Unstimulated monocytes rapidly undergo physiological changes resulting in programmed cell death (apoptosis) while stimuli promoting differentiation of these cells into macrophages were shown to inhibit apoptotic processes. In the present study, we report that the platelet-derived -chemokine platelet factor 4 (PF4) induces the differentiation of monocytes into macrophages, as is evident from morphological changes as well as from the up-regulation of differentiation markers (carboxypeptidase M/MAX1 and CD71). Significant alterations of the phenotype were observed after 72 hours of culture in the presence of the chemokine and required a minimal concentration of 625 nmol/L PF4. PF4-induced macrophages were characterized by a lack of HLA-DR antigen on their surface but showed a strong increase in the expression of the CD28 ligand B7-2. Furthermore, PF4 stimulation prevented monocytes from undergoing spontaneous apoptosis during 72 hours of culture as determined in an annexin-V–binding assay. Although PF4 induced the secretion of relevant amounts of TNF-, neutralizing antibodies directed against TNF- or granulocyte-macrophage colony–stimulating factor (GM-CSF) did not revert PF4-induced rescue from programmed cell death, suggesting that PF4 exerts its antiapoptotic effects in a TNF-– or GM-CSF–independent fashion. On the basis of these results, we propose a novel role for PF4 in the control of monocyte differentiation during an inflammatory process in vivo.
Platelets and many of their products not only are well characterized with respect to their function in hemostasis, but are now also recognized to play important roles in the immunoregulation and differentiation of various cell types. During acute vascular injury or chronic disease, activated platelets release a variety of mediators, including 3 members of the chemokine family, the connective tissue–activating peptide III, RANTES, and the platelet factor 4 (PF41).1-3 Although PF4 is a member of the α- or CXC-chemokine subfamily, sharing a high degree of similarity in structure and sequence with the other members of this group, the functional role of PF4 appears to be quite exceptional. Related CXC chemokines such as interleukin 8 (IL-8), neutrophil-activating peptide 2, or melanoma growth stimulatory activity (GROα), were shown to act as potent activators of polymorphonuclear granulocytes (PMN),4-6 inducing such biological responses as chemotaxis, degranulation, or adhesion through binding to common IL-8 receptors. In a recent report, we were able to show that highly purified PF4 lacks chemotactic activity for PMN, but in the presence of TNF-α, stimulates these cells to undergo such functions as exocytosis of secondary granule markers or tight adhesion to different surfaces.7 Investigating PF4-binding sites, we could demonstrate that PF4-induced functions were elicited not through binding to IL-8 receptors or another 7-transmembrane-domain molecule, but through interaction with an integral chondroitin sulfate proteoglycan expressed on the surface of human PMN.8 9
Unlike other CXC chemokines, which are predominantly active on PMN, PF4 affects a wide range of different cell types. Brindley and coworkers10 demonstrated the PF4-mediated release of histamine by basophils, and Hayashi et al11 reported a role for PF4 in the adherence of eosinophils. Besides eliciting these proinflammatory functions, which are induced rather rapidly, PF4 was also shown to be involved in long-term differentiation and regulatory processes. Han and coworkers reported that PF4 supports the survival of hematopoietic stem cells as well as of progenitor cells12and suppresses the development and maturation of cells from the megakaryopoietic lineage.13 Furthermore, an antiproliferative activity of the chemokine on endothelial cells and fibroblasts was reported by several authors.14-16 The impact of PF4 in the regulation of cell growth was shown in the work of Tanaka et al,17 who demonstrated the inhibition of tumor growth and tumor-associated angiogenesis in cells transfected with PF4 cDNA.
In this study, we focus on biological effects elicited by PF4 in human monocytes. We show that PF4 prevents monocytes from spontaneous apoptosis and induces their differentiation into macrophages. These results indicate a potential role for PF4 as a mediator of long-term effects in the regulation of inflammatory processes in vivo.
Materials and methods
Cytokines
Human natural PF4 was purified to homogeneity from release supernatants of thrombin-stimulated platelets in a 3-step procedure as previously described.7 The preparations contained less than 0.125 ng lipopolysaccharide (LPS) per mg PF4 (ie, below 4 pg/mL at 4 μmol/L PF4) as determined by the limulus amoebocyte lysate assay, ruling out possible side effects caused by contaminating LPS. PF4 was lyophilized, stored at −80°C, and reconstituted to stock solutions of 1 mg/mL in 0.1% trifluoroacetic acid (TFA) prior to use. Recombinant human IL-8 (72-residue isoform), recombinant human RANTES, recombinant human IP-10, as well as recombinant human fractalkine (76-residue chemokine domain) were purchased from Pepro Tech Inc (Rocky Hill, NJ). All recombinant chemokines contained 100 ng LPS/mg protein or less. Recombinant human macrophage colony–stimulating factor (M-CSF) was obtained from R&D Systems (Wiesbaden, Germany), and recombinant human granulocyte-macrophage colony–stimulating factor (GM-CSF) was purchased from Sandoz (Basel, Switzerland). Recombinant human TNF-α was from Schering (Berlin, Germany).
Antibodies to PF4 and immunoaffinity chromatography
A murine monoclonal antibody against PF4 (clone PF63.1) was generated in our laboratory following immunization of BALB/c mice with horse myoglobin–conjugated human PF4, according to standard protocols. The antibody (immunoglobulin [Ig] G1 isotype) specifically recognized PF4, as evidenced by total competition of its binding to solid phase–coated PF4 by excess soluble antigen. The antibody did not show crossreactivity with either bovine or rabbit PF4, or with the related human CXC chemokines β-TG Ag, IL-8, IP-10, or GROα as assayed by the same method. For the preparation of an immunocolumn, 2 mg of the antibody were coupled to 0.3 g cyanogen bromide (CNBr)-activated Sepharose (Pharmacia, Freiburg, Germany) according to the recommendations of the manufacturer to obtain a final gel volume of 1 mL. For depletion of PF4 from media spiked with 4 μmol/L PF4, the column was equilibrated with phosphate-buffered saline (PBS) followed by application of 5 mL culture medium. The respective flow-throughs contained less than 0.06 μmol/L PF4 and were used for stimulation following sterile filtration.
Cell preparation
Mononuclear cells were isolated from peripheral blood of healthy volunteer donors by Ficoll-Paque gradient centrifugation. Monocytes and lymphocytes were separated by counterflow centrifugation as described earlier.18 The resulting monocyte fraction consisted of more than 95% CD14+ cells as determined by immunofluorescence staining with anti–CD14-specific monoclonal antibody (Leu-M3, clone Mφ-P9, Becton Dickinson, Heidelberg, Germany).
Cell culture and stimulation
Cell cultures were routinely performed in RPMI-1640 (Biochrom, Berlin, Germany) supplemented with 100 U/mL penicillin G, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, and 5% heat-inactivated fetal calf serum (Biochrom). Monocytes (0.5 × 106/500 μL) were cultured in Nunclon polystyrene 24-microwell plates (Nunc, Roskilde, Denmark) at 37°C in a humidified atmosphere with 5% CO2 in the absence or presence of 4 μmol/L PF4. For control purposes, cells receiving no PF4 were always cultured either with medium alone or in the presence of an amount of TFA corresponding to that contained in PF4-treated samples. No differences between these samples were observed under any assay conditions or parameters measured. Further controls were performed by adding 20 μg/mL heparin (Sigma, Deisenhofen, Germany) to PF4-stimulated as well as to unstimulated cells and by culturing monocytes in immunodepleted medium that had been previously spiked with PF4. To determine whether potential contamination by endotoxin was responsible for the effects seen with PF4 and the other chemokine preparations used, blocking experiments with the LPS antagonist compound 406 were performed. For this, monocytes were preincubated for 10 minutes at 37°C in the presence or absence of 100 ng/mL compound 406 (kindly provided by Prof S. Kusomoto, Department of Chemistry, Faculty of Science, Osaka University, Osaka, Japan) and subsequently stimulated with the different chemokines at the concentrations indicated.
In some experiments, cells were cultured in the presence of recombinant human M-CSF (10 ng/mL), recombinant human GM-CSF (1 ng/mL), or recombinant human TNF-α (10 ng/mL). Stimulations in the presence of inhibitory antibodies were performed with 1 μg/mL neutralizing rat anti-human GM-CSF antibody (clone BVD2-23B6, IgG2a, PharMingen, Hamburg, Germany) or an IgG2a isotype control (clone R35-95, PharMingen), 3 μg/mL neutralizing mouse anti-human TNF-α antibody (clone 195, IgG3, Boehringer Mannheim, Mannheim, Germany) or an IgG3 isotype control (clone MIB13, a gift from Dr J. Gerdes, Research Center Borstel, Borstel, Germany). After a given period of time, the cells were kept on ice for about 1 hour, and culture plates were subsequently washed with ice-cold PBS to detach the adherent cells.
Immunofluorescence
Unconjugated murine monoclonal antibodies directed against the following human antigens were used for immunofluorescence labeling of monocytes: anticarboxypeptidase M/MAX1 (clone 7A2, IgG1), kindly provided by Dr R. Andreesen (Department of Hematology and Oncology, University of Regensburg, Germany), anti-CD80 (clone MAB104, IgG1, Coulter-Immunotech, Hamburg, Germany), anti-CD86 (clone FUN1), anti-CD95 (clone DX2, both IgG1, PharMingen), and IgG1 control antibody (Dako Diagnostika, Hamburg, Germany).
The following fluorescein isothiocyanate (FITC)–conjugated antibodies were used: anti-HLA-DR (clone L243, IgG2a), anti-CD14 (Leu-M3, clone Mφ-P9, IgG2b), and IgG2a control antibody (all purchased from Becton Dickinson), anti-CD14 (clone M5E2, IgG2a, PharMingen), IgG2b control antibody (Dako Diagnostika), and dichlorotriazinyl-amino-fluorescein (DTAF)–conjugated goat F(ab′)2anti-mouse IgG Fc (Dianova, Hamburg, Germany). Phycoerythrin (PE)–conjugated antibodies were anti-CD86 (clone IT2.2, IgG2b), anti-CD95 (clone DX2, IgG1), IgG2b control antibody (all purchased from PharMingen), and IgG1 control antibody (Dako Diagnostika).
For direct immunofluorescence labeling, cells were incubated with the respective FITC- or PE-conjugated antibodies at concentrations according to the manufacturers' advice for 15 to 30 minutes on ice in PBS, washed with PBS, and fixed with PBS containing 1.5% paraformaldehyde. For indirect immunofluorescence labeling, cells were incubated with the unconjugated antibodies and washed as described, followed by incubation with DTAF-conjugated goat anti-mouse antibody. After 15 to 30 minutes of incubation, the cells were washed again and finally resuspended and fixed. Flow cytometry analysis was performed with a FACStar Plus (Becton Dickinson).
Evaluation of cell viability and detection of apoptosis
Determination of apoptotic cells was done by double-labeling with annexin-V–FITC and propidium iodide (PI). Annexin-V binds to phosphatidylserine residues, which are translocated from the inner to the outer leaflet of the plasma membrane during the early stages of apoptosis.19,20 The amount of necrotic cells within the annexin-V–positive stained cells was determined by counterstaining with PI (final concentration 1 μg/mL).21 22
Labeling of apoptotic cells was performed by using an annexin-V kit (Bender MedSystems, Boehringer Ingelheim, Heidelberg, Germany). Briefly, monocytes were incubated with annexin-V–FITC in binding buffer (provided by the manufacturer) for 10 minutes on ice, washed, and resuspended in the same buffer as described by the manufacturer. Propidiumiodide was added immediately before flow cytometry analysis.
Measurement of monokine release into culture supernatants
After 24, 48, and 72 hours of cell culture, the supernatants were harvested and stored at −20°C until analysis. The concentration of TNF-α in the supernatants was determined by use of a quantitative enzyme-linked immunosorbent assay (ELISA), provided by Dr H. Gallati (Intex, Muttenz, Switzerland) and performed as recommended by the manufacturer. Quantitative ELISAs for the determination of IL-6, GM-CSF (both from PharMingen), and IL-1β (R&D Systems) were performed according to the manufacturers' advice.
Statistics
Wilcoxon matched pairs signed rank test was used for statistical analysis.
Results
PF4 induces morphological changes in human monocytes
To investigate the effects of PF4 on morphology and surface marker expression of monocytes, freshly isolated monocytes were cultured in the presence of PF4 or left unstimulated. Since we have shown previously that PF4 at concentrations of 4 μmol/L induces a strong biological response in human neutrophils,7this concentration was chosen for a first approach to investigate potential effects of the chemokine on the morphology of human monocytes.
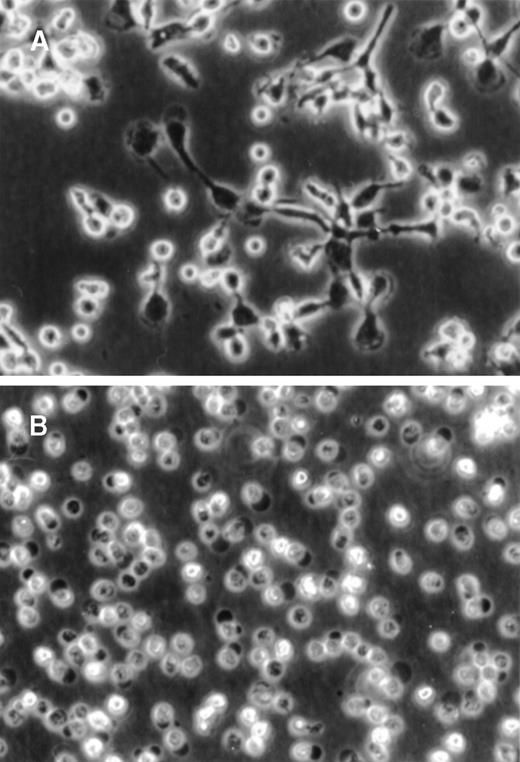
During 72 hours of culture, the PF4-stimulated monocytes showed a dramatic increase in total cellular size and acquired a macrophagelike morphology, including the formation of pseudopodia (Figure 1A). Furthermore, these cells became highly adhesive to the plastic surface. In contrast, unstimulated monocytes were far less adherent and appeared irregularly shaped, were of considerably lower size, and did not show any development of pseudopodia (Figure 1B). The increase in cell size in PF4-stimulated monocytes, as evident from microscopical examination, was confirmed by flow cytometry analysis, in which these cells gave rise to a significantly higher forward scatter signal as compared with unstimulated cells (data not shown).
PF4-induced changes in monocyte morphology.
Purified human monocytes were (A) cultured for 72 hours in the presence of 4 μmol/L PF4 or (B) left untreated. Photographs were taken by phase-contrast microscopy at the same magnification (× 20).
PF4-induced changes in monocyte morphology.
Purified human monocytes were (A) cultured for 72 hours in the presence of 4 μmol/L PF4 or (B) left untreated. Photographs were taken by phase-contrast microscopy at the same magnification (× 20).
PF4-mediated up-regulation of differentiation markers on human monocytes
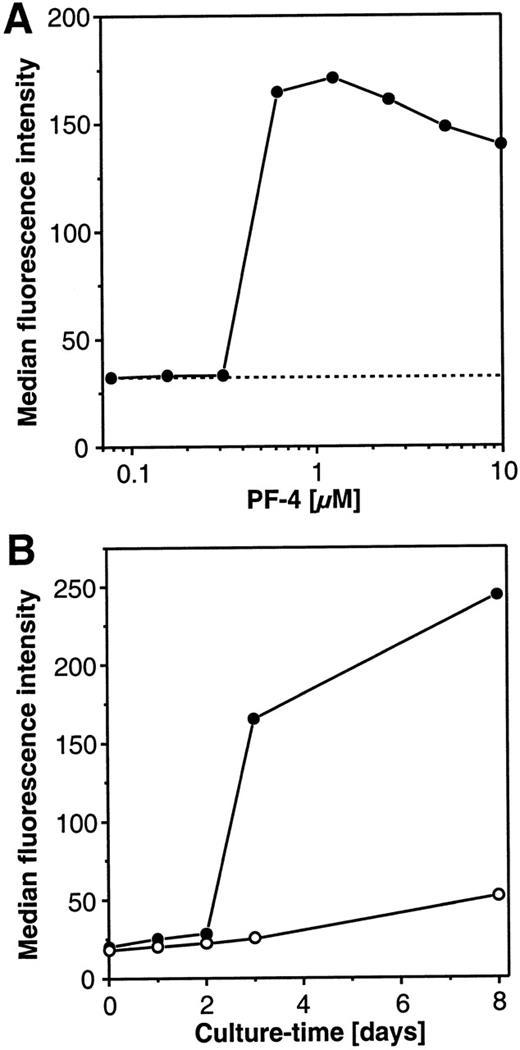
The PF4-induced changes in morphology of the cultured monocytes toward a macrophagelike cell type raised the question of whether this phenomenon represented a true cellular differentiation or simply a shape change of the cells during culture. Since carboxypeptidase M/MAX1 expression is known to increase during maturation of monocytes to macrophages,23 24 the expression of this differentiation antigen was used as a marker. In a first set of experiments, dose-response experiments with increasing amounts of PF4 were performed, and carboxypeptidase M/MAX1 expression was monitored after 72 hours of culture. As depicted in Figure2A, at concentrations of up to 312 nmol/L PF4, no difference was observed in the surface density of the marker (expressed as median fluorescence intensity [MFI]) in comparison with unstimulated cells (MFI of approximately 30). However, PF4 concentrations from 625 nmol/L or higher provoked about a 6-fold increase in the expression of carboxypeptidase M/MAX1 on monocytes. Surprisingly, the surface expression of the differentiation marker could not be further enhanced by increasing the dosage of PF4 up to 10 μmol/L, indicating that this phenomenon did not follow regular dose-response kinetics. Since a concentration of 4 μmol/L PF4 was found to be optimal, this dosage was chosen for all further experiments.
Concentration and time-dependent effects of PF4 on the surface expression of carboxypeptidase M/MAX1 on human monocytes.
(A) Concentration-kinetics. Monocytes were cultured for 72 hours in the presence of increasing concentrations PF4 (•) or left untreated (-----). Cells were subsequently analyzed by flow cytometry for carboxypeptidase M/MAX1 expression, given as median fluorescence intensity as described under “Materials and Methods.” (B) Time-kinetics. Monocytes were incubated for different time periods in the absence (○) or presence (•) of a constant concentration of PF4 (4 μmol/L) and analyzed as described above. Data from 1 representative experiment out of 3 are shown.
Concentration and time-dependent effects of PF4 on the surface expression of carboxypeptidase M/MAX1 on human monocytes.
(A) Concentration-kinetics. Monocytes were cultured for 72 hours in the presence of increasing concentrations PF4 (•) or left untreated (-----). Cells were subsequently analyzed by flow cytometry for carboxypeptidase M/MAX1 expression, given as median fluorescence intensity as described under “Materials and Methods.” (B) Time-kinetics. Monocytes were incubated for different time periods in the absence (○) or presence (•) of a constant concentration of PF4 (4 μmol/L) and analyzed as described above. Data from 1 representative experiment out of 3 are shown.
Next, monocytes were stimulated for different time periods with a constant concentration of PF4 (4 μmol/L) or left unstimulated, and expression of carboxypeptidase M/MAX1 was determined. As depicted in Figure 2B, the density of the marker remained low (greater than MFI 30) for up to 2 days of culture, showing only marginal variations between unstimulated and stimulated cells. When the cell culture period was extended to 3 days, significant differences became apparent: although only a rather low increase of the marker expression (up to MFI 25) could be observed in unstimulated cells, this increase was markedly higher in PF4-stimulated cells (up to MFI 170). Interestingly, at this time point, also the macrophagelike morphology became evident as controlled by microscopical examination. In the presence of the chemokine carboxypeptidase M/MAX1, levels increased further, up to 240 in MFI after 8 days, whereas marker expression remained low on unstimulated cells. It should be mentioned that in unstimulated cells the proportion of viable cells (as determined by trypan blue exclusion) decreased after 3 days of culture to approximately 70% and after 8 days was down to approximately 30%, while in PF4-treated samples, cell viability exceeded 95% at all time points.
Principally the same results for the time-and-dose response kinetics of monocytes stimulated with PF4 (increase after 72 hours at concentration greater than 312 nmol/L PF4) were obtained when we analyzed the expression of the transferrin receptor (CD71) as an alternative differentiation marker (data not shown). To test whether the observed effects were specific for PF4, control experiments were performed in which the PF4-containing culture medium (1) was supplemented with heparin and (2) was passed over an immunoaffinity column to deplete the added PF4. Neither morphological changes nor up-regulation of MAX1 expression were seen in cells stimulated with PF4 in the presence of 20 μg/mL heparin or in cells receiving medium immunodepleted from the added PF4 (data not shown).
Taken together, our data provide evidence that PF4 induces a change in the monocyte phenotype toward a macrophagelike cell type.
PF4 regulates changes in the expression of surface markers on human monocytes
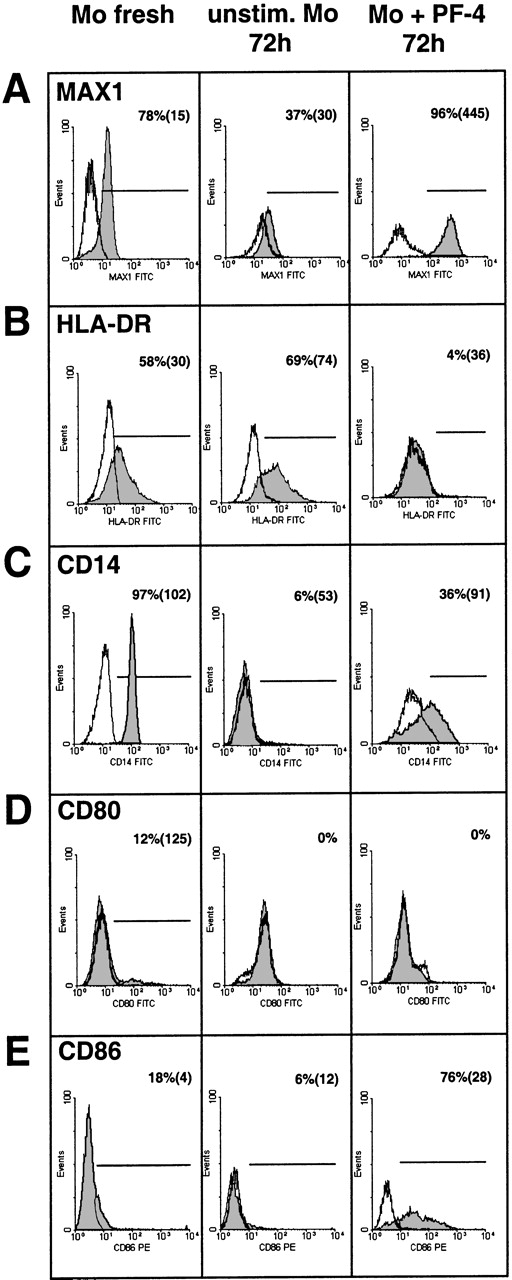
To further characterize the phenotype of PF4-treated monocytes, the cells were labeled with various monoclonal antibodies directed against cell-surface markers known to become modulated in response to inflammatory stimuli or during monocyte differentiation. Cells were stained immediately after isolation and then following 72 hours of culture in the presence or absence of PF4 and thereafter analyzed by flow cytometry. The expression of carboxypeptidase M/MAX1 was used as a functional positive control (Figure 3A). As already shown above, PF4 induced a strong up-regulation of the latter antigen after 72 hours of culture (up to MFI 445), as compared with unstimulated control cells cultured for the same time (MFI 30) or with freshly isolated cells (MFI 15). Just the opposite effect was observed when analyzing the expression of HLA-DR: although the density of this marker was slightly elevated after culture in the absence of PF4 (from 30 to 74 in MFI, Figure 3B) without relevant changes in the number of positive cells, PF4 stimulation resulted in a significant down-regulation of this marker to the levels of the isotype control. Analysis of the LPS/LPS-binding protein receptor CD14 (Leu M3), which was found to be present on all freshly isolated cells (MFI 102), revealed a decrease in its expression upon culture irrespective of whether PF4 was present or not (Figure 3C). However, in untreated cultures, CD14 expression was reduced to background levels whereas about 36% of the monocytes stimulated with the chemokine showed a significant residual expression (MFI 91).
Effects of PF4 on the surface expression of different monocyte markers.
Immunofluorescence-staining of human monocytes with antibodies directed against carboxypeptidase (A) M/MAX1, (B) HLA-DR, (C) CD14, (D) CD80, or (E) CD86 was performed either with freshly isolated cells (left column) or with cells cultured for 72 hours in the absence (middle column) or presence (right column) of 4 μmol/L PF4. Cells were analyzed for the respective surface marker expression (gray histograms) or isotype controls (open histograms) by flow cytometry. Percentage values refer to the relative number of positive cells and values within brackets to mean fluorescence intensity of these cells. Data are derived from 1 representative experiment out of at least 6. Significant differences between numbers of positive cells from PF4-treated and untreated cells after culture were observed in panel A (n = 10,P < .006), panel B (n = 9, P < .008), panel C (n = 9, P < .005), and panel E (n = 11,P < .006).
Effects of PF4 on the surface expression of different monocyte markers.
Immunofluorescence-staining of human monocytes with antibodies directed against carboxypeptidase (A) M/MAX1, (B) HLA-DR, (C) CD14, (D) CD80, or (E) CD86 was performed either with freshly isolated cells (left column) or with cells cultured for 72 hours in the absence (middle column) or presence (right column) of 4 μmol/L PF4. Cells were analyzed for the respective surface marker expression (gray histograms) or isotype controls (open histograms) by flow cytometry. Percentage values refer to the relative number of positive cells and values within brackets to mean fluorescence intensity of these cells. Data are derived from 1 representative experiment out of at least 6. Significant differences between numbers of positive cells from PF4-treated and untreated cells after culture were observed in panel A (n = 10,P < .006), panel B (n = 9, P < .008), panel C (n = 9, P < .005), and panel E (n = 11,P < .006).
B7-1 (CD80) and B7-2 (CD86) are costimulatory molecules that are required for the activation of T cells by monocytes. According to our data, these surface markers, which were only weakly expressed on freshly isolated monocytes, are differentially affected by PF4-treatment: while CD80 expression remained largely unchanged after culture in the presence and in the absence of PF4 (Figure 3D), stimulation with the chemokine mediated a strong up-regulation of the CD86 antigen from 18% to 76% positive cells with an MFI of 4 and 28, respectively (Figure 3E).
In summary, our data show that PF4-induced differentiation of human monocytes is accompanied by various changes of the phenotype of these cells. As will be discussed later, the PF4-mediated loss of HLA-DR, the preservation of CD14, as well as the up-regulation of CD86 may indicate a specific functional role of these cells.
PF4 prevents monocytes from undergoing spontaneous apoptosis
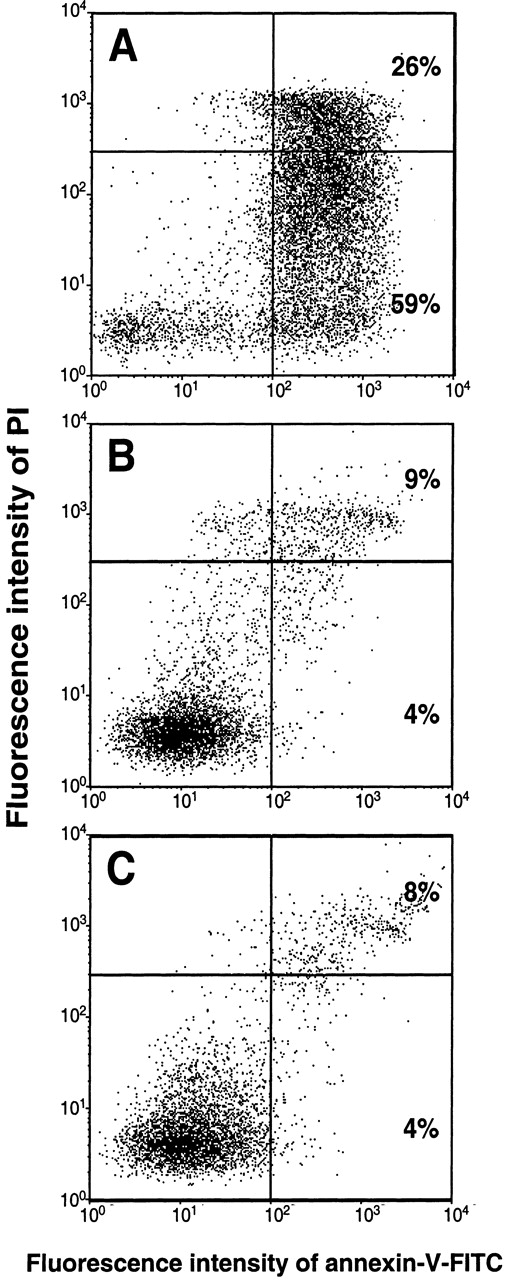
From our observation that considerably higher numbers of dead and damaged cells were present in unstimulated long-term cultures than in cultures run in the presence of PF4 (Figure 2B), the question arose as to how the chemokine would influence the survival of these cells. To assess the proportion of apoptotic cells, monocytes cultured for 72 hours in the presence or absence of PF4 were subsequently labeled with annexin-V. Necrotic cells were identified by counterstaining with PI as described in “Materials and Methods.” Recombinant human GM-CSF (1 ng/mL), which is known to inhibit spontaneous apoptosis in monocytes, was used as a positive control. As shown in Figure4A, in the absence of PF4, about 59% of the monocytes developed an apoptotic staining pattern (PIlow and annexin-Vhigh) whereas 26% of the population appeared to be necrotic (PIhigh and annexin-Vhigh). By contrast, PF4-stimulated monocytes were efficiently prevented from undergoing apoptosis. The number of apoptotic cells in PF4-stimulated cultures was reduced to 4%, whereas the number of necrotic cells was decreased to 9% (Figure 4B). Results almost identical to those obtained with PF4 were seen with GM-CSF (4% apoptotic cells and 8% necrotic cells) (Figure 4C). Comparable results were achieved following 7-amino actinomycin-D (7-AAD) labeling of monocytes or DNA-laddering in electrophoresis (data not shown). The effects induced by PF4 (but not those induced by GM-CSF) could be completely blocked by the addition of heparin (20 μg/mL) to cell cultures as well as by immunodepletion of PF4 from the medium (data not shown). Thus, our data clearly show that PF4 not only changes surface marker expression in monocytes but also prevents these cells from undergoing spontaneous apoptosis.
PF4 prevents monocytes from undergoing spontaneous apoptosis.
Monocytes were cultured for 72 hours (A) in medium alone, (B) in the presence of 4 μmol/L PF4, or (C) in 1 ng/mL GM-CSF. After simultaneous staining with annexin-V–FITC and PI, cells were analyzed by flow cytometry, as described under “Materials and Methods.” The upper right quadrant represents necrotic cells; the lower right quadrant represents apoptotic cells; and the lower left quadrant represents viable, nonapoptotic cells. Data are derived from 1 representative experiment out of 13. Statistical differences between numbers of apoptotic cells of untreated and PF4-stimulated or GM-CSF–stimulated cells were observed with P < .002 (n = 13).
PF4 prevents monocytes from undergoing spontaneous apoptosis.
Monocytes were cultured for 72 hours (A) in medium alone, (B) in the presence of 4 μmol/L PF4, or (C) in 1 ng/mL GM-CSF. After simultaneous staining with annexin-V–FITC and PI, cells were analyzed by flow cytometry, as described under “Materials and Methods.” The upper right quadrant represents necrotic cells; the lower right quadrant represents apoptotic cells; and the lower left quadrant represents viable, nonapoptotic cells. Data are derived from 1 representative experiment out of 13. Statistical differences between numbers of apoptotic cells of untreated and PF4-stimulated or GM-CSF–stimulated cells were observed with P < .002 (n = 13).
Since signaling via CD95 (Fas)/CD95 ligand represents one of the mechanisms known to induce apoptosis, we examined the expression of CD95 on human monocytes during culture in the presence or absence of PF4. Surprisingly, after 72 hours of culture, we found an efficient down-regulation of CD95 to levels of the isotype control in unstimulated cells, whereas the expression of the antigen remained unchanged on PF4-activated monocytes (about 80% positive cells with an MFI of 30; data not shown). The fact that CD95 expression was significantly higher in cells that were protected from apoptosis by PF4 as compared with the unstimulated controls (n = 9,P < .008) may indicate that signaling through CD95 does not play a central role in this regulatory process.
PF4-induced secretion of TNF- is not involved in the protection against spontaneous apoptosis
In principle, PF4-mediated inhibition of monocyte apoptosis could be induced either by direct action of the chemokine on the cells or indirectly by stimulating the release of secondary mediators acting in an autocrine manner. We and others have recently shown that low concentrations of exogenous TNF-α prevent monocytes from undergoing apoptosis.22,25 Similar effects have been described for hematopoietic growth factors, such as GM-CSF26 and M-CSF.27 In the following experiments, a potential role for autocrine acting factors was investigated first by direct measurement of different cytokines (TNF-α, IL-1β, GM-CSF) in the culture supernatants and then by the performance of blocking experiments using inhibitory monoclonal antibodies to these cytokines.
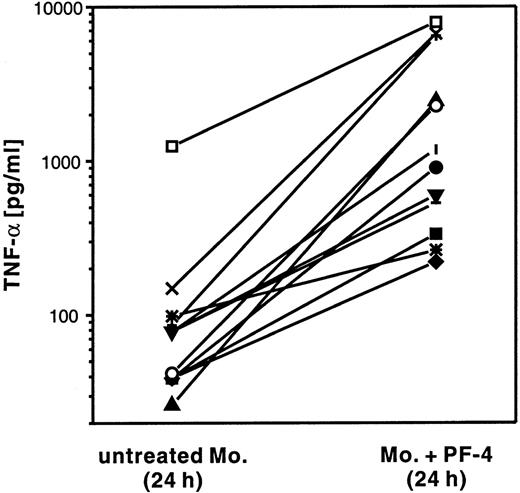
In a first set of experiments, human monocytes were stimulated for 24 hours with PF4 and the amount of TNF-α secreted into the culture supernatant was compared with that present in suspensions of unstimulated cells (Figure 5). Although the levels of TNF-α secreted displayed high variability between individual donors (from 30 to 1,250 pg/mL in unstimulated cells, and from 200 to 8,200 pg/mL in stimulated cells), the concentration of TNF-α from every single donor was always elevated in supernatants derived from cells treated with PF4 (2.7-fold to 93-fold) as compared with the respective unstimulated control. In the same supernatants, neither IL-1β nor GM-CSF was detectable (data not shown).
PF4 induces TNF-α release from monocytes.
Monocytes from 12 individual donors (indicated by different symbols) were cultured for 24 hours in the presence of 4 μmol/L PF4 or left untreated. TNF-α release in the supernatants was detected by ELISA.
PF4 induces TNF-α release from monocytes.
Monocytes from 12 individual donors (indicated by different symbols) were cultured for 24 hours in the presence of 4 μmol/L PF4 or left untreated. TNF-α release in the supernatants was detected by ELISA.
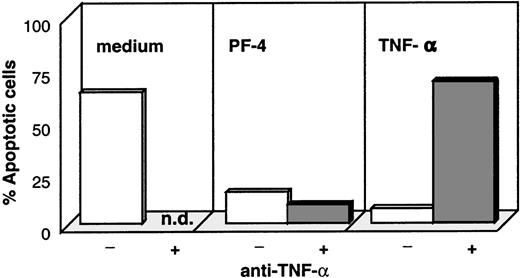
In the next step, we examined whether monocyte-secreted TNF-α was responsible for PF4-induced protection from spontaneous apoptosis. For this approach, TNF-α– and PF4-stimulated monocytes as well as unstimulated control cells were cultured in the presence and absence of inhibitory antibodies directed against TNF-α and were subsequently analyzed for apoptotic cells by annexin-V. As expected, stimulation with PF4 as well as with TNF-α significantly reduced the number of cells undergoing apoptosis (from about 70% in unstimulated cultures to 18% with PF4- and 10% with TNF-α stimulation, Figure6). Although this effect could be completely reverted by the addition of neutralizing anti–TNF-α antibodies in the TNF-α–stimulated control sample, these antibodies were without effect on PF4-activated cells. These results clearly show that PF4-mediated protection against spontaneous apoptosis was not due to the induced release of TNF-α. To exclude a potential effect of low concentrations of GM-CSF not detectable by ELISA, parallel experiments were performed with this growth factor in the presence or absence of respective neutralizing antibodies. Although we could confirm that GM-CSF effectively blocks spontaneous apoptosis in monocytes and that this could be reversed in the presence of the anti–GM-CSF antibody, these antibodies had no effect on PF4-stimulated cells (data not shown). Furthermore, analysis of the immune phenotype of cells stimulated with PF4, GM-CSF, or M-CSF revealed that all 3 stimuli induced an increased expression of carboxypeptidase M/MAX1 after 72 hours of stimulation (92% to 98% positive cells with an MFI between 150 and 250; data not shown). However, cells showed a strikingly different pattern in the expression of HLA-DR: although this surface marker was down-regulated on PF4-treated cells (Figure 3B), HLA-DR appeared to be highly expressed on GM-CSF– and M-CSF–activated monocytes (70% to 80% positive cells with an MFI between 80 and 180; data not shown).
Effect of neutralizing antibodies against TNF- on PF4- and TNF--mediated rescue from monocyte apoptosis.
Monocytes were cultured for 72 hours in medium alone (left panel), with 4 μmol/L PF4 (middle panel), or with 10 ng/mL TNF-α (right panel) in the presence (dark columns) or absence (white columns) of neutralizing antibodies directed against TNF-α. Cells were subsequently labeled with annexin-V and PI, and the percentage of apoptotic cells was determined by flow cytometry. Data from 1 representative experiment out of 6 are shown (n.d. = not determined).
Effect of neutralizing antibodies against TNF- on PF4- and TNF--mediated rescue from monocyte apoptosis.
Monocytes were cultured for 72 hours in medium alone (left panel), with 4 μmol/L PF4 (middle panel), or with 10 ng/mL TNF-α (right panel) in the presence (dark columns) or absence (white columns) of neutralizing antibodies directed against TNF-α. Cells were subsequently labeled with annexin-V and PI, and the percentage of apoptotic cells was determined by flow cytometry. Data from 1 representative experiment out of 6 are shown (n.d. = not determined).
PF4-mediated effects on human monocytes cannot be mimicked by other chemokines
To determine whether the induction of these cellular responses was unique to PF4 or instead represented a more general property of all chemokines, we tested several other chemokines representative of the ELR-CXC type (IL-8), non–ELR-CXC type (IP-10), CC type (RANTES), and CX3C type (fractalkine) at physiological (10 nmol/L) and pharmacological (100 nm and 1 μmol/L) concentrations for their ability to prevent spontaneous apoptosis in monocytes and to induce monocyte differentiation into macrophages. Data represented in Table 1 demonstrate that at concentrations of 10 and 100 nmol/L, none of the chemokines had any effect on these functions. However, at a pharmacological concentration of 1 μmol/L, a weak although statistically not significant reduction of monocyte apoptosis was seen in cells treated with IP-10 (48% apoptotic cells), RANTES (47% apoptotic cells), and fractalkine (62% apoptotic cells) as compared with the untreated control cells (74% apoptotic cells). To determine whether this effect was specifically induced by the chemokines or was rather due to potential endotoxin contamination of the preparations used, we performed control experiments in the presence of the potent endotoxin antagonist compound 406.28 In these assays, protection against apoptosis induced by all 3 stimuli was abolished by the endotoxin antagonist, indicating that the observed effects were caused by LPS contamination and not by the chemokines themselves (data not shown). However, our natural PF4 tested under the same conditions retained its activity in the presence of compound 406. IL-8 at a concentration of 1 μmol/L significantly reduced monocyte apoptosis to half of the level that was induced by PF4, in a way insensitive to the LPS antagonist. None of the chemokines tested (except for PF4) was able to induce monocyte differentiation or morphological changes of these cells.
Effect of different chemokines on monocyte differentiation and apoptosis
| Stimulus . | MAX1-Positive Cells, % . | Apoptotic Cells, % . | Necrotic Cells, % . |
|---|---|---|---|
| None | 9.3 ± 5.1 | 73.8 ± 10.9 | 7.0 ± 9.6 |
| PF4 | |||
| 4 μmol/L | 85.0 ± 6.2* | 13.1 ± 0.8* | 1.7 ± 2.0 |
| IL-8 | |||
| 10 nm | 14.7 ± 5.5 | 67.0 ± 8.8 | 3.7 ± 4.7 |
| 100 nm | 13.7 ± 4.7 | 69.7 ± 9.8 | 2.6 ± 3.0 |
| 1000 nm | 29.0 ± 16.5 | 32.0 ± 12.4* | 0.8 ± 0.3 |
| IP-10 | |||
| 10 nm | 18.7 ± 9.8 | 64.9 ± 5.2 | 4.4 ± 5.7 |
| 100 nm | 17.0 ± 5.6 | 60.7 ± 17.2 | 3.0 ± 2.9 |
| 1000 nm | 20.7 ± 13.6 | 48.1 ± 7.8 | 6.2 ± 7.7 |
| RANTES | |||
| 10 nm | 19.3 ± 7.8 | 65.5 ± 10.0 | 4.3 ± 5.5 |
| 100 nm | 19.7 ± 10.0 | 67.0 ± 11.7 | 3.1 ± 4.3 |
| 1000 nm | 23.0 ± 18.5 | 47.0 ± 9.9 | 2.6 ± 2.9 |
| Fractalkine | |||
| 10 nm | 14.0 ± 3.0 | 70.7 ± 12.7 | 5.5 ± 8.2 |
| 100 nm | 13.3 ± 4.2 | 68.4 ± 11.9 | 5.0 ± 6.2 |
| 1000 nm | 15.7 ± 8.1 | 61.5 ± 11.3 | 5.6 ± 8.6 |
| Stimulus . | MAX1-Positive Cells, % . | Apoptotic Cells, % . | Necrotic Cells, % . |
|---|---|---|---|
| None | 9.3 ± 5.1 | 73.8 ± 10.9 | 7.0 ± 9.6 |
| PF4 | |||
| 4 μmol/L | 85.0 ± 6.2* | 13.1 ± 0.8* | 1.7 ± 2.0 |
| IL-8 | |||
| 10 nm | 14.7 ± 5.5 | 67.0 ± 8.8 | 3.7 ± 4.7 |
| 100 nm | 13.7 ± 4.7 | 69.7 ± 9.8 | 2.6 ± 3.0 |
| 1000 nm | 29.0 ± 16.5 | 32.0 ± 12.4* | 0.8 ± 0.3 |
| IP-10 | |||
| 10 nm | 18.7 ± 9.8 | 64.9 ± 5.2 | 4.4 ± 5.7 |
| 100 nm | 17.0 ± 5.6 | 60.7 ± 17.2 | 3.0 ± 2.9 |
| 1000 nm | 20.7 ± 13.6 | 48.1 ± 7.8 | 6.2 ± 7.7 |
| RANTES | |||
| 10 nm | 19.3 ± 7.8 | 65.5 ± 10.0 | 4.3 ± 5.5 |
| 100 nm | 19.7 ± 10.0 | 67.0 ± 11.7 | 3.1 ± 4.3 |
| 1000 nm | 23.0 ± 18.5 | 47.0 ± 9.9 | 2.6 ± 2.9 |
| Fractalkine | |||
| 10 nm | 14.0 ± 3.0 | 70.7 ± 12.7 | 5.5 ± 8.2 |
| 100 nm | 13.3 ± 4.2 | 68.4 ± 11.9 | 5.0 ± 6.2 |
| 1000 nm | 15.7 ± 8.1 | 61.5 ± 11.3 | 5.6 ± 8.6 |
PF4 indicates platelet factor 4; IL-8, interleukin 8; IP-10.
Monocytes were cultured for 72 hours in the presence of various chemokines at the concentrations indicated or left untreated. Cells were subsequently analyzed by flow cytometry for carboxypeptidase M/MAX1 expression or stained with annexin-V-fluorescein isothiocyanate (annexin-V-FITC) in combination with propidium iodide. Results are given as the percentage of positively stained cells. Apoptotic and necrotic cells were determined as described in the legend to Figure 4. The data are expressed as mean ± SD of 3 independent experiments, recorded and analyzed with identical instrument settings for all assays.
Indicates statistically significant difference with the untreated control (P < .008).
Discussion
In the present study, we report on the discovery of novel biological activities of the platelet-derived CXC chemokine PF4 for human monocytes. Although rapidly inducible monocyte functions like chemotaxis or phagocytosis can be mediated by several chemokines,5,6 chemokines like MCP-1 and other chemotactic factors were shown to be incapable of rescuing monocytes from apoptosis.26 Here we show for the first time that PF4 elicits long-term biological effects in these cells, such as prolonged survival and differentiation into macrophages. Under physiological conditions, monocytes circulate for 3 to 4 days in the human blood and then emigrate into the tissue where they either differentiate into macrophages or undergo apoptosis.29 During maturation, monocytes increase in cell size and granularity and develop pseudopodia, reflecting a variety of functional changes within these cells.29-31 In general, the differentiation process takes about 7 to 10 days, but the cytokines M-CSF and GM-CSF are known to accelerate this development.27,32,33 Although platelets have been recognized to play an important role in the acute activation of monocytes, eg, by the release of pro-inflammatory cytokines such as IL-1 or the chemokine RANTES,3,34 their functional relevance for monocyte differentiation has remained ill defined and is only beginning to emerge. Recently Ammon et al35 described the development of macrophages upon coculture of monocytes with intact platelets, and further analysis revealed that the active components were undefined lipid constituents of the platelet membrane. We, however, show that a highly purified protein constituent, the PF4, is sufficient to induce differentiation of monocytes into macrophages and does so in a time- and concentration-dependent manner. PF4-treated monocytes exhibited an increased cell size, enhanced adhesiveness, and developed pseudopodia. Furthermore, diffentiation markers carboxypeptidase M/MAX1 and CD71 (transferrin receptor) were significantly up-regulated. Differentiation became visible after 72 hours of culture and required a dosage of at least 625 nmol/L PF4. Surprisingly, already moderately higher concentrations of the chemokine (about 1.25 μmol/L) did not lead to further enhancement of marker expression, indicating that the induction of this function did not follow classic dose-response kinetics but rather represented an on/off mechanism. The fact that PF4 (like GM-CSF) induces a differentiation within 3 days instead of 7 to 10 days as described for serum-derived macrophages, indicates that the chemokine actively accelerates the differentiation process. No other chemokine tested, including IL-8, IP-10, RANTES, and fractalkine, was able to induce monocyte differentiation even at pharmacological concentrations, indicating that within the chemokine family, this function may be a unique property of PF4. Although no data exist in the literature concerning PF4 concentrations at a site of acute platelet activation in vivo, normal serum concentrations of PF4 (1 to 2.5 μmol/L36; seen also in our own observations) are thus sufficient to induce a full monocyte response. Apart from this, concentrations near a thrombus, where platelet-monocyte interaction may occur, are likely to be much higher.
The mechanisms involved in the regulation of monocyte apoptosis are only partially understood. Today, several authors claim the existence of 2 distinct pathways: one by which inactivated monocytes are sentenced to programmed cell death and another by which activated cells undergoing differentiating processes are rescued from apoptosis. First reports by Mangan et al22,26 described the constitutive occurence of apoptotic monocytes in cultures with low serum content. Addition of LPS, GM-CSF, M-CSF,27 or the monokines TNF-α and IL-1β, which represent monocyte-activating factors, could inhibit this process and lead to prolonged survival of the monocytes. Since LPS and M-CSF are able to induce the release of monokines, it has been suggested that paracrine and autocrine mechanisms control monocyte apoptosis. Our results show that PF4, like GM-CSF or M-CSF, effectively blocks programmed cell death in human monocytes. Stimulation of these cells with other chemokines, such as IP-10, RANTES, or fractalkine, were without effect on monocyte apoptosis. Surprisingly, IL-8 at a concentration of 1 μmol/L significantly reduced monocyte apoptosis to half of the level induced by PF4. Since unlike PF4, which is found at micromolar concentrations, IL-8–induced effects occurred at dosages much higher than those that were reported to occur physiologically, it appears rather unlikely that IL-8 constitutes a physiologically relevant modulator of monocyte apoptosis. Considering this, the latter function will be physiologically monospecific for PF4 among chemokines. Furthermore, our observation that IL-8–mediated prevention of apoptosis occurred independently of monocyte differentiation indicates that PF4 and IL-8 may act through different signaling pathways.
PF4-mediated abrogation of apoptosis was accompanied by the secretion of TNF-α but not IL-1β or GM-CSF. However, the lack of the capability of neutralizing antibodies directed against TNF-α to revert PF4-induced protection against apoptosis clearly demonstrated that this monokine is not involved in the PF4-mediated process. Since we did not find IL-1β or GM-CSF in culture supernatants of cells stimulated with PF4, and anti–GM-CSF antibodies did not antagonize the PF4-mediated effect, a participation of these monokines in regulating PF4-induced functions appears unlikely. At present, we cannot decide whether still other factors are involved as autocrine effectors or whether PF4 is able to directly induce monocyte rescue from programmed cell death. A well-characterized pathway leading to apoptosis is mediated by interaction of Fas ligand (FasL) with Fas receptor (CD95).37,38 Both Fas and FasL are expressed on almost all freshly isolated monocytes.39,40 Therefore, monocyte apoptosis can be triggered by activating Fas on monocytes after interaction with FasL on other leukocytes, or, vice versa, monocytes can induce apoptosis in other Fas-expressing cells.39 However, our observation that CD95 is completely lost after 3 days of culture on untreated cells whereas its expression is conserved on PF4-treated cells argues against the involvement of the Fas/FasL pathway in our system. Our results are in line with results reported by others that various factors (IL-1β, TNF-α, GM-CSF, and LPS) that promote prolonged survival of monocytes do not mediate a down-regulation of CD95.38,39 Most recently, Heidenreich et al21 suggested that down-regulation of CD14 represents a trigger for the induction of monocyte apoptosis. Indeed we could confirm that the loss of this surface marker is accompanied by spontaneous apoptosis in unstimulated cells, but on the other hand, we also observed down-regulation (to a lesser extent) of CD14 in PF4-treated cells that showed prolonged survival. From our data, we are not able to decide whether the residual low expression of CD14 on the latter cells may be sufficient for their survival or whether the regulation of CD14 is not involved in PF4-mediated inhibition of apoptosis. Since it has long been known that high concentrations of human serum allow monocytes to survive in long-term culture, the question may be raised whether this could be due to PF4 present in the serum. We have investigated this by using PF4-immunodepleted human serum (at 20%) for 3-day monocyte cultures and found no difference in the numbers of apoptotic cells as compared with cultures performed with nondepleted serum (unpublished results). Therefore, the serum constituents protecting monocytes from apoptosis are apparently not identical to PF4.
Besides the up-regulation of differentiation markers, monocytes stimulated with PF4 undergo changes in the expression of several other surface molecules. Unlike unstimulated, M-CSF–stimulated, or GM-CSF–stimulated monocytes (data not shown), PF4-treated cells displayed a strong down-regulation of HLA-DR antigen, which could suggest a substantial loss of their antigen-presenting capacity for T cells. Simultaneously, the costimulatory molecule CD86 (B7-2), but not CD80 (B7-1), was up-regulated during culture with PF4. B7-1 and B7-2 are ligands for 2 receptors on T cells (CD28 and CTLA-4) that function as accessory molecules in monocyte-dependent T-cell activation.41,42 However, whether there is a regulatory role for B7-molecules in the absence of HLA-DR is not clear. Our data suggest that PF4 induces the differentiation of monocytes into a subtype of macrophages that is different from that induced in vitro by GM-CSF, since the latter cells showed a high expression of HLA-DR but no modulation of the B7-antigens.43 Current investigations are on the way to clarify the regulatory role of PF4-stimulated monocytes in the activation of T-cell functions.
In conclusion, our results provide direct evidence that platelet-derived PF4 prolongs monocyte survival and induces the differentiation of monocytes into macrophages. This indicates that defined platelet products are involved in long-term regulatory processes of these cells and might support the differentiation of infiltrating monocytes into macrophages in vivo during intermediate and late stages of an inflammatory process.
Acknowledgments
We wish to thank Drs C. Schlenke and H. Klüter (Institute of Immunology and Transfusion Medicine, Medical University of Lübeck, Germany) for the generous supply of platelet concentrates. We acknowledge Dr A. Petersen (Research Center Borstel, Borstel, Germany) for performing sequence analysis of the PF4 preparations. We thank Dr R. Andreesen (Department of Hematology and Oncology, University of Regensburg, Germany) for providing antibodies directed against carboxypeptidase M/MAX1. We especially thank Mrs R. Bergmann and Mrs E. Kaltenhäuser for perfect technical assistance and Dr L. Bock for the preparation of PF4.
Supported in part by Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 415, Projekt A5.
Reprints:Frank Petersen, Department of Immunology and Cell Biology,
Research Center Borstel, Parkallee 22, D-23845 Borstel, Germany; e-mail: fpeters@fz-borstel.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal