Chemotaxis in leukocytes is mediated through binding of soluble chemokines to transmembrane G-protein coupled receptors. The chemokine receptor CXCR3 has been previously shown to be widely expressed on activated T cells and to mediate T-cell chemotaxis on binding to various ligands, including Mig, IP-10, and ITAC. By using immunohistochemical and flow cytometric analysis, we report that CXCR3 is also expressed on a subset of peripheral blood B cells and in distinct subtypes of B-cell lymphoma. CXCR3 immunohistochemical or flow cytometric expression was seen in 37 of 39 cases of chronic lymphocytic leukemia/small lymphocytic lymphoma (diffusely positive in 33 cases), whereas mantle cell lymphoma (30 cases), follicular lymphoma (27 cases), and small noncleaved cell lymphoma (8 cases) were negative in all but 2 cases. Strong CXCR3 expression was also seen in splenic marginal zone lymphoma (14 of 14 cases) and in the monocytoid and plasmacytic cells in extranodal marginal zone lymphoma (15 of 16 cases). This differential expression of CXCR3 in B-cell tumors contrasts with that of another B-cell–associated chemokine receptor, BLR1/CXCR5, which we show here is expressed on all types of B-cell lymphoma tested. We also report that the CXCR3 ligand, Mig, is coexpressed on tumor cells in many cases of CLL/SLL (10 of 13 cases examined) with Mig expression less frequently seen in other B-cell lymphoma subtypes. Coexpression of CXCR3 and its ligand, Mig, may be an important functional interaction in B-CLL, as well as a useful diagnostic marker for the differential diagnosis of small cell lymphomas.
Chemotaxis and adhesion of leukocytes is regulated in part by binding of soluble chemokines to G-coupled transmembrane chemokine receptors containing either the CXC or CC structural motifs. Recently, a number of these chemokine receptors have been shown to mediate chemotaxis of B cells. CXCR3 was previously characterized as a chemokine receptor present on activated T cells, particularly those of the Th1 lineage.1-3 The high-affinity CXCR3 ligands, IP-10, Mig, and ITAC, are chemotactic for activated T cells and can stimulate integrin-mediated adhesion to endothelium and production of Th1-type cytokines.2,4-7 CXCR3 ligands are largely produced by activated endothelial cells and interferon-gamma stimulated macrophages.8
The chemokine receptors previously implicated in B-cell migration and proliferation include CXCR4, BLR1/CXCR5, CCR6, and CCR7.9-15 These receptors are expressed on B cells during most stages of maturation with chemotactic sensitivity varying, depending on the state of maturation or activation. We demonstrate here that CXCR3 is also expressed on a small number of normal circulating B cells. Its expression is highly associated with a subset of B-cell lymphomas, particularly B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and splenic marginal zone lymphoma (SMZL), but is not expressed in most other types of B-cell lymphoma.
Materials and methods
Material was obtained from cases at the Brigham and Women's Hospital, Boston, MA, after approval by an institutional review board. Diagnosis was based on the Revised European American Lymphoma Classification.16 Immunohistochemical characterization of cases was previously performed using monoclonal antibodies directed against the T-cell markers CD3 and CD45RO and the pan–B-cell marker CD20 (L26), as well as CD10, CD5, and CD23 (most cases) as previously described.17 In many cases, flow cytometric characterization was also performed with antibodies directed against CD19, CD20, CD3, CD5, CD10, CD23 (Becton Dickinson, San Jose, CA), and FMC7 (Coulter-Immunotech, Miami, FL). Minimal criteria for diagnosis of CLL/SLL included reactivity for CD20 and CD5, along with compatible cytomorphology. CD5-positive cases with unusual morphology or unusual immunophenotype (eg, CD23-negativity) were classified as “atypical” CLL/CD5-positive B-cell lymphoproliferative disorder. Criteria for diagnosis of SMZL included characteristic clinical presentation with a distinctive histologic pattern of splenic involvement and absence of expression of CD5 and CD10.
Immunoperoxidase studies were performed using a previously characterized mouse IgG1 antibody directed against CXCR3, 1C6, and a mouse IgG1 antibody directed against Mig, 4G10 (both generously provided by Dr Paul Ponath, Leukosite, Inc, Cambridge, MA). The specificity of the CXCR3 antibody had been previously demonstrated by its ability to specifically bind CXCR3-transfected L1.2 cell lines and to inhibit chemotaxis of these transfectants to IP-10.2CXCR3 immunostaining was performed with previously described methods for frozen material18 and paraffin embedded tissues after microwave antigen-retrieval.17 Strong staining was seen in biopsy specimens fixed in formalin, as well as the (mercury-based) B5 fixative. In 6 cases, we examined CXCR3 expression in both cryostat and paraffin-embedded tissue from the same biopsy specimen and saw similar levels of expression in both specimens. Reliable Mig staining was observed only on cryostat sections and therefore was only performed in those cases in which fresh tissue was available.
Flow cytometric analysis was performed on peripheral blood from normal volunteers and patient samples with CLL/SLL after lysis of red blood cells. Cells were incubated with a primary antibody directed against CXCR3 (FITC-conjugated mouse IgG clone 49 801.111) or BLR1/CXCR5 (biotinylated mouse IgG2B 51 505.111, both from R&D Systems, Minneapolis, MN), as well as fluorochrome-conjugated monoclonal antibodies directed against CD3, CD5, CD19, and CD20 (Becton Dickinson). CXCR5 was detected by secondary incubation with phycoerythrin-avidin (Becton Dickinson). Cells were analyzed on a FACS caliber flow cytometer (Becton Dickinson) with gating on the lymphoid cell population and quantitated by comparison with isotype control antibodies (Becton Dickinson). For 15 cases, staining for CXCR3 was assessed by both flow cytometric and immunohistochemical staining with different anti-CXCR3 antibodies.
Results
CXCR3 immunostaining in B cells and B-cell lymphoma
A previous preliminary study that used the CXCR3/1C6 monoclonal antibody had detected surface expression in a majority of circulating T cells as well as a small proportion of circulating peripheral B lymphocytes but not in neutrophils, monocytes, or eosinophils.2 Using flow cytometric analysis, we examined CXCR3 staining of peripheral blood lymphocytes in normal volunteers. As shown in Figure 1, variable expression of CXCR3 could be seen in approximately 50% of peripheral blood T cells as well as in a small population of circulating B cells (15% to 25% of total in different samples). Both CD5-positive and CD5-negative populations were present among CD19+CXCR3+ B cells as determined by a 3-color staining profile (Figure 1B).
CXCR3 expression in normal peripheral blood lymphocytes.
(A) Flow cytometric detection of CXCR3 in peripheral blood using the FITC-conjugated monoclonal antibody, 49801.111. Cytograms represent staining of cells within the lymphocyte gating for CD5 and CXCR3 (right panel) in comparison with an IgG1 isotype control antibody (middle panel). (B) CXCR3 expression in peripheral blood B cells as determined by CD19/forward scatter gating in a 3-color staining profile (CD19/CD5/CXCR3). Numbers correspond to percentage of total gated B cells in each quadrant.
CXCR3 expression in normal peripheral blood lymphocytes.
(A) Flow cytometric detection of CXCR3 in peripheral blood using the FITC-conjugated monoclonal antibody, 49801.111. Cytograms represent staining of cells within the lymphocyte gating for CD5 and CXCR3 (right panel) in comparison with an IgG1 isotype control antibody (middle panel). (B) CXCR3 expression in peripheral blood B cells as determined by CD19/forward scatter gating in a 3-color staining profile (CD19/CD5/CXCR3). Numbers correspond to percentage of total gated B cells in each quadrant.
Using immunohistochemistry, we examined expression of CXCR3 in reactive lymphoid tissues. CXCR3-expressing T cells were found predominantly in the interfollicular areas of lymph node and tonsil representing approximately 20% to 40% of the cellularity (Figure2 and data not shown). Examination of serial sections with the B-cell marker CD20 did not reveal detectable staining for CXCR3 in B cells of the primary and secondary follicles in either lymph node or spleen. Plasma cells in the medullary areas of lymph node and tonsil showed variable reactivity for CXCR3.
CXCR3 and Mig expression in low-grade B-cell lymphomas.
(A) Immunohistochemical detection on paraffin-embedded and cryostat sections using the 1C6/CXCR3 and 4G10/Mig monoclonal antibodies. Abbreviations: H&E, hematoxylin and eosin staining; CLL, nodal involvement by B-cell chronic lymphocytic leukemia; MCL, mantle cell lymphoma; FL, follicular lymphoma; SMZL, splenic marginal zone lymphoma. (B) Flow cytometric detection of CXCR3 in a representative case of B-CLL (3-color CD19/CD5/CXCR3 staining in panels 1 and 2) and mantle cell lymphoma (panel 3). Cytograms represent all cells in the sample within the lymphocyte gate.
CXCR3 and Mig expression in low-grade B-cell lymphomas.
(A) Immunohistochemical detection on paraffin-embedded and cryostat sections using the 1C6/CXCR3 and 4G10/Mig monoclonal antibodies. Abbreviations: H&E, hematoxylin and eosin staining; CLL, nodal involvement by B-cell chronic lymphocytic leukemia; MCL, mantle cell lymphoma; FL, follicular lymphoma; SMZL, splenic marginal zone lymphoma. (B) Flow cytometric detection of CXCR3 in a representative case of B-CLL (3-color CD19/CD5/CXCR3 staining in panels 1 and 2) and mantle cell lymphoma (panel 3). Cytograms represent all cells in the sample within the lymphocyte gate.
Given the presence of a small population of CXCR3-expressing peripheral blood B lymphocytes, we next examined tumors of B-cell derivation. A strong correlation was seen between CXCR3 expression and the subtype of B-cell lymphoma. Immunohistochemical staining of CXCR3 was seen in tumor cells in 34 of 36 cases of chronic lymphocytic leukemia/small lymphocytic lymphoma, with diffuse reactivity of most tumor cells seen in 30 cases (Figure 2A and Table 1). In most cases, the intensity of staining for CXCR3 in lymphoma cells, either in cryostat or fixed tissue sections, was less than that seen within admixed T cells, suggesting a lower level of expression. CXCR3 expression was seen in 1 of 3 cases of prolymphocytic leukemia (PLL). In cases of CLL-PLL (ie, tumors that showed varying degrees of prolymphocytic transformation), CXCR3 immunostaining was negative in 2 cases and showed variable expression in 4 others. Only 1 of 6 cases of “atypical” CLL (ie, unclassifiable CD5-positive B-cell lymphoproliferative disorder) showed CXCR3 reactivity.
CXCR3 and Mig expression in B-cell tumors
| Tumor Type . | CXCR3 by IHC . | CXCR3 by Flow . | Mig by IHC . |
|---|---|---|---|
| Lymphoblastic leukemia/lymphoma | 3/9 (2 focal, 1 diffuse) | nd | nd |
| Small noncleaved cell lymphoma (Burkitt's/L3 ALL) | 0/8† | nd | 1/4 |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 34/36 (30 diffuse) | 12/13 | 10/13 (5 diffuse) |
| CLL in transformation | 4/6 | 1/1 | 2/3 |
| Prolymphocytic leukemia | 1/3 | nd | 2/2 |
| Hairy cell leukemia | 5/7 | 1/1 | nd |
| Mantle cell lymphoma | 1/30† | 0/2 | 2/12 |
| Follicular lymphoma‡ | 1/27 (focal)† | 0/1 | 2/16 |
| Splenic marginal zone lymphoma | 14/14 | nd | 4/8 |
| Extranodal marginal zone lymphoma | 15/16* | nd | 3/3 |
| Plasmacytoma | 5/8 | nd | 1/1 |
| Large cell lymphoma | 5/33 (3 diffuse; all splenic) | nd | 2/3 |
| Tumor Type . | CXCR3 by IHC . | CXCR3 by Flow . | Mig by IHC . |
|---|---|---|---|
| Lymphoblastic leukemia/lymphoma | 3/9 (2 focal, 1 diffuse) | nd | nd |
| Small noncleaved cell lymphoma (Burkitt's/L3 ALL) | 0/8† | nd | 1/4 |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 34/36 (30 diffuse) | 12/13 | 10/13 (5 diffuse) |
| CLL in transformation | 4/6 | 1/1 | 2/3 |
| Prolymphocytic leukemia | 1/3 | nd | 2/2 |
| Hairy cell leukemia | 5/7 | 1/1 | nd |
| Mantle cell lymphoma | 1/30† | 0/2 | 2/12 |
| Follicular lymphoma‡ | 1/27 (focal)† | 0/1 | 2/16 |
| Splenic marginal zone lymphoma | 14/14 | nd | 4/8 |
| Extranodal marginal zone lymphoma | 15/16* | nd | 3/3 |
| Plasmacytoma | 5/8 | nd | 1/1 |
| Large cell lymphoma | 5/33 (3 diffuse; all splenic) | nd | 2/3 |
Focal staining corresponds to staining in 10% or fewer tumor cells.
Positive in plasma cells, lymphoplasmacytic forms and B cells with monocytoid appearance.
P < .0001 by Fisher's exact test, comparing CXCR3 expression to that seen in B-CLL.
Includes 16 small cell, 4 mixed small and large cell, and 7 large cell types.
To confirm the results of the tissue section data, flow cytometric analysis with a different CXCR3 antibody was also performed on samples from peripheral blood, bone marrow, and lymph node. With 3-color staining, CXCR3-positivity was seen in 12 of 13 B-CLL cases, with essentially all CD19+CD5+ tumor cells expressing CXCR3 (Figure 2B and Table 1). Immunohistochemical analysis performed on material from 10 of these patients showed a 100% correlation with the flow cytometric results.
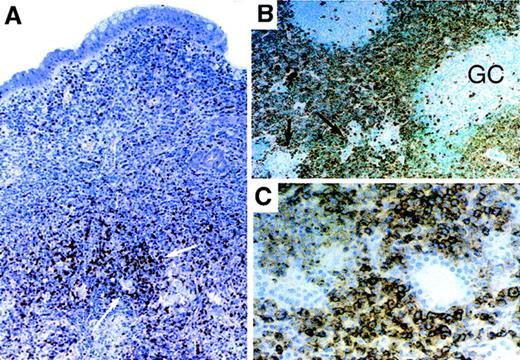
Among other B-cell tumors, low-grade splenic marginal zone lymphoma showed strong CXCR3 immunohistochemical staining in a vast majority of tumor cells in all 14 cases. In 15 of 16 cases of extranodal marginal zone lymphoma (ie, MALT lymphoma), involving stomach, thyroid, skin, lung, or salivary glands, CXCR3 immunostaining was seen in tumor cells that showed prominent plasmacytic differentiation or those with a monocytoid appearance (Figure 3). Admixed non-neoplastic elements (eg, reactive germinal centers) and the small cell B lymphocyte component of these tumors were typically negative for CXCR3 (Figure 3A and 3B). In 5 of 8 cases of plasmacytoma, most or all the tumor cells were strongly positive for CXCR3, with the remainder of cases showing staining in only a few cells (data not shown). Hairy cell leukemia also showed diffuse CXCR3 reactivity in 5 of 7 cases.
Expression of CXCR3 in B-cell lymphomas with varying degrees of plasmacytic differentiation.
(A) Low-grade extranodal marginal zone lymphoma (MALT lymphoma) of stomach showing CXCR3 in clusters of plasma cells (white arrows) and not in the majority of infiltrating small lymphocytes. (B) Salivary gland MALT lymphoma with sheets of CXCR3-positive interfollicular “monocytoid” neoplastic B cells. Admixed non-neoplastic follicles and associated mantle zones are negative for CXCR3, as are salivary ducts and glands (black arrows). (C) Diffuse CXCR3 staining in a low-grade MALT lymphoma of the lung with extensive plasmacytic differentiation. Epithelial elements are negative.
Expression of CXCR3 in B-cell lymphomas with varying degrees of plasmacytic differentiation.
(A) Low-grade extranodal marginal zone lymphoma (MALT lymphoma) of stomach showing CXCR3 in clusters of plasma cells (white arrows) and not in the majority of infiltrating small lymphocytes. (B) Salivary gland MALT lymphoma with sheets of CXCR3-positive interfollicular “monocytoid” neoplastic B cells. Admixed non-neoplastic follicles and associated mantle zones are negative for CXCR3, as are salivary ducts and glands (black arrows). (C) Diffuse CXCR3 staining in a low-grade MALT lymphoma of the lung with extensive plasmacytic differentiation. Epithelial elements are negative.
In contrast, among 30 cases of mantle cell lymphoma (MCL), 27 cases of follicular lymphoma (FL), 9 cases of lymphoblastic leukemia/lymphoma (ALL), and 8 cases of Burkitt's/small noncleaved cell lymphoma, tumor cells were negative for CXCR3 by immunochemistry in nearly all cases. CXCR3 staining was seen diffusely in only 1 case of mantle cell lymphoma and focally in 1 follicular lymphoma with a mixed large and small cell morphology. Two cases of mantle cell lymphoma and 1 case of follicular lymphoma were negative for CXCR3 expression by flow cytometry (Figure 2B and Table 1). Three cases of lymphoblastic lymphoma/leukemia showed CXCR3 staining, 2 of which were positive in only a small subset of cells (Table 1). The diffusely CXCR3-positive lymphoblastic lymphoma was unusual in that the tumor cells had a large cell morphology. Only a minority of diffuse large cell lymphomas examined were positive for CXCR3 (5 of 33 cases), including 3 splenic lymphoma cases, which were diffusely positive. By histologic features and clinical history, these positive cases apparently represented “de novo” large cell lymphoma and not transformations of an underlying low-grade tumor, such as SMZL.
Mig immunostaining
We also examined the expression pattern of 1 of the high-affinity ligands for CXCR3, Mig, by immunohistochemical staining of unfixed, frozen tissue. In reactive lymphoid tissues and many lymphomas, Mig expression was seen only in blood vessels and admixed macrophages with no appreciable staining of lymphocytes (Figure 2A). However, a number of B-cell tumors studied (29 of 65 cases; 45%) showed expression of Mig within variable numbers of lymphoma cells. This expression was particularly noted in cases of CLL/SLL in which 10 of 13 cases showed Mig staining, which was diffuse in 5 cases (Figure 2A and Table 1). Other types of B-cell lymphoma showed only occasional cases with Mig expression, usually in a minority of tumor cells. In follicular lymphoma, although only 2 of 16 cases showed Mig expression in the tumor cells, increased Mig staining was seen in macrophages surrounding the neoplastic tumor nodules (Figure 2A).
Expression of CXCR5
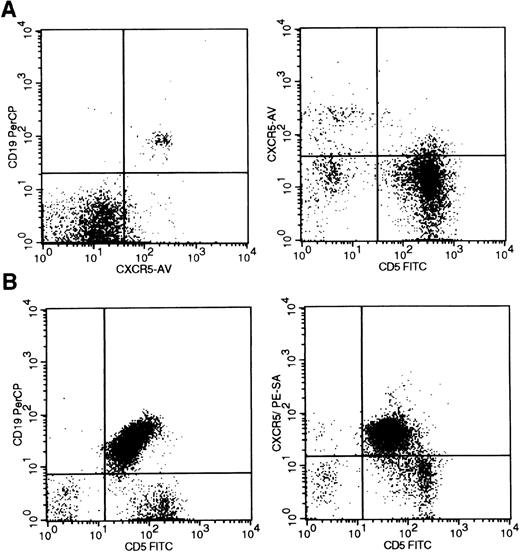
Flow cytometric analysis of B-cell tumors was also performed with a monoclonal antibody directed against the B-cell–associated chemokine receptor BLR1/CXCR5. In peripheral blood, the majority of B cells, but only a minority of T cells, were positive for BLR1/CXCR5 (Figure4A), as previously reported.19In non-neoplastic lymph node, tonsil, and spleen, the majority of both B and T cells were positive for BLR1/CXCR5, including both CD4 and CD8-positive T cells (Table 2 and data not shown). In contrast to CXCR3, strong BLR1/CXCR5 expression was seen in 28 cases of B-cell lymphoma/leukemia representing all stages of maturation, including 13 cases of CLL (Figure 4). BLR1/CXCR5 expression was seen in essentially all tumor cells (Table 2). CXCR5 expression was also seen in 2 T-cell lymphoproliferative disorders that were tested (data not shown).
BLR1/CXCR5 expression in B-cell chronic lymphocytic leukemia.
(A) BLR1/CXCR5 expression in peripheral blood of both CD19-positive B cells (left) and a subset of CD5-positive T cells (right). (B) BLR1/CXCR5 coexpression in the CD19/CD5-positive tumor cells of B-CLL. Flow cytometric detection used the 51505.111 CXCR5 monoclonal antibody with gating on the lymphocyte population by forward and side scatter properties.
BLR1/CXCR5 expression in B-cell chronic lymphocytic leukemia.
(A) BLR1/CXCR5 expression in peripheral blood of both CD19-positive B cells (left) and a subset of CD5-positive T cells (right). (B) BLR1/CXCR5 coexpression in the CD19/CD5-positive tumor cells of B-CLL. Flow cytometric detection used the 51505.111 CXCR5 monoclonal antibody with gating on the lymphocyte population by forward and side scatter properties.
BLR1/CXCR5 expression in B-cell tumors and the non-neoplastic T cells in these patients
| Tumor Type . | Cases Tested . | %-Positive B Cells (CD19-positive) . | %-Positive Non-Neoplastic T Cells (CD3-positive) . |
|---|---|---|---|
| L1 or L2 ALL | 8/8 | 95 | 74 |
| Burkitt's lymphoma/L3 ALL | 1/1 | 91 | 69 |
| CLL | 13/13 | 100 | 57 |
| Prolymphocytic leukemia | 1/1 | 99 | 17 |
| Hairy cell leukemia | 1/1 | 100 | 28 |
| Mantle cell lymphoma | 1/1 | 66 | 34 |
| Follicular lymphoma | 2/2 | 100 | 98 |
| Marginal zone lymphoma | 2/2 | 85 | 35 |
| Lymphoplasmacytic lymphoma | 1/1 | 91 | 74 |
| Reactive lymph node | 1/1 | 90 | 83 |
| Peripheral blood | 8/8 | 91 | 15 |
| Tumor Type . | Cases Tested . | %-Positive B Cells (CD19-positive) . | %-Positive Non-Neoplastic T Cells (CD3-positive) . |
|---|---|---|---|
| L1 or L2 ALL | 8/8 | 95 | 74 |
| Burkitt's lymphoma/L3 ALL | 1/1 | 91 | 69 |
| CLL | 13/13 | 100 | 57 |
| Prolymphocytic leukemia | 1/1 | 99 | 17 |
| Hairy cell leukemia | 1/1 | 100 | 28 |
| Mantle cell lymphoma | 1/1 | 66 | 34 |
| Follicular lymphoma | 2/2 | 100 | 98 |
| Marginal zone lymphoma | 2/2 | 85 | 35 |
| Lymphoplasmacytic lymphoma | 1/1 | 91 | 74 |
| Reactive lymph node | 1/1 | 90 | 83 |
| Peripheral blood | 8/8 | 91 | 15 |
Flow cytometric detection using 51505.111 CXCR5 monoclonal antibody with gating on the lymphocyte population by forward and side scatter properties.
Discussion
We report that the chemokine receptor CXCR3 is expressed in a small subset of normal circulating B lymphocytes as well as plasma cells, but not in the vast majority of lymph node and splenic B cells. The pattern of expression in normal B cells is paralleled by that seen in B-cell non-Hodgkin's lymphoma in which CXCR3 staining is seen in nearly all cases of B-cell chronic lymphocytic leukemia and SMZL. These are tumor types that nearly always show peripheral blood involvement. There is also variable CXCR3 staining of hairy cell leukemia and lymphoplasmacytic forms in extranodal marginal zone lymphoma and plasmacytomas. With rare exceptions, CXCR3 immunostaining is not seen in B-cell tumors of other types, including B-cell lymphoblastic leukemia or lymphomas of mantle cell, follicular, or small noncleaved cell types. The strong association of CXCR3 expression with B-CLL and SMZL and not with mantle cell lymphoma and follicular lymphoma suggests this receptor may be a useful marker in the differential diagnosis of mantle zone lymphoma and CLL/SLL. The staining for CXCR3 in difficult-to-classify cases (“atypical” CLL) clearly needs further study.
CXCR3 expression in leukocytes has been largely studied in activated T cells with some previous studies reporting an absence of CXCR3 expression in B cells from peripheral blood or lymph nodes.20 However, our results and those of 2 other groups conclusively demonstrate CXCR3 expression in a subset of circulating B cells.21 22 The specificity of the 1C6 antibody used here has been previously demonstrated by its ability to neutralize ligand bindings and chemotaxis to IP-102 and correlates well in our studies with expression detected using a different CXCR3 monoclonal antibody.
The selective expression of CXCR3 in a subset of B cell tumors contrasts with that seen with the other B-cell–associated chemokine receptors analyzed to date (Table 3). For instance, CXCR4 is expressed throughout B-cell development and in most B-cell tumor lines (with the exception of some myeloma lines),23-25 as well as in primary isolates of many types of B-cell lymphoma (DJ/DMD, unpublished data). BLR1/CXCR5 was previously shown to be expressed in most mature circulating B cells and in primary follicles and the mantle zone of lymph nodes with down-regulation of expression after antigen or cytokine activation.19,26-28 We demonstrate here that BLR1/CXCR5 is also widely expressed in B-cell lymphomas of all histologic types. Limited data currently exist on the expression pattern of other B-cell chemokine receptors, namely, CCR6 and CCR7, in B tumors. In normal B-cell subsets, CCR6 may be widely expressed9,11 and CCR7 expression parallels antigen activation and can mediate chemotaxis of a subset of both lymph node and peripheral blood B cells.10,13,14 29-31
Summary of expression patterns of some B-cell-associated chemokine receptors
| Receptor . | Expression in Normal B Cells . | Low/Absent Expression . | Expression in Lymphoma . | References . |
|---|---|---|---|---|
| CXCR3 | Subset of B cells in blood | Nodal/splenic B cells | CLL, SMZL, PC, MALT ± HCL | Table I, 21, 22 |
| CXCR4 | Pre-B and mature B cells | Plasma cells | ALL and other subtypes | 23-25, 34 |
| BLR1/CXCR5 | Mature/unstimulated B cells mantle, primary follicle | Immature B activated B cells | B cell lymphomas of all types | Table II, 12, 19, 26, 27, 35 |
| CCR6 | Most peripheral B cells | Not reported | 9, 11 | |
| BLR2/CCR7 | Mature B | Immature B | Not reported | 15, 28-31 |
| Receptor . | Expression in Normal B Cells . | Low/Absent Expression . | Expression in Lymphoma . | References . |
|---|---|---|---|---|
| CXCR3 | Subset of B cells in blood | Nodal/splenic B cells | CLL, SMZL, PC, MALT ± HCL | Table I, 21, 22 |
| CXCR4 | Pre-B and mature B cells | Plasma cells | ALL and other subtypes | 23-25, 34 |
| BLR1/CXCR5 | Mature/unstimulated B cells mantle, primary follicle | Immature B activated B cells | B cell lymphomas of all types | Table II, 12, 19, 26, 27, 35 |
| CCR6 | Most peripheral B cells | Not reported | 9, 11 | |
| BLR2/CCR7 | Mature B | Immature B | Not reported | 15, 28-31 |
PC, plasmacytoma, MALT, extranodal marginal zone lymphoma.
The expression of CXCR3 in tumors with a peripheral blood phase (ie, CLL, SMZL) and plasmacytic cells remains to be correlated with its functional activity. In other chemokine receptors, chemotactic response and activation cannot be predicted based solely on receptor expression pattern. For instance, although CXCR4 is present on nearly all B cells and cell lines, strong chemotactic responsiveness to its ligand, SDF-1, occurs only in B-cell lines with an immature B-cell phenotype.24,25 Furthermore, lymphocytes from mice deficient in CXCR4 display severe defects in early B lymphopoiesis but show largely normal localization of mature B cells.32,33These results suggest that desensitization to receptor signaling may occur during B-cell maturation.34 Likewise, although BLR1/CXCR5 is widely expressed in B cells, BLR1/CXCR5-deficient mice show a specific B-cell defect with absence of inguinal lymph nodes, Peyer's patches, and absence of germinal centers in splenic follicles.12,31,35,36 BLR1/CXCR5 is also expressed on a subset of T cells, including a majority of infiltrating cells in B-cell lymphomas, but has not been shown to mediate chemotaxis of T cells.12,19 36
Activation of CXCR3 occurs after binding of 1 of several high-affinity ligands, including Mig, I-TAC, and IP-10, which are produced by activated endothelial cells and macrophages.4,6,20 We report here that a relevant CXCR3 ligand within lymph nodes, Mig, is actually coexpressed in tumor cells in a number of cases of B-CLL/SLL as well as occasionally in other B-cell tumors. This suggests an autocrine mechanism of growth stimulation may operate in these tumors, in addition to probable paracrine CXCR3 activation through intermixed macrophages and adjacent blood vessels. In macrophages, induction of Mig expression is dependent on interferon-gamma (IFN-γ) as shown by the absence of Mig in IFN-γ deficient mice. The mechanism of induction of Mig expression in B cell tumors awaits further study but increased levels of IFN-γ are often seen in patients with CLL and T cells from B-CLL/SLL patients have been found to secrete increased IFN-γ.37
The consequences of Mig binding to CXCR3 in B-CLL could include regulation of tumor localization, chemotaxis, or adhesion of tumor cells to blood vessels or nodal and marrow microenvironments. The CXCR3 ligand IP-10 has even been shown previously to regulate growth in some hematopoietic progenitor cells.38 The factors promoting growth and survival of B-CLL cells are not known at present and the establishment of cell lines derived from these tumors has proven difficult.39-41 The role of chemokines, particularly Mig, in promoting B-CLL tumor growth and survival remains to be explored.
NOTE: Subsequent to our submission of this manuscript, Trentin et al also reported CXCR3 expression in B-CLL by flow cytometric methods.22 They showed chemotactic responsiveness of several primary B-CLL tumor samples to the chemokines Mig and IP-10. This work provides functional evidence for a role for this chemokine-receptor interaction in regulating B-cell chronic lymphocytic leukemia.
Acknowledgments
We thank G. Shaheen and A. O. Roma for flow cytometric expertise, and Dr Paul D. Ponath (Leukosite, Inc) for anti-CXCR3 and anti-Mig antibodies.
Research was presented preliminarily at the 1998 American Society of Hematology Meeting. Supported in part by grants from the Cancer Research Institute and the National Institutes of Health (D.J.).
Reprints:David M. Dorfman, Brigham and Women's Hospital, Department of Pathology, 75 Francis St, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal