Although hereditary spherocytosis (HS) is a common disorder of the red cell membrane, its clinical and biologic expression at birth and in early infancy has received little attention. In order to obtain insights into the natural history of HS during infancy, we studied 46 neonates, 39 from families in which 1 of the parents had previously been given a diagnosis of HS and 7 presenting with nonimmune hemolytic anemia and no family history of HS. Of these 46 neonates, 23 were subsequently confirmed to have HS and 23 were found to be healthy. The hematologic and biologic analyses carried out in this cohort of 46 newborns enabled us to develop guidelines for early diagnosis of HS. A careful clinical follow-up of 34 HS patients during the first year of life allowed us to define several important clinical features of HS during this period. Hemoglobin values are usually normal at birth but decrease sharply during the subsequent 20 days, which leads, in many cases, to a transient and severe anemia. The anemia is severe enough to warrant blood transfusions in a large number of infants with HS (26 of 34 in our series). The aggravation of anemia appears to be related to the inability of these infants to mount an appropriate erythropoietic response to anemia and to the development of splenic filtering function. These findings indicate that careful monitoring of infants with HS during the first 6 months of life is important for appropriate clinical management.
Hereditary spherocytosis (HS) is a common inherited hemolytic anemia involving cell-membrane alterations. Its prevalence in northern Europe is approximately 1 in 2000.1,2 Its clinical expression is heterogeneous, ranging from severe transfusion-dependent anemia to clinically silent forms with well-compensated chronic hemolysis. Because some patients, even those who later manifest either mild or moderate forms of the disease, can present with a very severe phenotype in early infancy, a reliable strategy for diagnosis of HS in neonates is needed. Well-defined criteria for diagnosis of HS at birth have not been firmly established. In fact, the disease is diagnosed in only one third of affected infants during the first year of life.3 Moreover, the natural history of the disease during the first year of life has received little attention.4 5 In order to fill these gaps in our understanding of HS, we undertook a study with the following goals: (1) to develop objective criteria for diagnosis of HS at birth, and (2) to describe the natural history of the disease during the first year of life.
Detailed biologic and hematologic evaluations of blood samples from 46 neonates with a presumptive diagnosis of HS enabled us to establish criteria for diagnosis of HS at birth. We found that in neonates, as in adults, a decrease in the surface area of the red cell membrane and increased red cell dehydration were hallmarks of HS. A careful clinical follow-up of a cohort of 34 HS patients during the first year of life enabled us to define several important clinical features of the disease during this period. Hemoglobin (Hb) values that were normal at birth decreased sharply during the subsequent 20 days, which led, in most cases, to a transient and often severe anemia. The anemia was severe enough to warrant blood transfusions in 26 of the 34 infants. During the first year of life, erythropoiesis increased and, by the age of 1 year, most of the children had well-compensated chronic hemolysis and no further need for blood transfusions. These findings indicate that children with HS have a severe phenotype early in life and that careful monitoring of infants with HS during the first 6 months of life is important for appropriate clinical management.
Patients and methods
The criteria for diagnosis of HS at birth were established on the basis of a study of 46 blood samples from neonates suspected of having HS. These samples were sent to our laboratory by various obstetric and pediatric services in order to confirm or rule out a diagnosis of HS.
Samples from neonates in whom a diagnosis of HS was suggested by familial history or the presence of icterus or anemia were assessed by osmotic gradient ektacytometry6 and red blood cell indices, as previously reported.7-9 Infants with associated other causes of neonatal hemolysis, such as isoimmune hemolytic anemia, as well as infants with a gestational age < 38 weeks, were excluded from the study.
Complete blood counts and red blood cell indices (mean corpuscular Hb concentration [MCHC], red cell volume distribution width [RDW], Hb concentration distribution width [HDW], and percentage of hyperdense cells) were obtained at the time of diagnosis by using an automated hematology analyser (H*2, Bayer Diagnostics, Tarrytown, NY).10 Reticulocyte counts were obtained with an automated hematology analyzer (H*3, Bayer Diagnostics) or by manual counting of reticulocytes on blood smears after staining with new methylene blue. Red cell deformability measurements were performed with an osmotic gradient ektacytometer (Bayer Diagnostics).8 Controls were age-matched healthy newborns or infants. Red cell membrane proteins in blood samples from probands and other affected family members were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, as previously described.11 12
To delineate the natural history of HS during the first year of life, we selected 34 patients with HS (from 26 families) who were available for regular follow-up, including 23 patients with a dominant mode of inheritance. For these 23 infants, either a parent or a close relative with HS had undergone splenectomy. Twenty-one patients with HS born between 1980 and 1992 were entered into the study retrospectively; the other 13 were entered into the study prospectively. Statistical analysis was performed with the unpaired Student t test.
Results
Neonatal diagnosis
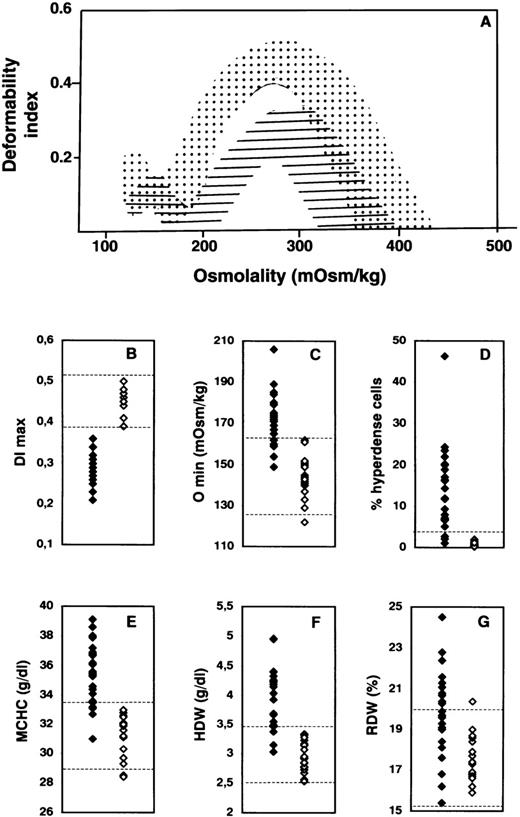
Detailed hematologic and red cell analyses were performed on blood samples from 46 neonates. Thirty-nine of these infants were from 31 families in which a parent had previously received a diagnosis of HS. The other 7 neonates had nonimmune hemolytic anemia but no familial history of HS. The analyses were carried out in either cord blood samples (15 infants) or venous blood samples (31 infants) drawn during the first month of life. The osmotic deformability profiles of the blood samples are illustrated in Figure 1A. These profiles clustered into 2 distinct groups on the basis of the maximum value attained by the deformability index (DImax). The first group of osmotic deformability profiles precisely matched the deformability profiles obtained with blood samples from healthy adults and children, whereas the second group had features similar to those obtained with blood samples from adults with HS. The DImaxvalues for the first group of blood samples (23 of the 46 samples) fell within the normal range for normal adult red cells, whereas those for the second group (23 of the 46 samples) were significantly lower (Figure 1B). Because the DImax value provides a direct measure of mean membrane surface area of red cells,6 these findings indicate that the group of blood samples with decreased DImax values contained red cells with reduced surface area, a hallmark of spherocytosis.
Hematologic and red cell analyses in hereditary spherocytosis (HS).
(A) Osmotic gradient ektacytometric profiles of blood samples. The deformability profiles of red cells from nonaffected newborns span the dotted area, whereas those of red cells from newborns with HS fall within the hatched area. DImax indicatesmaximum deformability index, a measure of red cell surface area; and Omin, the osmolality at which the deformability value reaches a minimum in the hypotonic region of the gradient, a measure of osmotic fragility of red cells. There is a decrease in DImaxvalues (B) in children with HS, reflecting membrane surface-area loss, and a right shift of the deformability curve in the hypotonic region (Omin), reflecting a decreased ratio of surface area to volume in the HS red cells (increased osmotic fragility) (C). Solid diamonds indicate affected children; and open diamonds, nonaffected children. The percentage of hyperdense cells (cell hemoglobin (Hb) concentration > 410 g/L) (D), mean corpuscular hemoglobin concentration (MCHC) (E), and hemoglobin concentration distribution width (HDW) (F) are higher in HS. In contrast, there is a large overlap between red cell volume distribution width (RDW) in newborns with HS and that in nonaffected newborns (G). The range of normal values in adults and children (B and C) or age-matched newborns (E-G) is indicated by dotted lines. The normal threshold value for percentage of hyperdense cells is indicated by the dotted line in D.
Hematologic and red cell analyses in hereditary spherocytosis (HS).
(A) Osmotic gradient ektacytometric profiles of blood samples. The deformability profiles of red cells from nonaffected newborns span the dotted area, whereas those of red cells from newborns with HS fall within the hatched area. DImax indicatesmaximum deformability index, a measure of red cell surface area; and Omin, the osmolality at which the deformability value reaches a minimum in the hypotonic region of the gradient, a measure of osmotic fragility of red cells. There is a decrease in DImaxvalues (B) in children with HS, reflecting membrane surface-area loss, and a right shift of the deformability curve in the hypotonic region (Omin), reflecting a decreased ratio of surface area to volume in the HS red cells (increased osmotic fragility) (C). Solid diamonds indicate affected children; and open diamonds, nonaffected children. The percentage of hyperdense cells (cell hemoglobin (Hb) concentration > 410 g/L) (D), mean corpuscular hemoglobin concentration (MCHC) (E), and hemoglobin concentration distribution width (HDW) (F) are higher in HS. In contrast, there is a large overlap between red cell volume distribution width (RDW) in newborns with HS and that in nonaffected newborns (G). The range of normal values in adults and children (B and C) or age-matched newborns (E-G) is indicated by dotted lines. The normal threshold value for percentage of hyperdense cells is indicated by the dotted line in D.
We identified 23 HS infants with a decreased surface area of red cell membranes (DImax < 0.39) in the cohort of 46 infants screened. Of the 23 blood samples from this group, 17 showed a shift to the right of the hypo-osmolar point (Omin) in the osmotic deformability profile (Figure 1C), indicating that the red cells had increased osmotic fragility, a distinct feature of spherocytes. But, in contrast to our findings with respect to reduced surface area, the increase in osmotic fragility was not a consistent feature of all HS blood samples: 6 of the 23 infants with HS had normal osmotic fragility. As has been documented of red cells in adults with HS, red cells in the newborns with HS were dehydrated.
Increased percentages of hyperdense cells (> 4% of red cells with a cell Hb concentration > 410 g/L) were noted in 20 of the 23 newborns with HS (Figure 1D). Interestingly, an increased percentage of hyperdense cells was a feature of blood samples from 5 of the 6 HS patients whose red cells had normal osmotic fragility, whereas increased osmotic fragility was a feature of blood samples from 2 of the 3 HS patients who did not have an increased percentage of hyperdense cells. MCHC was elevated (> 335 g/L) in 19 of the 23 HS newborns (Figure 1E), and HDW was increased (> 34.5 g/L) in 20 (Figure 1F). In contrast, there was a noticeable overlap in the RDW values in blood samples from healthy newborns and those from newborns with HS (Figure 1G). All these cellular features were reconfirmed on repeated assessment of blood samples obtained a year later from infants given a diagnosis of HS, as well as samples from those considered not to have HS.
HS during the first year of life
Thirty-four infants with HS (including 13 of the 23 given a diagnosis of HS at birth), from 26 families, were monitored regularly during early childhood and the following characteristics observed.
Clinical features.
The main clinical features observed during the first months of life were pallor, dyspnea (mild difficulties in breathing while feeding), or both, related to anemia in 31 of the 34 infants and to neonatal icterus in 27. Neonatal icterus occurred early in most patients (bilirubin level ranging from 168 to 462 μmol/L during the first 3 days of life) and led to phototherapy in 24. Splenomegaly was noted within the first 6 months of life in 23 of the 34 infants (range, 1-45 weeks; median, 5 weeks).
Hb values.
In 26 patients with HS who did not have a transfusion, one or several Hb measurements were available during the first month of life. At birth or during the first 96 hours of life, the mean Hb value was 150 g/L (range, 117-196 g/L) in the 19 newborns from whom samples were obtained. Hb values were above the normal threshold of 146 g/L in 63% of these infants and ranged from 117 to 142 g/L in 37%, suggesting anemia. Surprisingly, Hb values at birth were not statistically different from those in normal cord-blood samples or samples from nonaffected age-matched infants (Table 1). However, in the affected babies from whom samples were obtained after the age of 5 days, Hb values fell below the normal range (Figure2A). A similar pattern of Hb values was noted in all infants with HS, irrespective of initial clinical presentation. This decrease persisted throughout the following weeks, resulting in Hb levels < 80 g/L during the first month of life in 75% of the children (Figure 2B).
Hemoglobin values at birth in healthy full-term newborns and in newborns with hereditary spherocytosis (HS)
| . | Hemoglobin (g/L) . |
|---|---|
| Healthy newborns (n = 42) | 156 ± 11 (136-182) |
| Newborns with HS (cord blood) (n = 11) | 154 ± 23 (117-185) |
| Newborns with HS (venous blood) (n = 8) | 153 ± 28 (120-196) |
| . | Hemoglobin (g/L) . |
|---|---|
| Healthy newborns (n = 42) | 156 ± 11 (136-182) |
| Newborns with HS (cord blood) (n = 11) | 154 ± 23 (117-185) |
| Newborns with HS (venous blood) (n = 8) | 153 ± 28 (120-196) |
Values are mean ± SD (range).
Changes in Hb values.
(A) Temporal evolution of Hb values in 3 infants who did not have transfusions during the first weeks of life. Shaded area shows the range of Hb values in healthy infants.13 B shows histograms of Hb values (mean ± SD) before the first transfusion in infants with HS, as a function of postnatal age. The number of infants with HS (n) evaluated at various postnatal ages is indicated (a: n = 19; b: n = 12; c: n = 10; d: n = 8).
Changes in Hb values.
(A) Temporal evolution of Hb values in 3 infants who did not have transfusions during the first weeks of life. Shaded area shows the range of Hb values in healthy infants.13 B shows histograms of Hb values (mean ± SD) before the first transfusion in infants with HS, as a function of postnatal age. The number of infants with HS (n) evaluated at various postnatal ages is indicated (a: n = 19; b: n = 12; c: n = 10; d: n = 8).
Reticulocyte counts.
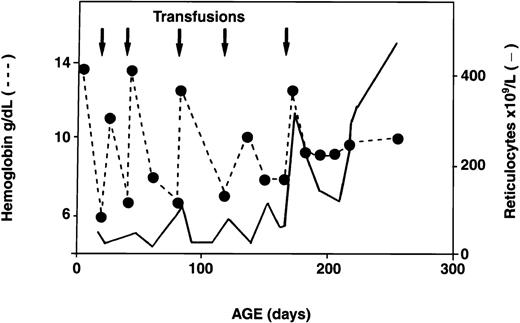
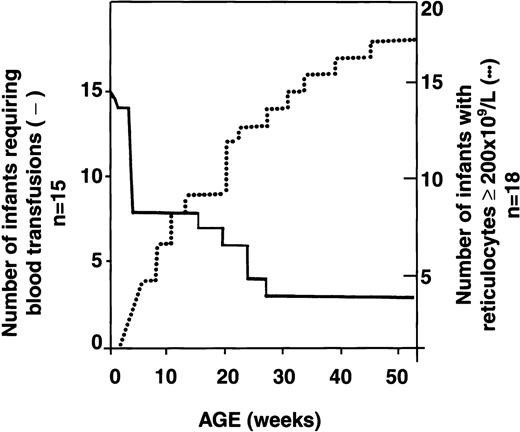
Serial reticulocyte counts were available for 18 of the 34 infants with HS. In 16 of the 18, reticulocyte counts were increased at birth, as is observed in normal newborns.13 Interestingly, however, subsequent reticulocyte counts decreased below the threshold value of 200 × 109/L and remained low for several weeks to months. During this period, erythropoiesis did not compensate for the low Hb values. Reticulocyte counts increased progressively with age, however, and by the time the infants reached 1 year of age, the counts were again > 200 × 109/L, resulting in better compensation for hemolysis. A representative example of the evolution of the reticulocyte response in an infant with HS is shown in Figure 3. Analysis of the reticulocyte data showed an inverse relation between transfusion requirements and reticulocyte counts ≥ 200 × 109/L (Figure 4). Among 18 HS newborns for whom sequential reticulocyte counts were available, 15 required blood transfusions. Over time, 12 of these 15 infants became transfusion independent, whereas the 3 other infants remained transfusion dependent (3, 7, and 10 transfusions, respectively, during the first year of life) in spite of having reticulocyte counts persistently above 200 × 109/L. Clearly, in these 3 infants, the increased reticulocytosis was unable to compensate for increased red cell destruction.
Temporal evolution of Hb values and reticulocyte counts in a patient with recurrent transfusion-dependent anemia in early infancy.
Arrows indicate transfusions; dotted line, Hb values; and solid line, reticulocyte counts.
Temporal evolution of Hb values and reticulocyte counts in a patient with recurrent transfusion-dependent anemia in early infancy.
Arrows indicate transfusions; dotted line, Hb values; and solid line, reticulocyte counts.
Inverse relation between reticulocyte count at least 200 × 109/L and transfusion requirements in infants with HS.
Dotted line indicates reticulocyte count at least 200 × 109/L; solid line, transfusion requirements. Although sequential reticulocyte counts were available for 18 infants with HS, only 15 of these patients required transfusions during the first year of life.
Inverse relation between reticulocyte count at least 200 × 109/L and transfusion requirements in infants with HS.
Dotted line indicates reticulocyte count at least 200 × 109/L; solid line, transfusion requirements. Although sequential reticulocyte counts were available for 18 infants with HS, only 15 of these patients required transfusions during the first year of life.
Red cell-membrane defects.
Red cell-membrane protein analyses were performed in either probands or other affected members of 17 families (25 patients). Eleven families presented with combined ankyrin and spectrin deficiency (probably reflecting a mutation of the ANK1 gene), whereas 6 other families presented with isolated spectrin deficiency (most likely reflecting a mutation in the SPTB gene). Four infants born into 3 HS families with band 3 deficiency during the course of this study were found to be unaffected by HS.
Transfusion requirements.
Of the 34 infants studied, 26 (76%) required transfusion therapy during the first year of life. Fourteen of the babies had several transfusions, whereas 12 received a single transfusion. A high bilirubin level associated with anemia required an exchange transfusion at the age of 2 days in 3 of the 26 infants. The first transfusion was prescribed during the initial 2 months of life in 24 (92%) of the 26 infants. The Hb values at which a first transfusion was prescribed varied widely; however, red blood cell transfusions were always prescribed when Hb values decreased below 100 g/L by the age of 8 days and below 85 g/L thereafter. Of the 24 children who required red cell transfusions during early infancy, only 8 continued to need transfusions between the ages of 6 and 12 months. A follow-up of 3 to 5 years showed that 6 of these 8 infants with HS remained transfusion dependent and underwent subtotal splenectomy14 between 2 and 5 years of age. The 2 others, who did not require splenectomy, received a single transfusion because of a pure red cell aplasia due to parvovirus B19.
Discussion
The increased rate of red cell destruction and the ability of erythropoiesis to compensate for this destruction are the 2 processes that determine the clinical severity of chronic hemolytic anemias. At birth and during the first month of life, major readjustments occur in both these processes. On the one hand, the splenic circulation is fully developed only after birth,15,16 resulting in an enhanced peripheral destruction of circulating abnormal red blood cells such as spherocytes. Consequently, hemolysis that is likely to be quiescent or mild in fetuses affected by HS (except in the very severe forms)17,18 increases dramatically after birth as the splenic microvascular filtration and phagocytosing function become fully effective. On the other hand, erythropoiesis, though highly stimulated during fetal life, abruptly enters a hypoplastic phase shortly after birth. The primary physiologic mechanism responsible for decreased erythropoiesis is a dramatic reduction in erythropoietin secretion, which is probably related to both the switch from hepatic to renal erythropoietin secretion and the marked elevation in oxygen levels resulting from the transition from fetal to lung respiration.19 A newborn with HS thus has to cope with both an increased rate of red cell destruction and an abruptly restricted ability to mount an appropriate erythropoietic response. Together, these 2 deleterious effects markedly aggravate anemia in patients with HS during the neonatal period and early infancy. The neonatal period has also been reported to be critical in murine models of HS, and it has been shown that neonatal transfusions can extend the lifespan of spectrin-deficient ja/ja pups.20
Our study of infants with HS during their first year of life highlights 3 facts: (1) a reliable diagnosis of HS can be made at birth, (2) there is a dramatic postnatal decrease in Hb values, and (3) an adequate reticulocyte response cannot be mounted for several months, which leads to a requirement for transfusion therapy that, in most cases, is transient.
In regard to the reliable diagnosis of HS in newborns, documentation of a decreased membrane surface area in red cells by osmotic gradient ektacytometry is the best indicator of spherocytosis, as previously reported for HS in adults. When osmotic gradient ektacytometry is not available, judicial assessment of data from different laboratory tests, including the osmotic fragility test (with a comparison to an age-matched control); red cell indices, particularly the percentage of hyperdense red cells; red cell morphologic evaluations; and careful assessment of family history can confirm most cases of HS in infants. It should be emphasized, however, that decreased membrane surface area is a hallmark of spherocytes of various origins and may also be transiently observed in acquired spherocytosis related to immune hemolytic anemia. Therefore, when HS is suspected at birth, it is important to test for maternal-fetal incompatibility. Repeated documentation of persistent cellular abnormalities, including decreased surface area, an increased osmotic fragility, and an increased percentage of hyperdense red cells, will confirm a diagnosis of HS, whereas the recovery of normal cellular features will be consistent with a diagnosis of immune hemolytic anemia.21
Our finding of near-normal Hb values at birth and a subsequent dramatic decrease in Hb levels during the first weeks of life (between days 5 to 30) in infants with HS is of major clinical relevance and has not previously been emphasized. This finding highlights the need for both an early and reliable diagnosis of HS and a careful follow-up in affected children. In infants considered to be at risk for HS, a normal Hb value at birth should not be used to rule out the diagnosis of HS or disregard the need for regular monitoring of hematologic status during the first weeks of life. Finally, a satisfactory erythropoietic response leading to well-compensated hemolysis, as indicated by persistently high reticulocyte counts and transfusion independence, was observed in most children only after several months of life. Moreover, the time it took to develop an appropriate erythropoietic response varied markedly from individual to individual.
On the basis of our findings, 4 groups of patients could be retrospectively defined in terms of transfusion requirements. The first group could be cared for without transfusions during the first year of life, despite low Hb values during the initial follow-up. Twenty-four percent of the patients in our study belonged to this group. For such patients, regular follow-up is mandatory until a trend toward an increase in reticulocyte counts leading to an acceptable Hb level can be documented. The second group of patients (34% of the total) needed only a single transfusion and, in most instances, this transfusion was administered before the age of 2 months. The third group (24% of patients) needed several transfusions and reached sustained transfusion independence only at an age ranging from 4 to 9 months. The fourth group required continuous transfusion support until a splenectomy was performed; 18% of our patients belonged to this group with severe forms of HS. Our findings regarding transfusion dependency are similar to those in an Italian pediatric survey.5
No reliable prognostic criteria for predicting transfusion requirements during infancy in patients with HS emerged from our study. Family history was not useful in predicting the severity of anemia in the infant. However, it appeared that in all children who required transfusion, the Hb value decreased by > 3 g/L per day after birth and during the first 3 weeks of life. Because of the small size of our patient population, no definite conclusion can be drawn, but our data strongly suggest that whenever HS is diagnosed in neonates, the evolution of Hb and reticulocyte values should be monitored carefully every other day during the first 10 days of life. Anemia at birth, or an abrupt decrease in Hb values during the first weeks of life, is a likely predictor of future transfusion needs. There appears to be a critical need to develop reliable predictive criteria for the transfusion needs of infants with HS. Alternative clinical management strategies to decrease or avoid transfusions during the first months of life in anemic infants should also be explored.22
Acknowledgments
We are grateful to J. A. Chasis for many helpful discussions and to M. Dehan for help in preparing the manuscript.
Supported by a grant from Direction de la Recherche Clinique, AP-HP, Paris (CRC 96082) and a grant from the National Institutes of Health (DK26263).
Reprints:Narla Mohandas, Mailstop 74-157, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94720; e-mail:mnarla@lbl.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal