Fetal liver kinase ligands (flk2L/flt3L) and stem cell factor (SCF) have been shown to promote natural killer (NK) cell differentiation from hematopoietic stem cell (HSC) precursors in vitro. However, the contribution of signaling through the receptors for these growth factors for in vivo NK cell development remains ill-defined. We have analyzed the role of the SCF receptor c-kit in NK cell differentiation by reconstituting NK-deficient mice with fetal liver (FL) HSCs of c-kit−/− (W/W) mice. Although c-kit−/−NK cells were generated inW/W chimeras, they were reduced in number, contained a lower percentage of CD45R (B220)+ cells, and were poorly cytolytic. In vitro experiments showed that generation of NK cells from FL precursors was reduced in the absence of c-kit signaling and that SCF promoted the survival of peripheral c-kit+ NK cells. We conclude that c-kit/SCF interactions in vivo are dispensable for the commitment of HSC to the NK lineage, but they provide essential signals for generating normal numbers of fully mature NK cells.
Natural killer (NK) cell differentiation is a multistep process that involves the commitment of hematopoietic stem cells (HSCs) to the NK cell lineage followed by further differentiation to yield functional mature NK cells. This process occurs primarily within the bone marrow (BM) microenvironment and requires cell-to-cell interactions and soluble factors derived from BM stromal cells. Based on a series of studies, models for NK cell development have been proposed whereby purified HSC populations could be made to differentiate into cells that can express NK markers and are capable of mediating natural cytotoxicity in vitro.1 William et al1 suggest that distinct phases in NK cell differentiation can be discerned. Early-acting cytokines, including interleukin-7 (IL-7), stem cell factor (SCF), and fetal liver kinase ligand (flk2L/flt3L), act on HSCs to commit them to the NK lineage with concomitant induction of the β subunit of the IL-15 receptor (IL2Rβ chain) expression. Subsequently, triggering of the IL-2Rβ/γc (common gamma chain) complex with IL-15 (or with high doses of IL-2) acts to further expand and differentiate these cells to fully mature NK cells.2 Of the above-mentioned cytokines or growth factors, none are capable of supporting NK cell development by themselves.2-4 However, certain early acting factors appear more potent in driving NK cell differentiation in vitro.4,5 This model provides a useful starting point to decipher NK cell development, although most of the data derive from in vitro experiments using cytokines at concentrations that may have little relevance to conditions normally encountered in vivo.
Administration of exogenous SCF, flk2L/flt3L, or IL-2 to humans or mice results in an augmentation in NK cell number and lytic activity,6-9 further supporting a role for these factors in promoting NK development in vivo. However, in these settings it is not clear whether the injected cytokines directly influence NK cell development or act indirectly by modifying the stromal cell microenvironment.
The analysis of natural mouse mutants and gene-targeted mice have made an important impact on our understanding of the in vivo roles of cytokines and their receptors in lymphoid development. IL-15 appears to play a major role in NK cell development. Mice deficient of any chains composing the receptor for IL-15, such as IL-15Rα, IL-2Rβ, or γc, are devoid of mature NK cells.10-12 In contrast, IL-2–deficient mice are capable of generating lytic NK cells.13 Thus, while both IL-15 and high doses of IL-2 can support NK development in vitro, only IL-15 appears to subserve this function in vivo. Targeted disruption of the interferon regulatory factor–1 causes a severe reduction of mature NK cells due to impaired IL-15 production within the BM microenvironment.14,15Treatment of mice with the BM localizing isotope strontium 89 (89Sr) has been reported to deplete NK cells by a similar mechanism.16 Thus blockade of IL-15 signaling in vivo results in defective NK differentiation and confirms the initial in vitro studies that suggest an important role for IL-15 in the generation, proliferation, and activation of human and murine NK cells.3,17 18
In contrast to IL-15, the role of flk2/flt3 and c-kit signaling for NK cell development in vivo is less well-defined. Flk2/flt3 and c-kit belong to a family of transmembrane receptor tyrosine kinases, which also includes the receptors for platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin, insulin growth factor–1 (IGF-1), and colony stimulating factor–1 (CSF-1).19 Both flk2/flt3 and c-kit are expressed by early multipotent HSCs and various subsets of lineage-committed cells including B, T, NK, erythroid, and mast cells.20-23 These 2 cytokines share some biological activities, but they maintain distinct and unique functions in the differentiation process of discrete cell lineages.24 For instance, targeted deletion of flk2/flt3 leads to deficiencies in primitive hematopoietic progenitors and early myeloid and B-lymphoid precursors.25 Preliminary observations inflk2L/flt3L−/− mice indicated an absence of a subset of NK cells bearing high levels of the NK1.1 marker,26 although the nature of this defect has not been defined. On the other hand, lack of c-kit/SCF interactions in mice homozygous for ac-kit−/− null allele (W/W) causes deficiencies in germ cells, melanocytes, HSCs, early lymphoid progenitors, mast cells, T cells, and red blood cells, which in turn can lead to severe anemia and death by day 10 of postnatal life.27 The lethal nature of the Wmutation has impeded any in-depth analysis of the role of c-kit on NK cell development, although mice with a viablec-kit mutation (W/Wv) have been shown to develop NK cells with reduced natural killing.28 Still, the Wv mutant receptor retains significant kinase activity (about 10% of wild type levels29), which precludes a definitive conclusion on the role of c-kit/SCF interactions in NK cell differentiation in vivo.
In this report, we address whether c-kit/SCF interactions are essential for NK cell differentiation by analyzing hematopoietic chimeras made by reconstituting alymphoid (T- B- NK-) RAG2/γc−/− mice with W/W c-kit−/− fetal liver cells. This complementation system is ideally suited for the analysis of genes involved in early lymphoid and NK cell differentiation, sinceRAG2/γc−/− mice (unlikeRAG2−/− mice) are severely depleted in lymphoid precursor cells and completely lack mature NK cells.30 With the data discussed below, we can conclude that signaling through c-kit is not required for commitment to the NK lineage, although c-kit plays a nonredundant role in the expansion of NK committed precursors and in the survival and final maturation of murine NK cells.
Materials and methods
Mice and generation of fetal liver hematopoietic chimeras
Mice with a null mutation in the γcchain10 were from the fourth generation backcross to the C57Bl/6 background. RAG2 mice31 were from the 10th generation backcross to C57Bl/6 (gift of B. Rocha, INSERM U345, Paris, France). Doubly deficient mice (RAG2/γc−/−) were obtained by intercrossing as described.30 We also used breeding stocks of WB-W/+ mice32(gift of Dr H.-R. Rodewald, Basel, Switzerland). Mice were intercrossed to generate the null allele W/W(c-kit−/−) and the control (c-kit+/+ or c-kit ±, hereafter referred to as wt) day 13 embryos. The morning of the vaginal plug discovery was designated as day 0. Fetal liver cell suspensions were obtained by passage of the tissue through a 23-gauge needle, and the genotypes were determined by fluorescence-activated cell sorter (FACS) analysis using an anti–c-kit monoclonal antibody (mAb). RAG2/γc−/− andRAG2−/− mice (more than 6 weeks of age) were irradiated with 0.3 Gy (gray) from a cobalt source, and 4 hours later they were injected intravenously with 4-8 × 105 FL cells as a source of HSCs. All mice received tetracycline and bactrim in the drinking water for the period following the FL cell transfer.
Flow cytometry
Single cell suspensions were prepared from spleen, thymus, liver, and BM cells. Erythrocytes were lysed in ammonium chloride, and the cells were resuspended in phosphate-buffered saline (PBS) with 3% fetal calf serum (FCS) and 0.01% sodium azide. The following mAbs (PharMingen, San Diego, CA) were directly conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), Tricolor (TRI), or biotin (biot) and were used for immunofluorescence analysis: CD117 (c-kit), CD122 (IL-2Rβ), CD3, CD4, CD8, TCRαβ, TCRγδ, CD24 (HSA), NK1.1, B220, CD90 (thymine [Thy-1]), DX5, CD2, Ly49A, Ly49C/I, Ly49D, CD43, immunoglobulin M (IgM), and IgD. Biotin conjugates were revealed by streptavidin-TRI (Caltag, San Francisco, CA). Cells (106) were first incubated with anti–fragment(Fc)γRII/III (hybridoma 2.4G2) and then stained with a mixture of biotinylated and fluorochrome-labeled mAbs at saturating concentrations, washed twice, and finally incubated with streptavidin-TRI. Analysis was performed on a fluorescence-activated cell sorter (FACS) flow cytometer (CellQuest software; Becton Dickinson, San Josè, CA). Dead cells were excuded by means of their forward and side scatters, and an electronic gate was set to acquire 5-10 × 103 lymphoid cells.
Lymphokine-activated killer cell cultures and cytotoxic assay
A chromium 51 (51Cr) release assay was used to measure NK lytic activity in vitro, as previously described.21 For natural cytotoxicity, mice were injected intraperitoneally with 200 μg of poly (I:C), and 40 hours later, to deplete macrophages, red cell–depleted splenocytes were incubated on plastic for 1 hour at 37°C. YAC-1 and EL-4 thymoma cells were maintained in complete medium (CM; RPMI-1640 with 10% FCS, 10-5 mol/L β-ME, 100 μg/mL streptomycin, and 100 units/mL penicillin). Target cells were labeled with 3.7 MBq (100 μCi) 51Cr (ICN Pharmaceutical, Costa Mesa, CA), and 2.5-5 × 103cells were incubated with graded numbers of effector cells in 200 units/L (μl) of medium for 4 hours. In some experiments, effector cells were CD122+DX5+ NK cells isolated from splenocytes by cell sorting.
To generate adherent–lymphokine activated killer (A-LAK) cells, red cell and macrophage-depleted splenocytes were cultured at 5 × 106 cells/mL in CM supplemented with 1000 units/mL of dihydrouridine IL-2 (huIL-2) (Peprotech, Rocky Hill, NJ) or with 0.5 μg of huIL-15 (Immunex, R&D Systems, Minneapolis, MN). After 3-4 days the nonadherent cells were removed, and the A-LAK cells were refed every 2-3 days and cultured until days 8-10. A-LAK cultures produced in this manner routinely contained more than 80% NK1.1+CD3−cells. For antibody-dependent cell cytotoxicity (ADCC), day 8-10 A-LAK cells were used as effectors, and target cells were EL-4 cells coated with anti-Thy 1.1 mAb, as previously described.21 Radioactivity released into the cell-free supernatant was measured, and the percentage of specific lysis was calculated as the following: 100 × (experimental release − spontaneous release) / (maximum release − spontaneous release).
Generation of NK cells in vitro from FL cells
Day 13 FL cells prepared from normal C57Bl/6 timed pregnancies were plated at 5 × 103 cells/mL in round-bottom microtitre plates in CM supplemented with 50 ng/mL muIL-7 (Peprotech), 100 ng/mL of huflk2L/flt3L (Immunex), and 100 ng/mL rat SCF (Peprotech). After 8 days, the cytokines were removed, and the cells were cultured with CM containing huIL-2 (1000 units/mL) for an additional 8-10 days.
Effects of SCF on mature NK cells in vitro
Purified splenic NK1.1+ c-kit+ and NK1.1+ c-kit− cells were isolated from RAG2−/−mice by cell sorting. Stringent sorting gates were applied to avoid cross-contamination of the 2 sorted subsets. Sorted cells (greater than 97% pure) were plated at 104 cells/well in round-bottom microtitre plates in 200 μL of CM supplemented with graded doses of huIL-2, in the presence or absence of 100 ng/mL muSCF. Cell viability was evaluated daily by trypan blue dye exclusion, and after 6 days of culture, cells were analyzed for cell-surface phenotype, cell cycle distribution, and cytotoxic activity. Cell- cycle analysis was performed using 7-aminoactinomycin-D (7 AAD) incorporation into saponin-permeabilized cells as described.32
Statistical analysis
Data were analyzed (Statview software, Version 4.5, SAS Institute, San Francisco, CA) applying the paired Student ttest. The null hypothesis was rejected, and the difference was assumed significant when P < .05.
Results
γc generation of FL → RAG/γc hematopoietic chimeras
We have recently developed a novel mouse strain harboring mutations in both the RAG2 and common cytokine receptorγc genes.30RAG2/γc−/−mice are alymphoid and have severely reduced numbers of BM and thymic lymphoid precursors, thereby making them ideal hosts for genetic complementation experiments analyzing lymphopoiesis. In order to define the role of the c-kit receptor tyrosine kinase in NK cell development, we reconstituted 0.3 Gy irradiatedRAG2/γc−/− mice with c-kit–deficient HSC precursors. W/W mice harbor a splice donor site mutation that causes deletion of the transmembrane region, thereby completely lacking cell-surface expression of c-kit molecules. The absence of c-kit results in severe anemia in these mice and death by day 10 after birth.27 In our experiments, c-kit–deficient embryos were identified by their extreme pallor, and day 13 FL cells were used as a source of HSCs. The genotypes were confirmed by flow cytometry using an anti–c-kit mAb (data not shown). While HSCs express c-kit, previous studies have shown that the content of HSCs in day 13 FL from SCF-deficient mice is no different from control mice.34 We therefore transferred equal numbers of FL cells from W/W or wt day 13 embryos, and 8 weeks posttransfer, analyzed thymic, splenic, and BM lymphoid populations in the RAG2/γc chimeras.
B- and T-cell development in hematopoietic chimeras
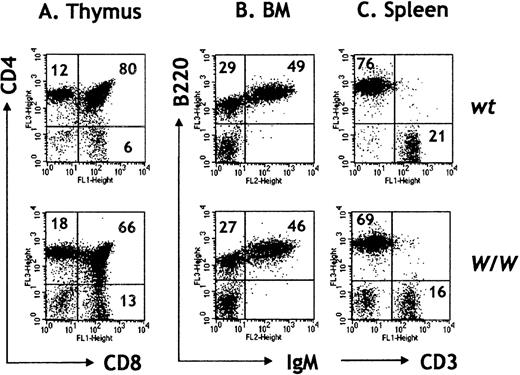
Consistent with previous reports,33 we found that B-cell development was permissive in the absence of c-kit (Figures1A-C). No obvious defects in BM B-cell development were observed, and the absolute numbers of pre-B (B220+IgM−) and mature B cells (B220+IgM+) were not significantly different between wt(more than RAG2/γc) and W/W (more thanRAG2/γc) chimeras (Table1, Figure 1B). In contrast, we found a 10- to 20-fold reduction in total thymocyte numbers in the absence of c-kit (Table 1). The diminished thymopoiesis after transfer of c-kit–deficient FL cells likely reflects the important role for c-kit/SCF interactions in the expansion of early thymic double-negative precursors, as described by Rodewald et al.35 Once past this critical stage, intrathymic development in the absence of c-kit appeared normal, and all mature thymocyte subsets could be identified in W/W chimeras, including single positive CD4 and CD8 αβ T cells, γδ T cells, and NK T cells (Figure 1A; data not shown). We confirmed previous results of Takeda et al33 demonstrating that c-kit–deficient FL cells fail to reconstitute the thymus of irradiatedRAG2−/− hosts (data not shown). The ability of W/W HSCs to repopulateRAG2/γc−/− but notRAG2−/− hosts can be explained by the severe reduction in both thymic and BM lymphoid precursors inRAG2/γc−/−mice30 (Table 1).RAG2/γc−/− mice thereby offer a less competitive host microenvironment thanRAG2−/− mice, where mutant (eg,c-kit−/−) HSCs can further differentiate. These results attest to the utility of theRAG2/γc strain for reconstitution experiments and the analysis of genes affecting HSCs and their immediate progeny.
T- and B-cell development in W/W chimeras.
Thymocytes and BM cells were depleted of red cells and stained with the indicated antibodies. Percentages are indicated within the dot plots. (A) Thymocyte numbers in W/W chimeras are much reduced, although thymopoiesis appears rather normal in the absence of c-kit. (B) BM pre-B (B220+ IgM−) and B cells (B220+ IgM+) are represented in normal percentages in W/Wchimeras. FACS profiles are representative of 4 independent experiments. (C) Reconstitution of splenic lymphoid populations with the presence of CD3+ T cells and B220+ B cells in W/W chimeras.
T- and B-cell development in W/W chimeras.
Thymocytes and BM cells were depleted of red cells and stained with the indicated antibodies. Percentages are indicated within the dot plots. (A) Thymocyte numbers in W/W chimeras are much reduced, although thymopoiesis appears rather normal in the absence of c-kit. (B) BM pre-B (B220+ IgM−) and B cells (B220+ IgM+) are represented in normal percentages in W/Wchimeras. FACS profiles are representative of 4 independent experiments. (C) Reconstitution of splenic lymphoid populations with the presence of CD3+ T cells and B220+ B cells in W/W chimeras.
Cell No (×105) in primary lymphoid organs of RAG2/γc chimeras reconstituted with W/W or wt FL cells
| Genotype of Fetal Liver . | Thymus Cells . | Bone Marrow Cells . | |||||
|---|---|---|---|---|---|---|---|
| Total . | Lymphocytes . | Total . | Lymphocytes . | NK1+CD3− . | Pre-B . | Mature B . | |
| Nonreconstituted (n = 4) | 0.4 ± 0.1 | ND | 72 ± 3 | 3.6 ± 1.7 | ND | ND | ND |
| wt (n = 7) | 452 ± 94 | 398 ± 94 | 106 ± 15 | 26 ± 5 | 0.31 ± 0.15 | 6.12 ± 2.2 | 11.9 ± 1.6 |
| W/W (n = 7) | 39 ± 24* | 32 ± 23* | 108 ± 8 | 23 ± 5 | 0.13 ± 0.04* | 4.72 ± 1.0 | 8.4 ± 1.9 |
| Genotype of Fetal Liver . | Thymus Cells . | Bone Marrow Cells . | |||||
|---|---|---|---|---|---|---|---|
| Total . | Lymphocytes . | Total . | Lymphocytes . | NK1+CD3− . | Pre-B . | Mature B . | |
| Nonreconstituted (n = 4) | 0.4 ± 0.1 | ND | 72 ± 3 | 3.6 ± 1.7 | ND | ND | ND |
| wt (n = 7) | 452 ± 94 | 398 ± 94 | 106 ± 15 | 26 ± 5 | 0.31 ± 0.15 | 6.12 ± 2.2 | 11.9 ± 1.6 |
| W/W (n = 7) | 39 ± 24* | 32 ± 23* | 108 ± 8 | 23 ± 5 | 0.13 ± 0.04* | 4.72 ± 1.0 | 8.4 ± 1.9 |
Eight to ten weeks posttransfer, thymus and BM (one femur) cells of transplanted mice and control nonreconstituted RAG2/γcchimeras were enumerated and stained with a combination of either α-CD8FITC and α-CD4TRIC, α-CD3FITC and α-NK1.1PE, or α-CD19FITC and α-IgMPE mAbs. Lymphoid gates were set based on forward and side scatter parameters, and the absolute numbers were calculated as follows: total cell No × percent of lymphoid cells × percent of cells stained positive for a given marker. Pre-B cells were defined as CD19+IgM−, and mature B cells were defined as CD19+ IgM+.
ND indicates not detected.
Indicates P < .05 for W/W versus wt chimeras.
IgM+/IgD+ B cells and CD4+ and CD8+ αβ T cells seed the peripheral lymphoid organs in W/W chimeras more thanRAG2/γc chimeras (Figure 1C; data not shown). Total splenic lymphocyte numbers were slightly reduced in the absence of c-kit, and this decrease was largely confined to the T-cell compartment. Thus, splenic CD3+ cells were 6.5 ± 1.8 × 106 in wt (n = 7) and 4.6 ± 0.7 × 106 in W/W chimeras (n = 7; P < .05) (Figure 1C). However, compared to the dramatic decrease in thymopoiesis (5%-10% of normal), peripheral T-cell numbers were about 70% of controls, suggesting that peripheral T-cell expansion had occurred. Together these results suggest that T lymphocytes do not require continual c-kit/SCF interactions for peripheral homeostasis.
NK cell development in the absence of c-kit
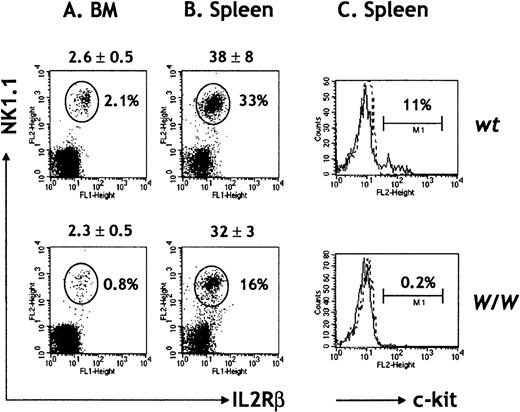
Since all NK cells in RAG2/γc chimeras are donor-derived,30,36 any abnormalities in NK cell differentiation in W/W chimeras result from cell intrinsic defects due to lack of c-kit signaling. The ability of W/W FL cells to fully replenish total BM lymphoid cellularity and BM and splenic B-cell compartments suggests that HSC function in the absence of c-kit was not a limiting factor.34 NK cells were detected in the BM and spleens of W/W FL chimeras (Figure2A and B), but absolute numbers of BM NK cells were only 40% of normal in the absence of c-kit. The difference was found to be statistically significant (P < .05). Thus, c-kit/SCF interactions are dispensable for NK lineage commitment but are required for normal NK cell differentiation (Table 1, Figure 2A). Total numbers of splenic NK cells were similarly decreased inW/W chimeras (Figure 2B), which suggests that either peripheral expansion is not a mechanism to regulate NK cell homeostasis or that this process normally relies on c-kit signaling pathways. In agreement with previous reports,1 we found that about 10% of peripheral NK1.1+ CD3-NK cells from wt chimeras expressed c-kit, while W/W NK cells were c-kit−, as expected (Figure 2C).
NK cells in W/W chimeras.
BM (A) and spleen cells (B) were red-cell depleted and stained with a combination of antibodies for IL-2RβFITC, NK1.1PE, TCRαβbiot, and CD19biot. Absolute cell numbers (×106) are indicated on top of each dot plot and refer to the mean ± SD of 7 chimeras per group. An electronic gate was set to exclude TCRαβ+ T cells and CD19+ B cells, and the percentage of IL-2Rβ+ NK1.1+ NK cells was calculated. (C) Spleen cells were stained for DX5FITC, TCRαβbiot, and c-kitPE. The percentages of c-kit+ DX5+ TCRαβ− NK cells are indicated. Results are representative of 5 independent experiments.
NK cells in W/W chimeras.
BM (A) and spleen cells (B) were red-cell depleted and stained with a combination of antibodies for IL-2RβFITC, NK1.1PE, TCRαβbiot, and CD19biot. Absolute cell numbers (×106) are indicated on top of each dot plot and refer to the mean ± SD of 7 chimeras per group. An electronic gate was set to exclude TCRαβ+ T cells and CD19+ B cells, and the percentage of IL-2Rβ+ NK1.1+ NK cells was calculated. (C) Spleen cells were stained for DX5FITC, TCRαβbiot, and c-kitPE. The percentages of c-kit+ DX5+ TCRαβ− NK cells are indicated. Results are representative of 5 independent experiments.
In light of the existence of a bipotent NK/T thymic precursor, the development of thymic NK cells and NK-T cells in the absence of c-kit was also evaluated. Both of these minor thymic lymphoid subsets could be detected in W/W chimeras, although their absolute numbers were clearly reduced compared to control chimeras (NK:wt = 5.95 ± 3.0 × 104 cells versusW/W = 0.5 ± 0.3 × 104 cells; NK-T:wt = 15.7 ± 5.6 × 104 cells versusW/W = 3.4 ± 1.7 × 104 cells; n = 4 for each genotype; P < .05). The reduction in absolute cell numbers of thymic NK and NK-T cells paralleled the overall reduction in thymic cellularity. These results suggest that thymic T, NK, and NK-T cells develop via a c-kit–dependent early thymic precursor, and the results argue against a major pathway of NK cells generated from a putative NK/T bipotent precursor.
Phenotype and function of c-kit−/−NK cells
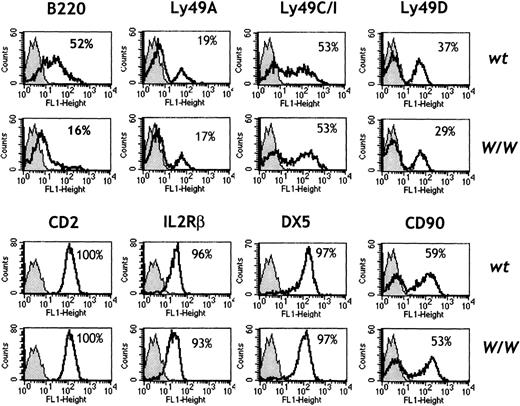
The phenotypic maturation of splenic NK cells from wt andW/W chimeras was characterized by flow cytometry using a panel of cell-surface markers (Figure 3). NK cells that were c-kit–deficient expressed normal levels of CD2, IL-2Rβ, NK1.1, DX5, and CD90 (Thy-1). Percentages and levels of Ly49 family members (including Ly49A, Ly49C/I, and Ly49D) were normal inW/W chimeras, which suggests that NK cell “education” with respect to inhibitory receptors for MHC class I molecules does not critically involve c-kit. Interestingly, c-kit–deficient NK cells showed markedly reduced expression of the B220 antigen (Figure 3). Similar results were obtained during analysis of BM NK cells (data not shown). As B220 expression increases on NK cells upon activation,16 we further compared the functional capacity of wt and c-kit–deficient W/W NK cells ex vivo.
Phenotype of W/W NK cells.
Spleen cells were red-cell depleted and stained with the indicated FITC-labeled antibodies in combination with NK1.1PE, TCRαβbiot, and CD19biot. An electronic gate was set to exclude TCRαβ+ T cells and CD19+B cells. The percentage of NK1.1+ NK cells positive for the indicated marker (white histograms) was calculated over the background of an isotype-matched control mAb (gray histograms). Results are representative of 3 independent experiments comprising 6 wt and 6 W/W chimeras.
Phenotype of W/W NK cells.
Spleen cells were red-cell depleted and stained with the indicated FITC-labeled antibodies in combination with NK1.1PE, TCRαβbiot, and CD19biot. An electronic gate was set to exclude TCRαβ+ T cells and CD19+B cells. The percentage of NK1.1+ NK cells positive for the indicated marker (white histograms) was calculated over the background of an isotype-matched control mAb (gray histograms). Results are representative of 3 independent experiments comprising 6 wt and 6 W/W chimeras.
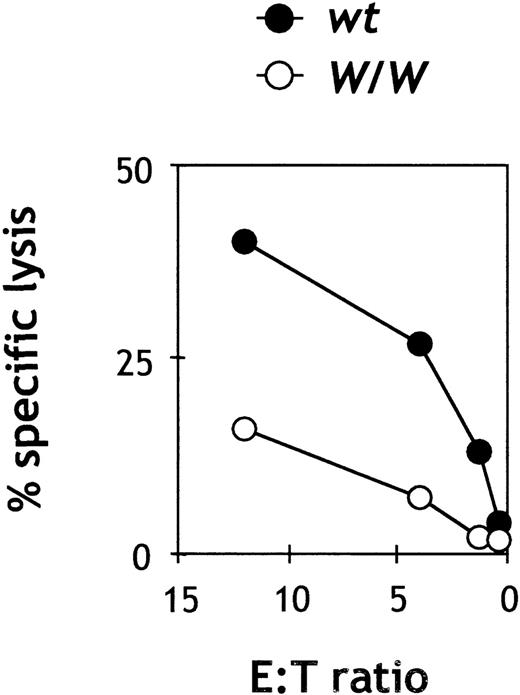
Previous studies had shown that unfractionated splenocyte populations from mice with a viable c-kit mutation (W/Wv) had a decreased level of natural killing.28 However, since absolute NK cell numbers were not determined in those studies, it was not possible to precisely define the nature of the functional defect. We found that total spleen cell preparations from W/W chimeras demonstrated less than one-third of the natural killing lytic activity as compared with wtsplenocytes at equivalent effector:target ratios (n = 5; data not shown), confirming the initial data by Seaman and Talal.28In order to determine the lytic ability ofc-kit−/−NK cells on a per-cell basis, we purified wt and W/W splenic NK cells by cell sorting. As shown in Figure 4, c-kit–deficient W/W NK cells showed less than half of the lytic capacity of wt NK cells against YAC-1 targets on a per-cell basis, thus confirming an intrinsic defect in natural killing of NK cells in the absence of c-kit.
Cytotoxic activity of purified W/W NK cells.
Spleen cells were depleted of red cells, B cells, and T cells and stained with IL-2Rβ and DX5. Double-positive NK cells were sorted and plated at the indicated E/T ratios with 2500-5000 YAC-1 target cells. All assays were done in duplicate or triplicate. Results are representative of 2 independent experiments. The spontaneous release was less than 10% of the maximal release.
Cytotoxic activity of purified W/W NK cells.
Spleen cells were depleted of red cells, B cells, and T cells and stained with IL-2Rβ and DX5. Double-positive NK cells were sorted and plated at the indicated E/T ratios with 2500-5000 YAC-1 target cells. All assays were done in duplicate or triplicate. Results are representative of 2 independent experiments. The spontaneous release was less than 10% of the maximal release.
Restoration of NK cell differentiation in vitro
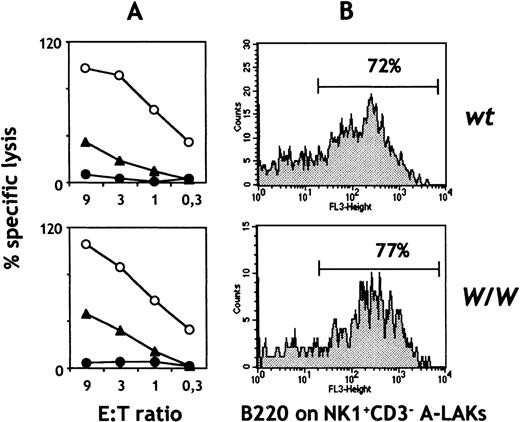
Combined signaling through c-kit and IL-2/IL-15 receptors has been shown to synergistically enhance NK cell expansion and lytic activity both in vitro and in vivo.8 We therefore tested whether exposure of c-kit–deficient NK cells to IL-2 or IL-15 in vitro (thereby generating A-LAKs) could correct their functional and phenotypic defects. In contrast to freshly isolated W/W NK cells, A-LAK cells from W/W chimeras lysed YAC-1 targets in a fashion indistinguishable from controls (Figure5A). A-LAK cells that were c-kit–deficient also mediated normal levels of ADCC. In parallel with the restoration of natural killing, A-LAK cells from W/W chimeras also upregulated B220 surface expression (Figure 5B). Similar results were obtained with IL-15 (data not shown). Taken together, these results suggest that the abnormal differentiation of c-kit–deficient NK cells in vivo can be corrected in vitro by signaling through the IL-2/IL-15 receptor.
Cytotoxic activity and phenotype of IL-2–activatedW/W NK cells.
(A) Spleen cells were depleted of red cells, B cells, and T cells, and the remaining cells were used to generate A-LAK cells. Day 7-10 A-LAK cells were used as effectors against YAC-1 cells (○) or EL-4 cells in the presence (▴) or absence of α-CD90 antibodies (•). All assays were done in duplicate or triplicate. (B) Day 7-10 A-LAK cells were stained with antibodies for CD3FITC, NK1.1PE, B220TRIC, and the expression of B220 on NK1.1+ CD3−NK cells was calculated. Data are representative of 3 independent experiments. Similar data were obtained with IL-15 (n = 2; data not shown).
Cytotoxic activity and phenotype of IL-2–activatedW/W NK cells.
(A) Spleen cells were depleted of red cells, B cells, and T cells, and the remaining cells were used to generate A-LAK cells. Day 7-10 A-LAK cells were used as effectors against YAC-1 cells (○) or EL-4 cells in the presence (▴) or absence of α-CD90 antibodies (•). All assays were done in duplicate or triplicate. (B) Day 7-10 A-LAK cells were stained with antibodies for CD3FITC, NK1.1PE, B220TRIC, and the expression of B220 on NK1.1+ CD3−NK cells was calculated. Data are representative of 3 independent experiments. Similar data were obtained with IL-15 (n = 2; data not shown).
SCF provides an essential signal for expansion of NK cell precursors
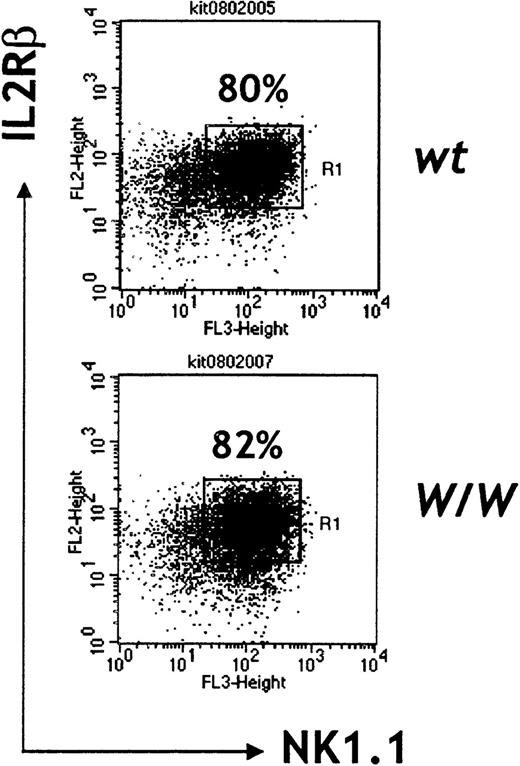
Our observations in W/W chimeras suggest that c-kit/SCF interactions in vivo are essential for generation of normal numbers of NK cells and for full differentiation of this lymphoid subset. To gain more insight into the stages at which c-kit signaling operates in NK cell development, the 2-step culture system described by Williams and colleagues2 was used to compare the cytokine requirements for differentiating NK cells from wt andW/W day 13 FL cells. When cultured in a mixture of IL-7, SCF, and flk2L/flt3L followed by high-dose IL-2, W/W FL cells developed similar frequencies of IL2Rβ+ NK1.1− and IL2Rβ+ NK1.1+ cells as compared with wt FL cells (Table2, Figure 6). However, total cell numbers were 3- to 4-fold reduced in W/Wcultures before the addition of IL-2 (data not shown). At the end of the culture period, c-kit−/−W/WNK cells were found to be less numerous than wt NK cells (Table2). These results confirm that SCF/c-kit interactions are not required for NK commitment, but they show that SCF provides an essential signal for the expansion of NK precursors, which possibly occurs during the acquisition of IL-15 responsiveness (up-regulation of IL2Rβ). NK cells generated from W/W FL cultures were as cytolytic aswt-derived NK cells (Table 2), which confirms that stimulation with high doses of IL-2 (or IL-15) in vitro can compensate for the lack of c-kit signaling and suggests that SCF may synergize with IL-15 in vivo to induce full lytic competence.
Effect of c-kit/SCF interactions on the expansion of NK cell precursors
| Genotype . | Total . | IL-2Rβ+ NK1.1− . | IL-2Rβ+ NK1.1+ . | Specific Lysis . |
|---|---|---|---|---|
| wt | 43.7 | 40.2 | 35.0 | 68% |
| W/W | 13.4 | 11.6 | 10.9 | 73% |
| Genotype . | Total . | IL-2Rβ+ NK1.1− . | IL-2Rβ+ NK1.1+ . | Specific Lysis . |
|---|---|---|---|---|
| wt | 43.7 | 40.2 | 35.0 | 68% |
| W/W | 13.4 | 11.6 | 10.9 | 73% |
Day 13 FL cells were plated at 1000 cells/well and cultured for 8 days in SCF (100 ng/mL), IL-7 (50 ng/mL), and flk2/flt3L (100 ng/mL). Thereafter, cytokines were removed, and cells were cultured for an additional 8 days in IL-2 (1000 units/mL). At the end of the culture period, cells were enumerated, stained with mAbs specific for IL-2Rβ and NK1.1, and tested in cytotoxic assays against YAC targets at an E:T ratio of 9:1. Cell numbers are expressed in ×103/well. Results are representative of two independent experiments.
Surface marker expression of in vitro–generated NK cells.
Day 13 FL cells were cultured in the presence of flk2L /flt3L, SCF, and IL-7 for a week followed by culture in IL-2 (1000 units/mL) alone, for an additional week. Representative of 2 independent experiments.
Surface marker expression of in vitro–generated NK cells.
Day 13 FL cells were cultured in the presence of flk2L /flt3L, SCF, and IL-7 for a week followed by culture in IL-2 (1000 units/mL) alone, for an additional week. Representative of 2 independent experiments.
Characterization of c-kit/SCF interactions in mature NK cells
Evidence for direct effects of SCF on mature murine NK cells has not been provided to date. Previous studies1,21 have shown that a subset of mature murine and human NK cells express c-kit, and human purified c-kit+ NK cells have been reported to show a lower cytotoxic activity, which suggests that this subset is somewhat less mature.21 In line with these studies, we found that freshly isolated c-kit+ murine NK cells have lower cytotoxic activity (70%-80%) than the c-kit–NK subset (data not shown). Studies by Caligiuri and colleagues8 have demonstrated that in vivo injection of SCF can enhance IL-2–mediated expansion of NK cells, which presumably acted at the level of NK cell precursors.
To characterize the potential mechanisms by which c-kit/SCF interactions act during NK cell differentiation, we analyzed the ability of SCF to promote survival, proliferation, and cytolytic activity of ex vivo purified c-kit+ and c-kit− NK1.1+cells from the pool of splenic NK cells. When c-kit+ NK cells were incubated with high doses of huIL-2 (1000 units/mL), the addition of SCF resulted in a modest (1.5- to 2-fold) increase in cell yield (Figure 7, Table3), although the cell viability (greater than 90%) and the percentage of cycling cells (about 30%) of c-kit+ cells cultured in high doses of IL-2 were not significantly different in the presence or absence of SCF (Table 3). In addition, the maximal lytic activity was comparable between c-kit+ and c-kit− NK cells cultured under conditions of high IL-2 concentrations (Table 3). These results are consistent with the ability of high doses of IL-2 to stimulate normal lytic activity in W/W NK cells.
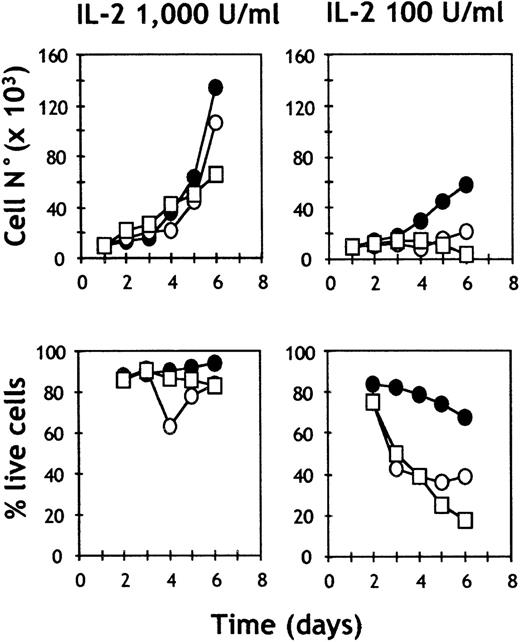
Proliferation and cell survival of purified c-kit− and c-kit+ NK cells.
Splenic NK cells were sorted by FACS in c-kit+ (○) and c-kit− (□) subsets, plated at 104 cells/well in CM supplemented with the indicated doses of IL-2 in the presence (•) or absence (○, □) of SCF (100 ng/mL). Plots indicate the numbers and percentages of live cells evaluated by trypan blue exclusion. Representative of 2 independent experiments.
Proliferation and cell survival of purified c-kit− and c-kit+ NK cells.
Splenic NK cells were sorted by FACS in c-kit+ (○) and c-kit− (□) subsets, plated at 104 cells/well in CM supplemented with the indicated doses of IL-2 in the presence (•) or absence (○, □) of SCF (100 ng/mL). Plots indicate the numbers and percentages of live cells evaluated by trypan blue exclusion. Representative of 2 independent experiments.
Survival, cell cycle progression, and lytic activity of splenic NK cells induced by SCF and IL-2
| c-kit Expression . | Culture Conditions3-150 . | Total Cell No3-151 . | Live (%) Cells . | % Apoptotis . | Cycling (%) Cells . | Specific (%) Lysis . | |
|---|---|---|---|---|---|---|---|
| SCF . | IL-2 . | ||||||
| + | + | high | 180 | 95 | 1.7 | 32 | 78 |
| + | − | high | 124 | 90 | 2.0 | 34 | 84 |
| − | − | high | 105 | 90 | 1.4 | 28 | 89 |
| + | + | low | 83 | 71 | 4.2 | 25 | 79 |
| + | − | low | 76 | 23 | 12 | 11 | 78 |
| − | − | low | 81 | 18 | 21 | 5.0 | 34 |
| c-kit Expression . | Culture Conditions3-150 . | Total Cell No3-151 . | Live (%) Cells . | % Apoptotis . | Cycling (%) Cells . | Specific (%) Lysis . | |
|---|---|---|---|---|---|---|---|
| SCF . | IL-2 . | ||||||
| + | + | high | 180 | 95 | 1.7 | 32 | 78 |
| + | − | high | 124 | 90 | 2.0 | 34 | 84 |
| − | − | high | 105 | 90 | 1.4 | 28 | 89 |
| + | + | low | 83 | 71 | 4.2 | 25 | 79 |
| + | − | low | 76 | 23 | 12 | 11 | 78 |
| − | − | low | 81 | 18 | 21 | 5.0 | 34 |
Splenic c-kit+ and c-kit− NK cells were purified by cell sorting from RAG2−/− mice and plated at 104 cells/well. After 6 days of culture, cells were harvested and enumerated. Dead cells were identified by trypan blue staining. A DNA content analysis was performed by flow cytometry of 7AAD staining, which distinguishes apoptotic cells (hypodiploid) from resting and cycling cells. A 51Cr release assay was performed to assess the lytic activity of effectors against YAC-1 targets at 9:1 E:T ratio. Data are mean values from two experiments.
Cytokine concentrations were 100 ng/mL SCF; 100-1000 units/mL IL-2.
The total cell numbers/well (×103) include both live and dead cells.
In contrast, when c-kit+ or c-kit− NK cells were cultured in a suboptimal concentration of IL-2 (100 units/mL), SCF had a clear effect on survival, cell cycle progression, and lytic activity of c-kit+ NK cells (Table 3). Survival in the presence of SCF was 70%, while in the absence of SCF, few c-kit+ (23%) or c-kit− (18%) NK cells were viable after the 6-day culture period. In the absence of SCF, fewer c-kit+ NK cells were cycling (11%), and more cells were apoptotic (12%), based on their DNA content. In contrast, a normal rate of cycling cells (25%) and apoptotic cells (4%) were detected in c-kit+ NK cells cultured in the presence of SCF. This results in the highest absolute cell yield (Table 3, Figure 7). Taken together, the results suggest that SCF can provide survival signals to c-kit+ mature NK cells.
Discussion
A number of soluble factors that play important roles in the generation of NK cells from hematopoietic precursors have been identified.1 It is now generally accepted that IL-15 is a dominant cytokine for NK cell development. Triggering of the IL-2Rβ/γc receptor complex by IL-15 (or mimicking this signal using high doses of IL-2) is required in vitro for NK cell differentiation from HSCs, while mice lacking IL-15 receptor components are NK deficient.3,10-12,14-16,18 Still, IL-15 alone only poorly promotes in vitro NK cell development,2,3 and additional factors may intervene in this process. The ligands for the receptor tyrosine kinases c-kit and flk2/flt3 (SCF and flk2L/flt3L, respectively) are 2 growth factors that play a role in the differentiation of multiple hematopoietic cell lineages.23,37 In vitro studies have shown that both SCF and flk2L/flt3L can significantly enhance the expansion of NK cells from HSCs in combination with IL-2 or IL-15.3,4Williams et al2 and Yu et al5 have demonstrated that flk2L/flt3L induce expression of the IL-2Rβ chain in murine and human stem cell precursors. Together, these results suggest a 2-stage model for NK cell differentiation, requiring first, c-kit and flk2/flt3 signaling, and second, IL-15 receptor signaling.1
The ability of either flk2L/flt3L or SCF to promote NK cell development in concert with IL-15 would be in agreement with presumed redundant roles for these 2 receptor tyrosine kinases in NK cell differentiation. However, while the in vitro data are compelling, little is known about the essential roles of these 2 pathways for NK development in vivo. Preliminary observations in mice with a null mutation in the flk2L/flt3L indicated an absence of NK1.1highcells,26 although the nature of this defect has not yet been fully explored. The NK cell deficiency inflk2L/flt3L−/−mice would suggest, however, that c-kit and flk2/flt3 signaling pathways are not entirely redundant in this regard. Seaman and Talal analyzed NK cells in viable (W/Wv) c-kit mutant mice and suggested that c-kit was involved in the acquisition of natural cytotoxicity.28However, these studies raised the question of whether c-kit was essential for NK cell development, since the Wvmutation retains significant kinase activity.29 We report here that c-kit−/−W/W FL cells generate reduced numbers of NK cells in vitro and only partially repopulate the NK cell pool in alymphoid RAG2/γc hosts. In addition, the W/W NK cells generated in vivo show defective killing. These results provide clear evidence for a nonredundant role of c-kit in the in vivo differentiation of murine NK cells. Together, the available data support the notion of unique (and perhaps additive4) roles for c-kit and flk2/flt3 signaling in NK cell differentiation.
While the precise action of SCF on developing NK precursors remains to be defined, a major role for c-kit/SCF interactions in the induction of IL-15 responsiveness appears unlikely, as IL2Rβ expression was comparable on wt and W/W NK cells both in vivo and in vitro (Figures 3 and 6). Assuming that this event restricts cells to the NK cell lineage, we can conclude that c-kit/SCF interactions are dispensable for NK cell commitment. Consequently, flk2L/flt3L might act as the dominant factor in NK cell determination in vivo. In contrast, c-kit signaling is required for generating normal numbers of NK cells. We would propose that c-kit/SCF interactions augment absolute numbers of BM and splenic NK cells by acting as a survival factor or (less likely) by inducing proliferation at the level of NK cell precursors. In this way, triggering of flk2/flt3 and c-kit on early lymphoid progenitors would provide distinct yet synergistic signals for NK cell differentiation.
Previous studies from Fehniger and colleagues8 suggested that SCF could synergize with IL-2 to promote proliferation of mature c-kit+ human NK cell subsets, although it did not appear that SCF had a direct effect on NK cell proliferation. The same group showed that in vivo SCF administration in mice also resulted in higher yields of NK cells, but studies done on bulk splenic populations did not show any direct effect of SCF on NK cell survival or proliferation.8Our in vitro data demonstrate that under conditions where other growth factors may be limiting, SCF can provide an essential signal for survival and cell cycle progression of the c-kit+ cells among the pool of mature NK cells. These c-kit/SCF interactions appear to act in an anti-apoptotic fashion and provide protection from cell death in part through the up-regulation of Bcl-2 levels.38 Taken together, these studies support a role for c-kit/SCF interactions in the survival of a subset of mature NK cells and their precursors in both mice and man.
The reduction in peripheral NK cell function in the absence of c-kit is noteworthy. Both lytic activity of freshly isolatedc-kit−/− W/W NK cells and their activation status (as evidenced by B220 expression) were reduced. These results would suggest that c-kit signals are required for the final maturation of NK cells, and they are in line with previous observations showing that human c-kit+ NK cells appear to be less mature.21 The functional defects inc-kit−/−NK cells could be reversed by exposure to IL-2 in vitro, which suggests that this phenotype could be overcome by compensatory pathways. These results are consistent with previous reports which have demonstrated that IL-2 and SCF can act synergistically to enhance NK cell expansion and lytic activity both in vitro and in vivo.8 We would propose that SCF not only expands the number of NK cell precursors, but that it also acts in synergy with IL-15 to drive full maturation of NK cells in vivo.
In summary, c-kit/SCF interactions are essential for the normal expansion of NK cell precursors and for the full maturation and survival of mature NK cells. In view of a role for c-kit/SCF interactions in generation of NK cell mediated functions, we are currently investigating the role of c-kit–deficient NK cells during in vivo responses to infectious pathogens and during tumor rejection.
Acknowledgments
We would like to thank Dr Hans Reimer Rodewald (Basel, Switzerland) for discussions and for kindly providing WB−W/+ mice; Drs Ana Cumano (Paris, France) and Emma Colucci-Guyon (Paris, France) for critically reviewing the manuscript; and the anonymous reviewers for their helpful comments.
Supported by grants from INSERM (Paris, France), Ligue Contre le Cancer (Paris, France), and Association pour la Recherche contre la Cancer (Paris, France) and by a fellowship from INSERM (F. Colucci).
Reprints:James P. Di Santo, Laboratory for Cytokines and Lymphoid Development, Institut Pasteur, 25, rue Dr. Roux, 75015 Paris, France; e-mail: disanto@pasteur.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal