Abstract
Delayed immune reconstitution after allogeneic bone marrow transplantation (BMT) with associated infection is a major cause of morbidity and mortality. We used third complementarity region (CDR3) size spectratyping as a tool for monitoring T-cell repertoire reconstitution in 19 patients over a median time of 40 months after T-cell–depleted allogeneic BMT for chronic myeloid leukemia (CML). Furthermore, the effect of donor lymphocyte infusions (DLI) for the treatment of relapse in 18 of the 19 patients was analyzed. All BMT recipients had irregular spectratypes in the first 3- to -6 months after transplant. These evolved to more normal patterns by 12 months after transplant and continued to improve thereafter. In approximately a third of the patients, it took 2 to 3 years for all spectratypes to normalize, whereas in the other two thirds, some abnormal spectratypes persisted even after several years. In 9 patients, there was no immediate change in the CDR3 size profiles after DLI. In 3 patients, spectratypes improved slightly after DLI, whereas in 6 patients, spectratypes became more restricted and irregular. Overall, T-cell spectratypes in BMT patients were characterized by instability over time and in patients with graft-versus-host disease (GVHD), this was even more exaggerated. Several factors, such as pre-BMT conditioning, T-cell depletion of the donor marrow, loss of thymic function in adults, exposure to infectious agents, GVHD, and immunosuppressive treatment, are likely contributors to the delay in T-cell–repertoire reconstitution.
Although allogeneic bone marrow transplantation (BMT) from HLA-matched donors can provide a curative therapy for chronic myeloid leukemia (CML)1 and other hematologic disorders, the long period of immune compromise experienced by BMT recipients after transplant is a major cause of morbidity and mortality in this patient group. T-cell immunity is affected most, because the T-cell pool is largely eliminated by the pretransplant conditioning, and the capacity for thymic output is very limited in adults.2,3Thus, T-cell immunodeficiencies render BMT recipients susceptible to a number of life-threatening opportunistic infections.4,5T-cell–repertoire reconstitution can be further delayed by the development of graft-versus-host disease (GVHD).6
Removal of T cells from the donor bone marrow before transplant reduces the risk of GVHD by limiting the number of allo-reactive T cells. An increased risk of relapse is, however, associated with the use of T-cell–depleted bone marrow compared with unmanipulated bone marrow, because T cells involved in graft-versus-leukemia (GVL) responses are also removed.7 8
Donor lymphocyte infusion (DLI) is an effective treatment for patients with relapsed CML. It induces GVL responses and results in complete remission in the majority of patients.9 However, DLI can also cause GVHD. Whether GVL occurs without GVHD depends to a large extent on the dose of donor lymphocytes infused.10
The technique of third complementarity region (CDR3) size analysis developed by Cochet et al11 and later called “spectratyping” by Gorski et al12 is a powerful tool to analyze T-cell receptor (TCR) diversity in health and disease. Reverse transcriptase-polymerase chain reaction (RT-PCR) products spanning the constant (C) /variable (V) region junctions of the CDR3 beta (B) chain in the TCR are separated according to size, giving rise to a spectrum of different size classes for each of the 22 BV gene families expressing functional rearrangements, thus providing a finer level of resolution than just analyzing BV gene family expression.
CDR3 beta chain size heterogeneity arises during developmental DNA rearrangement when a BC segment is joined with 1 of 22 BV segments via a diversity (D) and a joining (J) segment to give rise to a complete BV gene. During this process, random numbers of nucleotides are inserted and deleted enzymatically at the junctions between the gene segments.13 Successful rearrangements differ in size by multiples of 3 nucleotides. Any other rearrangements would be out of frame and are rarely detected in peripheral blood T cells.12 As the ratio of transcripts per cell is fairly constant,14 the amount of PCR product in each size class gives an indication of the number of clonotypes and thus T-cell repertoire diversity. Predominance of only a few size classes in a spectratype would indicate oligoclonality. Gaps in a spectratype indicate a lack of T cells expressing a certain CDR3 size class. However, sequencing would be required to determine the number of clones that make up each size class.
Several authors have successfully used CDR3 size spectratyping to study aspects of T-cell–repertoire reconstitution after BMT. Gorski et al12 related T-cell–repertoire complexity in BMT recipients to their state of immune function, Dietrich et al15 examined T-cell spectratypes in GVHD skin lesions, and Akatsuka et al16 and Roux et al17,18 looked at differences in T-cell–repertoire reconstitution in recipients of T-cell–depleted and –undepleted bone marrow. Finally, Claret et al19 characterized T-cell repertoires in BMT recipients with GVL responses after DLI. Limitations of these studies were the small number of patients included and short intervals of time after transplant over which patients were followed.
Here, the T-cell repertoires of 19 allogeneic T-cell–depleted BMT recipients were analyzed by CDR3 size spectratyping longitudinally for a median time of 40 months after BMT for CML. Furthermore, 18 of these patients underwent DLIs at some stage after BMT for treatment of relapse of their diseases. The effect of DLI on the T-cell repertoire was analyzed.
Patients, materials, and methods
Patients
Nineteen patients with CML in the first chronic phase were studied after allogeneic BMT. Donor-recipient pairs were matched at the human leukocyte antigen A, B, and DR loci (Table1). The patients were conditioned with fractionated total body irradiation 12 × 125 cGy + cyclophosphamide 120 mg/kg + thiotepa 10 mg/kg. All patients received a T-cell–depleted marrow graft. In vitro T-cell depletion was performed using soybean agglutinin (SBA) to produce an SBA-marrow fraction. This was further T-cell depleted by rosetting with sheep erythrocytes to give a SBA–E-marrow.20Eighteen of the patients received DLIs for the treatment of relapse as previously described.10
Patient and donor characteristics
| Patient number . | Sex . | Age . | Donor type . | Donor sex . |
|---|---|---|---|---|
| 1 | F | 18 | MUD | F |
| 2 | M | 38 | SIB | M |
| 3 | M | 35 | SIB | M |
| 4 | M | 53 | SIB | F |
| 5 | M | 40 | SIB | F |
| 6 | F | 13 | SIB | M |
| 7 | M | 33 | SIB | M |
| 8 | M | 40 | SIB | M |
| 9 | M | 26 | SIB | M |
| 10 | M | 38 | SIB | F |
| 11 | M | 46 | Mother | F |
| 12 | F | 47 | SIB | M |
| 13 | M | 43 | SIB | M |
| 14 | F | 41 | SIB | M |
| 15 | M | 43 | SIB | M |
| 16 | M | 45 | SIB | F |
| 17 | M | 56 | SIB | M |
| 18 | M | 63 | SIB | M |
| 19 | M | 46 | SIB | M |
| Patient number . | Sex . | Age . | Donor type . | Donor sex . |
|---|---|---|---|---|
| 1 | F | 18 | MUD | F |
| 2 | M | 38 | SIB | M |
| 3 | M | 35 | SIB | M |
| 4 | M | 53 | SIB | F |
| 5 | M | 40 | SIB | F |
| 6 | F | 13 | SIB | M |
| 7 | M | 33 | SIB | M |
| 8 | M | 40 | SIB | M |
| 9 | M | 26 | SIB | M |
| 10 | M | 38 | SIB | F |
| 11 | M | 46 | Mother | F |
| 12 | F | 47 | SIB | M |
| 13 | M | 43 | SIB | M |
| 14 | F | 41 | SIB | M |
| 15 | M | 43 | SIB | M |
| 16 | M | 45 | SIB | F |
| 17 | M | 56 | SIB | M |
| 18 | M | 63 | SIB | M |
| 19 | M | 46 | SIB | M |
F, female; M, male; MUD, matched unrelated donor; SIB, sibling donor.
RNA extraction
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood by density gradient centrifugation through Ficoll-Paque (Pharmacia Biotech, St Albans, UK). RNA was extracted from the cells using Ultraspec RNA (BiotecX Laboratories, Houston, TX), according to the manufacturer's protocol. Briefly, 5 to 10 million cells were homogenized in Ultraspec, followed by the addition of chloroform to separate the organic and aqueous phases. RNA was precipitated from the aqueous phase with isopropanol, followed by a wash with 70% ethanol. The resulting RNA pellet was resuspended in sterile water, and the yield of RNA and purity were determined by spectrophotometry.
Reverse transcriptase reaction
Complementary DNA (cDNA) was generated from 1 μg of RNA in a 20 μL reaction using random hexanucleotide primers for reverse transcription with reverse transcriptase (Superscript, Gibco, Paisley, UK).
Polymerase chain reaction primers
Each of 22 functionally rearranged BV gene subfamilies was amplified across the C/V junctions using the 24 BV subfamily-specific primers described previously by Maslanka et al,21 and a fluorescent dye (FAM, Perkin Elmer)-conjugated BC region-specific primer.12 Some of the BV primers amplify short, and others longer PCR products. “Short” and “long” BV primers were combined in multiplex PCRs to amplify 2 BV families in 1 reaction as follows: BV 5.1 + 1; BV2 + 12; BV8 + 3; BV4 + 5.4; BV13 + 7; BV9 + 14; BV11 + 20; BV17 + 15; BV16 + 21; BV18 + 23; and BV24 + 22. BV6.1 and BV6.2 were used unpaired.
Polymerase chain reaction
The total PCR volume was 20 μL containing Genamp PCR buffer (Perkin Elmer), 2 mmol/L MgCl2, 0.2 mmol/L each dNTP, 1 mmol/L of each primer, and 1 μL cDNA (equivalent to approximately 25 000 cells). For the hot start, 0.5 units of Amplitaq DNA polymerase were added last, after a 5-minute denaturation step at 95°C. Optimal cycling conditions were 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds, for 30 cycles, followed by a final extension at 72°C for 5 minutes.
T-cell spectratyping
One microliter of PCR product was denatured in 12 μL formamide and electrophoresed through Performance Optimized Polymer 4 (Perkin Elmer, Cambridge, UK) on an ABI 110 automated sequencer (Perkin Elmer) in the presence of Tamra 500 size standard (Perkin Elmer). Genescan software 2.1 (Perkin Elmer) was used to analyze the data. For the purpose of analyzing the T-cell repertoire of BMT recipients, a normal spectratype was defined as comprising at least 6 different size classes, at intervals of 3 nucleotides without any gaps.
T-cell dilution experiments
PBMCs from a healthy young donor were separated by density gradient centrifugation through Ficoll-Paque. The T2 lymphoblastic hybridoma cell line, which expresses TCR BV 9, and the Jurkat T-cell line, which expresses TCR BV8, were used to spike these polyclonal PBMCs in separate experiments. Serial dilutions of each cell line in PBMCs were made from 1:100 down to 1:100 000. A total of 1 × 107 cells of each dilution was used for RNA extraction.
Results
Definition of abnormal CDR3 size profiles and sensitivity of CDR3 spectratyping
TCR CDR3 size profiles in BMT recipients were compared with those in healthy adults. Although the amount of product in each size class in a normal spectratype is typically distributed in a Gaussian fashion with 6 to 10 different size classes at 3 nucleotide intervals, spectratype shapes frequently deviate markedly from this normal distribution pattern by having 1 or 2 predominant size classes.22 Hence, in this study, normal spectratypes were defined as consisting of at least 6 peaks spaced 3 nucleotides apart without any gaps.
T-cell numbers are often low in BMT recipients, especially shortly after transplant. In healthy individuals, l0 000 cells produce normal spectratypes (results not shown). In our PCRs, we used an amount of cDNA equivalent to approximately 25 000 PBMCs, which is in excess of the limiting cell number. The deviations from a normal Gaussian distribution in healthy individuals are accounted for by clonal expansions of T cells in the CD8+ subset.23Hence, a spectratype analysis of the CD8+ subset could have been more informative. However, because of the low cell numbers available at certain time points and additional losses that would have occurred during processing, it was decided not to separate samples into subsets for analysis.
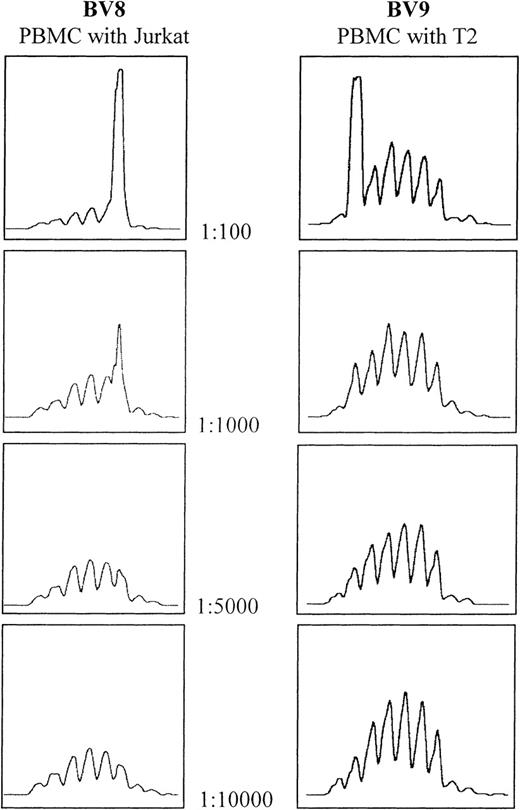
The sensitivity of the CDR3 spectratyping technique was determined by spiking experiments using the T2 and Jurkat cell lines. Analysis of the spectratype profile of spiked normal PBMCs showed that the clones can be detected at a dilution of 1:1000, but not 1:5000 or above (Figure1). This confirms published results.24 25
Determination of spectratype sensitivity to the addition of cells bearing clonally rearranged T-cell–receptor genes.
Serial dilutions of both Jurkat and T2 cell lines were made using PBMCs from the same healthy donor. In both cases, the threshold for detection lies between 1:1000 and 1:5000.
Determination of spectratype sensitivity to the addition of cells bearing clonally rearranged T-cell–receptor genes.
Serial dilutions of both Jurkat and T2 cell lines were made using PBMCs from the same healthy donor. In both cases, the threshold for detection lies between 1:1000 and 1:5000.
CDR3 size profile evolution in allogeneic T-cell–depleted bone marrow transplant recipients
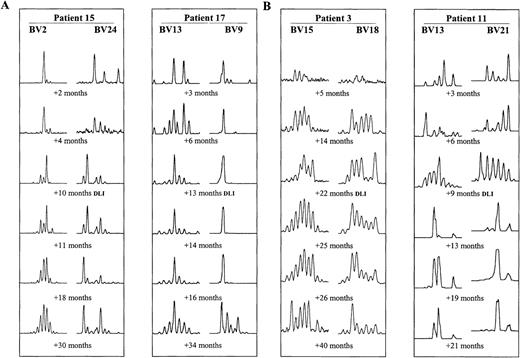
T-cell repertoires of 19 BMT patients were monitored by TCR spectratyping over a median time of 40 months after transplantation. The aim was to determine how long it takes after BMT for the T-cell repertoire to reach normal polyclonal complexity. At least 6 BV subfamilies were amplified and analyzed for each BMT recipient. The primer sets chosen differed among the various patients, however, subfamilies overrepresented and underrepresented within the normal repertoire were included for each patient. In most patients, all BV subfamilies comprised only very few size classes during the first 3 months after BMT (example in Figure 2A, first time points after BMT). After 6 months, T-cell repertoires gradually diversified. By 12 months, profiles of some BV subfamilies contained a full set of size classes in nearly all patients. However, transient changes in the amount of product in each CDR3 size class were common (eg, Figure 2, early time points). Furthermore, in all patients, some very abnormal spectratypes persisted by 12 months after BMT. Between 2 and 3 years after transplant, all patients demonstrated at least some normalization of CDR3 size profiles. However, there were marked differences in the progress of CDR3 size profile normalization between different patients as well as between the different BV subfamilies in the same patient. This is illustrated in Figure 2A, showing examples in 2 BMT recipients of normalizing (BV13 and 2) and abnormal (BV9 and 24) series of CDR3 size spectratypes.
Examples of CDR3 size pattern evolution after BMT.
Size profiles of 2 different BV subfamilies at 6 time points are shown for each patient. (A) Examples of interpatient and intrapatient variability in patients 15 and 17, who underwent DLIs +13 months and +14 months after BMT, respectively. (B) Examples of spectratype change after DLI. Improving spectratypes in patient 3 (DLI +22 months) and more abnormal spectratypes after DLI in patient 11 (DLI +9 months).
Examples of CDR3 size pattern evolution after BMT.
Size profiles of 2 different BV subfamilies at 6 time points are shown for each patient. (A) Examples of interpatient and intrapatient variability in patients 15 and 17, who underwent DLIs +13 months and +14 months after BMT, respectively. (B) Examples of spectratype change after DLI. Improving spectratypes in patient 3 (DLI +22 months) and more abnormal spectratypes after DLI in patient 11 (DLI +9 months).
In 6 patients, all spectratypes analyzed became similar to those of healthy adults. Figure 3 illustrates the normalization of the size profiles with 21 BV-specific primers between 13 and 37 months after BMT in patient 1. In the remaining 13 patients, some BV spectratypes normalized, whereas others were characterized by oligoclonal patterns that persisted until the end of the study, which meant up to 5 years after BMT in some patients.
CDR3 size profiles with 21 subfamily-specific primers at 2 time points after BMT in PBMCs from patient 1.
The patient underwent DLI +23 months after BMT, with subsequent normalization of spectratypes by +37 months after BMT.
CDR3 size profiles with 21 subfamily-specific primers at 2 time points after BMT in PBMCs from patient 1.
The patient underwent DLI +23 months after BMT, with subsequent normalization of spectratypes by +37 months after BMT.
Eighteen of the 19 patients underwent DLIs to treat relapses. Sixteen of these patients achieved remission after DLIs (Table2). In 9 patients, there was no detectable change in T-cell spectratypes between the pre-DLI and early post-DLI. In only 3 patients, the spectratypes became slightly more normal (example shown in Figure 2B, patient 3), whereas in 6 patients, the CDR3 size patterns appeared more abnormal after DLI (example shown in Figure 2B, patient 11). As described above, in the longer term, T-cell repertoires did improve, but whether this was the result of a delayed indirect effect of DLI or independent of DLI remains unclear. The timing of DLI after BMT did not seem to influence its effect on the T-cell repertoire. Whether an association exists between spectratype normalization and chimeric status was not clear. In the 2 patients who retained mixed chimerisms after DLI (Table 2), spectratypes became more abnormal in 1 (Figure 2B, patient 11), whereas no marked change in spectratypes after DLI occurred in the other.
Cytogenetic and molecular outcome after donor lymphocyte infusions
| Patient number . | Cytogenetic CR . | Molecular CR . | T-cell chimerism before DLIs . | T-cell chimerism after DLIs . |
|---|---|---|---|---|
| 1 | Y | N | Donor | Donor |
| 2 | Y | Y | Donor | N/A |
| 3 | Y | Y | Mixed | Donor |
| 4 | Y | Y | Mixed | Donor |
| 5 | Y | Y | Mixed | Donor |
| 6 | Y | Y | Mixed | Donor |
| 7 | Y | Y | Mixed | Donor |
| 8 | Y | Y | ND | ND |
| 9 | Y | Y | Donor | Donor |
| 10 | Y | Y | Mixed | Donor |
| 11 | N | N | Mixed | Mixed |
| 12 | Y | Y | Mixed | Donor |
| 13 | Y | N | ND | ND |
| 14 | Y | Y | Mixed | Donor |
| 15 | Y | Y | Mixed | Mixed |
| 16 | Y | Y | ND | ND |
| 17 | Y | Y | Mixed | Donor |
| 18 | Y | Y | Mixed | Donor |
| 19 | Y | Y | ND | ND |
| Patient number . | Cytogenetic CR . | Molecular CR . | T-cell chimerism before DLIs . | T-cell chimerism after DLIs . |
|---|---|---|---|---|
| 1 | Y | N | Donor | Donor |
| 2 | Y | Y | Donor | N/A |
| 3 | Y | Y | Mixed | Donor |
| 4 | Y | Y | Mixed | Donor |
| 5 | Y | Y | Mixed | Donor |
| 6 | Y | Y | Mixed | Donor |
| 7 | Y | Y | Mixed | Donor |
| 8 | Y | Y | ND | ND |
| 9 | Y | Y | Donor | Donor |
| 10 | Y | Y | Mixed | Donor |
| 11 | N | N | Mixed | Mixed |
| 12 | Y | Y | Mixed | Donor |
| 13 | Y | N | ND | ND |
| 14 | Y | Y | Mixed | Donor |
| 15 | Y | Y | Mixed | Mixed |
| 16 | Y | Y | ND | ND |
| 17 | Y | Y | Mixed | Donor |
| 18 | Y | Y | Mixed | Donor |
| 19 | Y | Y | ND | ND |
DLIs, donor lymphocyte infusions; Y, yes; N, no; CR, complete remission; N/A, not applicable; ND, not done.
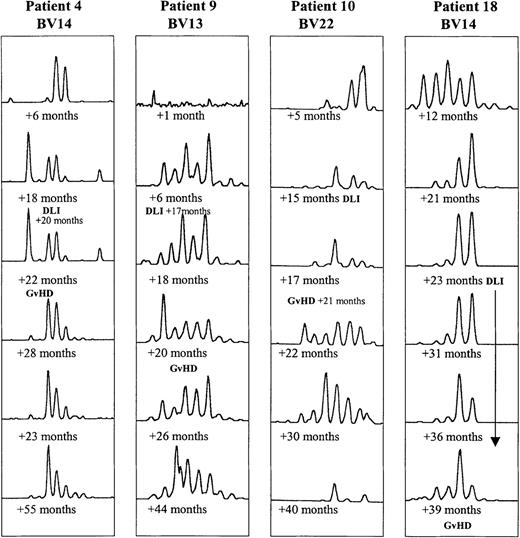
In 4 of the 18 BMT patients, clinical GVHD developed after DLI therapy. Their CDR3 profiles tended to undergo a larger number of transitory changes than those in the other BMT patients, as illustrated in Figure4.
Evolution of CDR3 size profiles in patients 4 (DLI +20 months), 9 (DLI +17 months), 10 (DLI +15 months), and 18 (multiple DLI treatments between +23 and +36 months after BMT), all in whom GVHD developed, as indicated.
Patterns for 1 BV subfamily are shown for each patient at 6 time points after BMT.
Evolution of CDR3 size profiles in patients 4 (DLI +20 months), 9 (DLI +17 months), 10 (DLI +15 months), and 18 (multiple DLI treatments between +23 and +36 months after BMT), all in whom GVHD developed, as indicated.
Patterns for 1 BV subfamily are shown for each patient at 6 time points after BMT.
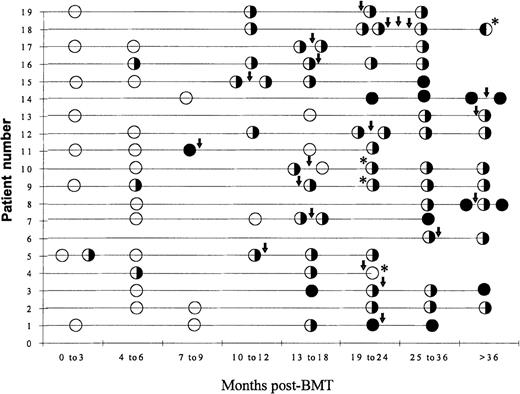
Figure 5 summarizes the evolution of CDR3 size patterns in all 19 patients. Overall, spectratypes in BMT recipients were much more irregular than those in healthy adults and at least some size classes were completely undetectable within 6 months of transplant. Also, unlike in healthy adults, in BMT patients some spectratypes changed considerably over time. However, on the whole, BMT patients' CDR3 profiles became more normal with increasing time from BMT (Figure 5). In 1 of the 3 patients who did not achieve molecular complete remission (CR) (Table 2), spectratypes completely normalized between 19 and 24 months post-BMT, whereas some spectratypes remained abnormal by the end of the study more then 36 months after BMT in the other 2 patients. Hence, these data do not support an association between molecular CR and spectratype normalization.
Summary of CDR3 size spectratyping results from 19 BMT recipients.
CDR3 size profiles were determined in PBMCs sampled at several time points after BMT for each patient. All patients underwent DLIs as indicated ↓. GvHD developed in 4 patients after DLI. ○ indicates that all spectratypes are abnormal; ✺ indicates that some spectratypes are normal; and • indicates that all spectratypes are normal. * indicates a patient in whom GVHD developed.
Summary of CDR3 size spectratyping results from 19 BMT recipients.
CDR3 size profiles were determined in PBMCs sampled at several time points after BMT for each patient. All patients underwent DLIs as indicated ↓. GvHD developed in 4 patients after DLI. ○ indicates that all spectratypes are abnormal; ✺ indicates that some spectratypes are normal; and • indicates that all spectratypes are normal. * indicates a patient in whom GVHD developed.
Discussion
In all our T-cell–depleted BMT recipients, T-cell repertoires showed restricted patterns for at least 1 year after BMT. During the first 3 to 6 months, their BV spectratypes comprised only a very limited number of size classes. This coincides with the time when BMT recipients are generally most susceptible to infections. One reason for the slow and usually incomplete development of complex repertoires is likely to be the reduced thymic output in adults.3 Roux et al17,18 discovered that, in the first several months after transplant, the circulating T-cell repertoire is essentially recruited from the progeny of the donor lymphocytes cotransplanted with the bone marrow, and possibly surviving recipient lymphocytes.26Hence, in patients receiving T-cell–depleted bone marrow, T-cell–repertoire reconstitution is delayed or incomplete compared with recipients of unmanipulated BMT. T-cell–repertoire recovery may depend to a greater extent on the few recipient T cells that may survive the preconditioning treatment.17,18 This limited size of the initial pool of T cells in combination with the length of time it takes them to repopulate the peripheral blood is likely to be a major factor contributing to the lack of complexity in T-cell repertoires in the first year after T-cell–depleted BMT. Recent evidence suggests that, contrary to earlier assumptions, the thymus may also contribute to immune reconstitution even in adulthood.3 However, the predicted slow tempo of thymic education in adulthood would similarly contribute to delays in restoration of a normal spectratype pattern.
There was an overall improvement of spectratypes in most patients after about 1 year after BMT. This timing coincides with the normalization of CD8+/CD4+ ratios and with the time when BMT recipients become less susceptible to opportunistic infections.4 5 CDR3 size profile normalization was by no means complete in the majority of patients even after several years and was highly variable between individual patients and between different BV families in the same patient. The normalization of all spectratypes in about a third of patients suggests that complete reconstitution of T-cell repertoires may be possible. However, this methodology provides no information about whether there are the same numbers of unique clones in these patients and their donors within each size class.
In approximately two thirds of the BMT recipients, some BV spectratypes were still abnormal after several years. Interestingly, despite these restricted T-cell spectratypes, BMT recipients did not show signs of impaired immune function, such as increased susceptibility to infections. There is the possibility that the amount of thymic selection persisting in adults is variable between individuals. However, the fact that some BV spectratypes normalize, whereas others remain abnormal in the same patient, suggests that other factors also play a part in shaping CDR3 size profiles. Such factors may be infectious agents encountered after BMT, activation of T cells by alloantigens, GVHD, and its treatment with immunosuppressive drugs. Activation of T cells by infectious agents or alloantigens have an immunosuppressive effect and inhibit the reconstitution of the normal underlying spectrum of T cells. GVHD can be immunosuppressive, and the drugs administered to treat GVHD are also toxic or inhibitory to lymphocytes.4 In agreement with this, none of the 4 patients in this study in whom GVHD developed were among those whose spectratypes completely normalized. Furthermore, the marked changes in the CDR3 size profiles observed in these patients over time may be a result of the combined effect of T-cell responses to the antigens involved in GVHD, and/or the immunosuppressive treatment.
Eighteen patients underwent DLI as a treatment for relapse of their diseases. As the donor lymphocytes given to BMT recipients during DLI were samples of a healthy donor T-cell repertoire, they might be expected to have an immediate normalizing effect on the patients' T-cell repertoires. However, if the bone marrow was of mixed chimeric status after transplant, host-versus-graft reactivity could possibly result in more abnormal spectratypes. Furthermore, in most patients, DLI gave rise to GVL responses, which eventually resulted in complete cytogenetic remission. This would require expansion of GVL or GVH T-cell clones, possibly resulting in a change in the CDR3 size profiles. Contrary to these predictions, no change in CDR3 size profiles was detected in half of the BMT recipients shortly after DLI. This suggests that the number of T cells infused was too small to immediately change spectratype shapes, and that expansions of clones mediating GVL or GVH were either too small to be detected or only occurred at later time points in these patients. Whether these expansions are demonstrable in terms of spectratype changes will depend on the relative size of the expansion, the level of expression of the particular BV family, possibly the level of TCR messenger RNA (mRNA) per cell, and the position of the CDR3 peak of the expanded clone within the BV spectratype. A small expansion of a single clone would be more easily visualized in a relatively underrepresented BV family, particularly if the CDR3 length was at one extreme of the length distribution represented in that family. Our data on the sensitivity of spectratype analysis for the detection of T-cell lines spiked into PBMCs from a healthy donor suggest that the clone may need to be represented at a level of 1 in 1000 to 5000 PBMCs to be detectable in well-represented BV families (and consequently higher relative frequencies within the CD4+ or CD8+ T-cell subsets). Clones mediating GVL or GVH may therefore be unrecognizable if present at lower levels. However, some caution is required in extrapolation of these results. The thresholds for detection using the cell lines cannot be directly equated with clonal expanded T cells in vivo, because TCR mRNA levels are generally higher in activated T cells than in resting T cells.27 Hence, a lower number of activated T cells may be detectable against a given background of resting T cells. Clonal expansion to antigens not involved with GVL or GVH would be predicted to occur with increasing frequency with increasing time after DLI, resulting in a gradual normalization of the spectratype appearance. Although some of the later changes may be correlated with the expansion of GVL T-cell clones, this methodology is not able to differentiate these from GVL-independent expansions, or to differentiate DLI-related spectratype changes from DLI-independent changes.
In only 1 patient did dramatic changes occur in all spectratypes after DLI (Figure 2B, patient 11), as they changed from normal patterns to restricted, clonal patterns. Changes in other patients' spectratypes were limited to a few BVs and were not as clear-cut.
In conclusion, T-cell spectratype analysis in 19 BMT patients sampled longitudinally for several years after BMT showed that T-cell–repertoire reconstitution differed greatly among patients. There was an overall improvement around 12 months after BMT, but the time to normalization in the different BV families in each patient was highly variable. Hence, it would not be possible, as suggested by Gorski et al,12 to use a few BVs as guideposts for detection of T-cell impairment. Although immunity residing in donor memory T cells was lost through T-cell depletion and T-cell spectratypes did not completely normalize in the majority of our BMT recipients even after years, this did not seem to affect the patients' general health.
In most patients, DLI did not result in an immediate change in BV spectratypes, and later changes could not be unequivocally assigned to DLI treatment.
Supported by the Leukemia Research Fund.
Reprints:S. Mackinnon, Department of Haematology, UCL, 98 Chenies Mews, London WC1E 6HX, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal