Abstract
Granulocyte colony-stimulating factor (G-CSF) is a glycoprotein believed to play an important role in regulating granulopoiesis both at steady state and during an “emergency” situation. Generation of G-CSF and G-CSF receptor–deficient mice by gene targeting has demonstrated unequivocally the importance of G-CSF in the regulation of baseline granulopoiesis. This study attempted to define the physiologic role of G-CSF during an emergency situation by challenging a cohort of wild-type and G-CSF–deficient mice with Candida albicans. Interestingly, after infection, G-CSF–deficient mice developed an absolute neutrophilia that was observed both in blood and bone marrow. In addition, 3 days after Candida infection increased numbers of granulocyte-macrophage (GM) and macrophage (M) progenitors were observed in the bone marrow of G-CSF–deficient mice. Of the cytokines surveyed, interleukin (IL)-6 levels in serum were elevated; interestingly, levels of IL-6 were higher and more sustained in G-CSF–deficient mice infected with C albicans than similarly infected wild-type mice. Despite the higher levels of serum IL-6, this cytokine is dispensable for the observed neutrophilia because candida-infected IL-6–deficient mice, or mice simultaneously deficient in G-CSF and IL-6, developed neutrophilia. Similarly, mice lacking both G-CSF and GM-CSF developed absolute neutrophilia and had elevated numbers of GM and M progenitors in the bone marrow; thus, G-CSF and GM-CSF are dispensable for promoting the emergency response to candidal infection.
Granulopoiesis is the process whereby mature granulocytes are produced from a small number of pluripotent hematopoietic stem cells. Despite the fact that a large number of granulocytes are produced daily to maintain homeostasis and a still higher number of granulocytes can be produced during an emergency response to infection, the process is highly regulated. Multiple cytokines including granulocyte colony-stimulating factor (G-CSF), granulocyte/macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-3 and IL-6 are thought to play a role in the regulation of granulopoiesis.1-3 The critical role of G-CSF in steady-state granulopoiesis has been clearly demonstrated by the generation of mice in which the G-CSF gene4 and subsequently G-CSF receptor gene have been disrupted by gene targeting in embryonic stem cells.5 These mice have 20% to 30% of circulating neutrophils, compared to wild-type mice. The existence of both immature and mature forms of neutrophils in mice lacking G-CSF indicates that neutrophil production can occur in a G-CSF-independent manner; thus a factor(s) other than G-CSF has the capacity to promote neutrophil production in the absence of G-CSF.
During an emergency, such as infection with pathogenic organisms, the response of the host involves a series of inflammatory events, with macrophages and neutrophils playing an important role in the cellular phase, followed by an acquired immunity specific to the pathogen. In such a situation, the hematopoietic system is triggered to meet the demand for production of appropriate cell types. The production of different cells is highly regulated, although the mechanism underlying emergency hematopoiesis remains poorly understood. On the basis of a large body of evidence it is thought that granulopoiesis during an emergency situation is regulated primarily by G-CSF; this notion, however, has so far been inferred rather than defined. Indirect evidence implicating G-CSF as an emergency granulopoietic factor includes its ability to stimulate neutrophil production when administered in pharmacologic doses6 and the coexistence, in some infective states, of high neutrophil levels and high serum levels of endogenously produced G-CSF.7
Infection of G-CSF–deficient mice with pathogenic organisms provides a valuable model to evaluate definitively the role of G-CSF in regulating neutrophil production during an emergency situation. Different pathogens evoke a distinct pattern of inflammatory cell responses; although the mechanisms underlying these differences remain largely unknown, it is believed to be due to the different sets of cytokines produced by the host. Because it is known that neutrophils predominate the first phase of a host's response to Candida albicans,8 we infected wild-type and G-CSF-deficient mice with the yeast to study regulation of emergency granulopoiesis. Intriguingly G-CSF–deficient mice, like similarly infected wild-type mice, developed profound and sustained neutrophilia, suggesting that G-CSF is dispensable for mounting an emergency granulopoietic response. Because serum levels of IL-6 were elevated in G-CSF–deficient mice, we infected mice simultaneously deficient in G-CSF and IL-6. Furthermore, to investigate the role of GM-CSF in candida-induced neutrophilia in G-CSF-deficient mice we also challenged mice simultaneously deficient in G-CSF and GM-CSF. Like G-CSF–deficient mice, both G-CSF−/−/GM-CSF−/− and G-CSF−/−/IL-6−/− mice developed a similar degree of neutrophilia indicating that in addition to G-CSF both IL-6 and GM-CSF are dispensable for emergency granulopoiesis in response to candidal infection.
Materials and methods
Mice
Eight- to 10-week-old wild-type, G-CSF–deficient (G-CSF−/−),9 G-CSF/GM-CSF-deficient (G-CSF−/−/GM-CSF−/−),4G-CSF/IL-6-deficient (G-CSF−/−/IL-6−/−) mice were used for these studies. G-CSF−/−/IL-6−/− mice were generated by intercrossing G-CSF–deficient mice with IL-6-deficient mice.10 The mice of mixed C57BL/6 and 129/OLA background were used for all studies. Within an experimental group mice were age and sex matched. All experiments were conducted according to the guidelines of The National Health and Medical Research Council of Australia, and the experiment protocols were approved by the Animal Ethics Committee of Royal Melbourne Hospital Campus (Victoria, Australia).
Candida albicans
Subcultures of Candida albicans (ATCC 18804) were maintained at −80°C in tryptone broth containing 10% glycerol. Cultures were plated on Sabouraud dextrose agar plates and grown at 37°C for 48 hours. The plates were stored at 4°C for a maximum of 4 weeks. Before each experiment, a colony was picked from a plate and grown in 5 mL of Sabouraud agar broth (SAB) at 37°C for 24 hours in a shaker. The cells were centrifuged and the pellet washed twice with pyrogen-free phosphate-buffered saline (PBS) and resuspended in PBS. The cell suspension was briefly sonicated, counted in a hemocytometer, and then adjusted to the desired concentration.
Experimental model
Disseminated candidiasis was produced by tail vein injection of 2.5 × 105 colony-forming units (CFUs) of C albicans blastoconidia on day 0. Animals were observed for 7 days for altered behavior and morbidity. On days 1, 3, and 7, subgroups of animals were bled through retro-orbital puncture and then killed by cervical dislocation. Animals were autopsied aseptically immediately after death. The right kidney was homogenized in 5 mL PBS. Dilutions of homogenate were plated on Sabouraud agar dextrose broth and incubated at 37°C. Colonies were counted after 48 hours. The left kidney was fixed in Bouin solution and stained with hematoxylin-eosin. For detection of fungi in tissues, sections were stained with Gomori methenamine silver.
Peripheral blood and bone marrow analysis
Mice were bled through retro-orbital plexus using EDTA-coated microhematocrit tubes (Clay Adams, Pasippany, NJ), diluted immediately 1:4 in PBS containing 2 mg/mL EDTA, and analyzed using a Sysmex-K1000 automated counter (Toa Medical Electronics Co, Kobe, Japan). Peripheral blood smears were stained with May-Grünwald-Giemsa, and manual differential cell counts of at least 200 nucleated cells were performed. In some cases, cytospins were prepared from peripheral blood after lysing the red blood cells (RBC) with RBC lysis buffer (0.16 mol/L ammonium chloride in Tris buffer; pH 7.2) and stained with May-Grünwald-Giemsa. Bone marrow cells were harvested from the femur and suspended in RPMI containing 5% fetal calf serum (FCS). Total bone marrow cells were counted in a hemocytometer using white blood cell (WBC) dilution buffer (3% glacial acetic acid in PBS). Differential blood cell counts were performed on cytospin preparations of bone marrow cells stained with May-Grünwald-Giemsa stain.
Phagocytosis and candidacidal activity of neutrophils
Mice were injected intraperitoneally with thioglycollate and after 4 hours the peritoneum was lavaged with PBS containing 5% FCS. Neutrophils were further purified on Percoll gradients (using gradients of 45%, 54%, 63%, and 72%) and centrifuged at 500g for 25 minutes. Polymorphonuclear cells were aspirated from the interphase of 63% and 72% Percoll gradients.
Phagocytosis
Briefly, 2.5 × 106 neutrophils were added to 1 × 106 heat-inactivated candida in Hanks' balanced salt solution (HBSS) containing 10% mouse serum in a total volume of 1 mL. The cells were incubated for 15 minutes at 37°C with shaking and then placed on ice. Trypan blue (1 mL) was added to each reaction mixture and a wet mount was prepared. Phagocytosed and extracellular yeast (including adherent but not internalized yeast) were distinguished and scored on the basis of trypan blue uptake (internalized yeast being colorless/clear).
Candidacidal assay
Neutrophils (1.5 × 106) were preincubated with HBSS containing 25% mouse serum at 37°C for 5 minutes before adding 1.0 × 107C albicans. Tubes containing the cells and the yeast were rotated end-over-end for 60 minutes at 37°C. Thereafter, 250 μL of 2.5% sodium deoxycholate was added and mixed, followed by 4.0 mL methylene blue; the tubes were then centrifuged at 900g for 15 minutes at 4°C. Wet preps were made and 300 yeast cells were counted under a microscope distinguishing the viable cells (unstained) versus dead (stained) cells.
Cytokine levels
Tumor necrosis factor-α (TNF-α) and IL-10 levels in serum were determined using an enzyme-linked immunoabsorbent assay (ELISA) (Genzyme Corp, Cambridge, MA and Pharmingen, San Diego, CA) specific for the cytokines under the conditions recommended by the suppliers. Absorbance values read at 450 nm were converted to concentrations (pg/mL) by comparison with the appropriate standard curve.
IL-6 assay
Serum IL-6 was registered indirectly by its capacity to promote proliferation of the IL-6–dependent mouse hybridoma cell line 7TD1 (obtained from Dr J. Van Snick, LICR, Brussels, Belgium). Test samples were serially diluted in 96-well flat-bottomed microtiter plates and incubated for 96 hours with 2000 7TD1 cells/well at 37°C in a humidified incubator containing 5% CO2 in air. Dilutions of murine IL-6 (a gift from Dr R. J. Simpson, LICR, Melbourne, Australia) were included as a standard. Cell proliferation was measured using the MTT (3-[4,5-dimethylthiazol-2-yl]-3,5-diphenyltetrazolium bromide [Sigma, St Louis, MO]) colorimetric method. After 96 hours of incubation, MTT was added to each well (5 mg/mL 1:20 v/v) and the cultures were incubated for a further 4 hours at 37°C at 5% CO2 in air. After incubation, the culture fluid was removed and the cells were lysed by adding 200 μL of acidified isopropanol (isopropanol with 0.04 N HCl). The plates were analyzed on a plate reader (Titertek Multiskan MCC 340; EFLAB, Helsinki, Finland) at a test wavelength of 560 nm and a reference wavelength of 690 nm. In assays using neutralizing antibody, samples were mixed with a 1:1 volume of a 1:100 dilution of polyvalent rabbit antimouse IL-6 antibody for 2 hours before analysis in the 7TD1 bioassay. These assays were performed in parallel with samples treated with control rabbit serum.
Flow cytometry
Blood was treated with RBC lysis buffer for 5 minutes at 37°C and then washed once with PBS containing 5% FCS. After lysing RBCs, WBCs were preincubated with 2.4G2 antibody (Pharmingen) for 10 minutes at room temperature to block Fc receptors (FcγR III/II) and were subsequently treated with indicated antibodies at 4°C for 30 minutes in PBS containing 2.5% heat-inactivated FCS. The following panel of antibodies were used: fluorescein isothiocyanate (FITC)-conjugated rat antimouse Mac-1 (CD 11b), biotinylated rat antimouse Gr-1 (Ly-6G), FITC-conjugated rat antimouse c-kit (CD117) (Pharmingen), and biotinylated rat antimouse c-fms (AFS98, kind gift from John Hamilton, Melbourne, Australia). Cells stained with biotinylated antibodies were finally developed with phycoerythrin-streptavidin (Pharmingen). All cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) using CellQuest software. Positive populations have been gated on the basis of staining profiles with isotype-matched control antibody.
Statistical analysis
Data are presented as mean ± SD unless otherwise stated. Organ loads of C albicans are expressed as the logarithm of the candida count per kidney. Comparisons were made using 2-tailed paired and unpaired Student t tests and the Mann-Whitney signed rank test as appropriate. A P value less than .05 was considered significant.
Results
Peripheral blood neutrophilia in candida-infected mice
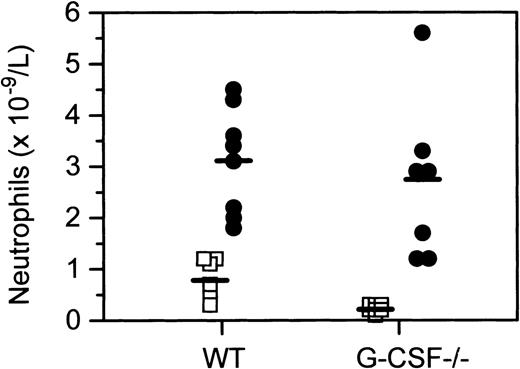
Morphologic examination of peripheral blood smears of wild-type and G-CSF–deficient mice revealed that mice of both genotypes developed significant neutropenia 24 hours after infection with C albicans(data not shown). However, an absolute neutrophilia was observed in both G-CSF–deficient and wild-type mice 3 days after infection. Neutrophilia in G-CSF–deficient mice was most pronounced on day 7 after infection (Figure 1). Due to baseline neutrophil depletion in G-CSF–deficient mice, the fold increase in neutrophil numbers was significantly higher in G-CSF–deficient mice than the wild-type mice. The striking neutrophilia in infected G-CSF–deficient mice was somewhat surprising in view of the prevailing view of a pivotal role for G-CSF in emergency granulopoiesis.
Peripheral blood neutrophil counts in wild type and G-CSF–deficient mice treated with C albicans.
Peripheral blood neutrophil levels in wild-type, G-CSF–deficient mice at baseline (□) and 7 days after candida infection (•), sampled identically.
Peripheral blood neutrophil counts in wild type and G-CSF–deficient mice treated with C albicans.
Peripheral blood neutrophil levels in wild-type, G-CSF–deficient mice at baseline (□) and 7 days after candida infection (•), sampled identically.
Changes in cellular composition of bone marrow after candidal infection
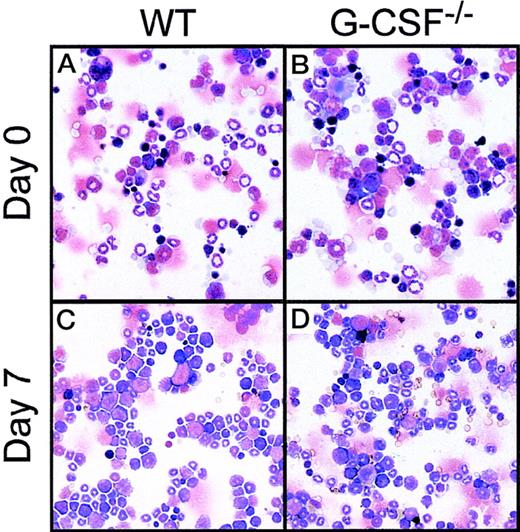
At baseline, both wild-type and G-CSF–deficient mice have similar numbers of nucleated bone marrow cells. However, G-CSF-deficient mice have a significantly lower number of neutrophils; the deficiency is most striking in the mature neutrophil compartment comprising metamyelocytes, “band” and segmented forms. To investigate whether the absolute neutrophilia following candidal infection was due to mobilization of neutrophils from bone marrow into circulation, or, that there was an increased production of neutrophilic cells, we examined cytospin preparations of bone marrow cells from femurs of mice challenged with C albicans. Despite the fact that the total number of bone marrow cells after infection in both G-CSF–deficient and control mice were similar to basal values, the increase in numbers of early and late neutrophil forms indicated preferential amplification within the neutrophil lineage. In G-CSF–deficient mice the observed increase was more marked for immature than for mature neutrophils. (Figure2 and Table 1).
Bone marrow cells.
Cytocentrifuge preparation of bone marrow cells at baseline (top panel) and 7 days after candida challenge (bottom panel) from wild-type (A, C) and G-CSF–deficient (B, D) mice. The cytospin preparations were stained with May-Grünwald-Giemsa stain.
Bone marrow cells.
Cytocentrifuge preparation of bone marrow cells at baseline (top panel) and 7 days after candida challenge (bottom panel) from wild-type (A, C) and G-CSF–deficient (B, D) mice. The cytospin preparations were stained with May-Grünwald-Giemsa stain.
Changes in bone marrow hematopoietic cells in response to candida infection in wild-type and G-CSF–deficient mice
| Genotype . | Days after candida infection* . | Total cellularity† . | Cell type (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Blast . | Nucleated erythrocyte . | Promyelocyte and myelocyte . | Metamyelocyte and polymorph . | Lymphocyte . | Monocyte . | Eosinophil . | |||
| Wild-type | 0 (9) | 3.8 ± 0.3 | 1 ± 0.6 | 27 ± 1 | 13 ± 1 | 23 ± 2 | 25 ± 2 | 9 ± 1 | 2 ± 1 |
| G-CSF−/− | 0 (8) | 3.3 ± 0.2 | 2 ± 1 | 31 ± 2 | 13 ± 2 | 10 ± 2 | 34 ± 3 | 8 ± 1 | 2 ± 1 |
| Wild-type | 3 (9) | 3.5 ± 0.2 | 3 ± 1 | 17 ± 1 | 10 ± 1 | 19 ± 1 | 23 ± 2 | 13 ± 1 | 6 ± 1 |
| G-CSF−/− | 3 (7) | 1.7 ± 0.1‡,1-153 | 11 ± 3 | 20 ± 2 | 18 ± 1‡ | 12 ± 2 | 30 ± 2 | 13 ± 2 | 6 ± 1 |
| Wild-type | 7 (10) | 4.5 ± 0.3‡ | 3 ± 1 | 11 ± 2 | 20 ± 1‡ | 35 ± 3‡ | 13 ± 2 | 15 ± 1‡ | 3 ± 1 |
| G-CSF−/− | 7 (7) | 3.7 ± 0.1 | 4 ± 1 | 19 ± 3 | 27 ± 3‡ | 15 ± 1‡ | 14 ± 2 | 21 ± 4 | 0 ± 1 |
| Genotype . | Days after candida infection* . | Total cellularity† . | Cell type (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Blast . | Nucleated erythrocyte . | Promyelocyte and myelocyte . | Metamyelocyte and polymorph . | Lymphocyte . | Monocyte . | Eosinophil . | |||
| Wild-type | 0 (9) | 3.8 ± 0.3 | 1 ± 0.6 | 27 ± 1 | 13 ± 1 | 23 ± 2 | 25 ± 2 | 9 ± 1 | 2 ± 1 |
| G-CSF−/− | 0 (8) | 3.3 ± 0.2 | 2 ± 1 | 31 ± 2 | 13 ± 2 | 10 ± 2 | 34 ± 3 | 8 ± 1 | 2 ± 1 |
| Wild-type | 3 (9) | 3.5 ± 0.2 | 3 ± 1 | 17 ± 1 | 10 ± 1 | 19 ± 1 | 23 ± 2 | 13 ± 1 | 6 ± 1 |
| G-CSF−/− | 3 (7) | 1.7 ± 0.1‡,1-153 | 11 ± 3 | 20 ± 2 | 18 ± 1‡ | 12 ± 2 | 30 ± 2 | 13 ± 2 | 6 ± 1 |
| Wild-type | 7 (10) | 4.5 ± 0.3‡ | 3 ± 1 | 11 ± 2 | 20 ± 1‡ | 35 ± 3‡ | 13 ± 2 | 15 ± 1‡ | 3 ± 1 |
| G-CSF−/− | 7 (7) | 3.7 ± 0.1 | 4 ± 1 | 19 ± 3 | 27 ± 3‡ | 15 ± 1‡ | 14 ± 2 | 21 ± 4 | 0 ± 1 |
Cell counts and 100-count manual leukocyte differentials were performed on mononuclear cells recovered from femurs of mice. Data are presented as mean ± SD.
Study day 0 is pretreatment; the value in the parentheses is the number of mice studied for that group.
Marrow cellularity is cells ×107/2 femurs.
P < .05 for comparison between mice of the same genotype at baseline and on the study day indicated.
P < .05 for comparison between wild type and G-CSF−/− on the same study day.
Role of GM-CSF and IL-6 in granulopoiesis in candida-infected G-CSF–deficient mice
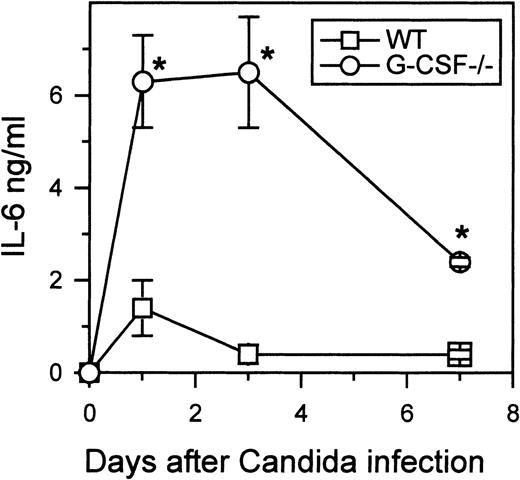
To understand the basis for the neutrophilia in candida-infected G-CSF–deficient mice, we analyzed the serum levels of various cytokines. In wild-type mice, at least, IL-3 and GM-CSF are thought to play an important role during emergency hematopoiesis.11However, neither IL-3 nor GM-CSF was detectable in the sera of either wild-type or G-CSF–deficient mice during the course of candida infection (data not shown). In addition to GM-CSF and IL-3, IL-6 is thought to be of importance in mounting a neutrophilic challenge in response to candida infection.12 In both wild-type and G-CSF–deficient mice, elevated levels of IL-6 were observed 24 hours after candida infection (Figure 3). However, in G-CSF-deficient mice, the level of IL-6 at 24 hours after candida infection was almost 4-fold higher than that observed in similarly infected wild-type mice. Moreover, unlike wild-type mice, in which IL-6 was undetectable in serum 7 days after candida infection, IL-6 levels remained elevated in G-CSF–deficient mice infected with C. albicans.
Kinetics of IL-6 production.
Wild-type and G-CSF–deficient mice were challenged with 2.5 × 105C albicans intravenously. At the indicated times, 6 mice of each genotype were killed. Serum samples from each mouse were tested for IL-6 as described in “Materials and methods.” Data are represented as mean ± SD. One of 2 separate experiments with similar results is shown.
Kinetics of IL-6 production.
Wild-type and G-CSF–deficient mice were challenged with 2.5 × 105C albicans intravenously. At the indicated times, 6 mice of each genotype were killed. Serum samples from each mouse were tested for IL-6 as described in “Materials and methods.” Data are represented as mean ± SD. One of 2 separate experiments with similar results is shown.
Although we were unable to detect GM-CSF in the serum of G-CSF–deficient and wild-type mice infected with candida, it still remained possible that because of its demonstrated action on GM and G progenitors, GM-CSF contributed to the candida-mediated neutrophilia. To address this possibility mice simultaneously deficient in G-CSF and GM-CSF were challenged with candida. Seven days after infection we observed an increase in neutrophil numbers in peripheral blood; the fold increase compared to baseline in each of the genotypes was similar in magnitude to that observed in G-CSF–deficient mice (Figure4). To investigate whether increased granulopoiesis in G-CSF–deficient mice in response to candida infection was mediated by IL-6, we challenged mice simultaneously lacking G-CSF and IL-6 with candida. At baseline, neutropenia in adult G-CSF−/−/IL-6−/− mice is comparable to that in G-CSF−/− mice (S.B. and A.R.D., manuscript in preparation); moreover, following candida infection, the increase in neutrophils in circulation was of a similar magnitude in G-CSF−/−/IL-6−/−and G-CSF−/− mice (Figure 4).
Peripheral blood neutrophil counts in wild type, G-CSF−/−, G-CSF−/−/GM-CSF−/− and G-CSF−/−/IL-6−/− mice treated with C albicans.
Peripheral blood neutrophil levels in wild type, G-CSF−/−, G-CSF−/−/GM-CSF−/−and G-CSF−/−/ IL-6−/−mice at baseline (empty bars) and 7 days after candida infection (shaded bars), sampled identically. Data are represented as mean ± SD. *There was a significant difference in the neutrophil count on day 7 compared to day 0 for each genotype of mice (P < .05).
Peripheral blood neutrophil counts in wild type, G-CSF−/−, G-CSF−/−/GM-CSF−/− and G-CSF−/−/IL-6−/− mice treated with C albicans.
Peripheral blood neutrophil levels in wild type, G-CSF−/−, G-CSF−/−/GM-CSF−/−and G-CSF−/−/ IL-6−/−mice at baseline (empty bars) and 7 days after candida infection (shaded bars), sampled identically. Data are represented as mean ± SD. *There was a significant difference in the neutrophil count on day 7 compared to day 0 for each genotype of mice (P < .05).
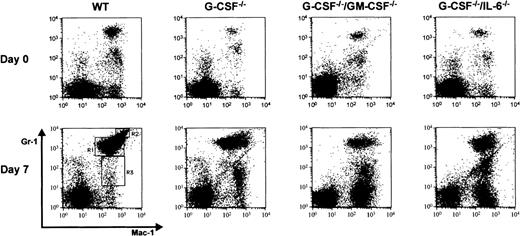
In addition to mature neutrophils, on day 7 after infection there were also immature neutrophils in the peripheral blood (left shift) of mice of all genotypes. However, this effect was more pronounced in mice deficient in growth factor compared to wild-type mice (Table2). G-CSF–deficient mice showed a higher proportion of myelocytes than that seen in the blood of wild-type mice. Also, in G-CSF–deficient mice, on day 7 after infection, the mature neutrophil compartment included a greater proportion of “band-forms” and only a few segmented neutrophils (Figure5). Similar observations were made in G-CSF−/−/GM-CSF−/− and G-CSF−/−/IL-6−/− mice. In addition to the observed neutrophilia in these mice, there was also a pronounced monocytosis (data not shown). To further characterize the immature myeloid cells in the periphery of knockout mice after candidal infection, we analyzed the cell types in blood from candida-infected mice by flow cytometry using surface markers specific for granulocytes and monocytes. At baseline, there were 2 populations of cells, which stained positive for Gr-1 and Mac-1, but could be distinguished on the basis of Gr-1 expression. One population of cells was Mac-1+Gr-1dull and the other population was Mac-1+Gr-1bright (Figure6, top panel). The forward and side scatter profile of Mac-1+Gr-1dull and Mac-1+ Gr-1bright cells suggested that they represented monocyte/macrophage and granulocytic cells, respectively. At baseline, the proportion of Mac-1+Gr-1bright cells was lower in growth factor(s)-deficient mice compared to wild-type mice, consistent with the neutropenic state of these mice. Seven days after candida infection, there were 3 populations of cells that could be distinguished based on Mac-1/Gr-1 staining pattern: Mac-1+Gr-1lo (region R3 on Figure 6, bottom panel), Mac-1+ Gr-1med (region R1 on Figure 6, bottom panel) and Mac-1+Gr-1high cells (region R2 on Figure 6, bottom panel). Consistent with the findings based on morphologic examination of WBCs, FACS analysis revealed that after candida infection, the proportion of Mac-1+Gr-1hi cells (representing mature neutrophils) was greater in wild-type mice compared to growth factor-deficient mice (Table 3). Furthermore, the proportion of Mac-1+ Gr-1locells in circulation of candida-infected growth factor(s)-deficient mice was significantly elevated, consistent with the monocytosis observed in these mice (Table 4). Interestingly, monocytes in candida-infected growth factor-deficient mice had higher expression of Mac-1 than monocytes from similarly infected wild-type mice (Table 4). Expression of Mac-1 is up-regulated on monocytes and neutrophils on activation.13 The presence of activated monocytes in candida-infected growth factor-deficient mice could be due to unresolved candida infection.
Presence of immature neutrophils in peripheral blood of mice after candida infection
| Genotype . | Leukocytes (109/L) . | Neutrophils (109/L) . | % Cell type (neutrophil lineage) . | |
|---|---|---|---|---|
| Promyelocyte and myelocyte . | Metamyelocyte, band, and polymorph . | |||
| Wild-type | 8.6 ± 2.8 | 2.1 ± 0.1 | 11 ± 5.0 | 87 ± 7.0 |
| G-CSF−/− | 7.9 ± 1.8 | 1.6 ± 1.0 | 31 ± 9.0 | 69 ± 13.0* |
| G-CSF−/−/GM-CSF−/− | 5.1 ± 0.5 | 1.1 ± 0.2 | 46 ± 14.0 | 54 ± 14.0* |
| G-CSF−/−/IL-6−/− | 6.2 ± 0.8 | 1.6 ± 0.3 | 35 ± 9.0 | 65 ± 9.0* |
| Genotype . | Leukocytes (109/L) . | Neutrophils (109/L) . | % Cell type (neutrophil lineage) . | |
|---|---|---|---|---|
| Promyelocyte and myelocyte . | Metamyelocyte, band, and polymorph . | |||
| Wild-type | 8.6 ± 2.8 | 2.1 ± 0.1 | 11 ± 5.0 | 87 ± 7.0 |
| G-CSF−/− | 7.9 ± 1.8 | 1.6 ± 1.0 | 31 ± 9.0 | 69 ± 13.0* |
| G-CSF−/−/GM-CSF−/− | 5.1 ± 0.5 | 1.1 ± 0.2 | 46 ± 14.0 | 54 ± 14.0* |
| G-CSF−/−/IL-6−/− | 6.2 ± 0.8 | 1.6 ± 0.3 | 35 ± 9.0 | 65 ± 9.0* |
Manual 100-count leukocyte differentials and further 100-count differentials of cells of neutrophil lineage were performed on blood smears from age- and sex-matched mice. A total of 6-8 mice of each genotype were analyzed on day 7 after candida infection. Data represent mean ± SD.
P < .05 compared to wild-type mice.
Two-color flow cytometric analysis of peripheral blood mononuclear cells at baseline and 7 days after candida infection.
Peripheral blood mononuclear cells from (A) wild-type, (B) G-CSF−/−, (C) G-CSF−/−/GM-CSF−/− , and (D) G-CSF−/−/IL-6−/−mice were incubated with Mac-1 and Gr-1 antibodies. An increase in proportion of granulocytic cells (Gr-1 and Mac-1 double positive cells) is seen in mice of all genotypes after candida infection.
Two-color flow cytometric analysis of peripheral blood mononuclear cells at baseline and 7 days after candida infection.
Peripheral blood mononuclear cells from (A) wild-type, (B) G-CSF−/−, (C) G-CSF−/−/GM-CSF−/− , and (D) G-CSF−/−/IL-6−/−mice were incubated with Mac-1 and Gr-1 antibodies. An increase in proportion of granulocytic cells (Gr-1 and Mac-1 double positive cells) is seen in mice of all genotypes after candida infection.
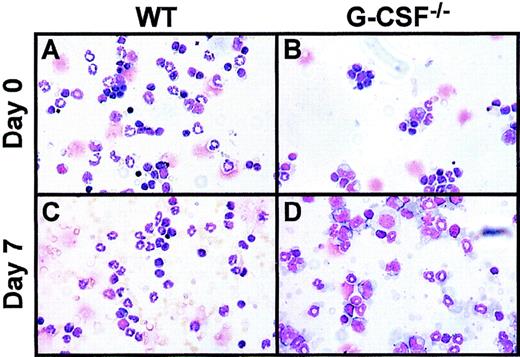
WBCs at baseline and after infection.
Cytocentrifuge preparation of WBCs at baseline (top panel) and 7 days after candida challenge (bottom panel) from wild-type (WT), G-CSF–deficient mice. The cytospin preparations were stained with May-Grünwald-Giemsa stain.
WBCs at baseline and after infection.
Cytocentrifuge preparation of WBCs at baseline (top panel) and 7 days after candida challenge (bottom panel) from wild-type (WT), G-CSF–deficient mice. The cytospin preparations were stained with May-Grünwald-Giemsa stain.
Frequency of Mac-1+Gr-1+-double positive cells in the peripheral blood of mice 7 days after candida infection
| Genotype . | WBC (109/L)3-150 . | % Cell type . | |
|---|---|---|---|
| Mac-1+Gr-1med . | Mac-1+Gr-1hi . | ||
| Wild-type | 8.4 ± 0.8 | 36.0 ± 7.0 | 14.0 ± 3.5 |
| G-CSF−/− | 6.6 ± 0.1 | 28.5 ± 5.8 | 4.1 ± 2.03-151 |
| G-CSF−/−/GM-CSF−/− | 5.3 ± 0.9 | 28.0 ± 2.4 | 6.0 ± 3.03-151 |
| G-CSF−/−/IL-6−/− | 6.2 ± 0.4 | 27.0 ± 4.0 | 6.0 ± 3.03-151 |
| Genotype . | WBC (109/L)3-150 . | % Cell type . | |
|---|---|---|---|
| Mac-1+Gr-1med . | Mac-1+Gr-1hi . | ||
| Wild-type | 8.4 ± 0.8 | 36.0 ± 7.0 | 14.0 ± 3.5 |
| G-CSF−/− | 6.6 ± 0.1 | 28.5 ± 5.8 | 4.1 ± 2.03-151 |
| G-CSF−/−/GM-CSF−/− | 5.3 ± 0.9 | 28.0 ± 2.4 | 6.0 ± 3.03-151 |
| G-CSF−/−/IL-6−/− | 6.2 ± 0.4 | 27.0 ± 4.0 | 6.0 ± 3.03-151 |
WBCs were incubated with antibodies against myeloid cell markers (Gr-1 and Mac-1) and analyzed on FACScan using CellQuest software. Data are represented as mean ± SD. Ten to 12 mice of each genotype were analyzed.
Total WBC count as determined by Sysmex-K1000.
P < .05 compared to wild-type mice.
P < .05 compared to wild-type mice.
C albicans infection results in higher frequency of Mac-1+Gr-1lo cells in peripheral blood of knockout mice compared to wild-type mice
| Genotype . | Leukocyte (109/L)4-150 . | Mac-1+Gr-1lo cells (%) . | Mac-1 expression (mean fluorescence intensity) . |
|---|---|---|---|
| Wild-type | 8.4 ± 0.8 | 2.3 ± 0.9 | 264 ± 31 |
| G-CSF−/− | 6.6 ± 0.1 | 6.2 ± 1.34-151 | 436 ± 62‡ |
| G-CSF−/−/GM-CSF−/− | 5.3 ± 0.9 | 8.2 ± 1.64-151 | 486 ± 95‡ |
| G-CSF−/−/IL-6−/− | 6.2 ± 0.4 | 6.9 ± 2.4 | 396 ± 50 |
| Genotype . | Leukocyte (109/L)4-150 . | Mac-1+Gr-1lo cells (%) . | Mac-1 expression (mean fluorescence intensity) . |
|---|---|---|---|
| Wild-type | 8.4 ± 0.8 | 2.3 ± 0.9 | 264 ± 31 |
| G-CSF−/− | 6.6 ± 0.1 | 6.2 ± 1.34-151 | 436 ± 62‡ |
| G-CSF−/−/GM-CSF−/− | 5.3 ± 0.9 | 8.2 ± 1.64-151 | 486 ± 95‡ |
| G-CSF−/−/IL-6−/− | 6.2 ± 0.4 | 6.9 ± 2.4 | 396 ± 50 |
WBCs were incubated with antibodies against myeloid cell markers (Gr-1 and Mac-1) and analyzed on FACScan using CellQuest software. Data are represented as mean ± SD. Ten to 12 mice of each genotype were analyzed.
Total WBC count as determined by Sysmex-K1000.
P < .05 compared to wild-type mice.
P < .05 compared to wild-type mice.
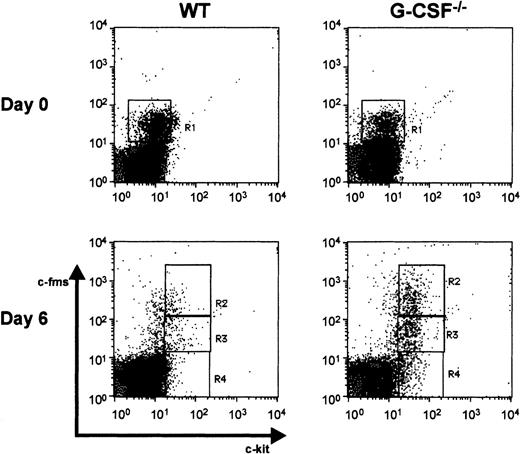
Because morphologic examination of peripheral blood smears 7 days after candida infection revealed the presence of an increased proportion of immature myeloid cells compared to baseline, we also stained WBCs with antibodies against c-fms and c-kit. The expression of these 2 markers is regulated during the differentiation and maturation of hematopoietic cells.14,15 It has been shown previously that both c-kit+c-fms− and c-kit+c-fms+ cells have colony-forming capacity in soft agar assay and represent progenitor cells.15However, only a small proportion of c-kit−c-fms+ cells has colony-forming capacity and most of these cells are lineage-committed myeloid cells. At baseline, in wild-type and growth factor-deficient mice there were primarily 2 classes of cells detected in circulation on the basis of c-kit and c-fms staining—most cells were c-kit−c-fms− representing lymphocytes and neutrophils; only a small proportion of cells were c-kit−c-fms+ (region R1), representing monocytic cells (Figure 7). However, on day 6 after candida infection, 3 populations of cells were identified based on their c-kit/c-fms staining pattern: c-kit−c-fms− (region R4), c-kit+c-fmslo (region R3), and c-kit+c-fmshi (region R2) (Figure 7). On the basis of the forward and side scatter profile it appeared that the c-kit+c-fmslo and c-kit+c-fmshi populations represented early granulocytes and monocytes, respectively. The frequency of c-kit+ c-fmslo cells in circulation was higher in the growth factor-deficient mice compared to wild-type mice following candida infection(Table 5). Moreover, in growth factor-deficient mice, c-kit expression was higher than that in wild-type mice suggesting that in wild-type mice these cells are more differentiated (data not shown).
Flow cytometric analysis of cell surface markers on wild-type and G-CSF–deficient mice.
Dot blots of peripheral blood leukocytes depicting c-fms and c-kit staining pattern at baseline (top panel) and 6 days after candida infection (bottom panel). Region R1 represents cells that are c-kit−c-fms+. On day 6 after infection, c-kit+ cells were seen in circulation, represented in regions R2 (c-kit+ c-fmshi), R3 (c-kit+c-fmslo), and R4 (c-kit+c-fms−).
Flow cytometric analysis of cell surface markers on wild-type and G-CSF–deficient mice.
Dot blots of peripheral blood leukocytes depicting c-fms and c-kit staining pattern at baseline (top panel) and 6 days after candida infection (bottom panel). Region R1 represents cells that are c-kit−c-fms+. On day 6 after infection, c-kit+ cells were seen in circulation, represented in regions R2 (c-kit+ c-fmshi), R3 (c-kit+c-fmslo), and R4 (c-kit+c-fms−).
Presence of c-kit+c-fms+cells in peripheral blood after candida infection
| Genotype . | Leukocytes (109/L) . | c-kit+c-fmslo cells (%) . |
|---|---|---|
| Wild-type | 9.2 ± 2.7 | 3.8 ± 1.8 |
| G-CSF−/− | 7.7 ± 1.8 | 12.1 ± 4.15-150 |
| G-CSF−/−/GM-CSF−/− | 6.8 ± 1.4 | 11.4 ± 1.15-150 |
| G-CSF−/−/IL-6−/− | 6.0 ± 0.7 | 10.3 ± 3.25-150 |
| Genotype . | Leukocytes (109/L) . | c-kit+c-fmslo cells (%) . |
|---|---|---|
| Wild-type | 9.2 ± 2.7 | 3.8 ± 1.8 |
| G-CSF−/− | 7.7 ± 1.8 | 12.1 ± 4.15-150 |
| G-CSF−/−/GM-CSF−/− | 6.8 ± 1.4 | 11.4 ± 1.15-150 |
| G-CSF−/−/IL-6−/− | 6.0 ± 0.7 | 10.3 ± 3.25-150 |
Presence of progenitor cells in circulation was studied by staining WBC with antibodies against c-fms and c-kit. Cells were analyzed on FACScan using CellQuest software and total WBC count was determined by Sysmex K-1000.
Data are represented as mean ± SD. Ten to 12 mice of each genotype were analyzed.
P < .05 compared to wild-type mice.
Increased numbers of colony-forming cells in the bone marrow of candida- infected mice
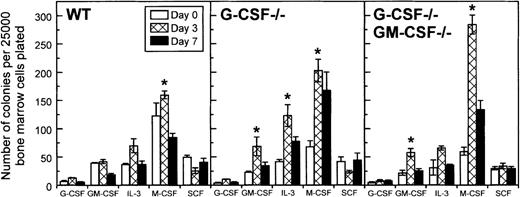
Using a soft agar assay, we examined whether increased myelopoiesis in the bone marrow of candida-infected G-CSF–deficient mice was associated with an increase in the frequency of myeloid progenitor cells. On day 1 after infection, there was a decrease in the frequency of myeloid progenitor cells in G-CSF–deficient mice (data not shown). However, on day 3 after infection, a significant increase in the frequency of myeloid progenitors, GM colony-forming cells (CFCs) and M-CFCs, in particular, was observed in G-CSF–deficient mice (Figure 8). Except for M-CFCs, which remained elevated, all other types of myeloid progenitors returned to basal values by day 7 after infection. A similar trend was noted in mice simultaneously deficient in G-CSF and GM-CSF (Figure 8). In wild-type mice the changes in the frequency of CFCs were less dramatic; only a slight increase in M-CSF progenitor cells was observed on day 3 after infection, and the frequency of CFCs, in general, were similar to basal values on day 7 after infection.
Changes in frequency of colony-forming cells in the bone marrow of wild-type, G-CSF−/− -deficient, and G-CSF−/−/GM-CSF−/− mice following candida infection.
Data are represented as mean ± SD. Data at each time point are from 9 mice per genotype. *P < .05 comparison between mice of same genotype at baseline and on the study day indicated.
Changes in frequency of colony-forming cells in the bone marrow of wild-type, G-CSF−/− -deficient, and G-CSF−/−/GM-CSF−/− mice following candida infection.
Data are represented as mean ± SD. Data at each time point are from 9 mice per genotype. *P < .05 comparison between mice of same genotype at baseline and on the study day indicated.
Candida load in kidneys of infected mice
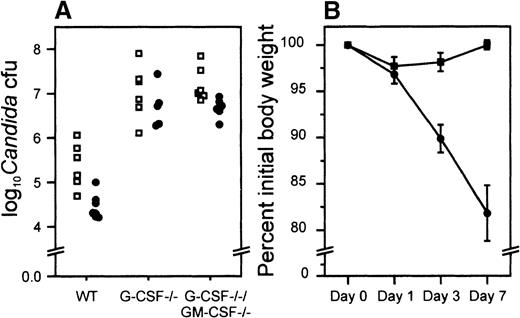
The initial clearance of candida from blood appeared to be similar in both G-CSF–deficient and wild-type mice and at 24 hours after infection no candida could be detected in mice of either genotype (data not shown). Because kidneys are the most representative organs for the determination of in vivo growth of candida16 and candida load in kidney underscores the severity of disseminated candidiasis,16 we determined the load of candida in the kidneys of infected mice. At 24 hours after infection the load of candida in kidneys of wild-type, G-CSF–deficient, and G-CSF/GM-CSF double-deficient mice was similar (data not shown). By day 3, the candida load had increased, and the numbers of viable organisms in wild-type and G-CSF–deficient mice was in the same range. However, by day 7, unlike wild-type mice where the candida load in kidney had declined and the infection appeared to be resolving, in G-CSF–deficient and G-CSF/GM-CSF-deficient mice the load of candida was greater than that observed on day 3 after infection (Figure9A). This trend was confirmed on microscopic inspection of kidney sections of infected mice stained with hematoxylin-eosin. Whereas in infected wild-type mice the acute inflammation appeared to be subsiding, candida-infected G-CSF–deficient mice showed signs of acute inflammation. Both suppurative and nonsuppurative granulomas associated with yeast and necrosis were observed in the knock-out mice. Analysis of kidney sections stained with Gomori methenamine stain further revealed that whereas only a few candida (mostly yeast forms) could be seen in the kidney of wild-type mice, both the mycelial and yeast forms of candida were seen more commonly in G-CSF–deficient mice (Figure10). G-CSF–deficient mice also appeared sick and had a greater loss of body weight than wild-type mice following infection (Figure 9B). Large numbers of G-CSF-deficient mice had to be killed during the course of infection due to acute illness.
Experimental C albicans infection of wild-type and G-CSF–deficient mice.
(A) Candida load per kidney (expressed as logarithm of the yeast count) in wild type (□) and G-CSF–deficient mice (•), 3 and 7 days after experimental infection with C albicans. (B) Body weight as a percent of pretreatment values after candida challenge. C albicans (2.5 × 105 CFUs ) was injected through lateral tail vein of wild-type (▪) and G-CSF–deficient mice (•). Body weights were recorded before and at the time points shown after challenge. Ten mice of each type were studied. Data are represented as mean ± SEM. *P < .05 for comparison of G-CSF–deficient and wild-type mice.
Experimental C albicans infection of wild-type and G-CSF–deficient mice.
(A) Candida load per kidney (expressed as logarithm of the yeast count) in wild type (□) and G-CSF–deficient mice (•), 3 and 7 days after experimental infection with C albicans. (B) Body weight as a percent of pretreatment values after candida challenge. C albicans (2.5 × 105 CFUs ) was injected through lateral tail vein of wild-type (▪) and G-CSF–deficient mice (•). Body weights were recorded before and at the time points shown after challenge. Ten mice of each type were studied. Data are represented as mean ± SEM. *P < .05 for comparison of G-CSF–deficient and wild-type mice.
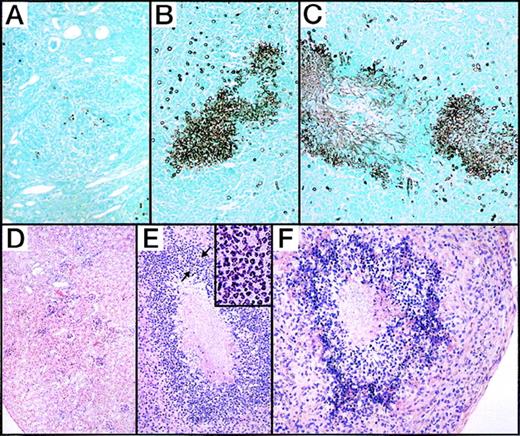
Tissue sections were prepared from kidneys of wild-type, G-CSF−/−, and G-CSF−/−/GM-CSF−/− mice 7 days after infection with C albicans.
Sections were stained with Gomori methenamine silver stain (top panel) and haematoxylin-eosin stain (bottom panel). (A, D) Wild-type mice show healing inflammatory lesions in the cortex and few yeast cells. (B-C, E-F) G-CSF–deficient and G-CSF/GM-CSF-deficient mice, respectively, show numerous abscesses throughout the section with large aggregates of fungal yeasts within abscesses. Inset shows polymorphonuclear neutrophils in an area of dense leukocytic infiltration surrounding fungal yeasts (marked by arrows).
Tissue sections were prepared from kidneys of wild-type, G-CSF−/−, and G-CSF−/−/GM-CSF−/− mice 7 days after infection with C albicans.
Sections were stained with Gomori methenamine silver stain (top panel) and haematoxylin-eosin stain (bottom panel). (A, D) Wild-type mice show healing inflammatory lesions in the cortex and few yeast cells. (B-C, E-F) G-CSF–deficient and G-CSF/GM-CSF-deficient mice, respectively, show numerous abscesses throughout the section with large aggregates of fungal yeasts within abscesses. Inset shows polymorphonuclear neutrophils in an area of dense leukocytic infiltration surrounding fungal yeasts (marked by arrows).
Because G-CSF is also known to modulate neutrophil function, we next investigated whether the neutrophils from G-CSF–deficient mice were functionally impaired in their capacity to control candida infection. We compared the thioglycollate-elicited peritoneal neutrophils from wild-type and G-CSF–deficient mice for phagocytic and candidacidal activity. As shown in Table 6, the phagocytic activity of the neutrophils from both G-CSF–deficient and wild-type mice was comparable. Although the candidacidal activity of neutrophils from G-CSF–deficient mice appeared to be somewhat lower than that from wild-type mice, statistical significance was not attained.
Phagocytic and candidacidal activity of neutrophils
| Genotype . | Phagocytic activity6-150 . | Candidacidal activity6-151 . |
|---|---|---|
| Wild type | 69 ± 9 | 37 ± 3 |
| G-CSF−/− | 67 ± 8 | 30 ± 4 |
| Genotype . | Phagocytic activity6-150 . | Candidacidal activity6-151 . |
|---|---|---|
| Wild type | 69 ± 9 | 37 ± 3 |
| G-CSF−/− | 67 ± 8 | 30 ± 4 |
Mice were injected with thioglycollate and after 4 h peritoneal cells were harvested. Peritoneal cells from mice of same genotype were pooled for neutrophils purification by Percoll gradient. Neutrophils were aspirated out from the interphase of 63% and 72% of Percoll gradient.
2.5 × 106 neutrophils were added to 1 × 106 heat-killed candida and incubated for 15 min at 37°C. Trypan blue (1 mL) was added to each mixture and wet mount was prepared. At least 500 yeasts (phagocytosed and extracellular) were scored on the basis of trypan blue intake.
1.5 × 106 neutrophils were preincubated with 25% mouse serum at 37°C for 5 min and then 1.0 × 107 candida was added to the cells. The tubes were rotated for 60 min at 37°C. Thereafter methoxycholate was added to stop the reaction followed by methylene blue. At least 300 yeast cells in wet preps were counted under microscope distinguishing the viable (unstained) versus dead (stained) cells.
Data are presented as mean ± SD of 3 experiments.
Levels of IL-10 and TNF- in circulation
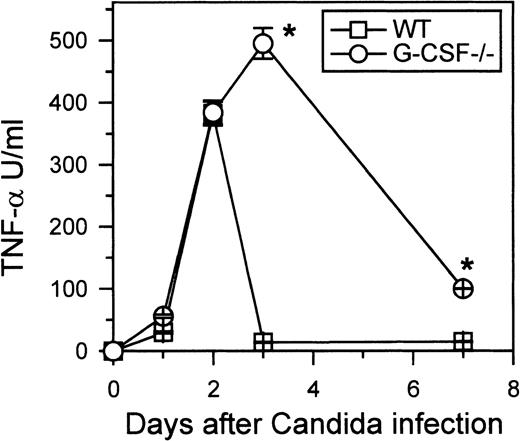
Despite developing neutrophilia, G-CSF–deficient mice had a greater load of candida in their kidneys and had higher morbidity than similarly infected control mice. Earlier reports have suggested that susceptibility of mice to candida can be due to increased production of IL-10, which in turn can suppress macrophage function.17 On the other hand, TNF-α is thought to be beneficial during candida infection.18 Indeed, TNF-deficient mice are more susceptible than wild-type mice to candida infection.19 To investigate whether the increased susceptibility of G-CSF–deficient mice to candida was due to an altered pattern of production of IL-10, TNF-α, or both, we measured the circulating levels of IL-10 and TNF-α in candida-infected G-CSF–deficient and wild-type mice. Low levels of IL-10 were detected in the sera of the infected mice of both genotypes (data not shown). However, levels of TNF-α were significantly elevated in the sera of candida-infected wild-type and G-CSF–deficient mice compared to basal levels (Figure 11). Until 48 hours, the levels of TNF-α in G-CSF–deficient and wild-type mice were similar. At 72 hours after candida infection, TNF-α was undetectable in circulation in wild-type mice; in G-CSF–deficient mice the level of TNF-α remained elevated and was still detectable 7 days after candida infection.
Kinetics of TNF- production.
Wild-type and G-CSF–deficient mice were challenged with 2.5 × 105C albicans intravenously. At the indicated times, 6 mice of each genotype were killed. Serum samples from each mouse were tested for TNF-α activity as described in “Materials and methods.” Data are represented as mean ± SD. One of 2 separate experiments with similar results is shown.
Kinetics of TNF- production.
Wild-type and G-CSF–deficient mice were challenged with 2.5 × 105C albicans intravenously. At the indicated times, 6 mice of each genotype were killed. Serum samples from each mouse were tested for TNF-α activity as described in “Materials and methods.” Data are represented as mean ± SD. One of 2 separate experiments with similar results is shown.
Discussion
The generation of mice lacking growth factors and cytokines has provided the means of definitively evaluating their normal physiologic role. Furthermore, experimental infection of factor-deficient mice with various pathogens provides the means of gauging the role played by these factors/cytokines in the host's response to infection.
Until recently, granulopoiesis, both at steady state and during emergency, has been believed to be regulated primarily by G-CSF. Studies with G-CSF–deficient and G-CSF receptor–deficient mice have led to the conclusion that although G-CSF plays an important role in steady-state granulopoiesis, there exist G-CSF-independent pathways for generation of granulocytes at steady state.4,5 Although historically G-CSF has been thought to play a critical role in emergency granulopoiesis, this remains largely inferred.6,20 An initial attempt to gauge the effect of G-CSF–deficiency on the host's capacity to mount an emergency granulopoietic response was evaluated by infecting G-CSF–deficient mice with Listeria monocytogenes.4 Compared to similarly infected control mice, G-CSF-deficient mice mounted only a modest neutrophilia; the load of organisms in spleen and liver was higher in infected G-CSF-deficient mice.4 This observation indicated that G-CSF is indispensable for mounting emergency granulopoietic response to infection with L monocytogenes in vivo. However, it is known that macrophages are the predominant cell type involved in host's response to infection with L monocytogenes.21,22 Earlier studies have demonstrated that neutrophils represent the first and most important line of host defense against C albicans.16,23,24 Moreover, neutrophilia has been observed in both humans and mice following candida infection.25 Because different pathogens evoke different cellular responses, we chose C albicans as a model pathogen to study emergency granulopoiesis and to investigate the role of G-CSF in this process.
Our observation that candida-infected G-CSF–deficient mice mount a profound and sustained neutrophilia in G-CSF–deficient mice following candida infection is intriguing. The observation indicates that in vivo factors other than G-CSF can promote neutrophil production during an infection. In addition to peripheral blood neutrophilia there was an increase in both early and late forms of neutrophils in the bone marrow of candida-infected G-CSF–deficient mice. The increase was, however, more marked in neutrophil precursors (promyelocyte and myelocyte) compared to late forms of neutrophils (metamyelocyte and bands). This may be due to increased trafficking of mature neutrophils from bone marrow to sites of infection in the tissues, due to prolonged candida infection in G-CSF–deficient mice. Alternatively, there are fewer cell divisions or a greater proportion of cells die between the early to late transition in neutrophil development. Because the candida-induced neutrophilia in G-CSF–deficient mice is sustained and is also accompanied by an increase in both precursor and mature neutrophils in bone marrow, this clearly indicates that this phenomenon is not merely due to mobilization of neutrophil “reservoir pool,” but involves neutrophil production per se. Furthermore, our observation that the increase in neutrophils in peripheral blood of candida-infected growth factor-deficient mice lagged behind the maximum increase of marrow CFUs seen on day 3, suggests that the increased mature elements observed in the periphery originated from marrow progenitors. Candida-infected G-CSF–deficient mice also develop monocytosis, which is more pronounced than that seen in wild-type mice. The presence of a higher proportion of immature neutrophils along with monocytosis in the peripheral blood of candida-infected G-CSF–deficient mice raises the possibility that less committed progenitor cells (at least bipotential progenitors) are undergoing amplification to generate neutrophils and monocytes in candida-infected G-CSF–deficient mice. Our study has shown the presence of cells coexpressing c-kit and c-fms in blood of candida-infected mice. These cells have been previously shown to represent myeloid progenitors with colony-forming capacity.15 Although we have not analyzed blood for the presence of progenitors, the presence of c-kit+c-fms+ cells in the blood suggests that myeloid progenitors are mobilized cells into the periphery after candida infection. There has been no report published so far regarding mobilization of hematopoietic progenitor cells in G-CSF–deficient mice; this phenomenon has, however, been found to be altered in G-CSF receptor-deficient mice.26 In G-CSF receptor–deficient mice, mobilization of hematopoietic progenitor cells in response to cyclophosphamide and IL-8 is impaired; however, Flt-3 ligand–mediated mobilization is unaffected.26 In addition to its capacity for mobilization, Flt-3 ligand has the capacity to expand early progenitor cells. It is therefore possible that the increased myelopoiesis in the growth factor(s)-deficient mice in response to candida infection is mediated by Flt-3 ligand. However, because Flt-3 ligand could not be detected in circulation in candida-infected wild-type or growth factor(s)-deficient mice, it is unlikely that this effect is being mediated by Flt-3 ligand (data not shown).
On the basis of the data presented here we conclude that both GM-CSF and IL-6 are dispensable for the observed neutrophilia. Clearly there exists in vivo a factor(s), other than G-CSF, GM-CSF, and IL-6, that can promote granulopoiesis. A similar conclusion was drawn by Zhang et al27 in their study on C/EBPα knockout mice. These mice do not express G-CSF and IL-6 receptors and have a complete blockade in granulocytic differentiation. However, in this study, the expression of these receptors on fetal liver hematopoietic cells by retroviral transduction resulted in only partial restoration of granulopoiesis in vitro. In the study the authors raised the possibility of existence of the other C/EBPα target genes, possibly cytokine receptors, that are also important for the block of granulocyte differentiation in C/EBPα-deficient mice.27
Despite developing neutrophilia, G-CSF–deficient mice were found to be more susceptible to C albicans infection than wild-type mice. In response to candida infection, the loss in body weight was greater in G-CSF–deficient mice than wild-type mice. Candida-infected G-CSF–deficient mice also had a greater load of candida in their kidneys and showed more extensive tissue damage. However, this was unlikely to be due to impaired neutrophil function because phagocytic and candidacidal activity of neutrophils from G-CSF–deficient mice was comparable to that of neutrophils from wild-type mice. The prolonged infection may be due to the basal neutropenic state of G-CSF deficiency rather than some functional deficiency of neutrophils in the absence of G-CSF.
Both TNF-α and IL-6 levels were elevated in candida-infected G-CSF–deficient mice as compared to similarly infected wild-type mice. These cytokines seem to have a dual role during infection with pathogens. Although the absence of TNF-α and IL-6 has been shown to aggravate candida infection in mice,19,28 high levels of these cytokines correlate with the fatal outcome following septicemia.29 30 Elevated levels of TNF-α and IL-6, coupled with a higher candida load in the kidney, are probably the key factors responsible for greater loss in body weight and higher mortality in candida-infected G-CSF–deficient mice than similarly infected wild-type mice.
Taken together these results clearly demonstrate that emergency granulopoiesis, particularly in response to candida infection, can occur in the absence of G-CSF. Furthermore, GM-CSF and IL-6 are dispensable for candida-driven neutrophilia in G-CSF–deficient mice. Our current efforts are focused on identifying factor(s) that account for residual neutrophils in G-CSF–deficient mice as well as those that underlie the G-CSF–independent neutrophilia after infection with C albicans.
Acknowledgments
We are thankful to T. Helman, B. Morrow, and M. Pitt for assistance in the animal house; J. Strickland for assistance with the artwork; and Prof A. W. Burgess for critically reviewing the manuscript.
Reprints:Sunanda Basu, Ludwig Institute for Cancer Research, PO Royal Melbourne Hospital, Victoria 3050, Australia; e-mail: sunanda.basu@ludwig.edu.au.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal