Abstract
Subsets of murine natural killer (NK) cells exist that express the Ly-49 family of molecules that recognize different major histocompatibility complex (MHC) determinants. Bone marrow transplantation studies were performed to examine the in vivo functions of 2 of these subsets. Subsets of Ly-49A and Ly-49G2 NK share specificity for the same MHC class 1 ligand, Dd, binding of which results in an inhibitory signal to the NK cell but allows them to lyse H2b targets in vitro. We therefore examined the ability of these subsets to reject H2b bone marrow cell allografts in lethally irradiated mice. Surprisingly, depletion of Ly-49A+ NK cells in BALB/c or B10.D2 mice (both H2d) had no effect on the rejection of H2b BMC. However, Ly-49A depletion did partially abrogate the ability of B10.BR (H2k) mice to reject H2ballografts. Although depletion of either Ly-49A+ or Ly-49G2+ NK cells alone had no effect on the ability of B10.D2 mice to reject H2b BMC, depletion of both subsets dramatically and synergistically abrogated rejection. Studies with various B10 congenic mice and their F1 hybrids indicate that this synergy between Ly49A and Ly4G2 depletion occurs in every instance. Thus, Ly-49A+ NK cells appear to play a role in the rejection H2b bone marrow allografts, but, in most strains of mice studied, Ly-49G2+ NK cells must also be eliminated. The putative roles of these NK cell subsets in clinical transplantation remains to be elucidated.
Natural killer (NK) cells are large granular lymphocytes that have the ability to lyse various tumor cells and virally infected cells.1-3 Murine NK cells also mediate the acute rejection of bone marrow cells in lethally irradiated mice.3-7 Recent studies have shown that NK cell cytotoxicity is regulated by a balance between specific activating and inhibitory receptors.8 Murine inhibitory receptors belong to C-type lectin superfamily.8-12 The cDNA sequences of 9 Ly-49 molecules, from A to I, have been reported.9-12Individual members of this receptor family recognize specific major histocompatibility complex (MHC) class 1 molecules and, on binding to these ligands, can transmit inhibitory intracellular signals through an immunoreceptor tyrosine-based inhibitory motif in their cytoplasmic domain.8,13 However, 2 of the Ly-49 members, D and H, lack this inhibitory motif. Subsequently, Ly-49D has been reported to be an activating receptor.14 We and others3-6,15 have shown that many of these NK subsets are responsible for the specific rejection of bone marrow cell (BMC) allografts in lethally irradiated mice. In addition, though subsets of T cells have also been demonstrated to express Ly-49 molecules, studies using T cell-deficient mice have shown that NK cells alone can mediate bone marrow allograft rejection.6 15
Of all these Ly-49 receptors, Ly-49A has been the most extensively studied in vitro.16-19 Ly-49A binds to Dd in vitro and sends inhibitory signals to NK cells after the interaction.17,18 Blocking Ly-49A antibodies allow Ly-49A+ NK cells to kill Dd-expressing targets, suggesting that Ly-49A is a Dd-specific receptor that delivers an inhibitory signal to NK cells.18,19 Ly-49A transgenic mice on an H2b background are unable to reject H2d bone marrow allografts, and NK cells from these mice poorly lysed tumor target cells expressing Dd, confirming that Ly-49A+ NK cells are inhibited by H2b in vitro and in vivo.20 However, evidence of an in vivo function of this subset has been surprisingly lacking. Another inhibitory Ly-49 member, Ly-49G2, also recognizes H2d on target cells in vitro.21 In agreement with the reported in vitro specificity, we have shown recently by bone marrow transplantation studies that in H2d BALB/c mice, Ly-49G2+ NK cells mediate the rejection of H2bbone marrow allografts.22 We have extended those studies to examine the in vivo function of Ly-49A+ NK cells, and we report that the effect of this subset alone can be detected only in mice bearing certain MHC haplotypes and that this subset can synergize with Ly-49G2+ NK cells in the rejection of H2bbone marrow allografts.
Materials and methods
Mice
BALB/c (H2d), C57BL/6 (H2b), BALB/c × C57BL/6 F1 (CB6F1, H2bxd), mice were obtained from the Animal Production Area (NCI–FCRDC, Frederick, MD). C57BL/10 (B10, H2b), B10.D2 (H2d), B10.BR (H2k), B10.BR × B10 (H2kxb), B10.BR × B10.D2 (H2kxd), and Bl0.D2 × B10 (H2dxb) mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were kept under specific pathogen-free conditions until use at 8 to 12 weeks of age.
Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH publication no. 86-23, 1985).
Natural killer cell isolation and cytometric analysis
NK cells from mice were enriched from spleens of 8- to 12-week-old animals, using a protocol that has been described previously.15 Flow cytometric analysis was performed to determine the percentage of Ly-49A+ and Ly-49G2+ NK cells. Cells were incubated with directly fluoresceinated monoclonal antibodies (mAbs) YE1-48 (anti-Ly-49A), 4D11 (anti-Ly-49G2), and NK pan marker DX5 (Pharmingen, San Diego, CA) for 30 minutes at 4°C. After 2 washes, the cells were fixed in 1% paraformaldehyde and analyzed on an EPICS flow cytometer (Coulter Electronics, Hialeah, HI).
Assays for bone marrow cell engraftment
Groups of 4 to 5 conventionally housed recipient mice were injected with 200 μg mAb 4D11 (anti-Ly-49G2), 200 μg mAb YE1-48 (anti-Ly-49A), 500 μg mAb PK136 (anti-NK 1.1), or 0.2 mL normal rat serum (NRS) 2 days before irradiation. The engraftment of donor bone marrow cells was assessed by an in vitro colony assay for hematopoietic cell growth.15 Two days after antibody injection, mice were lethally irradiated with a cesium 137 source (B10.D2 and B10.BR at 950 cGy; B10.BR × B10, B10.BR × B10.D2, Bl0.D2 × B10 at 1000 cGy, and BALB/c at 850 cGy) and later injected intravenously with 0.5 × 106 to 2 × 106 BMC in 0.2 mL Hanks' balanced salt solution. After 5 to 7 days, spleens were gently crushed in the medium, and single-cell suspensions were prepared. Spleen cells (106) were plated in 0.3% sea plaque agar in 35-mm dishes containing optimal concentrations of recombinant murine cytokines (IL-3 [10 ng/mL] and granulocyte-macrophage–colony-stimulating factors (GM-CSF) [10 ng/mL]). Cytokines were obtained from Biological Resources (Frederick, MD). Plates were incubated at 37°C for 7 days, and colonies of more than 50 cells were then counted (CFU-c). Data are presented as mean ± SD total CFU-c/spleen, which was obtained by multiplying the number of colonies by the cellularity. The Student t test was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 to 3 times with 4 mice per group.
Results
Expression of Ly-49A and G2 receptors on resting NK cells
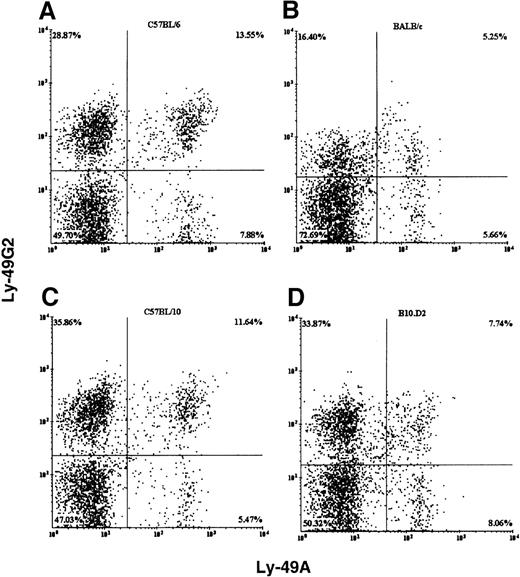
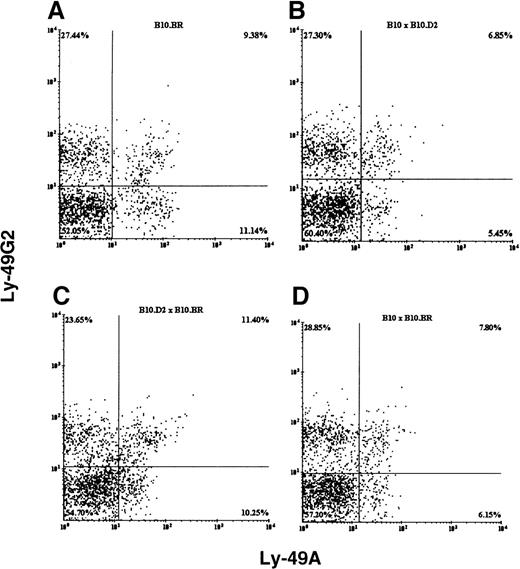
We examined the expression of Ly-49A and Ly-49G2 single-positive and double-positive resting splenic NK cells in mice (Figures1, 2). In all the mouse strains examined, both receptors were expressed on resting NK cells and all had comparable numbers of DX5+ NK cells (ranging from 1.3 to 2.4 × 106 cells) in the spleen. However, the percentage of single-positive Ly-49G2 NK cells was always higher than single-positive Ly-49A cells (Figures1, 2). In BALB/c (H2d) mice, which express the MHC class 1 ligand (Dd) for the above-mentioned Ly-49 receptors, the mean fluorescence intensity (MFI) of the expression of both receptors was lower than in C57BL/6 (H2b) NK cells (Figures 1A, 1B). The MFI for the Ly-49A and Ly-49G2 subsets in BALB/c mice were 509 and 452, respectively, whereas the MFI of these same subsets in C57BL/6 mice were 640 and 538. In contrast, in B10.D2—the other H2d-expressing strain (Figure 1D)—the mean fluorescence intensity of only Ly-49A was lower, but Ly-49G2 expression was comparable with that of C57BL/10 (H2b) NK cells. The MFI for the Ly-49A and Ly-49G2 subsets in B10.D2 mice was 527 and 517, whereas the MFI of these same subsets in C57BL/10 mice were 629 and 558. Additionally, in all the B10 hybrid strains (H2bxd, H2kxd, and H2kxb; Figures 2B to 2D), the MFIs of Ly-49A and Ly-49G2 expression were comparable. These data indicate that all C57BL/10 congenics and their F1 hybrids have both Ly-49A and Ly-49G2+ NK cells to a comparable extent.
Flow cytometric analysis of fresh splenic NK cells from different strains of mice.
C57BL/6 (H2b, A), BALB/c (H2d, B), C57BL/10 (H2b, C), and B10.D2 (H2d, D). NK cells were stained with pan NK marker DX5, anti-Ly-49A mAb YE1-48, and anti-Ly-49G2 mAb 4D11. The Y-axis and X-axis represent fluorescence intensity of phycoerythrin (PE)-conjugated 4D11 and fluorescein isothiocyanate (FITC)-conjugated YE1-48 antibodies, respectively.
Flow cytometric analysis of fresh splenic NK cells from different strains of mice.
C57BL/6 (H2b, A), BALB/c (H2d, B), C57BL/10 (H2b, C), and B10.D2 (H2d, D). NK cells were stained with pan NK marker DX5, anti-Ly-49A mAb YE1-48, and anti-Ly-49G2 mAb 4D11. The Y-axis and X-axis represent fluorescence intensity of phycoerythrin (PE)-conjugated 4D11 and fluorescein isothiocyanate (FITC)-conjugated YE1-48 antibodies, respectively.
Flow cytometric analysis of fresh splenic NK cells from different strains of mice.
B10.BR (H2k, A), B10 × B10.D2 (H2bxd,B), B10.D2 × B10.BR (H2dxk, C), and B10 × B10.BR (H2bxk, D). NK cells were stained with pan NK marker DX5, anti-Ly-49A mAb YE1-48, and anti-Ly-49G2 mAb 4D11. The Y-axis and X-axis represent fluorescence intensity of PE-conjugated 4D11 and FITC-conjugated YE1-48 antibodies, respectively.
Flow cytometric analysis of fresh splenic NK cells from different strains of mice.
B10.BR (H2k, A), B10 × B10.D2 (H2bxd,B), B10.D2 × B10.BR (H2dxk, C), and B10 × B10.BR (H2bxk, D). NK cells were stained with pan NK marker DX5, anti-Ly-49A mAb YE1-48, and anti-Ly-49G2 mAb 4D11. The Y-axis and X-axis represent fluorescence intensity of PE-conjugated 4D11 and FITC-conjugated YE1-48 antibodies, respectively.
Depletion of Ly-49G2+ but not Ly-49A+ NK subsets abrogates the rejection of H2b BMC in BALB/c (H2d) mice
We have demonstrated22 that depletion of the Ly-49G2+ NK subset promotes the engraftment of H2b bone marrow allografts in lethally irradiated BALB/c (H2d) mice. Because both Ly-49A+ and Ly-49G2+ NK cells show cytotoxicity toward H2btargets in vitro, it seemed likely that Ly-49A+ NK cells could also play a role in the rejection of H2b BMC in vivo. We first ascertained the extent of Ly-49A depletion in vivo. We have shown22 that anti-Ly-49G2 (mAb 4D11) eliminates Ly-49G2+ cells in vivo. Mice were injected with 0.2 mg anti-Ly-49A (mAb YEl-48) intraperitoneally; 48 hours later splenic NK cells were enriched and stained with anti-Ly-49A, and flow cytometric analysis was performed. The results presented in Figure3 demonstrate that the treatment of B10.D2 mice with anti-Ly-49A mAb eliminates 90% of Ly-49A+ NK cells. In addition, 20% to 30% of DX5+ cells were also depleted (control mice, 12% DX5+ cells; Ly-49A-depleted mice, 8.5% DX5+ cells). This suggests that the antibody treatment resulted in removal of the Ly-49A+ cells, not in down-regulation of the determinant. Intensive conditioning of the recipient before BMC infusion by irradiation reduces the numbers of NK cells even further.22 We next examined the effect of depletion of Ly-49A+ NK cells on the ability of mice to reject bone marrow allografts from different strains of mice compared with depletion of the Ly-49G2+ NK subset. We used the splenic CFU-c assay to assess BMC engraftment in mice. The CFU-c assay is more sensitive and specific than [125I]UdR incorporation assay for quantitating hematopoietic progenitor cell content in spleens of recipient mice.15 The results presented in Table1 indicate that, as expected and reported earlier, the depletion of Ly-49G2+ cells abrogates the rejection of H2b allografts by BALB/c recipient mice.22 However, the removal of Ly-49A+ NK cells had no effect on the rejection of H2b BMC. Hence, in BALB/c (H2d) mice that express the ligand for both Ly-49A and Ly-49G2 receptors, Ly-49G2+ cells appear to play a dominant role in the rejection of H2b allografts.
Effect of anti-Ly-49A treatment on Ly-49A+NK cell content in the spleens of B10.D2 mice.
Mice were treated as described in “Materials and methods.” Flow cytometric analysis of the spleen cells stained with anti-Ly-49A from untreated mice or mice treated with anti-Ly-49A.
Effect of anti-Ly-49A treatment on Ly-49A+NK cell content in the spleens of B10.D2 mice.
Mice were treated as described in “Materials and methods.” Flow cytometric analysis of the spleen cells stained with anti-Ly-49A from untreated mice or mice treated with anti-Ly-49A.
Effect of depletion of Ly-49A+ and Ly-49G2+ NK cells in BALB/c (H2d) on the rejection of H2b allografts
| BMC donor . | H2 . | BMC recipient . | H2 . | Recipient treatment . | Total CFU-c/spleen (×106) . |
|---|---|---|---|---|---|
| C57BL/6 | b | BALB/c | d | NRS | 43 ± 23 |
| 4D11 (anti-Ly-49G2) | 548 ± 180* | ||||
| YE1-48 (anti-Ly-49A) | 40 ± 18 | ||||
| ASGM.1 | 354 ± 67† |
| BMC donor . | H2 . | BMC recipient . | H2 . | Recipient treatment . | Total CFU-c/spleen (×106) . |
|---|---|---|---|---|---|
| C57BL/6 | b | BALB/c | d | NRS | 43 ± 23 |
| 4D11 (anti-Ly-49G2) | 548 ± 180* | ||||
| YE1-48 (anti-Ly-49A) | 40 ± 18 | ||||
| ASGM.1 | 354 ± 67† |
Mice were given various mAb treatments or NRS 2 days before lethal irradiation. Irradiated mice (4 mice/group) received 0.5 × 106 allogeneic BMC. Eight days later a soft-agar colony assay was grown on spleen cells to assess hematopoietic progenitor content.
Values significantly (P < .01) greater than in mice receiving NRS and mAb YE1-48.
Ly-49A NK subset mediates the rejection of H2ballografts in H2k mice
Next we examined the effect of depletion of either the Ly-49G2 or the Ly-49A subset in B10.BR (H2k) mice on H2bBMC allograft rejection. We reasoned that because Ly-49A has a stronger affinity for H2d than for H2k, it may be functionally down-regulated in mice of H2d haplotype. Therefore, H2k mice may be a better strain for assessing the function of Ly-49A.23 The data in Table2 demonstrate that the depletion of Ly-49A+ cells led to significant engraftment of H2b bone marrow allografts, whereas removal of Ly-49G2+ cells, in spite of their higher numbers (Figure2A) had less effect. In B10.BR (H2k) mice, then, Ly-49A+ NK cells play the dominant role in mediating the rejection of H2b allografts.
Effect of depletion of Ly-49A+ and Ly-49G2+ NK cells in B10.BR (H2k) mice on the rejection of H2b allografts
| BMC donor . | H2 . | BMC recipient . | H2 . | Recipient treatment . | Total CFU-c/spleen (×106) . |
|---|---|---|---|---|---|
| Experiment A | |||||
| C57BL/10 | b | B10.BR | k | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 56 ± 27 | ||||
| YE1-48 (anti-Ly-49A) | 371 ± 84* | ||||
| PK136 (anti-NK1.1) | 1057 ± 258* | ||||
| Experiment B | |||||
| C57BL/10 | b | B10.BR | k | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 123 ± 139 | ||||
| YE1-48 (anti-Ly-49A) | 453 ± 212 | ||||
| 4D11 + YE1-48 | 1622 ± 435† | ||||
| PK136 (anti-NK1.1) | 1732 ± 344† |
| BMC donor . | H2 . | BMC recipient . | H2 . | Recipient treatment . | Total CFU-c/spleen (×106) . |
|---|---|---|---|---|---|
| Experiment A | |||||
| C57BL/10 | b | B10.BR | k | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 56 ± 27 | ||||
| YE1-48 (anti-Ly-49A) | 371 ± 84* | ||||
| PK136 (anti-NK1.1) | 1057 ± 258* | ||||
| Experiment B | |||||
| C57BL/10 | b | B10.BR | k | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 123 ± 139 | ||||
| YE1-48 (anti-Ly-49A) | 453 ± 212 | ||||
| 4D11 + YE1-48 | 1622 ± 435† | ||||
| PK136 (anti-NK1.1) | 1732 ± 344† |
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 1 × 106 allogeneic BMC. Eight days later a soft-agar colony assay was grown to assess hematopoietic progenitor content.
Values significantly greater (P < .003) than in mice receiving NRS and mAb 4D11.
Values significantly greater (P < .005) than in mice receiving mAb 4D11 or YE1-48 in experiment B.
We simultaneously depleted the Ly-49A and Ly49G2 subsets in B10.BR mice. Surprisingly, depletion of both subsets resulted in significant (P < .005) abrogation of the rejection of H2ballografts when compared to depletion of either subset alone. Thus, there appears to be strong synergy between Ly-49A and Ly-49G2 in the rejection of H2b BMC by H2k recipients.
Simultaneous depletion of Ly-49A+and Ly-49G2+subsets inhibits rejection of H2bbone marrow allografts in H2d mice with B10 genetic background
B10.D2 (H2d) mice have the same MHC haplotype as BALB/c (H2d), but they differ in their background genes, including the NK gene complex on chromosome 6.8 This may account for why B10 background mice are strong rejectors of bone marrow grafts, as evidenced by the low background levels (NRS controls) in comparison with BALB/c mice (Tables 1, 3). To evaluate the effects of the background genes on allograft rejection, we examined the effect of depletion of either the Ly-49G2 or the Ly-49A subset in B10.D2 (H2d) mice on H2b BMC rejection. Flow cytometric analysis of splenic cells from B10.D2 mice indicated that, unlike in BALB/c, there was no significant down-regulation in the intensity or number of single-positive Ly-49G2+ NK cell (Figure 1). Surprisingly, BMT studies in B10.D2 mice revealed that individual depletion of either subset had absolutely no effect on the rejection of H2b allografts (Table 3). Increasing the bone marrow cell inoculum as high as 107 cells also did not result in any significant abrogation of rejection (data not shown). However, when both subsets were depleted together, there was a dramatic and highly significant (P < .003) abrogation of rejection of H2b BMC (Table 3B). This result contrasts with results found in BALB/c (H2d) mice, in which the depletion of Ly-49G2+ NK cells, but not Ly-49A+ NK cells, was sufficient to abrogate the H2b allografts rejection. Similar to what was seen in B10.BR mice, a strong synergy exists between the 2 subsets in the rejection of H2b BMC.
Effect of depletion of Ly-49A+ and Ly-49G2+ NK cells in B10.D2 (H2d) mice on the rejection of BMC allografts
| BMC donor . | H2 . | BMC recipient . | H2 . | Recipient treatment . | Total CFU-c/spleen (×106) . |
|---|---|---|---|---|---|
| Experiment A | |||||
| C57BL/10 | b | B10.D2 | d | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 0 ± 0 | ||||
| YE1-48 (anti-Ly-49A) | 0 ± 0 | ||||
| PK136 (anti-NK1.1) | 267 ± 423-150 | ||||
| Experiment B | |||||
| C57BL/10 | b | B10.D2 | d | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 9 ± 18 | ||||
| YE1-48 (anti-Ly-49A) | 0 ± 0 | ||||
| 4D11 + YE1-48 | 3244 ± 3493-151 | ||||
| PK136 (anti-NK1.1) | 885 ± 438 |
| BMC donor . | H2 . | BMC recipient . | H2 . | Recipient treatment . | Total CFU-c/spleen (×106) . |
|---|---|---|---|---|---|
| Experiment A | |||||
| C57BL/10 | b | B10.D2 | d | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 0 ± 0 | ||||
| YE1-48 (anti-Ly-49A) | 0 ± 0 | ||||
| PK136 (anti-NK1.1) | 267 ± 423-150 | ||||
| Experiment B | |||||
| C57BL/10 | b | B10.D2 | d | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 9 ± 18 | ||||
| YE1-48 (anti-Ly-49A) | 0 ± 0 | ||||
| 4D11 + YE1-48 | 3244 ± 3493-151 | ||||
| PK136 (anti-NK1.1) | 885 ± 438 |
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 1 × 106 (experiment A) or 2 × 106 (experiment B) allogeneic BMC. Eight days later a soft-agar colony assay was grown to assess hematopoietic progenitor content.
Values significantly greater (P < .008) than in mice receiving NRS in experiment A.
Values significantly greater (P < .003) than in mice receiving mAb 4D11 or YE1-48 alone in experiment B.
Both Ly-49A and Ly-49G2 subsets mediate the rejection of H2b allografts in F1 mice
We then performed BMT experiments with 3 different combinations of F1 hybrids on B10 backgrounds—B10.BR × B10 (H2kxb), B10.BR × B10.D2 (H2kxd), and Bl0.D2 × B10 (H2dxb)—to examine the role of MHC on the rejection capability of the subsets. Results presented in Table4 indicate that in all these F1hybrid mice strains, depletion of both Ly-49A+ and Ly-49G2+ NK cells was needed to abrogate completely the rejection of H2b BMC. When individual subsets were depleted in B10.BR × B10.D2 (H2kxd) F1 and B10 × B10.D2 (H2bxd) F1 mice, the removal of Ly-49G2+ cells abrogated rejection to a greater extent than with Ly-49A+ NK depletion. However, in B10.BR × B10 F1 (H2kxb) mice, depletion of Ly49A+ NK cells significantly inhibited BMC rejection, similar to what was seen with B10.BR recipients (Table 2). However, the results demonstrated again that a strong synergy exists when depleting both Ly-49A+ and Ly-49G2+ NK cells in all B10 background strains, regardless of their haplotype, to abrogate their ability to reject H2b allografts.
Effect of depletion of Ly-49A+ and Ly-49G2+ NK cells in different F1 mice on the rejection of BMC allografts
| BMC donor . | H2 . | BMC recipient . | H2 . | Recipient treatment . | Total CFU-c/spleen (×106) . |
|---|---|---|---|---|---|
| Experiment A | |||||
| C57BL/10 | b | B10.BR × B10.D2 | k × d | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 55 ± 30 | ||||
| YE-148 (anti-Ly-49A) | 2 ± 3 | ||||
| 4D11 + YE1-48 | 2946 ± 16654-150 | ||||
| PK136 (anti-NK1.1) | 2268 ± 549 | ||||
| Experiment B | |||||
| C57BL/10 | b | B10.D2 × B10 | b × d | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 97 ± 36 | ||||
| YE1-48 (anti-Ly-49A) | 41 ± 22 | ||||
| 4D11 + YE1-48 | 2941 ± 6584-151 | ||||
| PK136 (anti-NK1.1) | 1404 ± 406 | ||||
| Experiment C | |||||
| C57BL/6 | b | B10.BR × B10 | k × b | NRS | 10 ± 13 |
| 4D11 (anti-Ly-49G2) | 8 ± 7 | ||||
| YE1-48 (anti-Ly-49A) | 105 ± 41 | ||||
| 4D11 + YE1-48 | 267 ± 94‡ | ||||
| PK136 (anti-NK1.1) | 225 ± 37 |
| BMC donor . | H2 . | BMC recipient . | H2 . | Recipient treatment . | Total CFU-c/spleen (×106) . |
|---|---|---|---|---|---|
| Experiment A | |||||
| C57BL/10 | b | B10.BR × B10.D2 | k × d | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 55 ± 30 | ||||
| YE-148 (anti-Ly-49A) | 2 ± 3 | ||||
| 4D11 + YE1-48 | 2946 ± 16654-150 | ||||
| PK136 (anti-NK1.1) | 2268 ± 549 | ||||
| Experiment B | |||||
| C57BL/10 | b | B10.D2 × B10 | b × d | NRS | 0 ± 0 |
| 4D11 (anti-Ly-49G2) | 97 ± 36 | ||||
| YE1-48 (anti-Ly-49A) | 41 ± 22 | ||||
| 4D11 + YE1-48 | 2941 ± 6584-151 | ||||
| PK136 (anti-NK1.1) | 1404 ± 406 | ||||
| Experiment C | |||||
| C57BL/6 | b | B10.BR × B10 | k × b | NRS | 10 ± 13 |
| 4D11 (anti-Ly-49G2) | 8 ± 7 | ||||
| YE1-48 (anti-Ly-49A) | 105 ± 41 | ||||
| 4D11 + YE1-48 | 267 ± 94‡ | ||||
| PK136 (anti-NK1.1) | 225 ± 37 |
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 2 × 106 (experiments A and B) or 0.5 × 106(experiment C) allogeneic BMC. Eight days later a soft-agar colony assay was grown to assess hematopoietic progenitor content.
Values significantly greater (P < .04) than B10.BR × B10.D2 in mice receiving 4D11 or YE1-48 alone.
Values significantly greater (P < .003) than B10.D2 × B10 in mice receiving 4D11 or YE1-48 alone.
Values significantly greater (P < .03) than B10.BR × B10 in mice receiving 4D11 or YE1-48 alone.
Discussion
We conducted studies to delineate the role of various Ly-49 NK cell subsets in bone marrow allograft rejection. Our data indicate that analogous to results seen with Ly-49G2+NK cells, Ly-49A+ NK cells also appear to be capable of mediating the rejection of H2b bone marrow allografts in lethally irradiated mice. However, only in certain strains (ie, B10.BR) could this effect be detected. In each mice strain studied, both Ly-49 subsets had to be depleted completely to abrograte H2b bone marrow allograft rejection (Table5).
Effects of the depletion of Ly-49A and G2 NK subsets on the growth of H2b bone marrow allografts
| Strain . | H2 . | Ly-49A . | Ly-49G2 . | Ly-49A + G2 . |
|---|---|---|---|---|
| BALB/c | d | +/− | ++ | ++ |
| B10.D2 | d | − | − | ++++ |
| B10.BR | k | ++ | + | ++++ |
| B10.BR × B10.D2 | k × d | +/− | +/++ | ++++ |
| B10.D2 × B10 | d × b | + | ++ | ++++ |
| B10.BR × B10 | k × b | ++ | −/+ | +++ |
| Strain . | H2 . | Ly-49A . | Ly-49G2 . | Ly-49A + G2 . |
|---|---|---|---|---|
| BALB/c | d | +/− | ++ | ++ |
| B10.D2 | d | − | − | ++++ |
| B10.BR | k | ++ | + | ++++ |
| B10.BR × B10.D2 | k × d | +/− | +/++ | ++++ |
| B10.D2 × B10 | d × b | + | ++ | ++++ |
| B10.BR × B10 | k × b | ++ | −/+ | +++ |
Extent of marrow allograft engraftment graded − for no engraftment to ++++ for maximal growth.
In vitro cytotoxicity studies16-20 show that H2d on target cells inhibits Ly-49A+ and Ly-49G2+ NK cells and renders them unable to kill such targets. Interestingly, even in H2d B10.D2 mice, NK cells expressing either Ly-49A or Ly-49G2 inhibitory receptors are functional in vivo. Removal of either of the NK subsets singly resulted in no significant engraftment of H2b allografts (P > .05), suggesting that both subsets together can play a critical role in rejecting H2b bone marrow cells. As seen in all mice of B10 background, depletion of both these subsets in B10.D2 also resulted in significant synergy. In contrast, in BALB/c mice, the Ly-49G2 NK subset plays a dominant role in the rejection of H2b allografts, and the removal of Ly-49A+ NK cells had no effect on BMC rejection. Additionally, depletion of both A and G2 subsets in BALB/c mice did not produce synergistic effects on engraftment (data not shown), indicating that Ly-49A+ NK cells are most likely not able to reject H2b BMC in BALB/c mice. It is possible that in BALB/c mice, Ly-49A+ NK cells co-express inhibitory receptors for H2b (eg, Ly-49C or Ly-49I), whereas there is less co-expression of such receptors on Ly-49G2 cells. In these 2 H2d strains, BALB/c and B10.D2, we also observed a difference in the cell surface expression of these inhibitory receptors. It has been reported that the presence of MHC class 1 Dd ligand down-regulates the cell surface expression of Ly-49A and Ly-49G2 receptors in vivo.24 25 In BALB/c mice, but not in B10.D2 mice, the numbers of cells expressing Ly-49G2 and G2 and A double-positive cells were significantly reduced. Additionally, there may be differences in background genes in B10 background mice that alter their Ly-49 expression and their rejection capability. This observation supports the notion that the Ly-49A and Ly-49G2 subsets differ qualitatively and quantitatively between BALB/c (H2d) and B10.D2 (H2d) mice.
An activating Ly-49 receptor for H2d, Ly-49D, has been reported, but a similar receptor for H2b is not yet known.14 We have shown by BMT studies that the Ly-49D NK subset in H2b mice mediates the rejection of H2d homozygous or heterozygous bone marrow allografts.15 There may be other as yet unidentified Ly-49 activating receptors that recognize H2b. It is possible that in H2d mice, NK subsets with inhibitory Ly-49 receptors A and G2 reject H2b allografts because either has or both have a large fraction with a potential activating receptor for H2b.
Little is known concerning the in vivo nature of BMC recognition by the different NK cell subsets or the normal physiological significance of these subsets. It has been reported26-28 that NK cells from Fas ligand or perforin gene knockout mice, or from granzyme A-diphtheria toxin transgenic mice, have impaired cytotoxicity against tumor targets but retain the ability to eliminate bone marrow cells. This implies that direct cytotoxicity may not be the only, or even a major, mechanism of elimination of incompatible bone marrow cells. It is conceivable that differential cytokine production by NK subsets may play a role in bone marrow graft rejection or engraftment.29 For example, NK cells producing interferon (IFN)-γ are more myelo-inhibitory than NK cells producing GM-CSF. Alternatively, other “death”-inducing molecules (eg, TRAIL) may function in the absence of FasL or perforin.30 Our data suggest that a normal physiological role of these NK subsets may be in the homeostatic regulation of hematopoiesis in mice. We have observed that in H2d mice, Ly-49C/I+ NK cells promote hematopoiesis by producing greater amounts of growth-promoting cytokines (GM-CSF) rather than growth-suppressing cytokines (IFN-γ).29 Further studies with these Ly-49 NK subsets will help elucidate their normal physiological roles in hematopoiesis and in marrow rejection. In addition, though clinical evidence that NK cells can mediate rejection of marrow grafts has been lacking, reports have indicated an increased likelihood of graft failure in patients incompatible at HLA-C, a mismatch thought to influence NK activity.31
Our results indicate that the in vivo functions of NK subsets are more complex in that the single subset depletion or transfer may yield negative results because of overlap of specificities, compensation by other subsets, and synergistic interactions.
Acknowledgments
We thank Steve Stull for superb technical assistance and Laura Knott for excellent secretarial assistance.
Supported wholly or in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract no. N01-CO-56000.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Reprints:William Murphy, Building 567, Room 210, SAIC–Frederick, Frederick, MD 21702; e-mail: murphyw@ncifcrf.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal