PG27, an active fraction purified from an extract of a Chinese herb,Tripterygium wilfordii hook f, was used to prevent graft-versus-host disease (GVHD) in a murine model. Lethally irradiated BALB/c (H-2d) recipients of B10.D2 (H-2d) donor grafts were given daily intraperitoneal injections of PG27 (40 mg/kg per day) for the first 35 days after transplantation. Control mice were given daily injections of solvent vehicle (Ethanol and Cremophor EL). All the control recipients (15/15) died of GVHD within 90 days, but all the recipients given prophylactic treatment with PG27 (15/15) survived beyond 100 days without any signs of GVHD. Furthermore, the GVHD-free recipients were used as donors, and their bone marrow and spleen cells were transplanted into lethally irradiated normal BALB/c (same party) or lethally irradiated normal C3H (H-2k, third party) mice. Although 10 of 10 same-party recipients survived more than 100 days without any signs of GVHD, 10 of 10 third-party C3H recipients died of GVHD within 40 days. Further studies of PG27 in the murine BCL1 leukemia/lymphoma model demonstrated that animals treated with PG27 partially retained the graft-versus-leukemia (GVL) effect of the graft without GVHD. These results suggest that treatment with PG27 induces host-specific tolerance and retains the GVL effect of allogeneic marrow grafts.
Bone marrow transplantation (BMT) in humans is curative for certain malignant and nonmalignant diseases.1Graft-versus-host-disease (GVHD) represents a major barrier to successful allogeneic BMT. There have been many advances in the prevention and treatment of GVHD, including cyclosporine, FK506, and combination therapies. However, GVHD continues to account for significant morbidity and mortality after allogeneic transplantation. The incidence of GVHD varies from 26% to 76% (average, 30%), even though recipient and donor sibling pairs are matched at the major histocompatibility complex (MHC) level and intensive immunosuppression is used.2,3 The mortality rate can be as high as 50% in patients in whom GVHD develops.1 Therefore, the search for alternative immunosuppressive drugs effective in GVHD prevention continues.
The extracts of a Chinese herb, Tripterygium wilfordii hook f, have potent antiinflammatory and immunosuppressive properties and have been used successfully in traditional Chinese medicine for the treatment of rheumatoid arthritis.4 PG27, a refined extract of T wilfordii, prolongs heart and kidney allograft survival in rat transplantation models and displays synergy with cyclosporine in preventing cardiac and renal allotransplant rejection (Fidler J, manuscript in preparation). The combination of PG27 with cyclosporine substantially prolongs hamster cardiac xenograft survival in rat recipients and inhibits the production of serum antihamster IgM and IgG xenoantibodies, under conditions in which single drug therapies are ineffective. In this study, we used PG27 to prevent the development of GVHD in a murine bone marrow transplantation model. We demonstrated that PG27 treatment induced host-specific immune tolerance while it retained the GVL effect of allogeneic marrow grafts.Materials and methods
Animals
B10.D2/nSnJ (H-2d), BALB/c (H-2d), C57BL/6 (H-2b), and C3H (H-2k) mice were obtained from the breeding colony of the Department of Comparative Medicine at the Stanford University School of Medicine.
Primary mixed lymphocyte reactions across major histocompatibility barriers
2.5 × 105 BALB/c spleen cells were plated with 5 × 105 irradiated (30 Gy, cesium source) C57BL/6 spleen cell stimulators in the presence or absence of PG27. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine/well for an additional 16 hours. Results are mean count per minute (cpm) from triplicate cultures.5
Secondary mixed lymphocyte reactions across minor histocompatibility barriers
B10.D2/nSnJ mice were immunized intraperitoneally with 50 × 106 irradiated (30 Gy) BALB/c spleen cells. The primed spleen cells from these B10.D2/nSnJ mice were harvested 21 days later. In the presence or absence of PG272, 5 × 105primed spleen cells were plated with 5 × 105irradiated (30 Gy) BALB/c spleen cell stimulators. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine/well for an additional 16 hours. Results are mean count per minute (cpm) from triplicate cultures.6
Primary bone marrow transplantation
For the induction of GVHD, 2.5 × 106 B10.D2/nSnJ bone marrow cells and 10 × 106 B10.D2/nSnJ spleen cells were injected intravenously into lethally irradiated (8 Gy) BALB/c recipients' tail veins. B10.D2/nSnJ donor and BALB/c recipient mice (both H-2d) differ at multiple minor histocompatibility loci.7 Recipient mice were 12 to 13 weeks old at the time of transplantation. Each experimental group consisted of 5 to 10 animals, and each experiment was repeated 2 to 3 times.
PG27
PG27, a refined extract of the Chinese herb T. wilfordiihook f, was obtained from Pharmagenesis (Palo Alto, CA).
PG27 treatment
Recipient BALB/c mice received 40 mg/kg per day PG27 or solvent vehicle Ethanol (Gold Shield Chemical, Hayward, CA) and Cremophor EL (Sigma Chemical, St Louis, MO) intraperitoneally daily for the first 5 weeks after transplantation. Treatment was discontinued on day 36.
Assessment of GVHD
Polymerase chain reaction
Engraftment of donor bone marrow was documented by polymerase chain reaction (PCR) analysis of a polymorphic microsatellite region within the murine IL-1β gene. DNA was prepared from peripheral blood mononuclear cells or spleen cells 80 to 100 days after transplantation according to standard protocols.5,9 Primer sequences were 5′-CCAAGCTTCCTTGTGCAAGTA-3′ and 5′-AAGCCCAAAGTCCATCAGTGG-3′.10 Oligonucleotides were synthesized on a 391 DNA synthesizer (Applied Biosystems, Foster City, CA). Conditions for PCR were 25 μL total volume with 250 ng genomic DNA as template, 25 pmol primers, 0.4 mmol/L dNTP, 3 mmol/L Mg2+, 2.5 μL 10 × PCR buffer, 1 U AmpliTAQ DNA-polymerase (Perkin-Elmer, Emeryville, CA). Amplification was performed for 30 cycles with 1 minute denaturation at 94°C, 1 minute annealing at 57°C, and 1 minute elongation at 72°C.
Adoptive bone marrow transplantation
In the adoptive transplantation procedure, the PG27-treated GVHD-free recipients on day 100 after BMT were used as adoptive donors. Twelve- to 13-week-old BALB/c mice were lethally irradiated (8 Gy) and used as same-party adoptive recipients, and 12- to 13-week-old lethally irradiated (9.5 Gy) C3H mice were used as third-party adoptive recipients. Adoptive recipients were irradiated 12 hours before adoptive transplantation; 2.5 × 105 bone marrow cells and 10 × 106 spleen cells from the adoptive donors were injected intravenously into BALB/c or C3H adoptive recipients. In control groups, normal B10.D2/nSnJ mice were used as donors, and the same numbers of bone marrow and spleen cells were transplanted into lethally irradiated BALB/c or C3H mice. These adoptive recipients were observed for 100 days without any treatment after BMT.
BCL1 leukemia/lymphoma cells
Treatment of BCL1 leukemia/lymphoma with bone marrow and spleen cell transplantation and PG27
BALB/c mice were injected intraperitoneally with 10 × 106 BCL1 cells 24 hours before transplantation. On the day of transplantation, they were lethally (8 Gy) irradiated, then injected intravenously with B10D2 bone marrow cells (2.5 × 106) or bone marrow cells (2.5 × 106) plus spleen cells (10 × 106). These BCL1-bearing recipients were divided into 3 groups. Group A BCL1-bearing recipients were given bone marrow cells. Group B BCL1-bearing recipients were given bone marrow plus spleen cells. Group C BCL1-bearing recipients were given bone marrow (2.5 × 106) plus spleen cells (10 × 106) and PG27 (40 mg/kg per day) for the first 35 days after transplantation. Mortality rates were recorded daily. Moribund animals were killed and recorded as dead.13Pathologic specimens were examined, and gross abnormalities were recorded. The only abnormality that appeared to be specific for leukemic mice was marked splenomegaly.
Immunofluorescent staining for identification of BCL1 tumor cells in the spleen
Spleen cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-BCL1-Id monoclonal antibody (mAb) and FITC-conjugated rat IgG2a isotype control.12 Single-color analysis was performed with a FACStar (Becton Dickinson, Mountain View, CA).
Statistical analyses
Survival data were analyzed by Mann-Whitney U log-rank analysis. P < .05 was considered significant.
Results
Inhibition of primary mixed lymphocyte reactions across major histocompatibility barriers
The role of PG27 was tested in MLR across MHC barriers (Figure1). PG27 inhibited the MLR in a dose-dependent fashion; 100% inhibition was found at a concentration of 1.25 μg/mL.
Inhibition of mixed lymphocyte reactions across major histocompatibility barriers.
2.5 × 105 BALB/c spleen cell responders were plated with 5 × 105 irradiated (30 Gy) C57BL/6 spleen cell stimulators in the presence or absence of PG27. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine/well for an additional 16 hours. Results are mean cpm from triplicate cultures and are representative of 2 separate experiments.
Inhibition of mixed lymphocyte reactions across major histocompatibility barriers.
2.5 × 105 BALB/c spleen cell responders were plated with 5 × 105 irradiated (30 Gy) C57BL/6 spleen cell stimulators in the presence or absence of PG27. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine/well for an additional 16 hours. Results are mean cpm from triplicate cultures and are representative of 2 separate experiments.
Inhibition of secondary mixed lymphocyte reactions across minor histocompatibility barriers
Primed B10.D2 responder spleen cells were harvested 21 days after immunization with irradiated BALB/c spleen cells and plated with BALB/c stimulator cells. As shown in Figure 2, PG27 inhibited the secondary proliferative response in a dose-dependent fashion similar to the inhibitory effect across MHC barriers.
Inhibition of secondary mixed lymphocyte reactions across minor histocompatibility barriers.
Responder spleen cells were harvested from primed B10.D2 mice 21 days after intraperitoneal immunization with 50 × 106irradiated (30 Gy) BALB/c spleen cells. 2.5 × 105primed spleen cells were plated with 5 × 105irradiated (30 Gy) BALB/c spleen cell stimulators in the presence or absence of PG27. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine/well for an additional 16 hours. Results are mean cpm from triplicate cultures and are representative of 2 separate experiments.
Inhibition of secondary mixed lymphocyte reactions across minor histocompatibility barriers.
Responder spleen cells were harvested from primed B10.D2 mice 21 days after intraperitoneal immunization with 50 × 106irradiated (30 Gy) BALB/c spleen cells. 2.5 × 105primed spleen cells were plated with 5 × 105irradiated (30 Gy) BALB/c spleen cell stimulators in the presence or absence of PG27. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine/well for an additional 16 hours. Results are mean cpm from triplicate cultures and are representative of 2 separate experiments.
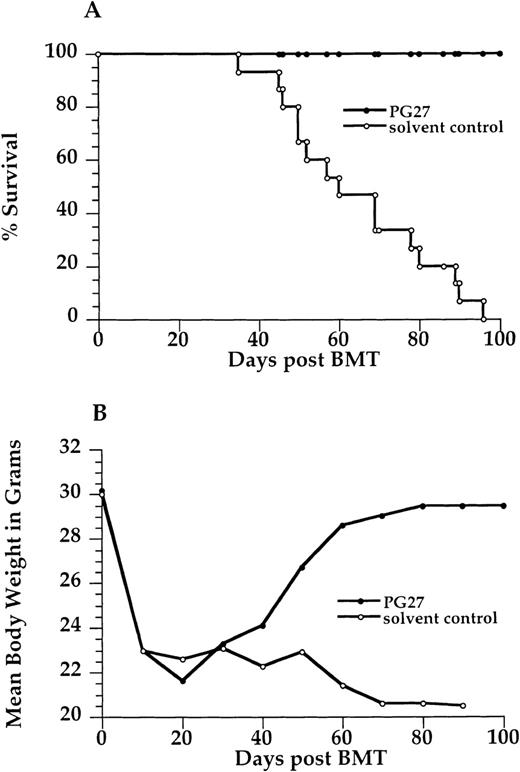
Effect of PG27 on severity of graft-versus-host disease and mortality rates
Treatment with PG27 for the first 5 weeks after transplantation significantly prevented GVHD, and it resulted in GVHD-free long-term survival in contrast to the treatment with solvent (P < .001). All recipients given PG27 (15/15) survived for more than 100 days without any signs of GVHD. Severe GVHD developed in all recipients given solvent only (15/15), and they died within 90 days. Figure 3 shows survival curves and mean body weight changes of the 2 groups.
Effect of PG27 on graft-versus-host-disease severity and mortality.
2.5 × 106 B10.D2/nSnJ bone marrow cells and 10 × 106 B10.D2/nSnJ spleen cells were injected intravenously into lethally irradiated (8 Gy) BALB/c recipients' tail veins. Recipient BALB/c mice received 40 mg/kg per day PG27 or solvent vehicle (Ethanol or Cremophor) intraperitoneally daily for the first 5 weeks after transplantation. (A) Survival curve. (B) Mean body weight curve. Data are pooled from 3 similar experiments.
Effect of PG27 on graft-versus-host-disease severity and mortality.
2.5 × 106 B10.D2/nSnJ bone marrow cells and 10 × 106 B10.D2/nSnJ spleen cells were injected intravenously into lethally irradiated (8 Gy) BALB/c recipients' tail veins. Recipient BALB/c mice received 40 mg/kg per day PG27 or solvent vehicle (Ethanol or Cremophor) intraperitoneally daily for the first 5 weeks after transplantation. (A) Survival curve. (B) Mean body weight curve. Data are pooled from 3 similar experiments.
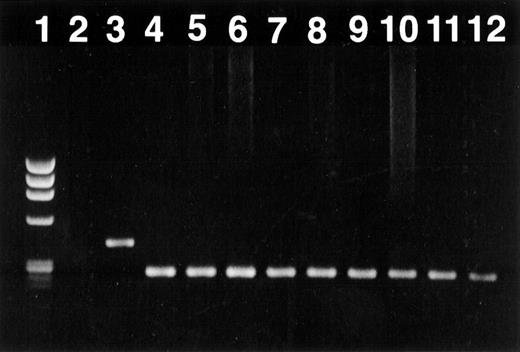
Documentation of chimerism
To document long-term engraftment of allogeneic bone marrow cells, PCR analysis was performed. DNA polymorphism, based on length variations in tandem repeat sequences of a microsatellite in the murine IL-1β gene, was used as a marker to differentiate between donor-derived B10.D2 and recipient BALB/c cells. All transplanted recipients were completely chimeric (Figure4).
Engraftment of donor bone marrow in the B10.D2 → BALB/c transplant documented by polymerase chain reaction amplification of a polymorphic microsatellite region within the IL-1β gene.
Long-term engraftment of allogeneic bone marrow was found in all transplanted mice. This figure demonstrates a representative analysis of an ethidium bromide-stained 1.5% agarose gel. (lane 1) DNA marker φ × 174 digested with Hae III (marker sizes 1353, 1078, 872, 603, 310, 281 and 271, and 243, 194 bp; GIBCO BRL). (lane 2) Negative control (PCR without DNA). (lane 3) Recipient (BALB/c) standard. (lane 4) Donor (B10.D2) standard. (lanes 5–8) Recipients given PG27 (ID nos. 602, 604, 2011, 2012). (lanes 9–12) Recipients given solvent control (ID nos. 606, 610, 2006, 2007).
Engraftment of donor bone marrow in the B10.D2 → BALB/c transplant documented by polymerase chain reaction amplification of a polymorphic microsatellite region within the IL-1β gene.
Long-term engraftment of allogeneic bone marrow was found in all transplanted mice. This figure demonstrates a representative analysis of an ethidium bromide-stained 1.5% agarose gel. (lane 1) DNA marker φ × 174 digested with Hae III (marker sizes 1353, 1078, 872, 603, 310, 281 and 271, and 243, 194 bp; GIBCO BRL). (lane 2) Negative control (PCR without DNA). (lane 3) Recipient (BALB/c) standard. (lane 4) Donor (B10.D2) standard. (lanes 5–8) Recipients given PG27 (ID nos. 602, 604, 2011, 2012). (lanes 9–12) Recipients given solvent control (ID nos. 606, 610, 2006, 2007).
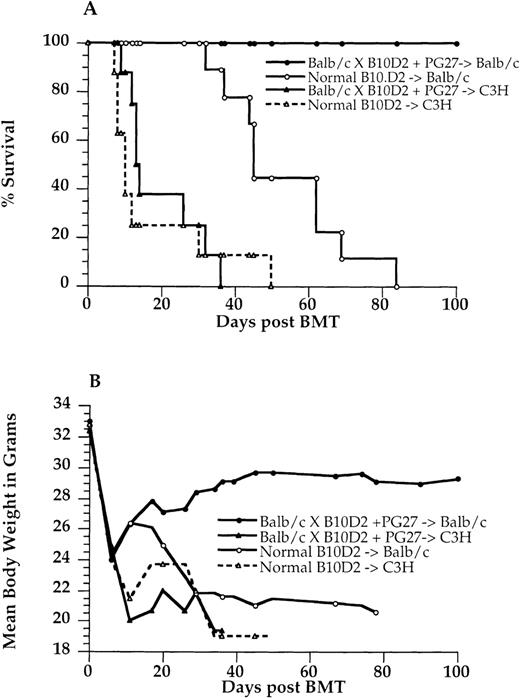
Results of adoptive transplantation
Because the recipients given PG27 were free of GVHD, it was of interest to explore whether PG27 induced antigen-specific tolerance in these recipients. These PG27-treated, GVHD-free BALB/c recipients were used as adoptive donors for an adoptive transfer experiment. Their bone marrow and spleen cells were rechallenged with same-party antigens and third-party antigens. Twelve- to 13-week-old BALB/c mice were lethally irradiated (8 Gy) and used as same-party adoptive recipients, whereas lethally irradiated (9.5 Gy) 12- to 13-week-old C3H mice were used as third-party adoptive recipients. In control groups, normal B10.D2 mice were used as donors, and their bone marrow and spleen cells were transplanted into lethally irradiated BALB/c mice as a same-party group control or to lethally irradiated C3H as a third-party group control. Severe GVHD developed in all the third-party C3H adoptive recipients (10/10), and they died within 40 days (Figure5). These results were similar to those for normal B10.D2 transplanted into C3H. In contrast, all same-party adoptive recipients (10/10) survived more than 100 days without any signs of GVHD, whereas severe GVHD developed in all recipients given normal B10.D2 (10/10), and they died within 90 days (P < .001) (Figure 5).
Adoptive transplantation.
The PG27 recipients treated 100 days after bone marrow transplantation were used as adoptive donors. Lethally irradiated (8 Gy) BALB/c mice were used as same-party adoptive recipients, and lethally irradiated (9.5 Gy) C3H mice were used as third-party adoptive recipients. 2.5 × 105 bone marrow cells and 10 × 106 spleen cells from adoptive donors were injected intravenously into BALB/c or C3H adoptive recipients. In control groups, normal B10.D2/nSnJ mice were used as donors, and the same numbers of bone marrow and spleen cells were transplanted in lethally irradiated BALB/c or C3H mice. These adoptive recipients were observed for 100 days without any treatment. (A) Survival curve. (B) Mean body weight curve. Data are pooled from 2 similar experiments.
Adoptive transplantation.
The PG27 recipients treated 100 days after bone marrow transplantation were used as adoptive donors. Lethally irradiated (8 Gy) BALB/c mice were used as same-party adoptive recipients, and lethally irradiated (9.5 Gy) C3H mice were used as third-party adoptive recipients. 2.5 × 105 bone marrow cells and 10 × 106 spleen cells from adoptive donors were injected intravenously into BALB/c or C3H adoptive recipients. In control groups, normal B10.D2/nSnJ mice were used as donors, and the same numbers of bone marrow and spleen cells were transplanted in lethally irradiated BALB/c or C3H mice. These adoptive recipients were observed for 100 days without any treatment. (A) Survival curve. (B) Mean body weight curve. Data are pooled from 2 similar experiments.
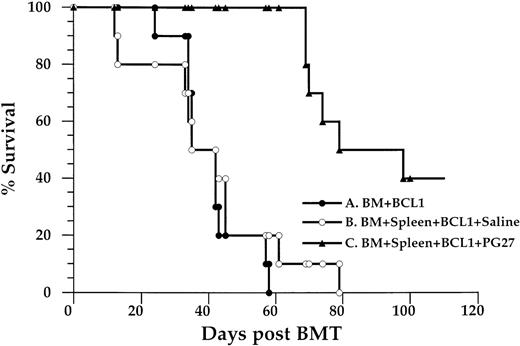
Treatment of BCL1 leukemia/lymphoma with bone marrow and spleen cell transplantation and PG27
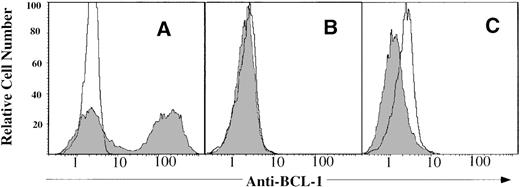
PG27 was tested in the murine BCL-1 leukemia/lymphoma model to study whether PG27 retained the graft-versus-leukemia effect while preventing the development of GVHD. The dose of BCL1 cells (10 × 106) was chosen in accordance with a previous study.13 As shown in Figure 6, 10 of 10 BCL-1–bearing recipients given B10D2 bone marrow cells (group A) died of tumor growth by approximately day 50 after BMT (median, 42 days; range, 24–58 days). This was similar to the result from 10 × 106 BCL1 cells injected intraperitoneally into unirradiated adult BALB/c mice (data not shown). Necropsy of moribund mice found that the spleens were 10 to 15 times heavier than normal spleens (data not shown), and 60% of spleen cells were BCL1-Id positive (Figure 7A). These data suggested that B10D2 bone marrow cells alone did not show GVL activity. Similar results were found when PG27 was administrated to irradiated BALB/c recipients given BCL1 cell and B10D2 bone marrow cells (data not shown). This suggested that PG27 (at doses of 40 mg/kg per day) did not rescue the mice from tumor without donor T cells or spleen cells. In group B, 10 of 10 BCL1-bearing recipients given B10D2 bone marrow cells and spleen cells (group B) died of severe GVHD by day 80 (median, 35 days; range, 12–79 days). They did not have splenomegaly, nor did they have detectable levels of residual BCL1 cells (Figure 7B). This indicates that transplanted B10.D2 T cells/spleen cells protected recipients from BCL1 tumor cells and that these cells were able to mediate a GVL effect. On the other hand, transplanted T cells/spleen cells induced severe GVHD from which they died. In group C, 10 of 10 BCL1-bearing recipients given B10D2 bone marrow cells plus spleen cells and treated with PG27 for the first 35 days did not have any signs of GVHD. Sixty percent of this group died of tumor, and they had splenomegaly rates similar to those in group A. Forty percent of the group survived for more than 100 days (median, 74 days; range, 69≥100 days), and there were no detectable levels of residual BCL1 cells in the spleen (Figure 7C). These data demonstrated that, in contrast to group B, PG27 completely prevented GVHD in group C. It also demonstrated that, in contrast to group A, there was a significant delay in leukemic mortality (P = .001) in PG27- and allogeneic T-cell–treated group C mice, suggesting that PG27 treatment partially spared allogeneic T cells capable of mediating a GVL effect.
Survival of BALB/c recipients given BCL-1 leukemia/lymphoma cells.
BALB/c mice were injected intraperitoneally with 10 × 106 BCL1 cells from BALB/c leukemic donors. Twenty-four hours later, they were lethally (8 Gy) irradiated, then injected intravenously with B10D2 bone marrow cells (2.5 × 106) or bone marrow cells (2.5 × 106) plus spleen cells (10 × 106). These BCL1-bearing recipients were divided into 3 groups. Group A (n = 10) BCL1-bearing recipients received bone marrow cells alone. Group B (n = 10) BCL1-bearing recipients received bone marrow plus spleen cells. Group C (n = 10) BCL1-bearing recipients received bone marrow plus spleen cells and PG27 for the first 35 days after transplantation. Data are pooled from 2 similar experiments.
Survival of BALB/c recipients given BCL-1 leukemia/lymphoma cells.
BALB/c mice were injected intraperitoneally with 10 × 106 BCL1 cells from BALB/c leukemic donors. Twenty-four hours later, they were lethally (8 Gy) irradiated, then injected intravenously with B10D2 bone marrow cells (2.5 × 106) or bone marrow cells (2.5 × 106) plus spleen cells (10 × 106). These BCL1-bearing recipients were divided into 3 groups. Group A (n = 10) BCL1-bearing recipients received bone marrow cells alone. Group B (n = 10) BCL1-bearing recipients received bone marrow plus spleen cells. Group C (n = 10) BCL1-bearing recipients received bone marrow plus spleen cells and PG27 for the first 35 days after transplantation. Data are pooled from 2 similar experiments.
Measurement of residual BCL1 tumor cells in the spleen by immunofluorescent staining.
Spleen cells from BCL-1 recipients after transplantation were stained with fluorescein isothiocyanate (FITC)-conjugated anti-BCL1-ID monoclonal antibody and FITC-conjugated rat IgG Za isotype control. (A) Recipients of bone marrow cells alone plus BCL1 50 days after bone marrow transplantation (BMT). (B) Recipients of bone marrow plus spleen cells and BCL1 cells 50 days after BMT. (C) Recipients of bone marrow plus spleen cells and BCL1 cells treated with PG27 100 days after BMT. Note that the far peak is markedly decreased, suggesting that there is no BCL1 detectable. One of 4 replicated experiments was shown.
Measurement of residual BCL1 tumor cells in the spleen by immunofluorescent staining.
Spleen cells from BCL-1 recipients after transplantation were stained with fluorescein isothiocyanate (FITC)-conjugated anti-BCL1-ID monoclonal antibody and FITC-conjugated rat IgG Za isotype control. (A) Recipients of bone marrow cells alone plus BCL1 50 days after bone marrow transplantation (BMT). (B) Recipients of bone marrow plus spleen cells and BCL1 cells 50 days after BMT. (C) Recipients of bone marrow plus spleen cells and BCL1 cells treated with PG27 100 days after BMT. Note that the far peak is markedly decreased, suggesting that there is no BCL1 detectable. One of 4 replicated experiments was shown.
Discussion
T cells play a central role in inducing GVHD. Across minor histocompatibility barriers, transplanted T cells that recognize multiple minor histocompatibility antigens of the host as “nonself” induce GVHD.1,9,14,15 Both CD4+and CD8+ T-cell subsets are responsible for GVHD induction in lethally irradiated BALB/c recipients, with CD4+ T cells the predominant subset.7 Activated T cells proliferate, leading to a cascade of events that includes upregulation of adhesion and costimulatory molecules, migration and dissemination of T cells throughout the body, recruitment of other effector cells, and activation of macrophages that release proinflammatory cytokines and cause tissue damage.16 17
The principal immunosuppressive substance in PG27 is triptolide.18,19 The triptolide content of 11 preparations of PG27 is 0.364% ± 0.038% assayed by high-performance liquid chromatography (Fidler J, manuscript in preparation). The mechanism of suppression of T-cell activation by triptolide has been intensively studied by Qiu et al,19 who demonstrate that triptolide completely inhibits IL-2 protein and mRNA expression by Jurkat T-cells stimulated with phorbol-12-myristate-13-acetate (PMA) and ionomycin. The inhibition of IL-2 expression and T-cell activation by triptolide occurs at the level of transcription. Triptolide inhibits early cytokine gene transcription by preventing activation by the DNA binding transcription factor, NF-κB, and NF-AT. The mechanisms through which triptolide suppresses T cell activation are different from those of cyclosporine and FK506.19 First, cyclosporine and FK506 inhibit transcription of the IL-2 gene through mechanisms that likely involve the serine–threonine protease phosphatase, calcineurin,19,20 whereas triptolide inhibition of IL-2 transcription does not involve calcineurin.19,21 Second, activation of IL-2 transcription triggered through costimulatory receptors such as CD28 involves NF-κB and is largely resistant to inhibition by cyclosporine.22,23 Triptolide can inhibit the cyclosporine-resistant pathway of T-cell activation by stimulation with PMA in combination with anti-CD28 monoclonal antibody.19Third, cyclosporine and FK506 inhibit transcription of the IL-2 gene through interference with the induction of sequence-specific DNA binding activity at the purine-box/NF-AT target DNA sequence.20
Triptolide inhibits transcription of the IL-2 gene by the inhibition of specific DNA binding activity and transcriptional activation at the purine-box/NF-AT target DNA sequence. For NF-κB, the inhibition of transcriptional activation occurs at a step after specific binding to DNA.19 The different mechanisms through which triptolide suppresses T-cell activation from cyclosporine and FK506 may provide PG27/triptolide, which is resistant to conventional therapies, with the potential for treating GVHD. Similar results in the prevention of GVHD and the induction of tolerance were observed using 100- or 200-fold lower doses of triptolide than of PG27 (Chen J, manuscript in preparation).
We have demonstrated that the administration of PG27 for a short period after BMT completely prevented the development of GVHD. It was of interest to determine whether PG27 was able to induce antigen-specific tolerance in these GVHD-free mice. Tolerance is the antigen-induced functional inactivation or death of specific lymphocytes that results in the inability of an organism to respond to that antigen.24 In most tolerance models, once tolerance is induced, it is robust and tends to dominate any tendency for the immune system to react against the same challenge.25 The data demonstrate that PG27-induced antigen-specific tolerance in GVHD-free recipients and treatment with PG27 did not result in generalized immune suppression.
The second goal of this study was to investigate whether PG27 preserved the beneficial GVL effect of allogeneic T cells while it inhibited GVHD across minor histocompatibility complex disparities. It has been shown that the GVL effect is caused by T-cell dependent immune responses of allogeneic T cells.26 Removing T cells from marrow has been associated with increased relapse rates for leukemia, though T-cell depletion can most reliably prevent GVHD.27,28 Studies of PG27 in the murine BCL1 lymphoma/leukemia model demonstrated that PG27 retained allogeneic T cells, which mediated a GVL effect. The exact mechanism by which PG27 was able to prevent GVHD while partially retaining a GVL effect of allogeneic T cells is not yet clear. It has been shown that triptolide, the active component of PG27, inhibits the proliferation of tumor cell lines, has antitumor activity in a murine breast cancer tumor model,29,30 and synergizes with tumor necrosis factor-α to induce apoptosis in tumor cells.31However, in our model, PG27 (at doses of 40 mg/kg per day) did not rescue the mice from the BCL1 tumor without T cells/spleen cells.
This differential response may be another example of partial GVL effect without GVHD.32 These results suggest the possibility that PG27/triptolide may act as a novel immunosuppressive agent.
Reprints:Nelson J. Chao, Division of Hematology/Oncology, Bone Marrow Transplantation Program, Duke University Medical Center, 2400 Pratt Street, Suite 1100, Durham, NC 27705.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Inhibition of mixed lymphocyte reactions across major histocompatibility barriers. / 2.5 × 105 BALB/c spleen cell responders were plated with 5 × 105 irradiated (30 Gy) C57BL/6 spleen cell stimulators in the presence or absence of PG27. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine/well for an additional 16 hours. Results are mean cpm from triplicate cultures and are representative of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.705/6/m_bloo00217001x.jpeg?Expires=1767236975&Signature=S-~~aeSvVVnum-S1St7gGPc0HfekXGxcxjaP-~7HqiwHPqc40eKxE39Wchdd3P4Fs0xONHfvJHbGcOVk9WaO9PVKy0PAB~Lm3NklPn3JReeg4t~jH0yKRJyc-suF2QtCUE0vBOlpo5Cl-5FP-Yflmf01S5jFIwUCNGJqL~IuUV1rFvDkhv0uGpLGzG3qdg1aGwhThP-mPJQrhh4iyEu~AhZ~XAmwPh6W45zF11aa1aG846TpBKb4mic6WDqfTNLJB0RZrs-ZJSTuCP8hT66KMRzNgb3NMq1Ph60W1GnCbIBWpZy6nbeZSy-B49EHlj29Gy7fd~pDBI8qSX3d-VRxiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Inhibition of secondary mixed lymphocyte reactions across minor histocompatibility barriers. / Responder spleen cells were harvested from primed B10.D2 mice 21 days after intraperitoneal immunization with 50 × 106irradiated (30 Gy) BALB/c spleen cells. 2.5 × 105primed spleen cells were plated with 5 × 105irradiated (30 Gy) BALB/c spleen cell stimulators in the presence or absence of PG27. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine/well for an additional 16 hours. Results are mean cpm from triplicate cultures and are representative of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.705/6/m_bloo00217002x.jpeg?Expires=1767236975&Signature=vbSRLM423vIF8gSldAd3MKrPJ77qh81-Vv5PL~rUCZFNEFNHUcg9gQZzIabBFNvZCeRmiIncim-E1EKKr54e7SgsB-53Ky0HXwqeMFr~eutADoe062NUvqKCD~OiB-SxCrIQALO-9NbHDKWNTURBu1ZfaVhWdihO03bL7nVUiqNp4PORaThFMZdhKz9g880Kfz6cr4ZDPo4pjus29S2c2hYlQZmsfs5~LYKSJIZRD0UDLEC2bLkUcGmFYToEWaAF43qAsTd4MkwNHRwBHQzhPv3P99VuBsiJ05j58bOTg-mHnfwLMMUY4ExdQjyRN-JIrIiC-k230EnHY3IEWSb1qw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal