased the number of mature myeloid cells as measured by the surface marker expression of CD11b and CD14.31
We believe that the hDll1 effects, including increased numbers of progenitor cells and cells in the S-phase of the cell cycle and decreased numbers of apoptotic cells in cultures with hDll1NDSL, result from the suppression of hematopoietic progenitor differentiation. It is known that mature granulocytes generated in culture readily undergo apoptosis within a day and that progenitor cells exit the cell cycle as they mature. However, we cannot rule out a mechanism by which hDll1 directly modulates the expansion, apoptosis, and cell cycle of hematopoietic cells. Data supporting the latter model have emerged recently. Jagged1 was shown to affect the proliferation of hematopoietic cells as measured by thymidine incorporation.32 The activated form of Notch1 expressed in HL-60 cells has been shown to enhance cell progression through the G1 phase of the cell cycle, which is associated with Notch1-induced delay of differentiation.31 It is also reported that an activated form of Notch1 provides significant protection of T-cell lines from TCR-mediated apoptosis.42
Most cytokines and their receptors have been identified at the molecular level.43 Binding of cytokines to their receptors elicits both proliferation and differentiation signals. The receptor functional domains have been mapped.44 The membrane-proximal domains of the Epo,45GM-CSF,46 G-CSF,47 and thrombopoietin48 receptors are sufficient for mediating mitogenic signals, whereas the membrane-distal domains are required only for generating differentiation signals. Cytokine-mediated receptor oligomerization juxtaposes and activates JAK kinases associated with the membrane-proximal box 1 and box 2 motifs of the receptor. Activated JAKs then phosphorylate and induce nuclear translocation of the STAT DNA-binding proteins.49 STATs appear to be involved in functional differentiation (as opposed to mitogenic) responses to cytokines.50 We speculate that the activated Notch receptor could inhibit hematopoietic differentiation in response to cytokine stimulation by interacting with molecules involved in the cytokine differentiation pathways. Recently, Bigas et al34 reported that mammalian Notch intracellular domain contains a Notch cytokine response region (NCR). Notch1 and Notch2 contain different NCR and, therefore, respond to different cytokine signals.
The functional domain of the fusion protein is the region of hDll1 sequences. This is demonstrated by GST control protein having no hematopoietic effect, antibodies against mDll1 blocking the GST-hDll1NDSL function, and hDll1NDSL released from the fusion proteins showing hematopoietic effects similar to those of the fusion protein. The potential physiological role of the secreted DSL proteins in vivo has recently been suggested because soluble forms of Delta do exist in Drosophila embryos.27 Given the general role of the Notch pathway in the inhibition of hematopoietic progenitor cell differentiation, the availability of soluble hDll1 ligand should facilitate the development of a new strategy for the ex vivo expansion of hematopoietic stem/progenitor cells.
Acknowledgments
We thank Dr Achim Gossler for providing the mDll1 cDNA probe and Lisa Wang for outstanding technical assistance.
Supported by grants from The Gar Reichman Fund of the Cancer Research Institute, The Bernard Mendik Fund, the Public Health Service (1-F32-HL10152-01), and the National Institutes of Health Cancer Center (CA-08748).
Reprints:Malcolm A. S. Moore, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue Mailbox 101, New York, NY 10021; email:m-moore@ski.mskcc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal