Fanconi anemia (FA) is an autosomal recessive disease characterized by congenital anomalies, aplastic anemia, and a susceptibility to leukemia. There are at least 8 complementation groups (A through H). Extensive analyses of the FA group C gene FANCC in Western countries revealed that 10% to 15% of FA patients have mutations of this gene. The most common mutation is IVS4 + 4 A to T (IVS4), a splice mutation in intron 4, which has been found only in patients of Ashkenazi Jewish ancestry. When we screened 29 Japanese patients (20 unrelated patients and 4 families) using polymerase chain reaction–single strand conformation polymorphism, we found 8 unrelated patients homozygous for IVS4. This is apparently the first non–Ashkenazi-Jewish population for whom this mutation has been detected. The Ashkenazi Jewish patients homozygous for IVS4 have a severe phenotype, in comparison with other FA patients. Our analyses of Japanese patients indicate no significant difference between IVS4 homozygotes and other patients with regard to severity of a clinical phenotype. Thus, ethnic background may have a significant effect on a clinical phenotype in FA patients carrying the same mutation.
Fanconi anemia (FA) is an autosomal recessive genomic instability syndrome characterized by progressive bone marrow failure, congenital anomalies, and cancer susceptibility.1,2 Cells derived from FA patients show hypersensitivity to DNA cross-linking agents, abnormal cell cycle progression, and reduced cell survival.3-5 FA patients constitute at least 8 different complementation groups (FA-A to FA-H), as defined by cell fusion analysis.5-8 The genes for group C (FANCC), group A (FANCA), and group G (FANCG) have been cloned by other investigators.9-12 While the cellular functions of proteins encoded by these genes are unknown, the proteins bind in a nuclear complex, suggesting that they cooperate in a nuclear function, such as DNA repair.13-15
The coding sequence of the FANCC gene is composed of 14 exons and encodes a protein of 558 amino acid residues with a molecular mass of 63 kd. Extensive analyses of the gene in Western countries revealed at least 9 mutations in this gene.16-19Mutations in most patients cluster in 3 regions of the gene: exon 1, intron 4, and exon 14. The most common mutation is IVS4 + 4 A to T (IVS4), a splice mutation in intron 4 resulting in deletion of exon 4.17,18 Less common mutations include 322delG, a frameshift generating a premature stop codon, and Q13X in exon 1, and R548X and L554P in exon 14.16,18 19
The percentages of group A and group C patients in Western countries are estimated to be 60% to 65% and 10% to 15%, respectively.20 However, it was reported that genetic abnormalities for FA vary among different ethnic groups. About 80% of Ashkenazi Jewish patients are assigned to group C.17On the other hand, the proportion of group C patients in non-Jewish populations is reported to be about 8%.16 Group A patients are more prevalent in Germany and Italy,21,22 whereas the majority of patients in the Netherlands belong to group C.21FANCC mutation types differ among distinct ethnic groups. The IVS4 mutation is unique to Jewish patients, whereas 322delG is often present in patients of Northern European ancestry.16,17 Genotype-phenotype analyses in FA patients revealed that Ashkenazi Jewish patients with the IVS4 mutation have the severe clinical phenotype, such as early onset of hematological diseases, multiple major malformations, and poor survival, in comparison with other group C patients with exon 1 mutations or non-C patients.18,23 24
Little is known of the genetic basis of FA patients in Asian countries. In the present work, we screened FANCC mutations in 29 Japanese patients and identified 8 patients homozygous for the IVS4 mutation. Our analyses show that these patients have a clinical phenotype similar to that in other patients, unlike Ashkenazi Jewish patients with the same mutation.23 The present findings provide important information for population-based screening forFANCC carriers in Japan and genotype-phenotype relationship in group-C FA patients.
Materials and methods
We diagnosed 29 Japanese patients with 25 probands on the basis of the presence of clinical manifestations of FA (hematological abnormalities and/or congenital anomalies typical of FA, as defined by Auerbach et al3) and on the basis of studies on the chromosomal breakage induced by diepoxybutane or mitomycin C in peripheral blood lymphocytes.
Onset of hematological abnormalities is defined as the time at which one of the following laboratory parameters was observed: platelet count below 100 × 109/L, hemoglobin (Hb) below 10 g/dL, or absolute neutrophil count below 1 × 109/L.
Patients were scored for the presence of major congenital abnormalities, defined as structural alterations that occur during embryogenesis, with medical and social consequences. The spectrum of major congenital malformations in FA patients included abnormalities of the kidney and urinary tract, genitalia, heart, gastrointestinal system, and central nervous system and skeletal abnormalities, including defects of the radius and thumb.
To screen for the FANCC gene, we used polymerase chain reaction (PCR)–single strand conformation polymorphism (SSCP). We obtained genomic DNA samples from the peripheral blood of our patients and from normal healthy volunteers. All of the subjects gave informed consent. Each coding exon of the FANCC gene was amplified by the PCR with the use of genomic DNA and primers flanking the individual exons and overlapping the intron-exon boundaries, as described by Gibson et al.25 PCR-SSCP analysis was performed according to the methods described by Orita et al.26 Briefly, a total of 10 μL of reaction mixture, containing 200 ng of genomic DNA, 0.4 pmol of each primer end-labeled with [γ-32P] ATP (Amersham), 100 μmol/L dNTPs, 10 mmol/L Tris-HCl(pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.001% gelatin, and 0.2 U of Taq polymerase (Perkin-Elmer) was used for PCR amplification. PCR reactions were performed for 1 step of denaturation (5 minutes at 94°C), followed by 35 cycles of denaturation (30 seconds at 94°C), annealing (30 seconds at 60°C), extension (40 seconds at 72°C), and a final step of extension (7 minutes at 72°C). Then, 5 μL of the PCR products was diluted in 45 μL of formamide loading buffer (90% formamide, 10 mmol/L ethylenediaminetetraacetic acid, 0.25% bromphenol blue, 0.25% xylene cyanol). The mixture was heated to 80°C for 5 minutes to denature the DNA, then rapidly chilled on ice. An aliquot of the sample (2 μL) was analyzed on a 6% nondenaturing polyacrylamide gel (49:1 acrylamide : bisacrylamide), with or without 10% glycerol in 0.5 × TBE buffer. Gels containing 10% glycerol were run at room temperature at 10 W for 14 hours and gels without glycerol were run for 4 hours at 4°C at 40 W. The gels were then dried and autoradiographed.
Mutations were characterized by direct sequencing of PCR products. When shifted bands were obvious on the SSCP gel, these bands were excised and eluted from the gel and then used as a template for PCR. Cycle sequencing reactions were performed with the use of Taq Dye Terminator Cycle Sequencing Kits (Perkin-Elmer). Sequencing was done with an automated sequencer (ABI model 310; Perkin-Elmer).
Allele-specific oligonucleotide (ASO) hybridization was performed as follows: Genomic DNA was used as template for PCR, with the following primers flanking exon 4:5′-GTAGGCATTGTACATAAAAG-3′ (forward reaction) and 5′-TGGCACATTCAGCATTAAAC-3′ (reverse reaction). The PCR product was dot-blotted onto nylon filters (Hybond N, Amersham) and hybridized with end-labeled oligonucleotides with [γ-32P] ATP corresponding to the wild-type or mutant sequence at intron 4, 5′-AAAATGTGAGTATTT-3′ or 5′-AAAATGTGTGTATTT-3′, respectively. Hybridizations were performed at 30°C for 3 hours, followed by washing for 10 minutes in 2 × SSPE, 0.1% sodium dodecyl sulfate (SDS) at room temperature, then by a 10-minute wash at 32°C in 0.2 × SSPE, 0.1% SDS.
For the statistical analysis, we used StatView 4.5 software. Unpaired Student t test was used to compare the age at onset of hematological abnormalities and the anomaly number between patients with IVS4 mutation and other patients.
Results
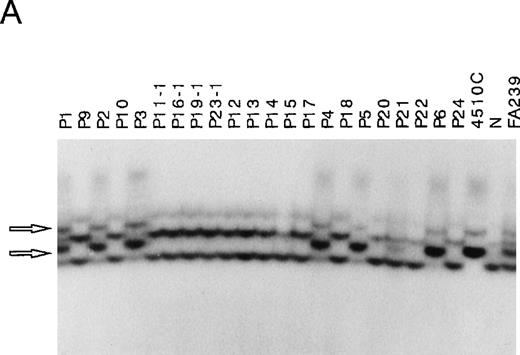
First, we amplified 14 segments covering each exon of FANCCfrom genomic DNA isolated from 29 FA patients and analyzed by SSCP. In samples from 8 patients (P1 through P8), PCR products corresponding to exon 4 and flanking intronic regions showed aberrant bands. Representative data of 6 patients (P1 through P6) are shown in Figure1A. Similar aberrant bands were seen in 4510 cells known to be homozygous for the IVS4 + 4A to T mutation, suggesting that 8 of the 29 patients carry this mutation. A sample from one patient, P21, showed both normal and aberrant bands, like a sample from FA239 cells known to be heterozygous for the IVS4 mutation. In none of the 29 samples did we detect mobility shifts of other segments (data not shown), while we did detect mobility shifts in control samples known to have 322delG and L554P mutations (data not shown).
Detection of IVS4 mutation by PCR-SSCP and subsequent direct sequencing.
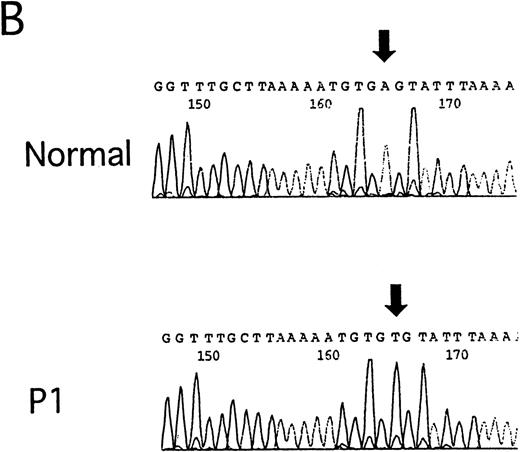
(A) Representative PCR-SSCP analyses using the primer set for exon 4 boundaries. 4510C cells are homozygous for the IVS4 mutation; FA239 cells are heterozygous (IVS4/wt) for this segment; and N indicates normal control. The patient numbers correspond to those in Table 1. Arrowheads indicate mobility shifts of the PCR products from the genomic DNA from the patients, P1, P2, P3, P4, P5, and P6. (B) Representative sequencing results. Direct sequencing of aberrant bands from P1 identified an A to T transition of the fourth base in intron 4 (indicated by arrows).
Detection of IVS4 mutation by PCR-SSCP and subsequent direct sequencing.
(A) Representative PCR-SSCP analyses using the primer set for exon 4 boundaries. 4510C cells are homozygous for the IVS4 mutation; FA239 cells are heterozygous (IVS4/wt) for this segment; and N indicates normal control. The patient numbers correspond to those in Table 1. Arrowheads indicate mobility shifts of the PCR products from the genomic DNA from the patients, P1, P2, P3, P4, P5, and P6. (B) Representative sequencing results. Direct sequencing of aberrant bands from P1 identified an A to T transition of the fourth base in intron 4 (indicated by arrows).
Direct sequencing of PCR products showing the mobility shift identified the IVS4 mutation in all of the samples from patients 1 through 8. Figure 1B shows representative data from a normal control and P1. A sample of P21 showed a normal sequence as well as the mutation (not shown), suggesting that this patient may be a compound heterozygote, although the other FANCC mutation was not detected.
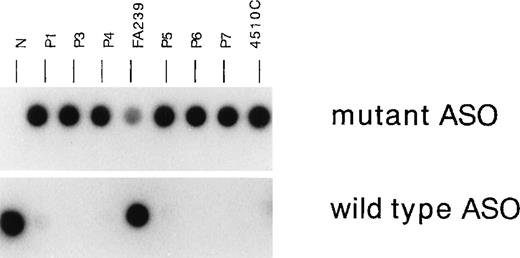
To confirm that the 8 patients (P1 through P8) are homozygous for the IVS4 mutation, we next carried out ASO hybridization (Figure2), as described.17 DNA samples from normal cells hybridized to the wild-type ASO but not to the mutant ASO, whereas a sample from 4510 cells homozygous for the IVS4 mutation only hybridized to the mutant ASO. A sample from FA239 heterozygous cells hybridized to both probes. DNA samples from P1, P3, P4, P5, P6, and P7 hybridized with the mutant oligonucleotide but not with the normal oligonucleotide (Figure 2). DNA samples from P2 and P8 gave similar results (data not shown). These results indicate that both alleles of the 8 patients have IVS4 mutation.
Allele-specific oligonucleotide hybridization for IVS4.
Hybridization of the wild-type or mutant oligonucleotide to DNAs from the FA patients with the IVS4 mutation (P1, P3, P4, P5, P6, and P7) and control cells. 4510C cells are homozygous for the IVS4 mutation; FA239 cells are heterozygous (IVS4/wt) for this segment; and N indicates normal control.
Allele-specific oligonucleotide hybridization for IVS4.
Hybridization of the wild-type or mutant oligonucleotide to DNAs from the FA patients with the IVS4 mutation (P1, P3, P4, P5, P6, and P7) and control cells. 4510C cells are homozygous for the IVS4 mutation; FA239 cells are heterozygous (IVS4/wt) for this segment; and N indicates normal control.
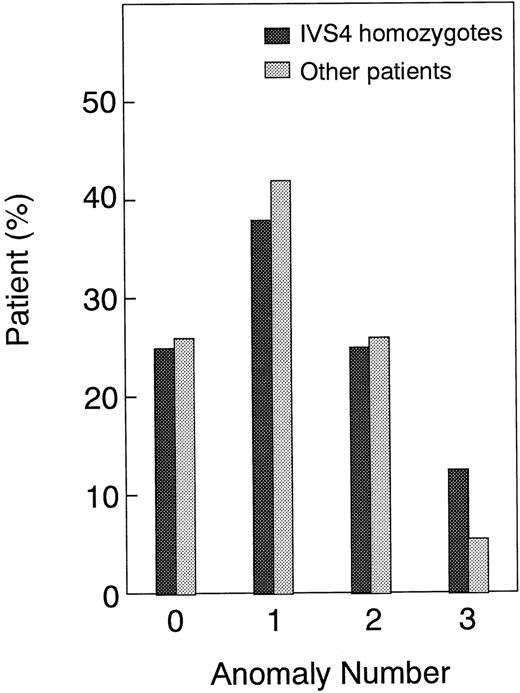
The clinical characteristics of the 29 patients, 8 patients homozygous for the IVS4 mutation (group I) and the other 21 patients (group II), are summarized in Table 1. There is no consanguinity or geographical isolation of the patients with IVS4. Previous studies showed that patients homozygous for IVS4 have a severe phenotype, as manifested by early onset of hematological disease and multiple major congenital anomalies.18,23,24 Therefore, we compared the age at onset of the hematological disease and the number of major congenital malformations between groups I and II. The median age at onset of hematological disease was 6.21 years (95% confidence interval [CI], 2.88 to 9.54 years) in group I and 5.79 years (95% CI, 4.14 to 6.44 years) in group II (Table2), suggesting no significant difference between the 2 groups. There was also no significant difference between the 2 groups in the mean number of major congenital anomalies (1.3 in group I versus 1.1 in group II) (Table 2) or the distribution of patients with each number of major anomalies (Figure3). Taken together, these results suggest that the severity of a clinical phenotype is similar between IVS4 homozygotes and other FA patients. Table 2 also shows the results of a previous study23 indicating that Ashkenazi Jewish IVS4 patients have an earlier onset of hematological abnormalities and a greater number of major congenital anomalies in comparison with other patients.
Characteristics of 29 FA patients in Japan
| Patient No. . | Sex . | Age at Onset of HA (yr) . | Hematological Data at Diagnosis . | Congenital Malformations . | Growth . | Treatment and Clinical Course . | Status (Age)* . | Genotype . | Birthplace (Prefecture) . | Consanguinity . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (×109/L) . | Hb (g/dL) . | Plt (×109/L) . | SP . | T/R . | S . | C . | KU . | G . | MC . | MP . | E . | Gl . | MDS/AML . | BMT . | ||||||||
| P1 | F | 4 | 3.1 | 10.1 | 25 | N | MDS | + | living (7) | IVS4/IVS4 | Nagasaki | − | ||||||||||
| P2 | M | 9 | 3 | 8.6 | 86 | + | + | R | + | living (15) | IVS4/IVS4 | Niigata | − | |||||||||
| P3 | M | 4 | 3 | 8.3 | 4 | + | + | + | + | R | + | died (6) | IVS4/IVS4 | Nagasaki | − | |||||||
| P4 | M | 4 | 5 | 6.7 | 33 | + | + | + | R | + | living (11) | IVS4/IVS4 | Tokyo | − | ||||||||
| P5 | M | 15 | 1.9 | 5.4 | 47 | + | + | N | MDS | + | living (11) | IVS4/IVS4 | Kyoto | − | ||||||||
| P6 | M | 10 | 1.5 | 7.4 | 22 | + | N | + | living (21) | IVS4/IVS4 | Kanagawa | − | ||||||||||
| P7 | M | 3 | + | + | + | + | N | + | living (23) | IVS4/IVS4 | Yamaguchi | − | ||||||||||
| P8 | M | 0.7 | 12.6 | 8.8 | + | + | R | + | living (17) | IVS4/IVS4 | Mie | − | ||||||||||
| P9 | F | 8 | + | R | AML (23 y) | + | living (26) | — | − | |||||||||||||
| P10 | F | 4 | 3.4 | 5.7 | 16 | + | + | R | living (8) | − | ||||||||||||
| P11-1† | F | 7 | 4.5 | 9.3 | 45 | R | living (16) | Kagoshima | − | |||||||||||||
| P11-2† | M | 7 | 2.3 | 10.8 | 23 | R | MDS | living (21) | Kagoshima | − | ||||||||||||
| P12 | M | 2 | 2.2 | 6.9 | 15 | + | + | N | MDS? | + | died (9) | — | ? | |||||||||
| P13 | F | 9 | 2.6 | 6.4 | 45 | + | N | living (11) | Hiroshima | + | ||||||||||||
| P14 | M | 3 | 3.5 | 4.2 | 41 | + | + | + | R | AML (10 y) | died (?) | Osaka | − | |||||||||
| P15 | F | 7 | 3 | 10.3 | 57 | + | + | R | AML (23 y) | + | died (24) | Hiroshima | − | |||||||||
| P16-1† | F | 11 | 2.1 | 4.8 | 18 | + | + | N | living (14) | Ibaraki | − | |||||||||||
| P16-2† | F | 4 | 3.7 | 5.7 | 29 | + | + | + | N | living (9) | Ibaraki | − | ||||||||||
| P17 | F | 5 | + | R | living (6) | — | + | |||||||||||||||
| P18 | M | 3 | 5.5 | 10.6 | 61 | + | N | living (18) | Shimane | − | ||||||||||||
| P19-1† | 6 | 3.4 | 8 | 27 | + | + | + | N | MDS | living (14) | — | − | ||||||||||
| P19-2† | F | 6 | 4.4 | 11.3 | 79 | + | R | living (7) | — | − | ||||||||||||
| P19-3† | F | 9.1 | 11.9 | 455 | + | + | R | living (2) | — | − | ||||||||||||
| P20 | F | 4 | 6 | 9.3 | 26 | + | + | + | R | + | living (20) | Kanagawa | − | |||||||||
| P21 | F | 7 | 2.6 | 4.8 | 19 | + | + | N | living (9) | IVS4/unknown | Gunma | − | ||||||||||
| P22 | F | 6 | 4.3 | 9.4 | 49 | + | R | living (13) | Hiroshima | − | ||||||||||||
| P23-1† | F | 0.8 | 2.7 | 4.8 | 14 | + | + | N | + | died (2) | — | − | ||||||||||
| P23-2† | F | 4 | 6.2 | 13.8 | 57 | + | + | + | R | living (15) | — | − | ||||||||||
| P24 | M | 2 | 2.9 | 11 | 60 | N | living (8) | Shimane | − | |||||||||||||
| Patient No. . | Sex . | Age at Onset of HA (yr) . | Hematological Data at Diagnosis . | Congenital Malformations . | Growth . | Treatment and Clinical Course . | Status (Age)* . | Genotype . | Birthplace (Prefecture) . | Consanguinity . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (×109/L) . | Hb (g/dL) . | Plt (×109/L) . | SP . | T/R . | S . | C . | KU . | G . | MC . | MP . | E . | Gl . | MDS/AML . | BMT . | ||||||||
| P1 | F | 4 | 3.1 | 10.1 | 25 | N | MDS | + | living (7) | IVS4/IVS4 | Nagasaki | − | ||||||||||
| P2 | M | 9 | 3 | 8.6 | 86 | + | + | R | + | living (15) | IVS4/IVS4 | Niigata | − | |||||||||
| P3 | M | 4 | 3 | 8.3 | 4 | + | + | + | + | R | + | died (6) | IVS4/IVS4 | Nagasaki | − | |||||||
| P4 | M | 4 | 5 | 6.7 | 33 | + | + | + | R | + | living (11) | IVS4/IVS4 | Tokyo | − | ||||||||
| P5 | M | 15 | 1.9 | 5.4 | 47 | + | + | N | MDS | + | living (11) | IVS4/IVS4 | Kyoto | − | ||||||||
| P6 | M | 10 | 1.5 | 7.4 | 22 | + | N | + | living (21) | IVS4/IVS4 | Kanagawa | − | ||||||||||
| P7 | M | 3 | + | + | + | + | N | + | living (23) | IVS4/IVS4 | Yamaguchi | − | ||||||||||
| P8 | M | 0.7 | 12.6 | 8.8 | + | + | R | + | living (17) | IVS4/IVS4 | Mie | − | ||||||||||
| P9 | F | 8 | + | R | AML (23 y) | + | living (26) | — | − | |||||||||||||
| P10 | F | 4 | 3.4 | 5.7 | 16 | + | + | R | living (8) | − | ||||||||||||
| P11-1† | F | 7 | 4.5 | 9.3 | 45 | R | living (16) | Kagoshima | − | |||||||||||||
| P11-2† | M | 7 | 2.3 | 10.8 | 23 | R | MDS | living (21) | Kagoshima | − | ||||||||||||
| P12 | M | 2 | 2.2 | 6.9 | 15 | + | + | N | MDS? | + | died (9) | — | ? | |||||||||
| P13 | F | 9 | 2.6 | 6.4 | 45 | + | N | living (11) | Hiroshima | + | ||||||||||||
| P14 | M | 3 | 3.5 | 4.2 | 41 | + | + | + | R | AML (10 y) | died (?) | Osaka | − | |||||||||
| P15 | F | 7 | 3 | 10.3 | 57 | + | + | R | AML (23 y) | + | died (24) | Hiroshima | − | |||||||||
| P16-1† | F | 11 | 2.1 | 4.8 | 18 | + | + | N | living (14) | Ibaraki | − | |||||||||||
| P16-2† | F | 4 | 3.7 | 5.7 | 29 | + | + | + | N | living (9) | Ibaraki | − | ||||||||||
| P17 | F | 5 | + | R | living (6) | — | + | |||||||||||||||
| P18 | M | 3 | 5.5 | 10.6 | 61 | + | N | living (18) | Shimane | − | ||||||||||||
| P19-1† | 6 | 3.4 | 8 | 27 | + | + | + | N | MDS | living (14) | — | − | ||||||||||
| P19-2† | F | 6 | 4.4 | 11.3 | 79 | + | R | living (7) | — | − | ||||||||||||
| P19-3† | F | 9.1 | 11.9 | 455 | + | + | R | living (2) | — | − | ||||||||||||
| P20 | F | 4 | 6 | 9.3 | 26 | + | + | + | R | + | living (20) | Kanagawa | − | |||||||||
| P21 | F | 7 | 2.6 | 4.8 | 19 | + | + | N | living (9) | IVS4/unknown | Gunma | − | ||||||||||
| P22 | F | 6 | 4.3 | 9.4 | 49 | + | R | living (13) | Hiroshima | − | ||||||||||||
| P23-1† | F | 0.8 | 2.7 | 4.8 | 14 | + | + | N | + | died (2) | — | − | ||||||||||
| P23-2† | F | 4 | 6.2 | 13.8 | 57 | + | + | + | R | living (15) | — | − | ||||||||||
| P24 | M | 2 | 2.9 | 11 | 60 | N | living (8) | Shimane | − | |||||||||||||
HA indicates hematological abnormality; N, normal; R, retarded; SP, skin pigmentation; T/R, thumb/radius; S, skeletal; C, cardiac; KU, kidney and urinary tract; G, genitalia; MC, microcephaly; MP, microphthalmia; E, ear; GI, gastrointestinal; BMT, bone marrow transplantation; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; IVS4, IVS4 + 4 A to T mutation.
Age is either age at death or age when reported as living.
P11-1 and P11-2 are siblings; P16-1, P16-2, and P16-3 are siblings; P19-1, P19-2, and P19-3 are siblings; and P23-1 and P23-2 are siblings.
Genotype-phenotype associations
| . | Present Study . | Previous Study (Gillio et al23) . | ||||
|---|---|---|---|---|---|---|
| Group I (N = 8, n = 8) . | Group II (N = 21, n = 17) . | P Value . | IVS4 (N = 26) . | Exon 1 (N = 17) . | Non-C* (N = 338) . | |
| Median age (y) at onset of hematological disease (95% CI) | 6.21 (2.88-9.54) | 5.79 (4.14-6.44) | .508 | 2.72 (2.32-3.12) | 7.60 (5.85-9.35) | 6.6 (6.17-7.03) |
| Mean number of congenital malformations | 1.3 | 1.1 | .496 | 3.7 | 0.6 | — |
| . | Present Study . | Previous Study (Gillio et al23) . | ||||
|---|---|---|---|---|---|---|
| Group I (N = 8, n = 8) . | Group II (N = 21, n = 17) . | P Value . | IVS4 (N = 26) . | Exon 1 (N = 17) . | Non-C* (N = 338) . | |
| Median age (y) at onset of hematological disease (95% CI) | 6.21 (2.88-9.54) | 5.79 (4.14-6.44) | .508 | 2.72 (2.32-3.12) | 7.60 (5.85-9.35) | 6.6 (6.17-7.03) |
| Mean number of congenital malformations | 1.3 | 1.1 | .496 | 3.7 | 0.6 | — |
N indicates number of affected individuals; n, number of families; CI, confidence interval.
Blank cell indicates a category that was not described in the study.
Comparison of anomaly number between IVS4 homozygotes and other patients.
Gillio et al also found that survival time of Ashkenazi Jewish patients with IVS4 is short in comparison with patients with the 322delG mutation of FANCC and non-C patients, partly because of the early development of leukemia.23 In the present study, 2 of the 8 patients (25%) in group I developed myelodysplastic syndrome, and 6 of 21 patients (28%) in group II had myelodysplastic syndrome or acute myeloid leukemia (Table 1). Thus, there is no significant difference with regard to disease progression. We cannot compare survival time of the 2 groups, partly because follow-up time was short. Another reason is the difference of treatment for the 2 groups; all the patients with IVS4 were treated with allogeneic bone marrow transplantation, whereas only 5 of the other patients underwent this treatment.
Discussion
In the first report describing IVS4 + 4A to T, the mutation was found in 5 Ashkenazi Jewish families and not found in any non-Jewish families.17 Analysis of patients in the International Fanconi Anemia Registry confirmed that this mutation is responsible for most cases of FA in Jewish patients; all of the families with this mutation have a Jewish heritage, and 16 of 20 Jewish FA families tested have this mutation.18 On the other hand, FA patients of non-Jewish ancestry did not have this mutation. Thus, the IVS4 mutation is referred to as the Ashkenazi Jewish mutation.27 In the current study, we found 8 homozygotes and 1 heterozygote with the IVS4 mutation among 29 Japanese patients. This is apparently the first report that the IVS4 mutation was identified in a population of non–Ashkenazi-Jewish origin.
In the current study, the IVS4 mutation was the only mutation found in our Japanese FA patients, although our screening method PCR-SSCP detects 70% to 80% of mutations26 and may have missed less common FANCC mutations. Two groups recently reported that most of Japanese FA patients have various types of FANCAmutations,28,29 suggesting that many of our non-C patients belong to group A. Possibly, the group A and group C patients in Japan have different ethnic backgrounds. However, it is difficult to address this question, because there is no obvious marker to identify ancestry in most of the Japanese. Modern Japanese populations have resulted from an intermingling of aboriginal people and immigrants from Korea or mainland China that started more than 2000 years ago.30Extensive screening in Asian countries using a rapid and readily facilitated assay to detect the specific mutation, such as the amplification refractory mutation system,27 may aid in identifying the origin of the mutation in Mongoloids.
It is likely that the IVS4 mutation derived from a founder and has expanded by a genetic drift in a small isolated population. However, there is no definite evidence for a founder effect in the Ashkenazi Jewish patients. In an attempt to search for a founder haplotype, we analyzed 4 microsatellite markers (D9S280, D9S1816, D9S1851, and D9S287) that are mapped within 1.8 cM around the FANCCgene31,32 but found no founder haplotype (data not shown). The Japanese IVS4 mutation is likely to have arisen independently of the Ashkenazi Jewish mutation. IVS4 carriers are detected in the Ashkenazi Jewish population but not in the Iraqi Jewish population, suggesting that the mutation in the Ashkenazi population arose after it separated from other Jewish populations as recently as 500 years ago.27 Thus, this mutation site appears to be a relative hot spot.
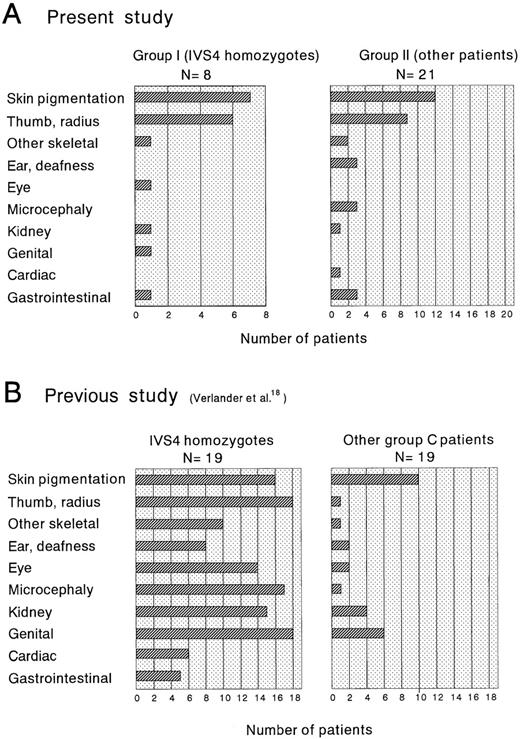
FA is characterized by a wide variety of clinical phenotypes.33 A clinical phenotype is associated, at least in part, with specific FANCC mutations within group-C FA patients.18,23 Patients with the IVS4 mutation or mutations in exon 14 (R548X or L554P) have a severe clinical phenotype, early onset of hematological abnormalities, multiple congenital anomalies, and short survival time, in comparison with patients with 322delG or non-C FA patients.18,23 On the other hand, our findings in the Japanese patients strongly suggest that the IVS4 mutation is not associated with a severe phenotype. The frequencies of congenital anomalies we noted were compared with results described by Verlander et al18 (Figure4). This figure clearly shows that frequencies of most major anomalies are much higher in Ashkenazi Jewish patients than in Japanese patients, whereas frequencies of skin pigmentation and thumb/radius anomalies are similar in the 2 ethnic groups. On the other hand, the frequencies of major anomalies, except for thumb/radius defects, are comparable between other FA patients (mostly non-C) in our study and patients with other FANCCmutations (mostly 322delG) in the study by Verlander et al.18 Therefore, the clinical phenotype of FA is affected by ethnic background even among patients with the same IVS4 mutation.
Frequencies of major anomalies in IVS4 homozygotes and other patients.
This compares (A) the present study and (B) a previous study.18 Part B was based on data shown in Table 3 in Verlander et al.18 However, patients with D195V and L554P were excluded because the pathogenic significance of D195V remains to be established16 and L554P was later shown to be associated with the severe clinical phenotype.23
Frequencies of major anomalies in IVS4 homozygotes and other patients.
This compares (A) the present study and (B) a previous study.18 Part B was based on data shown in Table 3 in Verlander et al.18 However, patients with D195V and L554P were excluded because the pathogenic significance of D195V remains to be established16 and L554P was later shown to be associated with the severe clinical phenotype.23
A possible interpretation of these results is that genetic or environmental factors protect against a severe phenotype in Japanese patients with IVS4. Conversely, a severe phenotype of Ashkenazi Jewish patients with IVS4 could be, at least in part, attributed to the ethnic background. Since patients with this mutation were confined to Ashkenazi Jews and the other patients in the previous studies were non-Ashkenazi Jews,17,18 23 it is difficult to determine whether a severe phenotype is due to the mutation, the ethnic background, or both.
There are many instances of identical mutations associated with phenotypic variation.34 This phenomenon was observed among unrelated individuals, within a family, and even in monozygotic twins. However, little has been documented regarding the phenotypic variation of genetic diseases among different ethnic groups. A similar event can be seen in case of Gaucher disease. Earlier reports demonstrated that a homozygous mutation resulting in an L444P transition in the glucocerebrosidase gene is closely associated with a neuronopathic form of Gaucher disease in the United States and a small population in Sweden.35,36 By contrast, homozygotes for this mutation are frequent in the nonneuronopathic form of patients in Japan.37 This discrepancy may be attributed to differences in ethnic backgrounds of the patients.
Several mechanisms may be involved in phenotypic variation among patients with an identical genotype: additional intragenic sequence alteration; modifier genes at other loci; genetic background in general, epigenetic mechanisms such as methylation and genomic imprinting; stochastic effects; influences of environment; and so forth.34 However, no specific molecular basis has been identified. To study cellular or molecular mechanisms for phenotypic variation of FA between the Ashkenazi Jewish population and the Japanese may elucidate the pathophysiologic basis of the clinical phenotype of FA.
Acknowledgments
The following institutions and investigators participated in this study: Ibaraki Children's Hospital (M. Tsuchida); Saitama Children's Hospital (R. Hanada); Chiba Children Hospital (Y. Okimoto, N. Kinugawa); Osaka University (J. Hara, Y. Nakanishi); Japan Red Cross Hiroshima Hospital and Atomic Bomb Survivors Hospital (K. Hamamoto); Hiroshima University (K. Ueda); Kyushu Cancer Center (S. Okamura); Kagoshima City Hospital (K. Muraoka). We are grateful to H. Joenje for providing the FA239 cell line, A. D. D'Andrea and J. Liu for helpful comments on the manuscript, and M. Ohara for language assistance.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and by grants from the Ministry of Health and Welfare of Japan.
Submitted May 25, 1999; accepted October 19, 1999.
Reprints:Takayuki Yamashita, Department of Hematology/Oncology, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639; Japan; e-mail:y-taka@ims.u-tokyo.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal