Antigen-induced eosinophil recruitment into the airways of sensitized mice is mediated by CD4+ T cells and their cytokines, especially IL-5. In this study, we found that the antigen-induced airway eosinophilia was diminished in Stat5a-deficient (Stat5a−/−) mice and Stat5b-deficient (Stat5b−/−) mice. We also found that antigen-induced CD4+ T-cell infiltration and IL-5 production in the airways were diminished in Stat5a−/− mice and Stat5b−/− mice. Moreover, antigen-induced proliferation of splenocytes was diminished in Stat5a−/− mice and Stat5b−/− mice, suggesting that the generation of antigen-primed T cells may be compromised in Stat5a−/−mice and Stat5b−/− mice and this defect may account for the diminished antigen-induced T-cell infiltration into the airways. Interestingly, IL-4 and IL-5 production from anti-CD3–stimulated splenocytes was diminished in Stat5a−/− mice and Stat5b−/− mice. However, antigen-specific IgE and IgG1 production was diminished in Stat5a−/− mice but not in Stat5b−/− mice, whereas antigen-specific IgG2a production was increased in Stat5a−/− mice, suggesting the enhanced Th1 responses in Stat5a−/− mice. Finally, we found that eosinophilopoiesis induced by the administration of recombinant IL-5 was also diminished in Stat5a−/− mice and Stat5b−/− mice. Together, these results indicate that both Stat5a and Stat5b are essential for induction of antigen-induced eosinophil recruitment into the airways and that the defects in antigen-induced eosinophil recruitment in Stat5a−/− mice and Stat5b−/− mice result from both impaired IL-5 production in the airways and diminished IL-5 responsiveness of eosinophils.
Allergic late-phase reactions provoked by specific antigens are associated with intense eosinophil infiltration into the site of antigen administration.1-3 In addition to the infiltration of eosinophils, there is an increase in CD4+ T cells and interleukin-5 (IL-5)–producing cells at the sites of late-phase reactions,4,5 suggesting that CD4+ T cells and IL-5 might be involved in antigen-induced eosinophil recruitment into the tissue. In a murine model of airway late-phase reaction, we and others have provided direct evidence that CD4+ T cells and IL-5 mediate antigen-induced eosinophil recruitment into the tissue of sensitized mice.6,7Conversely, endogenous IFN-γ production down-regulates antigen-induced eosinophil recruitment into the airways by inhibiting CD4+ T-cell infiltration.8 Taken together, these observations suggest that antigen-induced eosinophil recruitment into the airways is regulated by the balance between T helper 1 (Th1) and T helper 2 (Th2) cells, in which Th2 cells up-regulate but Th1 cells down-regulate the eosinophil recruitment. Although it is apparent that IL-5 plays an important role for antigen-induced eosinophil recruitment into the tissue, it remains unclear which signaling molecules mediate antigen-induced, IL-5–dependent eosinophil recruitment into the tissue.
IL-5 has been reported to activate a number of kinases, including Jak2, Lyn, Syk, and Raf-1, as well as the phosphatase, Shp2.9-12Among these molecules, Jak2 has been shown to be essential for eosinophil development13 and for the prevention of mature eosinophil apoptosis.10 In addition, administration of Jak2 inhibitor, AG490,14 prevents antigen-induced eosinophil recruitment into the airways (Kumano et al, in preparation), suggesting that Jak2 activation is required for IL-5–induced tissue eosinophilia. However, the interpretation of these experiments with AG490 must be evaluated with caution because AG490 inhibits Jak3 as well as Jak2.15
In the IL-5 signaling pathway, the most well-studied signaling molecules downstream of Jak2 are Stat5a and Stat5b, 2 highly related signal transducers and activators of transcription (STAT proteins).16-18 STAT proteins are cytosolic latent transcription factors that are rapidly activated after cellular exposure to interferons, cytokines, or growth factors.16-18Stat5 was originally identified as a mammary gland factor (MGF)–induced by prolactin.19 Subsequently, this protein was renamed Stat5a when a second, homologous gene, denoted Stat5b, was identified.18 Both Stat5a and Stat5b are activated not only by prolactin, but also by a very wide range of other cytokines, including IL-5.20 Although Stat5a and Stat5b are highly homologous, the specificities of their actions are demonstrated by the observations that Stat5a-deficient (Stat5a−/−) mice exhibit defective prolactin-related functions, with impaired lobuloalveolar outgrowth of mammary epithelium during pregnancy, resulting in defective lactation,21 whereas Stat5b-deficient (Stat5b−/−) mice exhibit defective growth similar to that found in Laron dwarfism.22In addition, Stat5a−/− and Stat5b−/− mice are also immunologically different each other. For example, basal as well as IL-2– and IL-15–mediated boosting of NK cytolytic activity is greatly diminished in Stat5b−/− mice but only partially diminished in Stat5a−/− mice.23 Moreover, although Stat5a−/− splenocytes exhibit only a partial defect in anti-CD3–induced proliferation that can be largely overcome by high-dose IL-2,24 defective proliferation in Stat5b−/− splenocytes cannot be corrected by this treatment.23 These different phenotypes underscore the distinctive roles of Stat5a and Stat5b. However, Stat5a and Stat5b may also have overlapping functions because Stat5a/Stat5b double-deficient mice exhibit a severe defect in T-cell proliferation.25
Although IL-5 is essential for antigen-induced eosinophil recruitment into the airways6,7 and IL-5 activates Stat5a and Stat5b,20 the role of Stat5a and Stat5b in allergic inflammation remains unclear. Therefore, we have now analyzed the allergic properties of Stat5a−/− and Stat5b−/− mice. Given the role of IL-5 in eosinophil development7,26,27 and allergic inflammation,6 7 we were particularly interested in analyzing whether the allergic inflammation is normally induced and whether IL-5 induces eosinophilopoiesis in Stat5a−/− mice and Stat5b−/− mice. We found that antigen-induced eosinophil and CD4+ T-cell recruitment was severely impaired in Stat5a−/− mice and Stat5b−/− mice. Furthermore, IL-5–induced eosinophilopoiesis was also impaired in Stat5a−/− mice and Stat5b−/− mice. The implications of these findings are discussed.
Materials and methods
Mice and genetic analysis
As the magnitude of antigen-induced eosinophil recruitment into the airways differs depending on a genetic background of mice (our unpublished data), Stat5a-deficient mice (Stat5a−/−, ref. 21) and Stat5b-deficient mice (Stat5b−/−, ref. 22) were back-crossed to BALB/c mice (Charles River Laboratories, Atsugi, Japan) for 4 generations. All mice were H-2d/d and littermate wild-type mice were used as a control. In preliminary experiments, we found that the littermate control wild-type mice exhibited indistinguishable responses to those seen with BALB/c mice in our assays; antigen-induced eosinophil and T-cell recruitment into the airways, titer of antigen-specific IgE antibody, and in vitro cytokine production and proliferation of splenocytes (data not shown). Mice were housed in microisolator cages under pathogen-free conditions. All experiments were performed according to the guidelines of Chiba University. The mice were genotyped by PCR of tail DNA, using the following primer pairs: To detect Stat5a wild-type gene, 5′-CTTTATTGATAACGATCTATCCCTCACCC-3′ and 5′-CTCCACTCTGCAGAGTCTATGGAATCC-3′; to detect Stat5a-deficient gene, 5′-GCTGACAGCCGGAACACGGCGG-3′ and 5′-GTGCAATCCATCTTGTTCAATGGCCG-3′; to detect Stat5b wild-type gene, 5′-CAGGAGGGATCCAGTGCCAGC-3′ and 5′-TGGCTCTACAGTGAGTTTGGT-3′; to detect Stat5b-deficient gene, 5′-GACTTGGAGATTGCCAACCCATATCTAAGT-3′ and 5′-TGAGCCGAAGGTGTAGTCGGAGTTTGCATT 3′.
Immunization
Mice (age 7-8 weeks) were immunized intraperitoneally twice with 4 μg of ovalbumin (OVA) (Sigma Chemical Co, St. Louis, MO) in 4 mg of aluminum hydroxide at a 2-week interval. Twelve to 14 days after the second immunization, the sensitized mice were challenged with aerosolized OVA as described below.
Antigen-induced eosinophil and T-cell infiltration in mouse airways
The eosinophil infiltration into the airways was induced by the inhalation of antigen in sensitized mice, and the number of eosinophils infiltrating into the submucosal tissue of trachea was evaluated as described previously.6 Briefly, the sensitized mice were given aerosolized OVA (50 mg/mL) dissolved in 0.9% saline by a DeVilbiss 646 nebulizer (DeVilbiss Corp, Somerset, PA) for 20 minutes. As a control, 0.9% saline alone was administered by the nebulizer. At 24 or 48 h after the inhalation, the tracheas were excised, fixed in 10% buffered-formalin, and embedded in paraffin. The specimens (3 μm thick) were stained with Luna solution and hematoxylin-eosin solution. The number of eosinophils in the submucosal tissue of trachea was counted in Luna-stained sections and expressed as the number of eosinophils per the length of the basement membrane of trachea, which was measured with a digital curvimeter.
The eosinophil and T-cell infiltration into the bronchoalveolar lavage fluid (BALF) was also evaluated as described previously.28In short, bronchoalveolar lavage was performed with 1.2 mL of phosphate-buffered saline (PBS) at 24 or 36 hours after saline or OVA inhalation. BALF was centrifuged at 400g for 5 minutes at 4°C, and cell differentials were determined by counting 500 cells stained with Wright-Giemsa solution. A fraction of the cells were subjected to a flow cytometric analysis for the lymphocyte surface phenotyping of CD4 and CD8, as described below.
IL-5 levels in BALF
The BALF was centrifuged at 400g for 5 minutes at 4°C, and the amount of IL-5 in the supernatant was measured by the enzyme immunoassay, according to the manufacturer's instruction (PharMingen, San Diego, CA). The assays were performed in duplicate.
Antigen-induced cytokine production and proliferation in splenocytes
The spleen was removed from OVA-sensitized mice and a single cell suspension of splenocytes was prepared. Splenocytes (2 × 105) were then suspended in 200 μL of RPMI 1640 medium supplemented with 10% FCS (GIBCO BRL, Rockville, MD), 10 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and were cultured in triplicate in the absence or presence of OVA (200 μg/mL) in a 96-well microtiter plate for 72 hours. In some experiments, splenocytes (2 × 105) were also cultured for 72 hours in a 96-well microtiter plate coated with 5 μg/mL of anti-CD3ε mAb (145-2C11, PharMingen). The culture supernatant was collected and the amounts of IL-4, IL-5, and IFN-γ were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (PharMingen). For proliferation assays, splenocytes (2 × 105) were cultured in the same conditions as described previously, with 1 μCi (0.037 MBq)3H] thymidine added for the final 16 hours.
Determination of antigen-specific IgE antibody in serum
Two weeks after the second immunization, the titer of OVA-specific IgE antibody in mouse serum was assessed by a 24-hour passive cutaneous anaphylaxis (PCA) reaction as described by Ovary.29
Determination of antigen-specific IgG1 and IgG2a antibodies in serum
The amount of OVA-specific IgG1 and IgG2a in serum was measured by ELISA as described elsewhere.30 In brief, ELISA plates were coated with OVA (250 μg/mL), washed 3 times with PBS containing 0.05% Tween20 (PBST), and blocked with blocking buffer (PBS containing 2% bovine serum albumin [BSA; Sigma Chemical Co]). Serum samples were added to the wells after 1:1000 or 1:3000 dilution in blocking buffer. As a control, serial dilution of pooled serum from OVA-sensitized wild-type mice were analyzed in each plate. After 1-hour incubation, wells were washed with PBST, added either biotinylated antimouse IgG1 (PharMingen) or biotinylated antimouse IgG2a (PharMingen) at 2 μg/mL in blocking buffer, and incubated for 1 hour. After washing, wells were incubated with 100 μL of ExtrAvidin alkaline phosphatase (1:2000 dilution, Sigma Chemical Co, St Louis, MO) for 45 minutes, washing with PBST, and the reaction was developed with pNPP (Sigma Chemical Co).
IL-5–induced eosinophilopoiesis
IL-5–induced eosinophilopoiesis was analyzed as described previously.26 In brief, mice were injected intraperitoneally with 20 000 U of recombinant murine IL-5 (rmIL-5)26 daily for 4 days. Forty-eight hours after the fourth administration of rmIL-5, mice were killed and cell differentials in bone marrow, peripheral blood, and peritoneal cavity were determined by counting 500 cells stained with Wright-Giemsa solution. A part of the cells in the peritoneal cavity were subjected to a flow cytometric analysis for the surface phenotyping of VLA-4 and Gr-1 as described below.
Flow cytometric analysis
Cells from the BALF and peritoneal cavity were stained and analyzed on a FACScaliber (Becton Dickinson, San Jose, CA) with CELLQuest software.24 For direct staining, the following conjugated antibodies were purchased from PharMingen: anti-CD4 FITC (H129.19), anti-CD8 APC, 53-6.7 anti-VLA-4 FITC (R1-2), and anti-Gr-1 APC (RB6-8C5). Before staining, Fc receptors were blocked with anti-CD16/32 antibody (2.4G2, PharMingen).
Data analysis
Data are summarized as mean ± SD. The statistical analysis of the results was performed by the unpaired t test. Pvalues < .05 were considered significant.
Results
Antigen-induced eosinophil recruitment into the airways is diminished in Stat5a−/− mice and Stat5b−/− mice
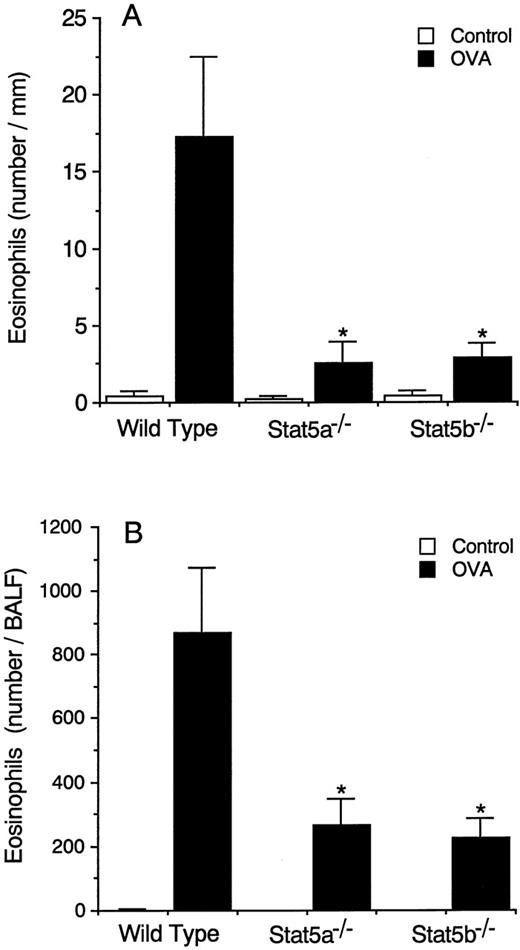
Because Stat5 is activated in response to IL-520 and IL-5 is known to be vital for antigen-induced eosinophil recruitment into the airways,6 we first determined whether antigen-induced eosinophil recruitment into the airways was normal in Stat5a-deficient (Stat5a−/−) mice and in Stat5b-deficient (Stat5b−/−) mice. As shown in Figure 1A, antigen-induced eosinophil infiltration into the trachea at 24 hours after antigen inhalation was severely diminished in both Stat5a−/− mice and Stat5b−/− mice by 85% and 84%, respectively (wild-type mice 17.2 ± 5.2, Stat5a−/−mice 2.5 ± 1.4, and Stat5b−/− mice 2.8 ± 1.0 eosinophils/mm, mean ± SD, n = 8 mice in each group, P < .001). Antigen-induced eosinophil infiltration into the trachea at 48 hours was also significantly diminished in Stat5a−/− mice and Stat5b−/− mice (data not shown). Consistent with diminished antigen-induced eosinophil infiltration in the trachea, the number of eosinophils recovered in BALF at 36 hours after antigen inhalation was also significantly decreased in Stat5a−/− mice and Stat5b−/− mice by 70% and 74%, respectively (n = 5, P < .005) (Figure 1B).
Antigen-induced eosinophil infiltration into the airways is diminished in both Stat5a−/− mice and Stat5b−/− mice.
(A) OVA-sensitized Stat5a−/− mice, Stat5b−/− mice, and littermate wild-type mice were challenged with the inhalation of OVA or saline (as control), and the number of eosinophils infiltrating into the submucosal tissue of trachea was evaluated at 24 hours after the inhalation. Data are means ± SD for 8 mice in each group. The mean values in Stat5a−/− mice and Stat5b−/− mice are significantly different from the mean value of wild-type mice, *P < .001. (B) Similar to A, the number of eosinophils in BALF was evaluated at 36 hours after the inhalation. Data are means ± SD for 5 mice in each group. *P < .005.
Antigen-induced eosinophil infiltration into the airways is diminished in both Stat5a−/− mice and Stat5b−/− mice.
(A) OVA-sensitized Stat5a−/− mice, Stat5b−/− mice, and littermate wild-type mice were challenged with the inhalation of OVA or saline (as control), and the number of eosinophils infiltrating into the submucosal tissue of trachea was evaluated at 24 hours after the inhalation. Data are means ± SD for 8 mice in each group. The mean values in Stat5a−/− mice and Stat5b−/− mice are significantly different from the mean value of wild-type mice, *P < .001. (B) Similar to A, the number of eosinophils in BALF was evaluated at 36 hours after the inhalation. Data are means ± SD for 5 mice in each group. *P < .005.
Antigen-induced T-cell recruitment into the airways is diminished in Stat5a−/− mice and Stat5b−/− mice
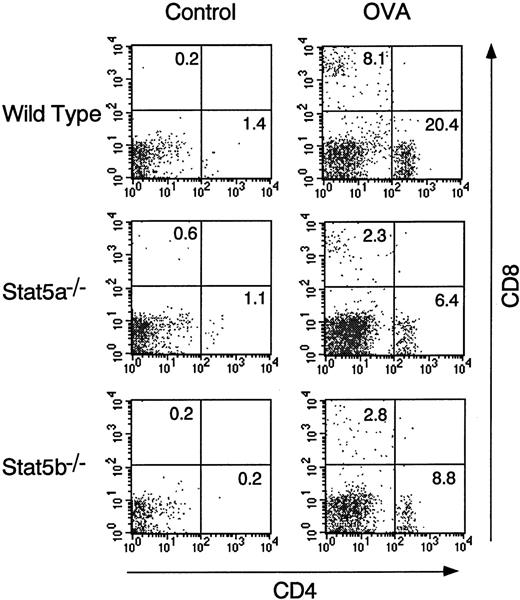
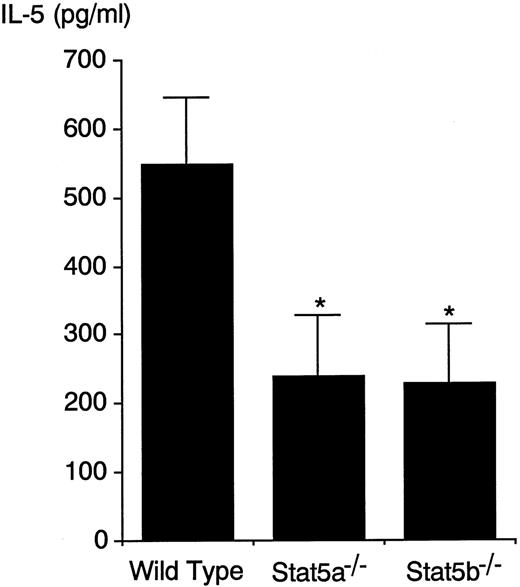
FACS analysis of BALF cells revealed that antigen-induced CD4+ T-cell infiltration into the airways at 24 hours was also decreased in both Stat5a−/− mice and Stat5b−/− mice by 64% and 61%, respectively (n = 5, P < .005) (Figure 2). In addition, consistent with diminished CD4+ T-cell infiltration in the airways, IL-5 levels in the BALF at 24 hours after antigen challenge were decreased in Stat5a−/−mice and Stat5b−/− mice by 57% and 59%, respectively (wild-type mice 549 ± 98, Stat5a−/− mice 239 ± 90, and Stat5b−/− mice 227 ± 86 pg/mL, n = 5,P < .005) (Figure 3). Taken together, these results suggest that both Stat5a and Stat5b are required for antigen-induced eosinophil and T-cell recruitment into the airways and that diminished antigen-induced eosinophil recruitment in Stat5a−/− mice and Stat5b−/− mice results in part from impaired IL-5 production in the airways.
Antigen-induced T-cell infiltration into the airways is diminished in Stat5a−/− mice and Stat5b−/− mice.
OVA-sensitized Stat5a−/− mice, Stat5b−/− mice, and littermate wild-type mice were challenged with OVA or saline as described in Figure 1. The T-cell infiltration in BALF was examined at 24 hours after OVA or saline inhalation. Shown are representative FACS profiles of CD4 versus CD8 staining on BALF cells using anti-CD4–FITC and anti-CD8–APC (n = 5 mice in each group).
Antigen-induced T-cell infiltration into the airways is diminished in Stat5a−/− mice and Stat5b−/− mice.
OVA-sensitized Stat5a−/− mice, Stat5b−/− mice, and littermate wild-type mice were challenged with OVA or saline as described in Figure 1. The T-cell infiltration in BALF was examined at 24 hours after OVA or saline inhalation. Shown are representative FACS profiles of CD4 versus CD8 staining on BALF cells using anti-CD4–FITC and anti-CD8–APC (n = 5 mice in each group).
Antigen-induced IL-5 production in BALF is decreased in Stat5a−/− mice and Stat5b−/− mice.
OVA-sensitized mice were challenged with inhaled OVA. At 24 hours after OVA inhalation, IL-5 levels in the BALF were determined by ELISA. Data are means ± SD for 5 mice in each group. IL-5 in the BALF was undetectable in mice that were challenged with inhaled saline (data not shown). *Significantly different from the mean value of wild-type mice, *P < .005.
Antigen-induced IL-5 production in BALF is decreased in Stat5a−/− mice and Stat5b−/− mice.
OVA-sensitized mice were challenged with inhaled OVA. At 24 hours after OVA inhalation, IL-5 levels in the BALF were determined by ELISA. Data are means ± SD for 5 mice in each group. IL-5 in the BALF was undetectable in mice that were challenged with inhaled saline (data not shown). *Significantly different from the mean value of wild-type mice, *P < .005.
Antigen-induced IL-4 and IL-5 production is decreased in Stat5a−/− mice and Stat5b−/− mice
We then examined the Th1 cell and Th2 cell development in Stat5a−/− mice and Stat5b−/− mice. As shown in Table1, IL-4 and IL-5 production from antigen (OVA)-stimulated splenocytes was undetectable in Stat5a−/− mice and Stat5b−/− mice, whereas antigen-stimulated wild-type splenocytes produced substantial amounts of IL-4 and IL-5 (n = 5, P < .01). Similarly, IFN-γ production from antigen-stimulated splenocytes was undetectable in Stat5a−/− mice and Stat5b−/− mice but was readily detected in antigen-stimulated wild-type splenocytes (n = 5, P < .01) (Table 1).
Cytokine production from OVA- or anti-CD3–stimulated splenocytes in Stat5a−/− mice and Stat5b−/− mice
| Cytokines . | Medium . | OVA . | anti-CD3 . |
|---|---|---|---|
| IL-4 (pg/mL) | |||
| Wild type | ND | 95.0 ± 37.0 | 2990.0 ± 598.3 |
| Stat5a−/− | ND | ND* | 328.0 ± 281.01-160 |
| Stat5b−/− | ND | ND* | 317.5 ± 173.51-160 |
| IL-5 (pg/mL) | |||
| Wild type | ND | 262.5 ± 68.7 | 4303.0 ± 1245.5 |
| Stat5a−/− | ND | ND* | 935.0 ± 535.51-160 |
| Stat5b−/− | ND | ND* | 931.4 ± 545.51-160 |
| IFN-γ (ng/mL) | |||
| Wild type | ND | 11.3 ± 4.8 | 401.6 ± 49.2 |
| Stat5a−/− | ND | ND* | 335.5 ± 91.4 |
| Stat5b−/− | ND | ND* | 236.5 ± 90.0* |
| Cytokines . | Medium . | OVA . | anti-CD3 . |
|---|---|---|---|
| IL-4 (pg/mL) | |||
| Wild type | ND | 95.0 ± 37.0 | 2990.0 ± 598.3 |
| Stat5a−/− | ND | ND* | 328.0 ± 281.01-160 |
| Stat5b−/− | ND | ND* | 317.5 ± 173.51-160 |
| IL-5 (pg/mL) | |||
| Wild type | ND | 262.5 ± 68.7 | 4303.0 ± 1245.5 |
| Stat5a−/− | ND | ND* | 935.0 ± 535.51-160 |
| Stat5b−/− | ND | ND* | 931.4 ± 545.51-160 |
| IFN-γ (ng/mL) | |||
| Wild type | ND | 11.3 ± 4.8 | 401.6 ± 49.2 |
| Stat5a−/− | ND | ND* | 335.5 ± 91.4 |
| Stat5b−/− | ND | ND* | 236.5 ± 90.0* |
Splenocytes from OVA-sensitized mice were stimulated with OVA (200 μg/mL) or plate-coated anti-CD3 mAb for 72 h, and the amount of IL-4, IL-5, and IFN-γ in the culture supernatant was determined by ELISA. Data are means ± SD for 5 mice in each group. The minimum significant values of these assays were 20 pg/mL of IL-4 and IL-5, and 0.1 ng/mL of IFN-γ. ND = not detectable.
P < .01,
significantly different from the mean value of wild-type mice,
P < .001.
Although similar numbers of T cells were found in splenocytes from wild-type mice, Stat5a−/− mice, and Stat5b−/− mice (data not shown), it was possible that antigen-specific T cells were decreased in Stat5a−/− mice and Stat5b−/− mice. To determine whether the defects in cytokine production from antigen-stimulated splenocytes in Stat5a−/− mice and Stat5b−/− mice resulted from the impaired generation of an antigen-specific T-cell pool, we analyzed cytokine production from anti-CD3–stimulated splenocytes. IL-4 production from anti-CD3–stimulated splenocytes was approximately 90% reduced in both Stat5a−/− mice and Stat5b−/− mice, compared with IL-4 production in wild-type mice (n = 5, P < .001) (Table 1). IL-5 production from anti-CD3–stimulated splenocytes was also similarly decreased in Stat5a−/− mice and Stat5b−/− mice by approximately 80% (n = 5,P < .001) (Table 1). In contrast, IFN-γ production from anti-CD3–stimulated splenocytes was normal in Stat5a−/− mice and but moderately diminished in Stat5b−/− mice by 41% (n = 5,P < .01) (Table 1).
Antigen-induced T-cell proliferation is decreased in Stat5a−/− mice and Stat5b−/− mice
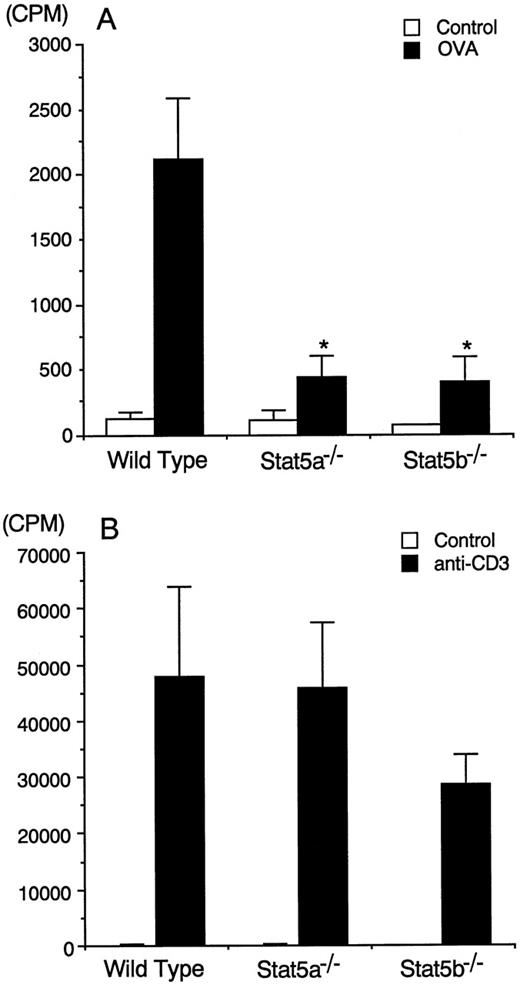
Given the importance of IL-2 in T-cell proliferation31and the ability of IL-2 to potently activate Stat5a and Stat5b,32-34 we next examined whether antigen-induced proliferation of splenocytes was normal in these mice. Antigen-induced proliferation of splenocytes was substantially observed in wild-type mice, whereas antigen-induced proliferation of splenocytes was significantly decreased in Stat5a−/− mice and Stat5b−/− mice by 79% and 81%, respectively (n = 5, P < .005) (Figure4A). This defect may account for the diminished antigen-induced T-cell infiltration into the airways (Figure2). In contrast to the diminished antigen-induced proliferation, anti-CD3–induced proliferation of splenocytes was normal in Stat5a−/− mice and slightly decreased (but not statistically significant) in Stat5b−/− mice (n = 5) (Figure 4B). These results suggest that Stat5a and Stat5b might be required for the generation of antigen-specific T-cell pools in the periphery.
Diminished antigen-induced proliferation of splenocytes in Stat5a−/− mice and Stat5b−/− mice.
(A) Splenocytes from OVA-sensitized mice were cultured in the presence or absence of 200 μg/mL of OVA for 72 hours. Data are means ± SD for 5 mice in each group. *Significantly different from the mean value of wild-type mice, *P < .005. (B) Splenocytes from OVA-sensitized mice were stimulated with or without plate-coated anti-CD3 mAb for 72 hours. Data are means ± SD for 5 mice in each group.
Diminished antigen-induced proliferation of splenocytes in Stat5a−/− mice and Stat5b−/− mice.
(A) Splenocytes from OVA-sensitized mice were cultured in the presence or absence of 200 μg/mL of OVA for 72 hours. Data are means ± SD for 5 mice in each group. *Significantly different from the mean value of wild-type mice, *P < .005. (B) Splenocytes from OVA-sensitized mice were stimulated with or without plate-coated anti-CD3 mAb for 72 hours. Data are means ± SD for 5 mice in each group.
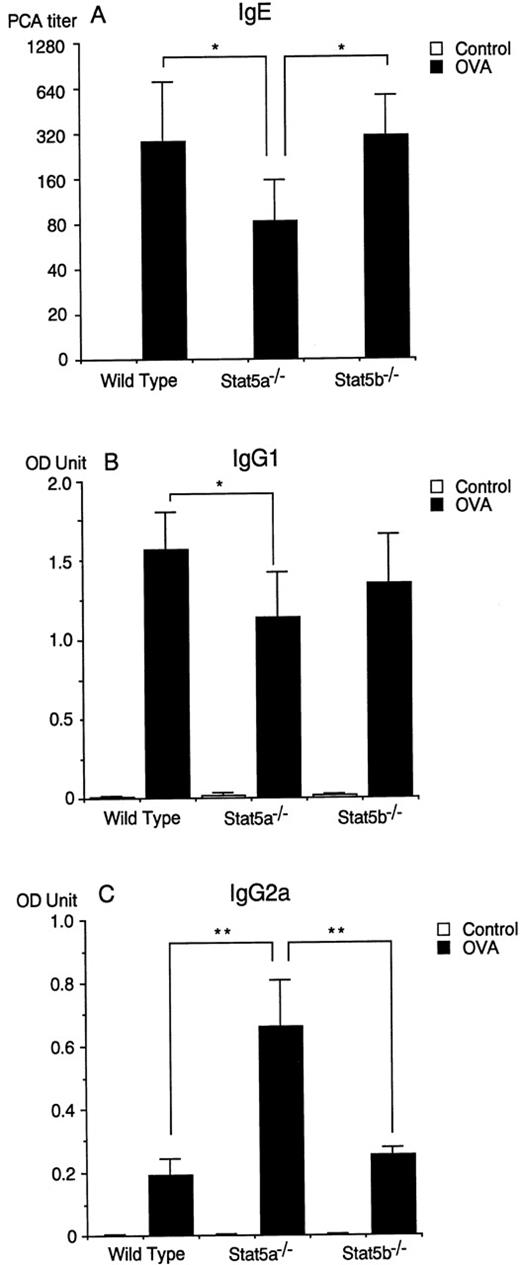
Antigen-specific IgE production is diminished in Stat5a−/− mice but not in Stat5b−/− mice.
It has been shown that antigen-specific IgE production is a marker of systemic Th2 responses,35 but it is not essential for antigen-induced airway eosinophilia in mice36 37 (and our unpublished data). Therefore, we examined antigen-specific IgE production in Stat5a−/− mice and Stat5b−/− mice at 2 weeks after the second immunization. Interestingly, Stat5a−/− mice exhibited decreased levels of antigen-specific IgE production (n = 10, P < .005), whereas antigen-specific IgE production was normal in Stat5b−/− mice (Figure5A). In addition, antigen-specific IgG1 production was also diminished in Stat5a−/−mice (n = 10, P < .01) (Figure 5B), suggesting that systemic Th2 response was diminished in Stat5a−/− mice but not in Stat5b−/− mice. In contrast to the diminished IgE and IgG1 production in Stat5a−/− mice, antigen-specific IgG2a production was increased in Stat5a−/− mice (n = 10, P < .005) (Figure 5C), suggesting the Th1-polarized immune responses in Stat5a−/− mice.
Antigen-specific IgE production is diminished in Stat5a−/− mice but not in Stat5b−/−mice.
(A) The titer of anti-OVA IgE antibody in sera was assessed at 2 weeks after the second immunization by a 24-hour passive cutaneous anaphylaxis (PCA) reaction. Data are means ± SD for 10 mice in each group. *P < .005. (B-C) Anti-OVA IgG1 (B) and IgG2a (C) antibodies in sera were assessed at 2 weeks after the second immunization by ELISA as described in “Materials and Methods.” Data are means ± SD for 10 mice in each group. *P < .01, **P < .005.
Antigen-specific IgE production is diminished in Stat5a−/− mice but not in Stat5b−/−mice.
(A) The titer of anti-OVA IgE antibody in sera was assessed at 2 weeks after the second immunization by a 24-hour passive cutaneous anaphylaxis (PCA) reaction. Data are means ± SD for 10 mice in each group. *P < .005. (B-C) Anti-OVA IgG1 (B) and IgG2a (C) antibodies in sera were assessed at 2 weeks after the second immunization by ELISA as described in “Materials and Methods.” Data are means ± SD for 10 mice in each group. *P < .01, **P < .005.
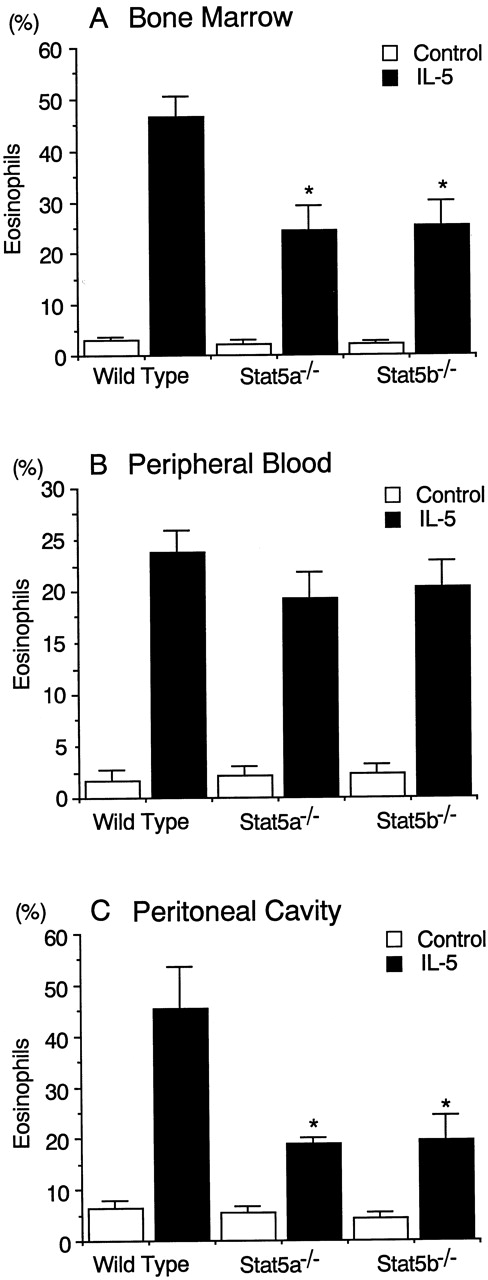
IL-5-induced eosinophilopoiesis is diminished in Stat5a−/− mice and Stat5b−/− mice.
Although the diminished antigen-induced eosinophil recruitment into the airways in Stat5a−/− mice and Stat5b−/− mice (Figure 1) presumably results in part from diminished IL-5 production in the airways (Figure 3), the defect in eosinophil recruitment into the airways appeared more severe than the defect in IL-5 production. We therefore investigated whether IL-5-dependent eosinophilopoiesis was normal in Stat5a−/− mice and Stat5b−/− mice. To address this issue, Stat5a−/− mice and Stat5b−/− mice were injected intraperitoneally with recombinant murine IL-5 (rmIL-5; 20 000 U/d) for 4 days and the eosinophil numbers in the bone marrow, peripheral blood, and peritoneal cavity were determined at 48 hours after the last injection. The administration of rmIL-5 significantly increased eosinophil numbers in the bone marrow in wild-type mice (n = 5) (Figure6A). In Stat5a−/−mice and Stat5b−/− mice; however, the magnitude of the IL-5-induced eosinophilia in the bone marrow was approximately half of that in rmIL-5–treated wild-type mice (n = 5,P < .005) (Figure 6A). These results indicate that both Stat5a and Stat5b play a role in IL-5-dependent eosinophilopoiesis.
IL-5–induced eosinophilopoiesis is diminished in Stat5a−/− mice and Stat5b−/− mice.
Stat5a−/− mice, Stat5b−/− mice, and wild-type mice were injected intraperitoneally daily for 4 days with 20 000 U of recombinant murine IL-5. Forty-eight hours after the last injection, mice were killed and the numbers of eosinophils in bone marrow (A), peripheral blood (B), and peritoneal cavity (C) were evaluated. Data are means ± SD for 5 mice in each group. *Significantly different from the mean value of wild-type mice, *P < .005.
IL-5–induced eosinophilopoiesis is diminished in Stat5a−/− mice and Stat5b−/− mice.
Stat5a−/− mice, Stat5b−/− mice, and wild-type mice were injected intraperitoneally daily for 4 days with 20 000 U of recombinant murine IL-5. Forty-eight hours after the last injection, mice were killed and the numbers of eosinophils in bone marrow (A), peripheral blood (B), and peritoneal cavity (C) were evaluated. Data are means ± SD for 5 mice in each group. *Significantly different from the mean value of wild-type mice, *P < .005.
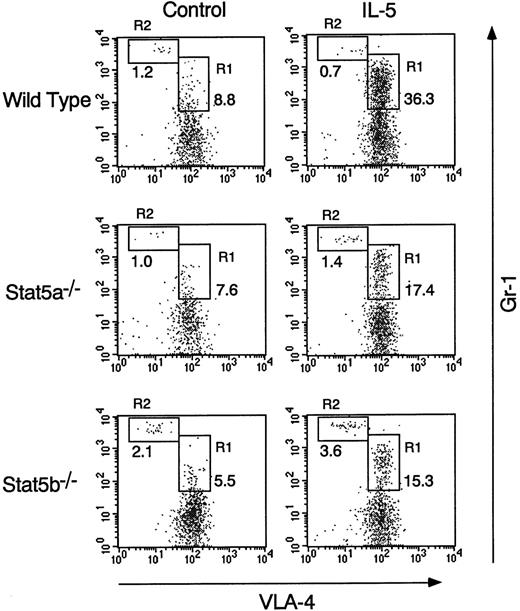
The eosinophil numbers in the peripheral blood and the peritoneal cavity were also significantly increased by the intraperitoneal injection of rmIL-5 in wild-type mice (Figure 6B and C). Although eosinophil numbers in the peripheral blood were similarly increased by the administration of rmIL-5 among the 3 groups of mice (Figure 6B), however, the eosinophil numbers in the peritoneal cavity were significantly decreased in Stat5a−/− mice and Stat5b−/− mice by 59% and 57%, respectively (n = 5, P < .005) (Figure 6C). Consistent with increased eosinophils in the peritoneal cavity in rmIL-5–treated wild-type mice, FACS analysis revealed that the Gr-1mid VLA-4+population (R1 region), which most likely represents eosinophils,38 was selectively increased in the peritoneal cavity after rmIL-5 administration (Figure7). In contrast, the Gr-1highVLA-4− population (R2 region), which most likely represents neutrophils,38 was not increased in the peritoneal cavity on rmIL-5 administration (Figure 7). Consistent with the diminished eosinophils in the peritoneal cavity of rmIL-5–treated Stat5a−/− mice or Stat5b−/− mice, the fraction of Gr-1mid VLA-4+ cells in the peritoneal cavity was also diminished in Stat5a−/− and Stat5b−/− mice (Figure 7). These results suggest that, in addition to the role in IL-5–induced eosinophilopoiesis, Stat5a and Stat5b may be necessary for IL-5–induced chemotaxis of eosinophils.
Gr-1mid VLA-4+ population is diminished in IL-5–treated Stat5a−/− mice and Stat5b−/− mice.
Mice were injected intraperitoneally with rmIL-5 as described in Figure6. Forty-eight hours after the last injection, mice were killed and cells in the peritoneal cavity were stained with anti-VLA-4 FITC and anti-Gr-1 APC. Shown are representative FACS profiles from 5 mice in each group.
Gr-1mid VLA-4+ population is diminished in IL-5–treated Stat5a−/− mice and Stat5b−/− mice.
Mice were injected intraperitoneally with rmIL-5 as described in Figure6. Forty-eight hours after the last injection, mice were killed and cells in the peritoneal cavity were stained with anti-VLA-4 FITC and anti-Gr-1 APC. Shown are representative FACS profiles from 5 mice in each group.
Discussion
It has been shown that antigen-induced eosinophil recruitment into the airways of sensitized mice is mediated by the Th2 subset of CD4+ T cells and subsequent secretion of IL-5.6 In this study, we showed that both Stat5a and Stat5b were required for antigen-induced eosinophil and T-cell recruitment into the airways. We found that antigen-induced eosinophil infiltration in the airways and antigen-induced CD4+ T-cell infiltration and IL-5 production in the airways were diminished in both Stat5a−/− mice and Stat5b−/− mice. Moreover, we found that antigen-induced proliferation of splenocytes was diminished in Stat5a−/− mice and Stat5b−/− mice, suggesting that the generation of antigen-primed T cells might be compromised in Stat5a−/− mice and Stat5b−/− mice. This defect may be due to diminished IL-2–induced proliferation of T cells in Stat5a−/− mice24 and Stat5b−/− mice23 and may account for the diminished antigen-induced T-cell infiltration into the airways. Finally, we found that IL-5–dependent eosinophilopoiesis was impaired in Stat5a−/− mice and Stat5b−/− mice. Therefore, these results indicate that the diminished antigen-induced eosinophil recruitment into the airways in Stat5a−/− mice and Stat5b−/− mice results from both impaired IL-5 production in the airways and impaired IL-5 responsiveness of eosinophils.
Given that Stat5a and Stat5b are highly homologous and exhibit overlapping functions,18,25 it was surprising that antigen-induced eosinophil and T-cell recruitment into the airways was severely impaired in mice lacking either Stat5a or Stat5b. Because Stat5b DNA binding activity is not dependent on the presence of Stat5a,24 it is possible that the residual eosinophil recruitment in Stat5a−/− mice is regulated by intact Stat5b. However, it is also possible that the residual eosinophil recruitment in Stat5a−/− mice or Stat5b−/− mice is regulated by Stat5-independent signaling pathway(s). This can be further assessed when Stat5a/Stat5b double-deficient mice in the BALB/c background are available. Nevertheless, it may be predicted that antigen-induced eosinophil recruitment into the airways, which is regulated by CD4+ T cells,6 is greatly diminished in Stat5a/Stat5b double-deficient mice, because TCR-mediated proliferation of CD4+ T cells from Stat5a/Stat5b double-deficient mice is defective.25
We also found that IL-5-induced in vivo eosinophilopoiesis was diminished in Stat5a−/− mice and Stat5b−/− mice. IL-5 acts on eosinophil precursors, resulting in induction of proliferation and differentiation into mature eosinophils.39 It has been reported that bone marrow cells from IL-5Rα−/−mice26 or IL-5−/−mice7,27 fail to form eosinophilic colonies in response to IL-5 or parasite infection. GM-CSF also plays an important role in the production of eosinophils in bone marrow.40IL-5, GM-CSF, and IL-3 all share common β chain (βc) as a receptor component41 and activate Jak2 and Stat5a/Stat5b.16-18,20 Recently, Feldman et al42 demonstrated that GM-CSF-induced proliferation of bone marrow-derived macrophages is diminished in Stat5a−/− mice, suggesting that Stat5a plays a role in βc-dependent proliferation. In contrast, it has been reported that in vitro IL-5–induced colony formation from bone marrow cells is normal in Stat5a−/− mice and Stat5b−/− mice.43 At present, the precise mechanism(s) underlying the discrepancy between the in vivo and the in vitro requirement for Stat5a/Stat5b in IL-5–induced eosinophilopoiesis is unclear. However, it is likely that the compensatory role of Stat5a by Stat5b or Stat5b by Stat5a is not sufficient for in vivo IL-5–induced eosinophilopoiesis, because IL-5–dependent in vitro colony formation is diminished in Stat5a/Stat5b double-deficient mice.43 In addition, our finding of diminished eosinophil numbers in the peritoneal cavity after IL-5 administration in Stat5a−/− mice and Stat5b−/− mice suggest that Stat5a and Stat5b may also be necessary for IL-5–induced chemotaxis of eosinophils.44
Interestingly, we found that IL-4 production from anti-CD3–stimulated splenocytes was diminished in Stat5a−/− mice. We also found that antigen-specific IgE and IgG1 production was diminished in Stat5a−/− mice, whereas antigen-specific IgG2a production was increased in Stat5a−/− mice. Therefore, in Stat5a−/− mice, the balance between Th1 and Th2 cell activation is biased toward a Th1 profile, suggesting that Stat5a may be involved in the IL-4–dependent Th2 cell development. Consistent with this finding, it has recently been shown that IL-4 signaling activates Stat5 as well as Stat6.45 In contrast, in Stat5b−/− mice, the situation may be more complicated. In these mice, although anti-CD3–induced IL-4 production from splenocytes was diminished, antigen-specific IgE production in vivo was normal. Because anti-CD3–induced IFN-γ production was also slightly diminished in Stat5b−/− mice, it is possible that the balance between IL-4 and IFN-γ rather than the amount of IL-4 may be critical for in vivo IgE production. Alternatively, it is conceivable that the discrepancy may result from compensatory role(s) of other cytokines such as IL-13.46
As discussed previously, diminished antigen-induced eosinophil recruitment into the airways is likely to result from both impaired IL-5 production by T cells and impaired IL-5 responsiveness of eosinophils in Stat5a−/− mice and Stat5b−/− mice. However, other cell populations may also be involved in the diminished antigen-induced eosinophil recruitment into the airways. Recently, it has been shown that γδ T cells47 and NK cells48,49 play important role(s) in allergic inflammation. Because γδ T cells are diminished in Stat5a−/− mice24 and NK cell function is particularly impaired in Stat5b−/−mice,23 these defects may be responsible for the diminished antigen-induced eosinophil recruitment into the airways.
In this study, we provide the evidence that Stat5a and Stat5b are important for allergic inflammation. Recently, several lines of evidence have indicated that other STAT proteins besides Stat5 also play important immunopathogenic roles in the development of allergic inflammation. Stat6 has been shown to play an important role in causing allergic inflammation,50,51 probably by generating antigen-specific Th2 cells.52-54 These observations may be consistent with the finding that IL-13, which activates Stat6,46 plays a role in allergic inflammation.55,56 In contrast, because Stat4 is activated by IL-12 and is essential for Th1 cell differentiation57,58and because IL-12 is shown to exhibit inhibitory effects on allergic inflammation,28,59 Stat4 may play a role in prevention of allergic inflammation. More recently, Sampath et al60demonstrated that Stat1 was activated in epithelial cells in patients with asthma, correlating with ICAM-1 expression and T-cell infiltration. Although the target genes of these STAT proteins for allergic inflammation remain unclear, more detailed mechanism(s) may be revealed using these mutant mice.
In conclusion, from the analysis of Stat5a−/−mice and Stat5b−/− mice, we have established several important points. First, both Stat5a and Stat5b are vital for antigen-induced T-cell infiltration and IL-5 production in the airways and thereby play an important role in causing antigen-induced eosinophil recruitment into the airways. Second, our data provide in vivo evidence that both Stat5a and Stat5b are required for IL-5–induced eosinophilopoiesis. Third, although the precise mechanism(s) is at present unclear, Stat5a is important for the determination of Th1/Th2 balance on antigen sensitization. Finally, our results suggest that specific inactivation of Stat5 proteins in the tissue could potentially represent a basis for the treatment of allergic inflammation.
Acknowledgments
We thank Drs L. Hennighausen and X. Liu for Stat5a-deficient mice and K. Kurasawa, M. Nishimura, T. Sato, K. Hirano, T. Matsui, and M. Watanabe for technical help.
Supported in part by grants from the Ministry of Education, Science and Culture, Japan, and from ASTRA Japan Co.
S-i.K. and H.N. contributed equally to this work.
Reprints:Dr Hiroshi Nakajima, Department of Internal Medicine II, Chiba University School of Medicine, 1-8-1 Inohana, Chiba City, Chiba 260-8670, Japan; e-mail:nakajimh@intmed02.m.chiba-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal