Because of their hypervariable regions and somatic mutations, the antigen receptor molecules of lymphomas (idiotypes) are tumor-specific antigens and attractive targets for antilymphoma immunotherapy. For the optimal induction of human idiotype-specific cytotoxic T cells (CTL), idiotype was presented to CD8+ peripheral blood mononuclear cells by monocyte-derived autologous dendritic cells (DC) after the endocytosis of idiotype protein or by idiotype-expressing DC. Recombinant idiotype was obtained as a functionally folded Fab fragment by periplasmic expression in Escherichia coli. Idiotype-expressing DC were generated by transduction with recombinant Semliki forest virus vectors encompassing heavy- or light-chain idiotype genes. Autologous lymphoblastoid cell lines stably transfected with Epstein-Barr virus-based idiotype expression vectors were used as target cells to detect idiotype-specific lysis. CTL stimulated with idiotype-loaded DC showed strong specific, CD8-mediated, and major histocompatibility complex (MHC) class I-restricted cytotoxicity against autologous heavy- and light-chain idiotype. In contrast, stimulation with idiotype-transduced DC resulted in only moderate natural killer cell activity. These data confirm the existence of idiotype-specific CTL in patients with lymphoma, define a “good manufacturing practice”-compatible protocol for the generation of these cells without the requirement of viable lymphoma cells, and favor the processing of exogenous antigen over DC transduction for the induction of MHC I-restricted CTL against idiotypes with unknown antigenicity.
During the past decade, dendritic cells (DC) have been identified as the most potent antigen-presenting cells of the immune system.1,2 Their particular efficiency to induce specific immune responses is based on several specialized functions. Immature DC, also referred to as Langerhans-type DC (LC), have a high capacity for antigen uptake and processing.3,4 Processed oligopeptides of protein antigens are efficiently loaded onto major histocompatibility complex (MHC) class II molecules through an endosomal pathway and onto MHC class I molecules by a less understood mechanism known as cross-presentation.2,5 After antigen uptake, DC migrate to regional lymph nodes and undergo terminal maturation. Consequently, they acquire an outstanding capacity to induce T cells specific for the antigens presented by DC. The expression of human leukocyte antigen (HLA) molecules and costimulatory molecules on the surface of DC is up-regulated, and T-cell stimulatory cytokines, predominantly IL-12, are released.1 2
After their immunostimulatory potential became recognized, DC have attracted interest as a therapeutic tool to activate the immune system against antigens that usually fail to elicit sufficiently strong immunity for protection of the host organism. This applies in particular to tumor-associated antigens (TAA), to which potentially reactive T cells may be infrequent6 or may be in an anergic state.7,8 Laboratory techniques to obtain DC in sufficient quality and quantity from monocytes9,10 or hematopoietic progenitor cells11,12 have been developed and have facilitated clinical trials for tumor vaccination. Indeed, objective tumor regression has been reported after DC-based immunization against the immunoglobulin expressed by malignant lymphomas13 and against melanoma-associated antigens.14
Although it is widely accepted that TAA-specific cytotoxic T cells (CTL) are the most important immune effector cells against malignant melanoma,15,16 the main effector mechanism against lymphoma cells has been more difficult to identify. Hypervariable sequences and somatic mutations of the lymphoma immunoglobulin variable regions result in individual tumor-specific antigens. Therefore, the antigen receptor of a given lymphoma, also referred to as an idiotype, represents a likely target structure for antitumor immunity. Tailor-made anti-idiotypic monoclonal antibodies alone can induce tumor remissions in vivo.17 In addition, lymphoma-specific CTL have been detected in patients.18,19 After the anti-idiotype vaccination of patients with malignant lymphoma, humoral and T-cell responses have been described.20-22 In animal models, the main effector mechanism appears to vary depending on the vaccination protocol and on the tumor model used.23-26
As a prerequiste to define further the role of idiotype-specific CTL in antilymphoma immunity, we performed a series of experiments to determine the efficiency of DC in inducing human idiotype-specific T cells in vitro. In addition to providing further evidence for the existence of these effector cells in patients pretreated for lymphoma, we sought to define optimal conditions for the induction of idiotype-specific CTL and to develop a protocol for the generation of these cells for adoptive transfer treatment strategies that could meet “good manufacturing practice” (GMP) criteria.
Methods
Patient samples
Frozen lysates from nonsterile, single-cell suspensions prepared from biopsy specimens with histologically and immunophenotypically confirmed infiltrations of malignant lymphoma cells were obtained from the diagnostic immunophenotyping laboratory of our institution and stored at −70°C. Patients gave their informed consent for the use of their cryopreserved tumor cells and for the acquisition of additional heparinized blood samples from routinely performed phlebotomies during complete clinical remission. The experiments were approved by the local ethics committee.
Patient 1 was a 43-year-old man diagnosed with a nonsecreting polymorphic immunocytoma, stage IIIB. The surface immunoglobulin of the tumor cells was IgMκ. The patient was treated successfully by polychemotherapy, including high-dose BEAM chemotherapy with autologous peripheral stem cell transplantation. He was in clinical complete remission for 11 months when the first peripheral blood samples were taken. His HLA type was A 0201, B 07, C 07 as determined by DNA typing.
Patient 2 was a 57-year-old woman with IgMκ-positive follicular lymphoma, stage IIA, involving the cervical and submandibular lymph nodes. She underwent extended-field radiotherapy (40 Gy) with curative intent and was in complete remission for 6 months when the first blood samples were obtained. Her HLA type was A 0205/31, B 40/49, C 02/07.
Production of lymphoma-derived idiotype protein as recombinant Fab fragment
Total RNA was isolated from lymphoma cells by centrifugation-driven chromatography through a silica matrix (RNeasy columns; Qiagen, Hilden, Germany) and converted to cDNA with RNaseH-deficient reverse transcriptase (Gibco BRL, Paisley, UK) and an oligo-dT primer. cDNA of more than 400 bp was purified by spun-driven chromatography through Sepharose CL-4B (Pharmacia, Uppsala, Sweden) and tailed at the 3′ end with dGTP by terminal transferase (Boehringer Mannheim, Mannheim, Germany). Immunoglobulin transcripts were amplified by polymerase chain reaction (PCR) with recombinant Taq polymerase (Perkin Elmer, Norwalk, CT) using nested μ or κ constant region-specific primers and an oligo-dC-containing anchor primer.27 PCR products were cloned into a TA-PCR cloning vector (Invitrogen, Carlsbad, CA) and sequenced with an automated DNA sequencer (model 373; Applied Biosystems, Foster City, CA).
Clonally rearranged, bona fide lymphoma-derived heavy- and light-chain variable region sequences were cloned into the dicistronic periplasmic expression pFAB.γκ27 after reamplification with primers adding the appropriate restriction sites and Vent polymerase (New England Biolabs, Beverly, MA). Escherichia coli JM83 were transformed with the resultant individual Fab expression vectors, grown to an OD550nm of 0.5, and induced with 0.2 mg/L anhydrotetracycline (Fisher Scientific, Nidderau, Germany) for 3 hours. The periplasmic cell fraction was isolated by lysis with 100 mmol/L Tris-HCl (pH 8)/0.5 mol/L sucrose/1 mmol/L EDTA, followed by centrifugation for 10 minutes at 4000g. After dialysis overnight against 0.5 mol/L betaine monohydrate (Fluka, Deisenhofen, Germany)/50 mmol/L Na phosphate (pH 7.5), recombinant Fab fragments were purified from these periplasmic cell fractions by conventional affinity chromatography on a Zn2+ column (chelating Sepharose fast flow; Pharmacia).28 29 The purity and integrity of the Fab fragments were assessed by standard 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. Endotoxin contamination of purified recombinant proteins was measured with a limulus amebocyte assay (BioWhittaker, Walkersville, MD).
Generation of idiotype-expressing lymphoblastoid cell lines
Peripheral blood mononuclear cells (107; PBMC) were resuspended in 2 mL Epstein-Barr virus (EBV)-containing supernatant (provided by Fisch P). After a 2-hour incubation at 37°C, 4 mL RPMI 1640 supplemented with 10% fetal calf serum (PAA Laboratories, Linz, Austria), 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, MEM vitamins 1:200, MEM nonessential amino acids 1:100, 0.1 μmol/L 2-mercaptoethanol, 50 U/mL penicillin, 50 μg/mL streptomycin (all Gibco BRL), and 1 μg/mL cyclosporin A (Novartis, Basel, Switzerland) were added. Fresh medium without cyclosporine was added once a week until outgrowth of EBV-transformed permanent cell lines occurred.
To obtain EBV-replicon-based idiotype expression vectors, individual clonally rearranged IgL genes and heavy-chain Fd segments (ie, the heavy-chain variable region with the first constant domain) were amplified from the respective pFab.γκ clones with Ventpolymerase. In addition to sequences complementary to the cDNA, the 5′ (forward) primers contained an SfiI restriction site, a Kozak consensus sequence, and an ATG start codon (Table1), whereas the 3′ (reverse) primers incorporated the necessary codons for a hexahistidine tag, followed by 2 stop codons and a BamHI site (Table 1). The PCR products were inserted into the SfiI/BamHI sites of pCEP4 (Invitrogen). With an electric discharge of 250 V and 960 μF in 0.4-cm cuvettes (Bio-Rad, Hercules, CA), 2 × 107vigorously proliferating lymphoblastoid cell line (LCL) cells were electroporated with 20 μg pCEP4 vector. Successfully transfected cells were selected with 50 μg/mL hygromycin (Boehringer Mannheim) for 1 week and with 100 μg/mL thereafter.
Sequences of PCR primers used for subcloning
| Primer . | Patient . | Ig Chain . | 5′-3′ Sequence . |
|---|---|---|---|
| CEP.1Hfor | 1 | Fd | ATCTGGCCGGCAAGGCCACCATGcaggtgcagctacagcagtg |
| CEP.2Hfor | 2 | Fd | ATCTGGCCGGCAAGGCCACCATGgagctgcagctggtggag |
| CEP.Hrev | 1, 2 | Fd | AGCTGGATCCATTATGCTCAATGATGATGATGATGATGgcaagatttgggctcaactttc |
| CEP.1Lfor | 1 | IgL | AGTCAGATCTGGCCGGCAAGGCCACCATGgacatccagatgacccagtctc |
| CEP.2Lfor | 2 | IgL | ATCTGGCCGGCAAGGCCACCATGgaaattgtattgagacagtctccag |
| CEP.Lrev | 1, 2 | IgL | AGCTGGATCCATTATGCTCAATGATGATGATGATGATGacactctccgcggttgaag |
| Primer . | Patient . | Ig Chain . | 5′-3′ Sequence . |
|---|---|---|---|
| CEP.1Hfor | 1 | Fd | ATCTGGCCGGCAAGGCCACCATGcaggtgcagctacagcagtg |
| CEP.2Hfor | 2 | Fd | ATCTGGCCGGCAAGGCCACCATGgagctgcagctggtggag |
| CEP.Hrev | 1, 2 | Fd | AGCTGGATCCATTATGCTCAATGATGATGATGATGATGgcaagatttgggctcaactttc |
| CEP.1Lfor | 1 | IgL | AGTCAGATCTGGCCGGCAAGGCCACCATGgacatccagatgacccagtctc |
| CEP.2Lfor | 2 | IgL | ATCTGGCCGGCAAGGCCACCATGgaaattgtattgagacagtctccag |
| CEP.Lrev | 1, 2 | IgL | AGCTGGATCCATTATGCTCAATGATGATGATGATGATGacactctccgcggttgaag |
SfiI and BamHI restriction sites are underlined. Kozak consensus sequences, translation start codons, and stop codons are depicted in bold. Regions annealing to the immunoglobulin cDNA are in small letters. CEP.1Lfor contains an additional BglII site immediately upstream of the SfiI site. CEP.Hrev and CEP.Lrev contain six histidine codons immediately upstream of the first stop codon.
Generation of human monocyte-derived dendritic cells
Fresh PBMC were prepared by Ficoll density centrifugation, suspended in RPMI/0.5% human serum albumin (HSA fraction V; Calbiochem, La Jolla, CA), and allowed to adhere to tissue culture flasks for 2 hours. Nonadherent cells were removed by 3 × washing with PBS. Adherent cells were differentiated into Langerhans-type cells (LC) in serum-free monocyte medium (Cellgenix, Freiburg, Germany) supplemented with 100 ng/mL granulocyte-macrophage colony-stimulating factor (Novartis), 50 ng/mL IL-4, and 5 ng/mL transforming growth factor-β1 (both Genzyme, Cambridge, MA) for 6 days (Garbe A et al, manuscript submitted for publication). Final differentiation to mature DC was induced by the addition of 100 ng/mL lipopolysaccharide (LPS; Pyroquant Diagnostik, Walldorf, Germany). Twenty-four hours later, the cell population was used for stimulation of CTL (see below). Flow cytometry analyses of LC and DC were performed with antibodies specific for CD1a, CD14, and CD86 (Becton Dickinson, Mountain View, CA).
Generation and analysis of protein-loaded DC
For loading of DC with idiotype protein, 1 mg/mL solutions of recombinant Fab fragment were denatured by heating to 80°C in a microwave oven. This material was added to immature DC at a concentration of 50 μg/mL on day 5 of culture.
To analyze the uptake of recombinant Fab fragments by immature DC, 10 μL fluorescent dye FLUOS (Boehringer Mannheim) was incubated with 660 μg recombinant Fab fragment for 2 hours at room temperature. The protein was purified from unbound chromophore by gel filtration through Sephadex G-25 (Pharmacia). Photometric analysis allowed calculation of the ratio of fluorescein molecules per protein by the formula 3.053 × OD495nm/OD280nm − 0.255 × OD495nm. In typical experiments, the FLUOS/protein ratio was approximately 15:1.
Six hours after the addition of FLUOS-labeled denatured Fab protein to LC, 105 cells per spot were pipetted onto a polylysine-coated, 12-spot glass slide and allowed to adhere for 30 minutes at 4°C. After fixation with 3% paraformaldehyde, cells were analyzed by confocal laser microscopy (LSM 410 Invert microscope; Zeiss, Oberkochen, Germany).
Construction of individual Semliki forest virus idiotype expression vectors and generation of idiotype-transduced DC
Plasmids pSFV1, pSFVhelper1, and the cell line BHK-2130were kindly supplied by P. Fisch. The variable light chain gene of patient 1 was reamplified from the respective pFab.γκ vector with primers CEP.1Lfor and CEP.1Lrev (Table 1). The resultant PCR product was digested with BglII and BamHI and inserted into the singular BamHI site of pSFV1. A pSFV1-subclone with the insert in correct orientation was designated pSFV.1H. The coding region of pSFV.1H could then be replaced with the Fd or IgL genes from the individual pCEP expression constructs (see above) through SfiI and BamHI.
For the production of infectious Semliki forest virus (SFV) vector particles, idiotype sequence-carrying pSFV plasmids and plasmid pSFVhelper1 were linearized with SpeI. Approximately 1 μg linearized plasmid was transcribed in vitro with 30 U SP6 RNA polymerase (Boehringer Mannheim) in the presence of 1 mmol/L dithiothreitol, 1 mmol/L capping analogue m7G(5′)ppp(5′)G (Gibco BRL), 1 mmol/L rNTP, and 60 U RNasin (Promega, Madison, WI) in a total of 50 μL SP6 buffer (Boehringer Mannheim) for 2 hours at 37°C.
The packaging cell line BHK-21 was grown in DMEM with 5% FCS (PAA Laboratories), 1:50 tryptose phosphate, 1 mmol/L L-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin (all Gibco BRL). When the culture reached 90% confluence, the cells were harvested by trypsinization and resuspended at a concentration of 107/mL in PBS, and 0.8 mL cell suspension was mixed with 10 μg transcript of a pSFV idiotype construct and 10 μg pSFVhelper1 transcript. After a 5-minute incubation on ice, the cells were electroporated with a double discharge of 850 V and 960 μF in a 0.4 cm cuvette and thereafter recultured in 10 mL medium. The supernatant was harvested after 36 hours and cryopreserved at −70°C until it was used for transduction.
After terminal differentiation of DC by LPS, cells were washed twice with PBS and resuspended in 1:10 diluted infectious BHK-21 supernatants for 1 hour at 37°C. Immediately afterward, cells were used for the stimulation of PBMC or analyzed by Western blot for the expression of idiotype protein.
Western blot analysis for expression of recombinant idiotype
5 × 106 SFV-transduced DC or 5 × 107 pCEP4-transfected LCL were harvested and lysed in 100 μL 1% (vol/vol) Nonidet P40 in 50 mmol/L Tris-HCl (pH 7.6)/150 mmol/L NaCl/2 mmol/L EDTA. After a short centrifugation to separate particulate material, 15 μL lysate was electrophoresed by 12% SDS-PAGE under reducing conditions. Protein was transferred to a polyvinylidene difluoride membrane (Hybond-P; Amersham Buchler, Braunschweig, Germany) by electroblotting with 40 mA for 90 minutes (Transblot semidry; Bio-Rad). The membrane was blocked with 5% milk powder in PBS/0.1% Tween 20 (blocking buffer) for 1 hour at room temperature. The primary antibody incubation was performed with a mouse anti-His (C-terminal) horseradish peroxidase-conjugated antibody (Invitrogen) diluted 1:2500 in blocking buffer. After 2 washes with 0.1% Tween 20 in PBS, the membrane was incubated with a sheep antimouse IgG-alkaline phosphatase conjugate (Amersham) 1:4000 in blocking buffer for 1 hour. After 3 washes with 0.1% Tween 20 in PBS and 1 wash with PBS, specific binding was detected with the chemiluminescence Western blot detection system (Amersham) following the instructions of the manufacturer.
Isolation of CD8+ PBMC and stimulation of CTL with DC
Using Ficoll density centrifugation, we isolated PBMC from heparinized blood samples. CD8+ cells were purified by depletion of CD4+, CD11b+, CD16+, CD19+, CD36+, and CD56+ cells with hapten-coupled antibodies against these antigens. Antibody-loaded cells were removed with a secondary hapten-specific antibody coupled to magnetic microbeads (MACS CD8+ T-cell isolation kit; Miltenyi Biotech, Bergisch Gladbach, Germany).
The culture medium for T-cell stimulation consisted of RPMI supplemented with 2 mmol/L L-glutamine, 0.55 mmol/L L-arginin, 0.24 mmol/L L-asparagine, 1 mmol/L sodium pyruvate, MEM vitamins 1:200, MEM nonessential amino acids 1:100, 0.1 μmol/L 2-mercaptoethanol, 50 U/mL penicillin, 50 μg/mL streptomycin (all Gibco BRL), and 5% (vol/vol) autologous human plasma. In round-bottom microtiter wells, 5 × 104 CD8+ PBMC were stimulated with 2 × 104 mature DC in 210 μL medium supplemented with 1000 U/mL IL-6 and 10 ng/mL IL-12 (both Boehringer Mannheim). T cells were pooled and restimulated with DC at the same cell ratio at weekly intervals. After restimulations, cells were cultured in medium supplemented with 20 U/mL IL-2 and 5 ng/mL IL-7 (both Boehringer Mannheim).31 During the entire stimulation period, 70 μL respective culture medium was renewed every 2 to 4 days.
Detection of cytotoxic activity
To obtain appropriate target cells, 106 LCL or K562 cells were incubated with 10 to 40 μl Na251CrO4 (Amersham) for 1 hour at 37°C. After 2 washes with RPMI, 2000 target cells were plated per well into V-bottom microtiter plates. With the exception of blocking experiments with antibodies, experiments with LCL as target cells were performed as cold-target inhibition assays by adding 105unlabeled K562 per well to exclude natural killer (NK) activity. Effector cells were added at effector-to-target ratios of 50:1, 10:1, and 2:1 in duplicate or triplicate. For maximal lysis, 0.15% Triton-X in medium was added. After a 4-hour incubation at 37°C, 50 μL supernatant was counted in a scintillation counter (Lumaplate; Canberra-Packard, Dreiech, Germany). Specific lysis was calculated by the formula (specific release − spontaneous release)/(maximum release − spontaneous release).
Blocking experiments were performed with effector cells preincubated for 1 hour at 37°C with antibodies against CD3 (OKT-3; Novartis), CD4, or CD8 (both Biotrend, Cologne, Germany). In the case of antibodies against HLA-A, HLA-B, HLA-C, HLA-DR, HLA-DP, and HLA-DQ (all Biotrend), the target cells were incubated under otherwise identical conditions. The concentration of all antibodies was 100 μg/mL during the following lysis reaction. Antibody-blocking experiments were performed without the addition of cold K562 target cells.
Results
Preparation of idiotype-presenting autologous dendritic cells from patients with malignant lymphoma
Clonally rearranged immunoglobulin genes from patients 1 and 2 were amplified from frozen lymphoma biopsies by anchored PCR, inserted into a TA cloning vector, and sequenced. Consensus sequences for the variable segments of the heavy- and light-chain genes of both patients are given in Table 2.
After reamplification to add the appropriate restriction enzymes for subcloning, the variable regions were inserted into the dicistronic prokaryotic expression vector pFab.γκ. This vector carries a single tetracycline-inducible expression cassette for Fd and IgL genes with specialized bacterial leader sequences exporting the polypeptides into the periplasmic space.28 Because of the special cellular milieu in the bacterial periplasma, IgL and Fd chains assume correct conformation and are assembled into functionally folded Fab fragments, including the interpeptide disulfide bond.28 Addition of a hexahistidine tag at the C terminus of the Fd fragment allows purification of the recombinant Fab fragment by metal affinity chromatography.29 This rapid and convenient expression system permitted the production of virtually pure idiotype protein in both patients (Figure1). The protein concentration after purification was adjusted to 1 mg/mL, and determination of the residual endotoxin content demonstrated less than 0.2 U endotoxin/μg protein.
SDS-PAGE analysis of recombinant, lymphoma-derived Fab fragments.
Immunoglobulin transcripts from lymphoma biopsies of both patients were amplified by PCR, sequenced, and inserted into pFab.γκ. Recombinant Fab fragments were expressed from pFab.γκ vectors in E. coli. Periplasmic fractions of induced bacteria were prepared and purified by affinity chromatography. The resultant protein eluate was analyzed by standard 12% SDS-PAGE under reducing and nonreducing conditions. Protein bands were visualized by silver staining. The Fd and IgL chains of patient 2 had almost identical migration properties under reducing conditions. Even when the gel was run with 6 mol/L urea, the separation of the bands could not be improved (not shown). SM, high-molecular weight marker.
SDS-PAGE analysis of recombinant, lymphoma-derived Fab fragments.
Immunoglobulin transcripts from lymphoma biopsies of both patients were amplified by PCR, sequenced, and inserted into pFab.γκ. Recombinant Fab fragments were expressed from pFab.γκ vectors in E. coli. Periplasmic fractions of induced bacteria were prepared and purified by affinity chromatography. The resultant protein eluate was analyzed by standard 12% SDS-PAGE under reducing and nonreducing conditions. Protein bands were visualized by silver staining. The Fd and IgL chains of patient 2 had almost identical migration properties under reducing conditions. Even when the gel was run with 6 mol/L urea, the separation of the bands could not be improved (not shown). SM, high-molecular weight marker.
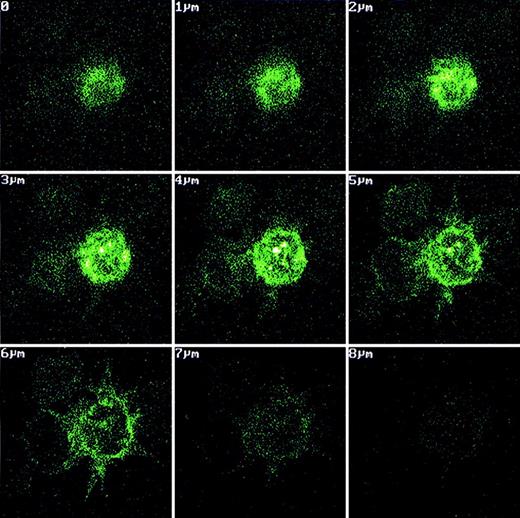
Monocytes enriched from peripheral blood by adhesion to tissue culture flasks were differentiated in vitro to immature DC in serum-free medium supplemented with IL-4, granulocyte-macrophage colony-stimulating factor, and transforming growth factor-β1 for 6 days. One day before the induction of maturation with LPS, recombinant Fab fragments were added to the culture at a final concentration of 50 μg/mL. To analyze the uptake of recombinant idiotype by DC, an aliquot of the cells was incubated with FLUOS-labeled Fab fragment for 6 hours under otherwise identical conditions. These cells were analyzed by confocal microscopy to assess the location of fluorescence within the cells. In cells with DC morphology, high fluorescence was detected in rounded compartments with shape and location typical for endosomes (Figure 2). The immunophenotype of DC was routinely verified by flow cytometry. More than 80% of cells recovered from day 7 cultures expressed CD1a and CD86 and stained negative for CD14, thus exhibiting the phenotype of mature DC since the down-regulation of CD1a proceeded slowly after the induction of maturation (data not shown).
Uptake of fluorescein-labeled recombinant Fab fragments by monocyte-derived dendritic cells.
Fluorescence-labeled recombinant Fab fragment was added to monocyte-derived LC at a concentration of 660 μg/mL immediately before terminal differentiation to DC with LPS. After DC differentiation, protein-loaded cells were pipetted onto adhesion slides and fixed with 3% paraformaldehyde. The location of fluorescence within the cells was assessed by confocal microscopy. In cells with DC morphology, bright fluorescence was present in cellular subcompartments with the typical shapes and localization of endosomes.
Uptake of fluorescein-labeled recombinant Fab fragments by monocyte-derived dendritic cells.
Fluorescence-labeled recombinant Fab fragment was added to monocyte-derived LC at a concentration of 660 μg/mL immediately before terminal differentiation to DC with LPS. After DC differentiation, protein-loaded cells were pipetted onto adhesion slides and fixed with 3% paraformaldehyde. The location of fluorescence within the cells was assessed by confocal microscopy. In cells with DC morphology, bright fluorescence was present in cellular subcompartments with the typical shapes and localization of endosomes.
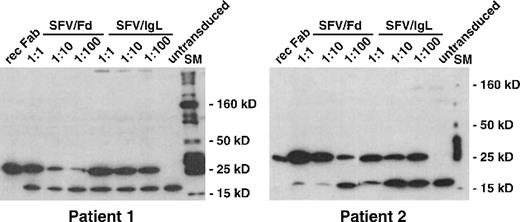
To generate idiotype-expressing antigen-presenting cells, terminally differentiated DC were incubated with infectious supernatants of idiotype SFV vector-producing BHK-21 cells. To verify the efficient transduction of DC with SFV vectors, transduced DC were harvested 24 hours after exposure to the vector supernatant and were analyzed by Western blot. Blotting with an anti-His-tag antibody permitted detection of recombinant IgL and Fd chains in DC lysates because PCR-mediated subcloning (Table 1) introduced hexahistidine tags into all IgL and Fd inserts of the SFV vectors (Figure3). Strong signals of the expected molecular weights were observed in all transduced DC lysates, even when the vector supernatant was diluted 100-fold. The intensity of the signal indicated very efficient transduction of DC by SFV vectors, corresponding to independent data showing a transduction efficiency of 80% on a per cell basis as determined with a GFP reporter gene (Albrecht B, Fisch P, unpublished observations). A second band of 18 kd was observed in untransduced and transduced DC. This band appeared to represent a cellular protein with nonspecific binding of the anti-His antibody and allowed us to compare the amount of lysate loaded onto each lane. No changes in viability and immunophenotype of transduced DC were detected for at least 3 days.
Expression of recombinant idiotype in SFV-transduced DC.
Lymphoma-derived immunoglobulin genes were inserted with an attached hexahistidine tag into the SFV vector plasmid pSFV1. In vitro transcripts were synthesized from idiotype SFV vector plasmids, and the helper plasmid pSFVhelper1. BHK-21 packaging cells were electroporated with a mixture of helper and idiotype vector transcripts. Twenty-four hours later, infectious BHK-21 supernatants were harvested and used for transduction of terminally differentiated DC at 1:1, 1:10, and 1:100 dilutions. Twenty-four hours after transduction, DC were lysed and analyzed by Western blot with an antihistidine antibody. Bound antibody was detected by chemiluminescence. rec Fab, recombinant Fab fragment loaded as control; SFV/Fd, SFV/IgL, transduction with SFV vectors encoding the heavy- or light-chain idiotype of the respective patient's lymphoma; SM, 10- to 250-kd molecular weight marker.
Expression of recombinant idiotype in SFV-transduced DC.
Lymphoma-derived immunoglobulin genes were inserted with an attached hexahistidine tag into the SFV vector plasmid pSFV1. In vitro transcripts were synthesized from idiotype SFV vector plasmids, and the helper plasmid pSFVhelper1. BHK-21 packaging cells were electroporated with a mixture of helper and idiotype vector transcripts. Twenty-four hours later, infectious BHK-21 supernatants were harvested and used for transduction of terminally differentiated DC at 1:1, 1:10, and 1:100 dilutions. Twenty-four hours after transduction, DC were lysed and analyzed by Western blot with an antihistidine antibody. Bound antibody was detected by chemiluminescence. rec Fab, recombinant Fab fragment loaded as control; SFV/Fd, SFV/IgL, transduction with SFV vectors encoding the heavy- or light-chain idiotype of the respective patient's lymphoma; SM, 10- to 250-kd molecular weight marker.
Induction of idiotype-specific CTL with idiotype-presenting DC
CD8+ cells were obtained from the peripheral blood of both patients with lymphoma during clinical remission. All other major cell types were depleted with monoclonal antibodies. As confirmed by FACS analysis, more than 85% of the recovered population stained positive for CD3 and CD8. These cells were stimulated in the presence of IL-12 and IL-6, with autologous DC loaded with recombinant Fab fragment derived from lymphoma cells of the respective patient. Alternatively, DC transduced with either the Fd or the IgL idiotype segment were also used for T-cell stimulation. T cells were restimulated in the presence of IL-2 and IL-7 with freshly prepared DC, presenting the same antigen as during the initial stimulation at weekly intervals. Vigorous cell proliferation with a 2- to 3-fold expansion per week was observed in wells with Fab-loaded DC. The cell proliferation rate in wells with idiotype-transduced DC was approximately half the growth rate achieved with Fab-loaded DC.
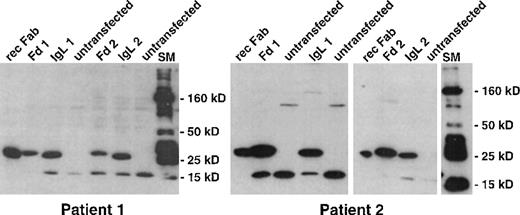
One week after the third restimulation, stimulated T cells were tested for idiotype-specific cytotoxicity. Because the entire stimulation protocol was designed to be feasible without the necessity of viable autologous lymphoma cells, autologous LCL of both patients served as surrogate target cells. These target cells were transfected with pCEP vectors, and they stably expressed IgL or Fd molecules cloned from autologous lymphoma cells. Idiotype expression by transfected LCL was confirmed with the same anti-His-tag Western blot used to analyze idiotype-expressing DC (Figure4).
Expression of recombinant idiotype in stably transfected LCL.
Lymphoma-derived immunoglobulin genes were inserted with an attached hexahistidine tag into the EBV-replicon vector pCEP4. Well-proliferating LCL were electroporated and selected with hygromycin. Hygromycin-resistant LCL were lysed and analyzed by Western blot with an antihistidine antibody. Bound antibody was detected by chemiluminescence. rec Fab, recombinant Fab fragment loaded as control; Fd1, IgL1, LCL transfected with pCEP4 vectors encoding the heavy- and light-chain idiotype gene of patient 1; Fd2, IgL2, LCL transfected with pCEP4 vectors encoding the heavy- and light-chain idiotype gene of patient 2; SM, 10- to 250-kd molecular weight marker.
Expression of recombinant idiotype in stably transfected LCL.
Lymphoma-derived immunoglobulin genes were inserted with an attached hexahistidine tag into the EBV-replicon vector pCEP4. Well-proliferating LCL were electroporated and selected with hygromycin. Hygromycin-resistant LCL were lysed and analyzed by Western blot with an antihistidine antibody. Bound antibody was detected by chemiluminescence. rec Fab, recombinant Fab fragment loaded as control; Fd1, IgL1, LCL transfected with pCEP4 vectors encoding the heavy- and light-chain idiotype gene of patient 1; Fd2, IgL2, LCL transfected with pCEP4 vectors encoding the heavy- and light-chain idiotype gene of patient 2; SM, 10- to 250-kd molecular weight marker.
Specific lysis of cells expressing the idiotype derived from each patient's lymphoma cells was demonstrated using51Cr-labeled autologous, idiotype-transfected LCL as target cells. Autologous LCL expressing the other patient's idiotype chains served as controls. These 51Cr release assays were performed as cold target inhibition assays with an excess of the typical NK target cell line K562.
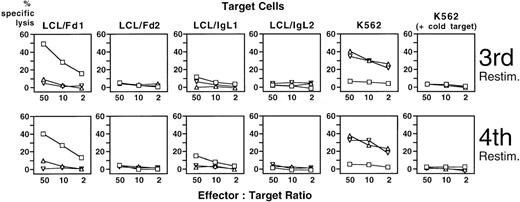
T cells of patient 1 exhibited strong specific cytotoxicity (49% of maximum release) against autologous Fd-expressing LCL after stimulation with Fab-loaded DC (Figure 5). Weak lytic activity (12% release) of the same T-cell population was detected against IgL-expressing LCL. In contrast, lytic activity for autologous IgH idiotype was marginal (9% release) after stimulation with appropriately transduced DC and practically absent for IgL (5% release). Moderate NK activity was detected when K562 were used as target cells (41% and 35%, respectively), whereas T cells stimulated with Fab-loaded DC had no detectable NK activity (less than 7% release). The experiment was confirmed with virtually identical results after a fourth restimulation with idiotype-presenting DC (Figure 5).
Cytotoxicity of PBMC of patient 1 against idiotype-expressing autologous LCL after stimulation with idiotype-presenting autologous DC.
CD8+ PBMC of patient 1 were stimulated repetitively with autologous DC loaded or transduced with idiotype derived from the patient's lymphoma cells. Cytotoxic activity was assessed in a standard 51Cr release assay with idiotype-expressing autologous LCL or the NK target cell line K562 as target cells. An excess of unlabeled K562 cells was added to LCL target cells to inhibit NK activity. □: DC loaded with recombinant Fab fragment derived from the patient's lymphoma; ▵ and ▿: DC transduced with SFV vectors encoding autologous lymphoma-derived immunoglobulin heavy or light chain; LCL/Fd1, LCL/IgL1, LCL of patient 1 stably transfected with vectors encoding the idiotype heavy or light chain of the lymphoma of patient 1; LCL/Fd2, LCL/IgL2, LCL of patient 1 stably transfected with vectors encoding the idiotype heavy or light chain of the lymphoma of patient 2.
Cytotoxicity of PBMC of patient 1 against idiotype-expressing autologous LCL after stimulation with idiotype-presenting autologous DC.
CD8+ PBMC of patient 1 were stimulated repetitively with autologous DC loaded or transduced with idiotype derived from the patient's lymphoma cells. Cytotoxic activity was assessed in a standard 51Cr release assay with idiotype-expressing autologous LCL or the NK target cell line K562 as target cells. An excess of unlabeled K562 cells was added to LCL target cells to inhibit NK activity. □: DC loaded with recombinant Fab fragment derived from the patient's lymphoma; ▵ and ▿: DC transduced with SFV vectors encoding autologous lymphoma-derived immunoglobulin heavy or light chain; LCL/Fd1, LCL/IgL1, LCL of patient 1 stably transfected with vectors encoding the idiotype heavy or light chain of the lymphoma of patient 1; LCL/Fd2, LCL/IgL2, LCL of patient 1 stably transfected with vectors encoding the idiotype heavy or light chain of the lymphoma of patient 2.
Equivalent results with respect to autologous IgH-idiotype-specific lysis and NK activity were obtained with DC-stimulated T cells from patient 2 (Figure 6). The main difference with respect to the antigens between both patients was found for IgL-idiotype-expressing target cells: T cells of patient 2 killed IgL-expressing LCL almost as efficiently as Fd-expressing target cells (38% and 50% release, respectively).
Cytotoxicity of PBMC of patient 2 against idiotype-expressing autologous LCL after stimulation with idiotype-presenting autologous DC.
CD8+ PBMC of patient 2 were stimulated repetitively with autologous DC loaded or transduced with idiotype derived from the patient's lymphoma cells. Cytotoxic activity was assessed in a standard 51Cr release assay with idiotype-expressing autologous LCL or the NK target cell line K562. An excess of unlabeled K562 cells was added to LCL target cells to inhibit NK activity. □: DC loaded with recombinant Fab fragment derived from the patient's lymphoma; ▵ and ▿: DC transduced with SFV vectors encoding autologous lymphoma-derived immunoglobulin heavy or light chain; LCL/Fd1, LCL/IgL1, LCL of patient 2 stably transfected with vectors encoding the idiotype heavy or light chain of the lymphoma of patient 1; LCL/Fd2, LCL/IgL2, LCL of patient 2 stably transfected with vectors encoding the idiotype heavy or light chain of the lymphoma of patient 2.
Cytotoxicity of PBMC of patient 2 against idiotype-expressing autologous LCL after stimulation with idiotype-presenting autologous DC.
CD8+ PBMC of patient 2 were stimulated repetitively with autologous DC loaded or transduced with idiotype derived from the patient's lymphoma cells. Cytotoxic activity was assessed in a standard 51Cr release assay with idiotype-expressing autologous LCL or the NK target cell line K562. An excess of unlabeled K562 cells was added to LCL target cells to inhibit NK activity. □: DC loaded with recombinant Fab fragment derived from the patient's lymphoma; ▵ and ▿: DC transduced with SFV vectors encoding autologous lymphoma-derived immunoglobulin heavy or light chain; LCL/Fd1, LCL/IgL1, LCL of patient 2 stably transfected with vectors encoding the idiotype heavy or light chain of the lymphoma of patient 1; LCL/Fd2, LCL/IgL2, LCL of patient 2 stably transfected with vectors encoding the idiotype heavy or light chain of the lymphoma of patient 2.
To gain a better estimate of the overall degree of idiotype-specific lysis, allogeneic T cells from a voluntary donor were stimulated simultaneously by the untransfected LCL of both patients. Lysis of LCL by these allogeneic responder cells was measured in the same experiments in which idiotype-specific lysis was determined, and it amounted to 74% to 78% (data not shown). Therefore, this allogeneic response was only moderately stronger that the specific lysis of autologous idiotype-expressing LCL.
Characterization of idiotype-specific CTL
The composition of the effector cell populations was assessed by flow cytometry (Table 3). A high percentage of cells had phenotypic features of activated T cells with coexpression of CD3 and HLA-DR. As expected for cultures initiated with a highly enriched CD8+ population, most cells (56%-93%) expressed CD8 independent of the stimulation conditions. Notable differences, however, were observed in the remaining cells. Corresponding to the NK activity, a sizable fraction of cells in cultures stimulated with SFV-transduced DC expressed the NK marker CD56 or CD16 (25%-44%). In contrast, these markers were expressed only by a minority of cells (4%-7%) after stimulation with protein-pulsed DC. Another striking and unexpected difference existed with regard to the numbers of CD4+CD8+ double-positive T cells. Only cultures obtained with Fab-loaded DC contained a significant proportion of effector cells expressing both CD4 and CD8 (22-27%).
Immunophenotype of CD8+ PBMC after repetitive stimulation with idiotype-presenting dendritic cells
| Stimulation . | Patient 1 . | Patient 2 . | ||||
|---|---|---|---|---|---|---|
| DC/Fab (%) . | DC/Fd (%) . | DC/IgL (%) . | DC/Fab (%) . | DC/Fd (%) . | DC/IgL (%) . | |
| CD3+ | 77.7 | 70.1 | 66.7 | 84.3 | 90.5 | 90.8 |
| CD4+ | 40.0 | 7.4 | 9.4 | 35.9 | 5.0 | 6.1 |
| CD8+ | 67.4 | 69.9 | 55.8 | 89.9 | 92.5 | 92.1 |
| CD8+/CD4+ | 22.0 | 3.2 | 4.0 | 27.5 | 2.7 | 2.1 |
| CD8+/CD4− | 36.3 | 62.8 | 41.6 | 60.3 | 87.7 | 87.6 |
| CD4+/CD8− | 6.7 | 4.7 | 7.3 | 2.4 | 2.2 | 2.9 |
| CD3+/HLA-DR+ | 55.0 | 40.5 | 26.0 | 75.7 | 82.6 | 81.1 |
| CD56+ or CD16+ | 6.9 | 25.0 | 28.2 | 4.3 | 44.3 | 34.8 |
| γ/δ-TCR+ | 1.3 | 1.1 | 1.2 | 0.3 | 0.6 | 0.4 |
| Stimulation . | Patient 1 . | Patient 2 . | ||||
|---|---|---|---|---|---|---|
| DC/Fab (%) . | DC/Fd (%) . | DC/IgL (%) . | DC/Fab (%) . | DC/Fd (%) . | DC/IgL (%) . | |
| CD3+ | 77.7 | 70.1 | 66.7 | 84.3 | 90.5 | 90.8 |
| CD4+ | 40.0 | 7.4 | 9.4 | 35.9 | 5.0 | 6.1 |
| CD8+ | 67.4 | 69.9 | 55.8 | 89.9 | 92.5 | 92.1 |
| CD8+/CD4+ | 22.0 | 3.2 | 4.0 | 27.5 | 2.7 | 2.1 |
| CD8+/CD4− | 36.3 | 62.8 | 41.6 | 60.3 | 87.7 | 87.6 |
| CD4+/CD8− | 6.7 | 4.7 | 7.3 | 2.4 | 2.2 | 2.9 |
| CD3+/HLA-DR+ | 55.0 | 40.5 | 26.0 | 75.7 | 82.6 | 81.1 |
| CD56+ or CD16+ | 6.9 | 25.0 | 28.2 | 4.3 | 44.3 | 34.8 |
| γ/δ-TCR+ | 1.3 | 1.1 | 1.2 | 0.3 | 0.6 | 0.4 |
DC/Fab, idiotype-loaded DC; DC/Fd, DC/IgL, idiotype heavy- or light chain-transduced DC.
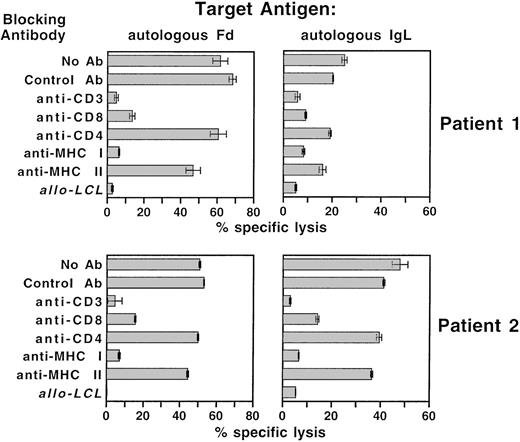
To determine the nature of the T cell–target cell interaction in T cells stimulated with Fab-loaded DC, specific lysis was measured after the blockade of T-cell coreceptors and MHC molecules with monoclonal antibodies (Figure 7). These experiments were performed without the addition of cold K562 target cells. Idiotype-specific lysis could be reduced from more than 50% to 18% with an antibody directed against CD8 and to less than 5% with CD3-or MHC I-specific antibodies. Anti-MHC II and anti-CD4 antibodies had negligible effects on idiotype-specific lysis. The overall pattern of killing served as an additional confirmation of the idiotype-specific lysis shown in Figures 5 and 6.
Inhibition of idiotype-specific cytotoxicity with specific antibodies.
Autologous DC were repetitively stimulated against the idiotype of autologous lymphoma cells with Fab-loaded dendritic cells. Specific cytotoxicity was assessed in a standard 51Cr release assay with idiotype heavy or light chain expressing autologous LCL as target cells. For the inhibition of lytic activity, effector cells were preincubated with anti-CD3, anti-CD4, or anti-CD8 antibody, and target cells were preincubated with anti-MHC class I or anti-MHC class II antibodies. AlloLCL, allogeneic LCL included as a negative control target.
Inhibition of idiotype-specific cytotoxicity with specific antibodies.
Autologous DC were repetitively stimulated against the idiotype of autologous lymphoma cells with Fab-loaded dendritic cells. Specific cytotoxicity was assessed in a standard 51Cr release assay with idiotype heavy or light chain expressing autologous LCL as target cells. For the inhibition of lytic activity, effector cells were preincubated with anti-CD3, anti-CD4, or anti-CD8 antibody, and target cells were preincubated with anti-MHC class I or anti-MHC class II antibodies. AlloLCL, allogeneic LCL included as a negative control target.
A computerized search for HLA class I-binding motives within the idiotype sequences of both patients32 merely yielded the following peptides: NQFSLKLSSV (HLA-A 0201, Patient 1, heavy chain, FR3), KLLIYAAST (HLA-A 0201, Patient 1, light chain, FR2-CDR2), and IVLRQSPATL (HLA-A 0205, Patient 2, light chain, FR1). These peptides, however, had low disassociation scores below 100.
Discussion
The results of our experiments defined a standardized and highly efficient protocol for the induction of anti-idiotype cytotoxic T cells from patients with lymphoma. In daily clinical practice, an autologous lymphoma cell line may not be available for most patients with malignant lymphoma. We therefore developed a method based on frozen biopsies and blood samples only. The protocol relies on a convenient and rapid expression system for the immunoglobulin transcripts of frozen lymphoma biopsies.27 The lymphoma-derived idiotype genes were used to compare 2 distinct ways to present antigens to T cells by dendritic cells—uptake, processing, and presentation of soluble protein antigen by DC and expression of the idiotype antigen within the dendritic cells themselves by transduction with a viral vector system.
To obtain a sufficient quantity of idiotype protein antigen from each patient, recombinant, functionally folded lymphoma-derived Fab fragments were generated by a periplasmic expression system in E. coli and purified with 1-step affinity chromatography.27 With this strategy, idiotype protein could be produced routinely within a period of 2 to 3 weeks after a biopsy was performed. The relatively short production time and the purity of the recombinant material (Figure 1) offer a valuable alternative to other idiotype production methods, such as somatic cell fusion of lymphoma cells with a heterohybrid cell line or production of scFv fragments in plant cells.33,34 As expected,3 4 monocyte-derived LC efficiently endocytosed the recombinant idiotype Fab fragment.
For the endogenous antigen presentation strategy, the SFV vector system allowed the efficient transduction of DC with idiotype genes and did not lead to appreciable changes in DC viability or morphology for several days. When compared to stable transfection of LCL cell lines with cytomegalovirus promoter-driven idiotype genes, only 10% of SFV-transduced DC were necessary to obtain specific bands of similar intensity in Western blot analyses (Figure 3, 4). Even at a 1:100 dilution, infectious SFV supernatants gave rise to easily detectable recombinant protein. According to these analyses, efficient presentation of the idiotype antigen in the following T-cell stimulation experiments could be assumed for both techniques used in this study—exogenous idiotype loading and endogenous idiotype expression.
Presentation of exogenous idiotype to autologous T cells by DC induced strong cytotoxic activity against the heavy immunoglobulin chain of the lymphoma cells in both patients. These patients were chosen on the basis of the IgMκ isotype of their lymphoma B-cell receptor and because of the availability of fresh blood samples at regular intervals, but they represented otherwise unselected patients. The overall level of specific lysis reproducibly approached an order of magnitude not much lower than lysis in an allogeneic reaction performed simultaneously within the same experiment.
Specific lysis could be detected against the idiotype light chain, but the level of recognition was much higher in patient 2 than in patient 1. Nevertheless, this finding proved that immunoglobulin light chains can also harbor lymphoma-specific antigens. Idiotype vaccines composed of both immunoglobulin variable regions may thus be superior to single CDR III-derived peptides for anti-idiotype vaccination.35
All specific cytotoxic activity in these experiments could be inhibited by antibodies against the T-cell receptor, the CD8 coreceptor, and the MHC class I molecules. Therefore, the main effector cells were classic cytotoxic CD8+ T cells that recognized their antigens in an HLA class I-restricted fashion.
Background lysis levels against autologous target cells transfected with idiotype genes of the other patient proved that the target antigen indeed resides in the variable immunoglobulin region of the lymphoma. Neither constant regions nor the variable regions of the other patient's lymphoma or of the polyclonal receptors constitutively expressed by the LCL target cells were recognized. The cytotoxic response was highly specific; even strongly immunogenic EBV-derived antigens expressed by LCL cells did not raise the 51Cr release above background.
Since the initial report of idiotype-specific CTL in a patient with follicular lymphoma,18 lymphoma-specific T cells have been described in various studies. In the human system, there is compelling though indirect evidence that the critical antigen recognized by CTL is indeed the idiotype of the lymphoma cells. Proliferative responses of T cells in response to CDR III peptides have been observed after anti-idiotype vaccination.13,21,22,35 Lymphoma-specific CTL could be expanded in vitro by stimulation with CD40 ligand-activated lymphoma cells, but the target antigen was not formally identified.19 The detection of lymphoma-specific T cells in patients after vaccination with idiotype protein strongly suggests, but does not prove, idiotype-specific cytotoxicity.13,20 In addition, idiotype-specific T cells secreting γ-interferon, a feature characteristic of CTL responses, could be detected after idiotype protein vaccination.22 Because our study relied on heterologous idiotype expression by transfected target cells, the results unequivocally demonstrated that the tumor-specific antigen recognized by CTL was indeed the lymphoma idiotype.
Despite efficient immunization with SFV vectors in experimental systems in vivo,36 no idiotype-specific cytotoxicity could be induced by idiotype SFV-transduced DC. A toxic or immunosuppressive effect of transduction with SFV in vitro appears unlikely because the transduced DC remained essentially intact, proliferation of T cells after stimulation with SFV-transduced DC occurred, and NK activity could be demonstrated. Thus, endogenous expression of idiotype appears to be less efficient than processing of endocytosed protein antigen for the induction of MHC class I-restricted lysis. Consistent with findings in experimental systems with well-defined antigens,37-39our results suggest an additional requirement for MHC class II-restricted presentation of exogenously acquired epitopes for the induction of maximum idiotype-specific cytolytic activity. This may be of special importance because the antigenicity of idiotypes of individual patients is a priori unknown. When additional presentation of idiotype epitopes by MHC class II complexes is absent, it appears that the recently recognized potential of DC for direct activation of NK cells40 overrides the stimulation of idiotype-specific CTL.
In the context of preferential stimulation of MHC class I-restricted CTL, the emergence of a distinct CD4/CD8 double-positive T-cell population after stimulation with protein-loaded DC may play a role. Little information is available about the function of peripheral T cells with this phenotype, but a critical helper function could be possible in our experimental setting.
Understanding the requirements for optimal induction of idiotype-specific cytotoxic T cells may help to design improved vaccination strategies for B-cell lymphoma. Our data suggest that the transfection of DC may not be the most efficient vaccination strategy, and therefore presentation of the protein idiotype by activated dendritic cells should be sought. Moreover, our data defined a GMP-compatible protocol for the generation of idiotype-specific CTL for adoptive transfer. Starting from 10 microtiter plates, the high proliferation rate of stimulated T cells should permit the generation of up to 109 idiotype-specific CTL. The transfer of lymphoma-specific effector cells may be especially valuable after allogeneic stem cell transplantation because donor lymphocyte infusion (DLI) can have dramatic therapeutic efficacy for patients with relapsed lymphoid malignancy.41 To avoid the potentially deleterious effects of DLI-associated graft-versus-host disease, the adoptive transfer of idiotype-specific immunity after immunization of the donor has been proposed.42 43 Active donor immunization, however, may not be possible for unrelated donors. For unrelated donors, a standardized and broadly applicable protocol for in vitro stimulation of idiotype-specific donor T cells by donor-derived DC against the patient's idiotype may be especially valuable.
Acknowledgments
We thank Roland Mertelsmann for financial support and assignment of laboratory space. We also thank Marie Follo for DNA sequence analysis, Helmut Lang for performing HLA typing, and Ozan Alkan and MathiasÖlke for helpful discussions.
Supported by Sonderforschungsbereich 364 of the Deutsche Forschungsgemeinschaft.
Reprints:Hendrik Veelken, Department of Hematology/Oncology, Freiburg University Medical Center, Hugstetter Strasse 55, D-79106 Freiburg, Germany; e-mail:veelken@mm11.ukl.uni-freiburg.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal