Abstract
The aim was to better understand the function of von Willebrand factor (vWF) A1 domain in shear-induced platelet aggregation (SIPA), at low (200) and high shear rate (4000 seconds-1) generated by a Couette viscometer. We report on 9 fully multimerized recombinant vWFs (rvWFs) expressing type 2M or type 2B von Willebrand disease (vWD) mutations, characterized respectively by a decreased or increased binding of vWF to GPIb in the presence of ristocetin. We expressed 4 type 2M (-G561A, -E596K, -R611H, and -I662F) and 5 type 2B (rvWF-M540MM, -V551F, -V553M, -R578Q, and -L697V). SIPA was strongly impaired in all type 2M rvWFs at 200 and 4000 seconds-1. Decreased aggregation was correlated with ristocetin binding to platelets. In contrast, a distinct effect of botrocetin was observed, since type 2M rvWFs (-G561A, -E596K, and -I662F) were able to bind to platelets to the same extent as wild type rvWF (rvWF-WT). Interestingly, SIPA at 200 and 4000 seconds-1 confirmed the gain-of-function phenotype of the 5 type 2B rvWFs. Our data indicated a consistent increase of SIPA at both low and high shear rates, reaching 95% of total platelets, whereas SIPA did not exceed 40% in the presence of rvWF-WT. Aggregation was completely inhibited by monoclonal antibody 6D1 directed to GPIb, underlining the importance of vWF-GPIb interaction in type 2B rvWF. Impaired SIPA of type 2M rvWF could account for the hemorrhagic syndrome observed in type 2M vWD. Increased SIPA of type 2B rvWF could be responsible for unstable aggregates and explain the fluctuant thrombocytopenia of type 2B vWD.
Under static conditions, platelet activation induced by agents such as adenosine 5′-diphosphate or thrombin involves an interaction between fibrinogen and the activated αIIbβ3 integrin (GPIIb/IIIa). Shear-induced platelet aggregation (SIPA) occurs in high shear conditions and involves binding of von Willebrand factor (vWF) to platelet glycoprotein (GP) Ib. High molecular weight multimers (HMWM) of vWF are required to induce SIPA.1 Studies of platelet-rich plasma from patients with severe von Willebrand disease (vWD), Glanzmann thrombasthenia, or Bernard-Soulier syndrome have established that vWF binds to both GPIb and αIIbβ3.2,3 With the use of a Couette viscometer, we have confirmed that SIPA involves predominantly vWF-GPIb and partially vWF-αIIbβ3interaction.4 Atomic force microscopy studies suggested that high shear allows a conformational change of vWF from a globular form to an extended chain.5
vWF binding to GPIb involves a complex mechanism, which is reproduced in the presence of nonphysiological agents, such as ristocetin or botrocetin, and involves different sequences of vWF A1 domain, extending from amino acids 497 to 716.6,7 Ristocetin binds to 2 proline-rich, negatively charged regions (amino acids 474-488 and 695-708) flanking the disulfide bridge between Cys 509 and Cys 695. Botrocetin binds to predominantly positively charged sequences in the A1 loop (amino acids 514-542, 539-553, 569-583, and 629-643).7-9 Interestingly, both inducers may act through different mechanisms.10 Binding of ristocetin to vWF may relieve the effect of inhibitory sites responsible for maintaining vWF in an inactive conformation, thus indirectly inducing vWF binding to GPIb.10 11 In contrast, botrocetin favors direct binding of vWF to GPIbα without a need for relieving inhibitory sites.
vWD is a heterogeneous, hereditary bleeding disorder that results from the quantitative or qualitative deficiency in vWF. Type 2 vWD consists of qualitative variants; type 1 refers to a partial quantitative defect; while type 3 refers to the absence of detectable vWF in plasma. Type 2 vWD comprises 3 types of variants with impaired binding to GPIb: 2A, 2B, and 2M.12 Type 2A and type 2M (multimer) vWD patients have decreased platelet-dependent functions but differ by the multimeric composition of vWF: Type 2A is associated with the absence of the HMWM, whereas type 2M has all multimers. Until now, several type 2M variants have been described and confirmed by expression of mutant recombinant vWF (rvWF). Mutations F606I, I662F, and G561S are responsible for an impaired ristocetin-mediated but normal botrocetin-mediated binding to platelets.13-15 In contrast, type 2MMilwaukee-1 (deletion between amino acids 629 and 639) and the mutation of R611H are characterized by a decreased ristocetin-mediated and botrocetin-mediated binding to platelets.16,17 However, in some cases, the classification has been debated and the rvWF-R611H reported as unclassified.17 (For the sake of simplicity, we have included the rvWF-R611H into type 2M.)
Type 2B vWD is characterized by a gain-of-function phenotype (aggregation induced by lower ristocetin concentrations than normal plasma) because of increased affinity for platelet GPIb. Loss of HMWM is attributed to their enhanced binding to platelets and their removal from the circulation.18 Type 2B vWD mutations are localized in the A1 loop of vWF between amino acids 540 and 578, on a small fragment that overlaps botrocetin and heparin binding sites. Studies of crystal structure have demonstrated that most of the type 2B mutations are located at the interface between the N- and C-terminal parts of the A1 domain and are responsible for disruption of salt bridges or hydrophobic packing.19 Thus, type 2B mutations may change the conformation of the molecule, resulting in a gain of function.10
Expression of type 2B rvWFs has shown that they display the whole range of multimers. Several groups20,21 have reported on the ability of type 2B plasma or rvWF to induce platelet aggregation in the absence of shear. Interestingly, several type 2B rvWFs (R543Q, V553M, L697V, and A698V) were shown to induce different extents of activation and aggregation.21 Mutations located inside the C509-C695 loop were more efficient than those located outside of the loop.21
Despite an increased affinity of vWF for platelet GPIb, type 2B vWD is characterized by a hemorrhagic disease and a fluctuating thrombocytopenia. Platelet adhesion studies at high shear rates have contributed to a better understanding of the physiopathology of this bleeding disorder. A defect of adhesion and thrombus formation at 2600 seconds-1 has been observed, compared with normal blood, using a parallel-plate perfusion chamber exposing type III collagen to type 2B vWD blood.22 The discrepancy between the gain-of-function phenotype of type 2B mutation and the impaired adhesion has been confirmed, using type 2B R543Q and R543W rvWFs exhibiting a normal multimeric pattern.23 It was hypothesized that in type 2B vWD, the mutated vWF inhibits normal vWF function. Occupancy of GPIb by soluble type 2B rvWF because of its increased affinity for this receptor resulted in a decreased platelet adhesion to collagen type III, compared with wild type (WT). However, the adhesive capacity of immobilized type 2B vWF was not impaired, demonstrating the importance of the conformation of vWF in shear-dependent functions.23
In our study, we have attempted to better understand the involvement of vWF mutated amino acids on GPIb interaction in shear conditions at 200 and 4000 seconds-1 using rvWF reproducing type 2M or type 2B mutations. We report for the first time on the effect on SIPA of 5 type 2B and 4 type 2M mutations located in the A1 domain.
Materials and methods
Purification of protein
Botrocetin was purified from crude Bothrops jaraca as described by Christophe et al20 and was stored at −80°C.
Characterization of antibodies
Monoclonal antibodies (MoAbs) directed against human vWF were produced in mice and used as immunoglobulin (Ig)G fractions.24,25 Several different MoAbs were pooled for measurement of vWF antigen levels. MoAb 505, which was used for indirect labeling of vWF in platelet-binding assays, is directed against the collagen-binding domain, and its epitope is within a region between aa 927 and 1114.26 We have determined that MoAb 505 interacts with mutated rvWFs in a similar way as with rvWF-WT and does not inhibit binding of rvWFs to platelets.
We also used MoAb 6D1, directed against vWF binding site on platelet GPIb-IX that inhibits the binding of vWF to GPIb (kind gift of Dr B.S. Coller, SUNY Stony Brook, NY).
Radiolabeling of IgG
IgG was labeled with Na125I (Amersham, Les Ulis, France) and Iodogen (Pierce Chemical Co, Rockford, IL) as described.27 Specific radioactivity varied from 3 to 10 μCi/μg. Labeled antibodies were stored at 4°C and used within 2 months.
Plasmid constructs
Plasmids with full-length complementary DNA (cDNA) coding for rvWF-WT (pSVL-WT, pSVvWFA, pCDNA3--WT)10,28 or mutated rvWF with either duplication of methionine in position 540, or substitution of phenylalanine in position 551 for valine (pSVL-M540MM, -V551F), were constructed as previously described.29,30Plasmids coding for substitution of histidine in position 611 for arginine, of methionine in position 553 for valine, of alanine in position 561 for glycine, of glutamine in position 578 for arginine, or valine in position 697 for leucine (pSVvWF-R611H, -V553M, -G561A, -R578Q, -L697V) were a kind gift from Drs C. Mazurier and L. Hilbert (LFB, France).15,17,31 32 The new plasmid with full-length cDNA mutated in position 662 of phenylalanine for isoleucine (pSVvWF-I662F) was obtained by site-directed mutagenesis using QuickChange kit (Stratagene, CA) and the other one in position 596 substituted of lysine for glutamic acid (pCDNA3--E596K) was obtained by Transformer Site-Directed Mutagenesis Kit (Clontech Laboratories, CA).
Cell culture and transfection
COS-7 cells were cultured with Dulbecco modified essential medium containing l-glutamine (GIBCO-BRL, Cergy Pontoise, France), penicillin (100 U/mL), streptomycin (100 mg/mL), and 10% (v:v) fetal calf serum (Boehringer, Mannheim, Germany). Cells were transfected, using the electroporation method33 or the diethylaminoethyl-dextran method as previously described.31
Briefly, transfected cells were washed with phosphate-buffered saline and cultured in serum free-MCDB 105 medium (Sigma, St Louis, MO). The rvWFs secreted in the medium was collected 72 hours later. The conditioned medium was centrifuged for 10 minutes at 7000g to remove cell debris, and rvWFs were stored at −80°C. Conditioned medium from cells transfected with plasmid pcDNA3- without full-length cDNA of vWF was used as negative control (mock-transfected cell medium, referred to as mock). In some experiments, before storage at −80°C, medium was concentrated twofold to threefold, using polyethylene glycol 6000 (Sigma) to obtain vWF antigen (vWF:Ag) levels between 1.4 and 1.6 μg/mL. The recombinant proteins (rvWFs) are respectively referred to as rvWF-WT, -M540MM, -V551F, -I662F, -V553M, -L697V, -G561A, -R578Q, -R611H, and -E596K. We also used rvWF deleted in its A1 domain between amino acids 478 and 716 (rvWF-ΔA1), obtained by stable expression in baby hamster kidney cells (kind gift from Dr J.J. Sixma, Utrecht, The Netherlands).34
rvWF:Ag determination
The amount of vWF:Ag in conditioned media after transfection was determined by enzyme-linked immunoadsorbent assay (ELISA) using a pool of 12 MoAbs to vWF (5 μg/mL) for coating wells of Maxi-sorp Nunc-Immuno Plates (A/S Nunc, Roskilde, Denmark) for 2 hours at 37°C, and a pool of 35 MoAbs to vWF coupled to horseradish peroxidase as a second antibody.28 In both pools, epitopes were distributed along the vWF subunit with an approximate ratio of 2:1 in the amino-terminal versus carboxy-terminal region. Levels of vWF:Ag present in the media of transfected cells were expressed relative to a control pool plasma of 15 healthy donors that had been calibrated against the Third International Standard for Willebrand factor in plasma (code 91/666, National Institute for Biological Standards and Control). Data were expressed in μg/mL.
rvWF multimer analysis
Multimeric analysis of rvWFs was performed by 0.1% SDS-1% agarose gel electrophoresis, as previously described, except that a different source of agarose was used (IEF Pharmacia Fine Chemical, Uppsala, Sweden).35
Preparation of washed platelets
Blood was obtained from healthy individuals who had not ingested any medication for 2 weeks before donation. The blood was drawn into 15% (v/v) acid citrate dextrose pH 5.8. Washed platelets were prepared from isolated platelet-rich plasma in the presence of apyrase (1 U/mL) (Sigma) and acid citrate dextrose (1 mL for 40 mL) as described.4 Briefly, after washing, platelets were resuspended in Hepes buffer,10 mmol/L Hepes (N-[2-hydroxyethyl]piperazine-N′-[ethanesulfonic acid]), 0.136 mol/L NaCl, 2.7 mmol/L KCl, and 2 mmol/L MgCl2 pH 7.5 containing BSA 0.15%, and they were used after 1 hour of incubation at 37°C. CaCl2 (1 mmol/L) was added after the incubation period. Platelets were counted with an electronic particle counter (Model 1, Coulter Electronics, Margency, France), and the concentration was adjusted to 3.5 × 108 platelets/mL.
Shear-induced platelet aggregation
The rotating device is a Couette type viscometer used as previously described4 with the following minor modifications. Washed platelet suspensions (0.5 × 108/mL) were exposed for 5 minutes at 20°C to a continuous shear rate of 200 or 4000 seconds-1 in the presence of rvWF (1 μg/mL) diluted in culture medium with 0.15% BSA, in a final volume of 210 μL. In some experiments, MoAb 6D1 (20 μg/mL) was pre-incubated with platelets for 5 minutes at 20°C. Following exposure to shear, samples were fixed with 1% paraformaldehyde by addition of a 10-fold concentrated solution and mixed for 30 seconds. An aliquot (10 μL) of the sheared or control platelet sample as defined above was diluted in 1 mL of Facs-flow buffer (Becton Dickinson, Le Pont-de Claix, France). SIPA was measured in a FACScan flow cytometer (Becton Dickinson) as reported.4 Data acquisition was performed by counting the particle number during a constant time (50 seconds) to measure identical volumes in different samples. In each sample, 1000 events were at least counted. Washed platelets were analyzed by forward light scatter and side light scatter. As negative control, platelet suspensions were incubated with mock-transfected cell medium in the cylinder gap for 5 minutes without exposure to shear, followed by fixation as above. This sample was used as the reference for gating the region of single platelets in the absence of shear. SIPA in the sheared samples was calculated by counting the gated population of single platelets, and results were expressed as the percentage of disappearance of single platelets: DSP = [(no − n)/no] × 100, where no represents the single platelet population of the negative control platelet sample and n represents the sheared sample containing WT or mutated rvWF. Means ± SEM were calculated from 3 experiments performed in duplicate.
Binding of 125I-MoAb/vWF complexes to platelets
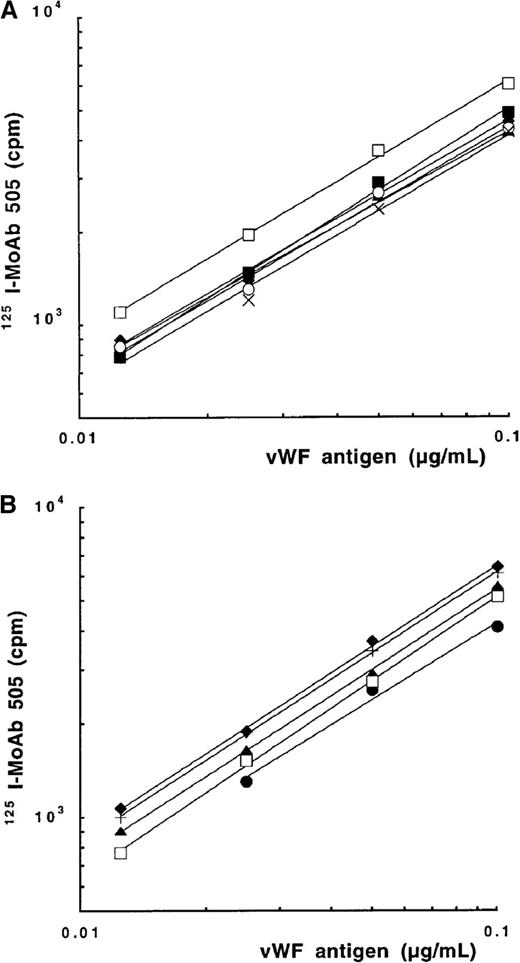
Binding of rvWF to platelets was performed as described with some modifications.36 Platelets were isolated from outdated platelet concentrates by centrifugation at 200g for 15 minutes and fixed with paraformaldehyde (2%) in 0.15 mol/L NaCl, 25 mmol/L Tris-HCl buffer, pH 7.4 (TBS) containing 0.1% BSA. rvWF (0.4 μg/mL) diluted in TBS containing 1% BSA was pre-incubated with125I-MoAb 505 (10 ng/mL, 900 000 cpm/mL) during 30 minutes at 20°C. The final mixture contained 108 platelets/mL,125I-MoAb 505/rvWF complexes and varying concentrations of ristocetin (ABP, New York, NY) (0-1 mg/mL) or botrocetin (0-1 μg/mL). After 1 hour of incubation at 20°C, duplicate aliquots (100 μL) were layered onto 200 μL of 25% sucrose in Microfuge tubes and centrifuged for 3 minutes at 10 000g. The tube tip containing bound ligand was separated from the supernatant. Bound and free radioactivities were counted in a γ counter (LKB Instruments SA, Bromma, Sweden). The percentage of total bound radioactivity was calculated as bound/(free + bound) radioactivity. Nonspecific binding was obtained by incubating platelets with 125I-MoAb 505 and ristocetin or botrocetin in the absence of rvWF. Specific binding was obtained by subtracting nonspecific binding from total binding. Means ± SEM were calculated from 3 experiments performed in duplicate. We verified that the binding of MoAb 505 to mutated and rvWF-WT, which was used for indirect labeling of rvWF, was similar to that of a pool of 35 MoAbs. Figure 1 shows that MoAb 505 binds to type 2M or 2B rvWF with a similar affinity as a pool of MoAbs.
Binding to rvWFs of 125I-MoAb 505.
Correlation between vWF:Ag levels measured with a pool of monoclonal antibodies, plotted on the x-axis, and the bound radioactivity of MoAb 505, directed against the A3 vWF domain, plotted on the y-axis. (A) Plasma pool (♦), rvWF-WT (▴), or mutated rvWF-I662F (x), -E596K (•), -G561A (○), and -R611H (□). (B) Mutated rvWF-L697V (+), -R578Q (▴), -V553M (●), -M540MM (♦), and -V551F (□).
Binding to rvWFs of 125I-MoAb 505.
Correlation between vWF:Ag levels measured with a pool of monoclonal antibodies, plotted on the x-axis, and the bound radioactivity of MoAb 505, directed against the A3 vWF domain, plotted on the y-axis. (A) Plasma pool (♦), rvWF-WT (▴), or mutated rvWF-I662F (x), -E596K (•), -G561A (○), and -R611H (□). (B) Mutated rvWF-L697V (+), -R578Q (▴), -V553M (●), -M540MM (♦), and -V551F (□).
Statistical analysis
Means ± SEM were calculated from 3 experiments performed in duplicate. Two statistical approaches were carried out, depending on the experimental design. The first approach was performed when the WT and all mutated rvWFs were analyzed in the same series of experiments and was applied to binding studies. Comparison of the 6 groups (1 WT and 5 type 2B rvWFs) was performed by using analysis of variance (ANOVA). Each ANOVA was performed at 4 different concentrations of agonist, ristocetin, or botrocetin. When the global F test was significant (P < .05), multiple comparison procedures were performed to define which recombinant differed from the other. To this end, we used the Student-Newman-Keuls multiple-comparison test with a 5% significance level. The analysis was performed using the procedure proc GLM type III of the SAS software (SAS Institute).
The second approach was elected for experiments when a mutated rvWF was compared with the WT in 1 series of experiments and when different experiments were required to study the different mutants. Significance of differences was evaluated using the Student t test for paired samples by comparing mutated rvWF versus rvWF-WT. Because each mutated rvWF was tested against the rvWF-WT in a different set of experiments, we used a substantial Bonferroni correction to theP value obtained from each paired t test (multiplication by 5 for the comparison of 5 mutated rvWFs against the WT). P < .05 was considered significant. This correction was needed to avoid the risk of excessive false-positive results from the multiple comparisons that were made. The analysis was performed using StatView software (Abacus, Berkeley, CA).
Results
Characterization of rvWFs
Expression levels of COS-7 cells transfected with rvWF-WT, mutated type 2M rvWF-I662F, -G561A, -E596K, and -R611H, or mutated type 2B rvWF-V551F, -M540MM, -V553M, -R578Q, and -L697V ranged between 1.1 and 4.9 μg/mL, depending on the transfection method. Conditioned medium from mock-transfected cells was used as negative control in each set of experiments. The multimeric structure of the recombinant proteins was analyzed by 0.1% SDS-1% agarose gel electrophoresis as shown in Figure 2. All multimeric forms were present in type 2M mutated rvWF (-E596K, -G561A, and -I662F) compared with rvWF-WT (Figure 2). As previously reported, HMWM of rvWF-R611H were present but displayed a decreased intensity compared with rvWF-WT.17 All type 2B rvWFs exhibited a normal multimeric pattern as depicted in Figure 2 for a representative type 2B rvWF (L697V).
Multimer analysis of rvWF-WT, type 2M and type 2B rvWFs.
rvWF multimers were resolved by 0.1% SDS/1% agarose gel electrophoresis and detected by 125I-MoAb anti-vWF. rvWF-WT, type 2M rvWF (-I662F, -G561A, -E596K) and type 2B rvWF-L697V exhibited a normal multimeric pattern, whereas rvWF-R611H displayed a normal multimeric composition but a decreased intensity of the highest molecular weight multimers. NP indicates normal plasma.
Multimer analysis of rvWF-WT, type 2M and type 2B rvWFs.
rvWF multimers were resolved by 0.1% SDS/1% agarose gel electrophoresis and detected by 125I-MoAb anti-vWF. rvWF-WT, type 2M rvWF (-I662F, -G561A, -E596K) and type 2B rvWF-L697V exhibited a normal multimeric pattern, whereas rvWF-R611H displayed a normal multimeric composition but a decreased intensity of the highest molecular weight multimers. NP indicates normal plasma.
Type 2M rvWFs interaction with GPIb induced by nonphysiological agonists
To test the functional characteristics of the mutated rvWF, platelet-binding assays were performed with rvWF-G561A, -E596K, -I662F, and -R611H in the absence or in the presence of varying concentrations of ristocetin or botrocetin. In the absence of agonist, neither rvWF-WT nor mutated type 2M rvWF was able to bind to platelets. Binding of rvWF-WT to platelets reached 24% at 1 mg/mL of ristocetin, whereas rvWF-G561A, -E596K, -I662F, and -R611H were not able to bind to platelets in these conditions (data not shown). In these samples, the percentage of specific binding was similar to that of the mock-transfected cell medium sample, reaching 2%.
In contrast to ristocetin, substantial binding was obtained in the presence of varying concentrations of botrocetin (Figure3). Binding to platelets increased in a dose-dependent manner with botrocetin concentrations. In the presence of 1 μg/mL of botrocetin, rvWF-G561A, -E596K, or -I662F binding to fixed platelets reached a value of approximately 50%, similar to that of rvWF-WT. In contrast, rvWF-R611H was not able to bind to platelets even at the highest concentration of botrocetin of 1 μg/mL.
Botrocetin-induced binding of rvWF-WT and type 2M rvWFs to platelets.
rvWF-WT (□) or mutated rvWF (I662F (♦), G561A (x), E596K (•), and R611H (●; 0.4 μg/mL) labeled with 125I-MoAb 505 were incubated with paraformaldehyde-fixed platelets (108/mL) for 1 hour at room temperature in the presence of varying concentrations of botrocetin (0-1 μg/mL). Results were expressed as the specific binding of 125I-MoAb 505/rvWF complexes to platelets as described in the “Materials and methods” section. Binding estimated using mock-transfected cell medium was < 5%. Results are from a typical experiment performed in duplicate.
Botrocetin-induced binding of rvWF-WT and type 2M rvWFs to platelets.
rvWF-WT (□) or mutated rvWF (I662F (♦), G561A (x), E596K (•), and R611H (●; 0.4 μg/mL) labeled with 125I-MoAb 505 were incubated with paraformaldehyde-fixed platelets (108/mL) for 1 hour at room temperature in the presence of varying concentrations of botrocetin (0-1 μg/mL). Results were expressed as the specific binding of 125I-MoAb 505/rvWF complexes to platelets as described in the “Materials and methods” section. Binding estimated using mock-transfected cell medium was < 5%. Results are from a typical experiment performed in duplicate.
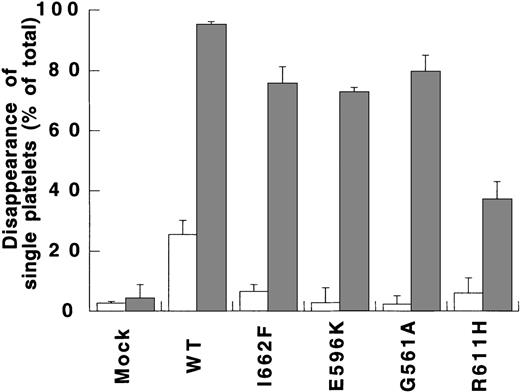
SIPA of type 2M rvWFs
To determine the involvement of mutated amino acids in type 2M vWD on SIPA, the effect of rvWF-G561A, -E596K, -I662F, or -R611H on platelet aggregation was studied at 4000 seconds-1 (Figure4). Whereas in the presence of 1 μg/mL rvWF-WT, aggregation reached a DSP of 25.4% ± 4.8%, it was strongly impaired in the presence of each type 2M rvWF, yielding DSP varying between approximately 2% and 6%, as compared with 2.7% ± 0.5% in mock-transfected cell medium, used as a negative control. When higher concentrations of rvWF-G561A, -I662F, and -R611H were tested ranging from 1.5 to 3.7 μg/mL, none of them was able to induce any aggregation at 4000 seconds-1 (data not shown).
Effect of botrocetin on shear-induced platelet aggregation in the presence of type 2M rvWFs.
Mock-transfected cell medium, rvWF-WT, or type 2M rvWF (-I662F, -E596K, -G561A, -R611H) were preincubated without (open bars) or with (solids bars) 1 μg/mL botrocetin. The mixture was added to washed platelets (0.5 × 108/mL) in the shearing device and exposed to 4000 seconds-1 for 5 minutes. The single platelet region was determined in the absence of shear and in the presence of mock-transfected cell medium and used as the reference for calculation of disappearance of single platelets (DSP). At 4000 seconds-1, botrocetin was a potent inducer of SIPA in rvWF-WT. Type 2M rvWF-I662F, -E596K, and -G561A were increased to a similar extent as WT. In contrast, SIPA in rvWF-R611H reached 40%, in agreement with decreased botrocetin-induced binding to GPIb.
Effect of botrocetin on shear-induced platelet aggregation in the presence of type 2M rvWFs.
Mock-transfected cell medium, rvWF-WT, or type 2M rvWF (-I662F, -E596K, -G561A, -R611H) were preincubated without (open bars) or with (solids bars) 1 μg/mL botrocetin. The mixture was added to washed platelets (0.5 × 108/mL) in the shearing device and exposed to 4000 seconds-1 for 5 minutes. The single platelet region was determined in the absence of shear and in the presence of mock-transfected cell medium and used as the reference for calculation of disappearance of single platelets (DSP). At 4000 seconds-1, botrocetin was a potent inducer of SIPA in rvWF-WT. Type 2M rvWF-I662F, -E596K, and -G561A were increased to a similar extent as WT. In contrast, SIPA in rvWF-R611H reached 40%, in agreement with decreased botrocetin-induced binding to GPIb.
Because botrocetin was able to potentiate the interactions between vWF and platelets in static conditions, it was of interest to assess its effect on SIPA. Shear-induced experiments were performed in the presence of 1 μg/mL of botrocetin and rvWF-WT or type 2M rvWF (1 μg/mL). The effect of botrocetin was clearly apparent when aggregation was measured at a shear rate of 4000 seconds-1(Figure 4). In samples containing rvWF-WT, botrocetin was able to induce an aggregation of 95.3% ± 0.9%, whereas DSP of rvWF-I662F, -E596K, and -G561A reached approximately 75%, and DSP of rvWF-R611H was lower at approximately 40% (Figure 4). In contrast, DSP of negative control (mock-transfected cell medium) did not exceed 4.4% ± 4.4% in the presence of botrocetin at 4000 seconds-1 (Figure 4). The effect of botrocetin was also assessed at a lower shear of 200 seconds-1. Whereas, in the absence of botrocetin, rvWF-WT was unable to induce any aggregation, it was increased up to 40% in the presence of botrocetin. Consistently lower DSP was obtained in the type 2M rvWF samples reaching approximately 15% in the presence of botrocetin (data not shown). These values were higher than those obtained in the mock-transfected cell medium sample in the same conditions.
To ascertain the effect of botrocetin on the interaction between vWF A1 domain and GPIb in type 2M rvWF, we measured the effect of MoAb 6D1 on SIPA in the presence of botrocetin. At 4000 seconds-1 MoAb 6D1 completely abolished SIPA mediated by either rvWF-WT or -I662F, as shown by DSP of 6.5% and 5.6%, respectively. Moreover, vWF deleted in its A1 domain (rvWF-ΔA1) was unable to mediate SIPA at 4000 seconds-1 even in the presence of botrocetin (data not shown). These results suggest that botrocetin and shear act on different pathways on vWF A1 domain/GPIb interaction.
Type 2B rvWFs interaction with GPIb induced by nonphysiological agonists
We compared the ability of different type 2B rvWF to bind to platelets in the absence or in the presence of varying concentrations of ristocetin or botrocetin (Figure5). Although binding of rvWF-WT to platelets only occurred at the highest concentration of ristocetin (1 mg/mL), different responses were observed among the mutants at lower concentrations. ANOVA indicated that differences between the groups were significant in the absence of agonist, in the presence of ristocetin at all concentrations, and in the presence of the lowest botrocetin concentration, whereas there was no significant difference at higher concentrations of botrocetin (0.5 and 1 μg/mL).
Ristocetin- and botrocetin-induced binding of rvWF-WT and type 2B rvWFs to platelets.
rvWF-WT (open bars) or mutated 2B rvWF-L697V (diagonal hatched bars), -R578Q (black bars), -V553M (gray bars), -M540MM (vertical hatched bars), and -V551F (horizontal hatched bars) labeled with125I-MoAb 505 were incubated with paraformaldehyde-fixed platelets for 1 hour at room temperature in the presence of varying concentrations of ristocetin (0-1 mg/mL; panel A) or botrocetin (0-1 μg/mL; panel B). Results were expressed as outlined in the legend to Figure 4. Means ± SEM from 3 experiments were performed in duplicate.
Ristocetin- and botrocetin-induced binding of rvWF-WT and type 2B rvWFs to platelets.
rvWF-WT (open bars) or mutated 2B rvWF-L697V (diagonal hatched bars), -R578Q (black bars), -V553M (gray bars), -M540MM (vertical hatched bars), and -V551F (horizontal hatched bars) labeled with125I-MoAb 505 were incubated with paraformaldehyde-fixed platelets for 1 hour at room temperature in the presence of varying concentrations of ristocetin (0-1 mg/mL; panel A) or botrocetin (0-1 μg/mL; panel B). Results were expressed as outlined in the legend to Figure 4. Means ± SEM from 3 experiments were performed in duplicate.
In the absence of agonist, rvWF-WT and rvWF-L697V were not able to bind to platelets, whereas binding of rvWF-V553M, -M540MM, and -V551F ranged from 17.4% to 26.4% (Figure 5A). Multiple-comparison test indicated that all mutants, except -L697V, differed from WT and that they all differed from each other except for the pair -M540MM and -V551F.
When higher ristocetin concentrations of 0.2 or 0.4 mg/mL were used, rvWF-WT was unable to bind to platelets, whereas all type 2B rvWF bound to platelets. Their binding was different according to the mutation: at 0.2 mg/mL ristocetin, rvWF-L697V had the lowest binding capacity (21.7% ± 1.3%); rvWF-R578Q, -V553M, -M540MM, and -V551F were able to bind to platelets up to 26.5% ± 3.1%, 32.3% ± 2.8%, 35.1% ± 3.2%, and 41.3% ± 3.1%, respectively. In these conditions, statistics showed that each mutant without exception differed from WT, whereas the only difference between the mutants was within the pair -L697V and -V551F. Interestingly, this tendency was even reinforced at the highest ristocetin concentration (1 mg/mL), whereby we found that all type 2B mutants bound to platelets to a similar extent (approximately 45%). This binding was twofold higher than that of rvWF-WT. Multiple-comparison test indicated that binding of each mutant was different from that of WT but that mutants did not differ from each other.
The ability of type 2B rvWF to bind to platelets in the presence of low botrocetin concentrations was also studied and compared with that of rvWF-WT (Figure 5B). At 0.5 μg/mL of botrocetin, rvWF-WT was able to bind to platelets up to 39.1% ± 3.3% that corresponds to the plateau as no increase of binding was observed at 1 μg/mL of botrocetin. All type 2B rvWFs were able to bind to platelets at the lowest botrocetin concentration (0.1 μg/mL). Interestingly, multiple-comparison test indicated, as found for ristocetin, that all mutants differed from the WT, whereas the mutants did not differ from each other. According to the mutation, binding increased in the following order: rvWF-L697V < -R578Q < -V553M < -M540MM < -V551F. At 0.5 μg/mL or 1 μg/mL of botrocetin, rvWF-WT as well as all type 2B rvWFs bound to platelets to a similar extent (ranging from 38% to 42.4%). At these botrocetin concentrations, we did not find statistical differences between them by ANOVA.
Effect of type 2B rvWFs on SIPA
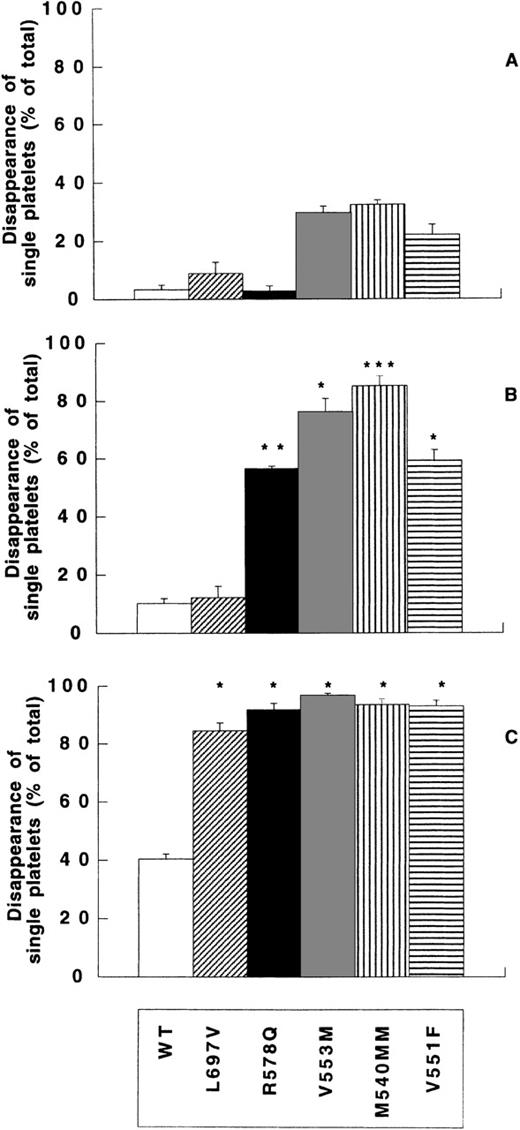
To determine the involvement of mutated amino acids in type 2B rvWFs on SIPA, we tested the capacity of each type 2B rvWF to induce platelet aggregation at different shear rates (0, 200, and 4000 seconds-1) (Figure 6). When the percentage of DSP in the single platelet region was plotted as a function of shear rate in type 2B rvWF samples, SIPA increased with shear.
Shear-induced platelet aggregation of rvWF-WT and type 2B rvWFs.
Washed platelets (0.5 × 108/mL), rvWF-WT (open bars), -L697V (diagonal hatched bars), -R578Q (black bars), -V553M (gray bars), -M540MM (vertical hatched bars), or -V551F (horizontal hatched bars) were incubated without shear (panel A) or were exposed to 200 seconds-1 (panel B) or to 4000 seconds-1(panel C) for 5 minutes at 20°C. DSP was expressed as outlined in the legend to Figure 4. Means ± SEM were calculated from 3 experiments performed in duplicate. P values were calculated by Student t test on paired samples of mutant versus rvWF-WT, as described in the “Materials and methods” section. *P < .05, **P < .001, ***P < .005. DSP of all type 2B rvWFs increased with shear, reaching similar values at 4000 seconds-1 twofold to threefold higher than rvWF-WT.
Shear-induced platelet aggregation of rvWF-WT and type 2B rvWFs.
Washed platelets (0.5 × 108/mL), rvWF-WT (open bars), -L697V (diagonal hatched bars), -R578Q (black bars), -V553M (gray bars), -M540MM (vertical hatched bars), or -V551F (horizontal hatched bars) were incubated without shear (panel A) or were exposed to 200 seconds-1 (panel B) or to 4000 seconds-1(panel C) for 5 minutes at 20°C. DSP was expressed as outlined in the legend to Figure 4. Means ± SEM were calculated from 3 experiments performed in duplicate. P values were calculated by Student t test on paired samples of mutant versus rvWF-WT, as described in the “Materials and methods” section. *P < .05, **P < .001, ***P < .005. DSP of all type 2B rvWFs increased with shear, reaching similar values at 4000 seconds-1 twofold to threefold higher than rvWF-WT.
As observed above in binding experiments, some of the rvWF (rvWF-V551F, -V553M, or -M540MM) induced a spontaneous aggregation, reaching 22.1% ± 3.5%, 29.9% ± 2.2%, and 32.6% ± 1.5%, respectively, whereas rvWF-L697V or -R578Q did not induce any measurable aggregation, as was observed for rvWF-WT (3.4% ± 2.3%) (Figure 6A). However, statistical analysis of paired samples (mutated versus rvWF-WT) indicated that none of these differences appeared significant (Bonferroni corrected P value > .05). In addition, the DSP value of rvWF-WT, -L697V, and -R578Q was close to that of the control mock-transfected cell medium sample (data not shown).
Interestingly, at 200 seconds-1 comparison versus rvWF-WT indicated significant differences of mutated type 2B-rvWFs with the exception of the rvWF-L697V mutant (Figure 6B). Whereas DSP of -L697V did not exceed 12.2% ± 3.9%, DSP of all other type 2B-rvWFs reached significantly higher DSP than WT (10.2% ± 1.8%), namely 56.7% ± 0.9% for rvWF-R578Q (P < .01), 76.2% ± 4.6% for -V553M (P < .05), 85.3% ± 3.3% for -M540MM (P < .005), and 59.3% ± 3.6% for -V551F (P < .05). Thus, SIPA at 200 seconds-1 clearly indicated some differences in the ability of mutated type 2B rvWFs to increase platelet aggregation: Whereas rvWF-L697V was unable to induce any measurable aggregation, M540MM and -V553M induced a very high aggregation of approximately 80%.
At 4000 seconds-1 DSP of rvWF-WT was enhanced up to 40.4% ± 1.6%. This value was significantly lower than the DSP of each type 2B rvWF that increased up to approximately 95% (Pvalue of each mutated rvWF versus WT < .05) (Figure 6C). Moreover, the size of aggregates in type 2B rvWF samples increased with the shear (data not shown).
Therefore, our data indicate that SIPA allows established differences between 2B rvWF mutants, some being more potent than others in this process.
Effect on SIPA of MoAb to GPIb interfering with platelet-vWF interaction
To test the specificity of the interaction between A1 domain of type 2B rvWFs and GPIb in SIPA, we studied the effect of MoAb 6D1 (anti-GPIb), known to block the vWF-GPIb interaction. The results were expressed as the percentage of DSP in the absence or in the presence of MoAb 6D1 (Table 1). At 200 and 4000 seconds-1, a complete inhibition of SIPA was observed in the presence of MoAb 6D1 (Table 1). Furthermore, MoAb 6D1 completely prevented the formation of large as well as smaller aggregates. At 4000 seconds-1, the aggregation induced by rvWF-WT was also completely abolished by MoAb 6D1. In the absence of shear, spontaneous aggregation induced by rvWF-V551F, -V553M, or -M540MM was inhibited by MoAb 6D1; DSP of the samples was 5.2% ± 3.3%, 7.4% ± 5.7%, and 10% ± 6.5%, respectively (results not shown). These results suggest that platelet aggregation induced by rvWF-WT or type 2B rvWF involves predominantly GPIb.
Effect of MoAb 6D1 on shear-induced platelet aggregation in the presence of type 2B rvWFs and rvWF-WT
| . | Disappearance of single platelets (%) . | |||
|---|---|---|---|---|
| 200 seconds−1 . | 4000 seconds−1 . | |||
| . | None . | MoAb 6D1 . | None . | MoAb 6D1 . |
| WT | 13.3 ± 5.9 | ND | 40.4 ± 1.6 | 6.4 ± 4.7 |
| L697V | 12.2 ± 5.6 | ND | 83.5 ± 3.8 | 3.5 ± 0.7 |
| R578Q | 62.5 ± 10.1 | 2.3 ± 3.2 | 91.7 ± 3.2 | 8.9 ± 3 |
| V551F | 59.1 ± 5.4 | 8.7 ± 1.6 | 89.5 ± 4.8 | 7.8 ± 6.5 |
| V553M | 76.6 ± 5.7 | 6.1 ± 2.5 | 95.9 ± 1.7 | 16.2 ± 3.8 |
| M540MM | 85.9 ± 4.3 | 9.9 ± 2.9 | 94.8 ± 1 | 10.2 ± 2.5 |
| . | Disappearance of single platelets (%) . | |||
|---|---|---|---|---|
| 200 seconds−1 . | 4000 seconds−1 . | |||
| . | None . | MoAb 6D1 . | None . | MoAb 6D1 . |
| WT | 13.3 ± 5.9 | ND | 40.4 ± 1.6 | 6.4 ± 4.7 |
| L697V | 12.2 ± 5.6 | ND | 83.5 ± 3.8 | 3.5 ± 0.7 |
| R578Q | 62.5 ± 10.1 | 2.3 ± 3.2 | 91.7 ± 3.2 | 8.9 ± 3 |
| V551F | 59.1 ± 5.4 | 8.7 ± 1.6 | 89.5 ± 4.8 | 7.8 ± 6.5 |
| V553M | 76.6 ± 5.7 | 6.1 ± 2.5 | 95.9 ± 1.7 | 16.2 ± 3.8 |
| M540MM | 85.9 ± 4.3 | 9.9 ± 2.9 | 94.8 ± 1 | 10.2 ± 2.5 |
Washed platelets (0.5 × 108/mL) were preincubated with MoAb 6D1 (20 μg/mL), rvWF (1 μg/mL) was added, and the mixture was subjected to a shear rate of 200 seconds−1 or 4000 seconds−1. The results were expressed as the percentage of disappearance of single platelets in the absence or in the presence of MoAb 6D1. Means ± SEM from 3 experiments were performed in duplicate.
MoAb 6D1 was able to inhibit SIPA induced either by type 2B rvWF or by rvWF-WT-containing samples relative to the sample in the absence of antibody, both at 200 and 4000 seconds−1. ND indicates not done.
Discussion
With the use of a rotating device to apply shear rates ranging from 0 to 4000 seconds-1, we report on the effect on SIPA of 9 different mutations localized in the A1 domain of vWF that reproduce type 2B or type 2M vWD, characterized by an increased or a decreased affinity of vWF for GPIb, respectively.
Type 2M vWD phenotype is characterized by a decreased platelet-dependent function of plasma vWF, associated with a normal multimeric pattern. We have studied 4 rvWFs, 3 of which (rvWF-G561A, -R611H, and -I662F) have been already described14,15,17 and an additional new rvWF, the -E596K mutant. For all of them, we found the presence of HMWM associated with impaired ristocetin-induced binding to platelet GPIb, indicating that they fulfilled the major criteria of type 2M vWD. The normal botrocetin-induced binding of rvWF-G561A, -E596K, and -I662F was in agreement with previous findings13-15 on type 2M rvWF. In contrast, we found a strong impairment of the platelet-binding capacity of rvWF-R611H in the presence of botrocetin, confirming the 10% residual binding obtained in the presence of 2 μg/mL of botrocetin.17
We report for the first time on the effect of type 2M rvWF on SIPA. At 4000 seconds-1, none of the type 2M rvWFs was able to induce any measurable aggregation compared with rvWF-WT. Our data, showing that type 2M rvWFs respond in a similar way to ristocetin and shear, suggest that type 2M rvWFs are responsible for a modification of vWF conformation insensitive to high shear rates and to ristocetin. It is not yet clear whether type 2M mutations affect residues involved in direct binding to GPIb rather than in the folding of the A1 domain of vWF.10
To determine whether type 2M rvWFs were functional using a different agonist than shear and because botrocetin was able to induce platelet GPIb binding of rvWF-G561A, -E596K, and -I662F, we studied its effect on SIPA at 4000 seconds-1. In these conditions, botrocetin was able to increase SIPA of these type 2M rvWF samples as well as of rvWF-WT. Thus, our results suggest that ristocetin- rather than botrocetin-dependent platelet binding reflects the interaction between vWF and GPIb under shear conditions. Our results are confirmed by the finding that MoAb SZ2, directed to the Y276-R293 sequence of GPIb, which inhibits botrocetin but not ristocetin-induced platelet binding and aggregation, was unable to inhibit shear-dependent rolling on vWF of mammalian cells expressing the GPIb-IX-V complex.37
Altogether, our experimental data support the hypothesis that the lack of platelet aggregation in response to high shear rate may provide a physiopathological basis for the hemorrhagic syndrome observed in type 2M vWD patients.
To address the importance of type 2B mutation independently of HMWM, we designed this study of SIPA in 5 type 2B rvWFs expressing the full range of multimers, in contrast to the corresponding patients' plasma vWF that lack their HMWM. This study may help to better understand the physiopathology of type 2B vWD, because the gain-of-function property is paradoxically associated to the absence of a thrombotic tendency in these patients. In the present study, we have demonstrated that the higher ability of type 2B rvWFs to aggregate platelets at 200 or 4000 seconds-1 compared with rvWF-WT was related to the type 2B mutations. This aggregation was completely inhibited by MoAb 6D1 directed to GPIb, underlining the importance of vWF-GPIb interaction in type 2B rvWFs.
Interestingly, we found that the ability of rvWF to induce spontaneous aggregation (rvWF-M540MM, -V551F, or -V553M) did not predict the extent of SIPA. At 200 seconds-1, 4 of 5 type 2B rvWFs induced an increased SIPA, in contrast to rvWF-WT and rvWF-L697V that were unable to induce any platelet aggregation. In agreement with published data,21 38 we have now extended to SIPA the findings that mutations inside the loop are more efficient regulators of the interaction with GPIb than those outside the loop, such as L697V.
At 4000 seconds-1, all type 2B rvWFs were able to aggregate platelets up to approximately 95%, a twofold to threefold higher aggregation value than with rvWF-WT. Our data are in agreement with numerous reports on the type 2B gain-of-function mutations in the absence of shear. Furthermore, they clearly indicate that the complete aggregation observed at 4000 seconds-1 requires the full range of multimers. Thus, our results may help clarify some surprising findings that a similar extent of platelet aggregation has been found in type 2B vWD and in normal platelet-rich plasma at high shear rates (approximately 1000 seconds-1),39 40 whereas the gain-of-function phenotype may have been concealed by the loss of HMWM.
Interestingly, mutations V551F and V553M, which both induced an increased platelet aggregation at low and high shear, have been previously reported to be responsible for a 2A phenotype in 2 patients, associated to a 2B genotype since the rvWF-V551F or -V553M had the binding characteristics of a type 2B.30,41 It has been suggested that this discrepancy between phenotype and genotype could be related to a loss of both high and intermediate multimers bound to platelets and that such mutations may correspond to more severe forms of type 2B vWD.30 41 However, we have found that SIPA did not allow to further discriminate such rvWF from other type 2B rvWFs.
Altogether, our results underline the major involvement of vWF and GPIb in platelet aggregation at 200 and 4000 seconds-1 and suggest that type 2B vWF mutations induce a regulated conformation of vWF A1 domain, sensitive to lower shear rates than rvWF-WT. Despite this active vWF conformation, these type 2B vWD patients do not have any thrombotic tendency. Instead, they display a variable thrombocytopenia, which could be attributed to the formation of unstable platelet aggregates.
Therefore, we propose the following model: Platelet aggregation, which is fully supported by vWF-GPIb interaction, is not irreversible. According to the dual-step model of platelet adhesion to vWF in high shear conditions, activation of the αIIbβ3 integrin is required for irreversible adhesion leading to thrombus formation.42An important question will be to determine the involvement of αIIbβ3 activation in the physiopathology of type 2B vWD.
In conclusion, our findings provide new insights into the physiopathology of type 2M and 2B vWD. Although vWD is always associated to a hemorrhagic disorder, we have observed that SIPA was abolished in type 2M rvWF or increased in type 2B rvWF. Thus, the absence of platelet aggregation in high shear conditions in type 2M rvWF suggests a correlation between bleeding symptoms and SIPA. In contrast, the enhancing effect of type 2B rvWF on platelet aggregation could be responsible for unstable aggregates and may explain the fluctuant thrombocytopenia without thrombosis observed in these patients.
Acknowledgments
We thank Drs L. Hilbert and C. Mazurier from LFB, Lille, France, for the kind gift of plasmids coding for V553M, G561A, R578Q, R611H, and L697V. We thank B. Obert and P. Legendre for expert technical assistance. Dr C. Mazurier is gratefully acknowledged for critical reading of the manuscript. We would like to thank Dr J. Warszawski for help in statistical analysis.
Supported by an INSERM fellowship (Poste d'accueil) to N.A. and a fellowship from Ministère de l'Education Nationale, de la Recherche et de la Technologie to G. R.-L.
Reprints:Dominique Baruch, INSERM U143, 84 rue du Général Leclerc, 94276 Bicêtre Cedex, France; e-mail:baruch@infobiogen.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal