Abstract
Peripheral blood cells are increasingly used in place of bone marrow as a source of hematopoietic stem cells for allogeneic transplantation. The relative efficacy of these 2 approaches is unknown. This retrospective multivariate analysis compared results of 288 HLA-identical sibling blood stem cell transplantations with results of 536 HLA-identical sibling bone marrow transplantations. No transplants were T-cell depleted. Median follow-up was 12 months, and analyses focused on 1-year outcomes. Recipients of blood stem cell transplants had more rapid recovery of neutrophils to at least 0.5 × 109/L (median time to recovery, 14 days, compared with 19 days for marrow transplants;P < .001) and of platelets to at least 20 × 109/L (median time, 18 days, compared with 25 days for marrow transplants; P < .001). There was no significant difference in the incidence of grades II to IV acute graft versus host disease (GVHD). The incidence of chronic GVHD was significantly higher after blood stem cell transplantation (1-year probability [95% confidence interval], 65% [56%-72%] compared with 53% [47%-59%]; P = .02) Relapse incidence in the 2 transplant groups did not differ significantly. Treatment-related mortality rates were lower and leukemia-free survival rates were higher with blood stem cell transplants in patients with advanced leukemia (acute leukemia in second remission or chronic myelogenous leukemia in accelerated phase) but not in early leukemia (acute leukemia in first remission or chronic myelogenous leukemia in chronic phase). The median time from transplantation to hospital discharge was 23 days after blood stem cell transplantation and 28 days after bone marrow transplantation (P = .003). Further study with longer follow-up is necessary to definitively establish the role of blood stem cells for allogeneic transplantation, especially in patients with good-risk disease.
Bone marrow transplantation is an accepted treatment for many patients with malignant and nonmalignant diseases. Since the early 1990s, peripheral blood progenitor cells collected by apheresis have largely replaced bone marrow as a source of hematopoietic stem cells for autologous transplantation.1Peripheral blood cells produce more rapid hematopoietic recovery, thereby leading to reduced costs.2-5 For allogeneic transplantation, blood stem cells collected from healthy donors given granulocyte colony-stimulating factor are now often used as an alternative to bone marrow.6-15 The mobilization, collection, and safety of allogeneic blood stem cell donors has been reviewed.16 Preliminary results of allogeneic blood stem cell transplantation in many single centers and a small randomized trial indicate more rapid hematopoietic recovery with this method than with allogeneic bone marrow transplantation.6-15 17
Collections of blood stem cells contain approximately 1 log more lymphocytes than bone marrow harvests.10,18 This could possibly affect immune reconstitution, graft versus host disease (GVHD), and graft versus leukemia effects. In most studies, the incidence of acute GVHD with blood stem cell transplants is similar to that reported for bone marrow transplants. Some studies do not indicate a difference in chronic GVHD,14,19 but others suggest a substantially higher incidence of chronic GVHD with blood stem cell transplantation.20,21 Use of allogeneic blood stem cells reduced early morbidity and mortality in comparison with results of bone marrow transplantation in some22 but not all17 studies. However, all these studies were small, with fewer than 100 patients, and had relatively short follow-up periods. It is still unclear whether blood stem cells are as good as (or better than) bone marrow as a source of hematopoietic stem cells for allogeneic transplantation. We report a nonrandomized comparison of 288 HLA-identical sibling blood stem cell transplantations and 536 HLA-identical sibling bone marrow transplantations for leukemia.
Patients and methods
Patients
The study includes data for 288 patients 20 years of age or older who received non–T-cell depleted, HLA-identical sibling blood stem cell transplants for acute myelogenous leukemia (AML) or acute lymphoblastic leukemia (ALL) in first or second remission or chronic myelogenous leukemia (CML) in chronic or accelerated phase in 1995 or 1996 and whose transplantation was reported to the International Bone Marrow Transplant Registry (IBMTR) or the European Group for Blood and Marrow Transplantation (EBMT) (Table 1). Controls were 536 patients 20 years of age or older who received non–T-cell depleted, HLA-identical sibling bone marrow transplants for the same indications during the same period and whose transplantations were reported to the IBMTR. Data were reported by 105 transplant centers in 34 countries and include more than half of the allogeneic blood stem cell transplantations for leukemia done in 1995 and 1996. Cases were reviewed to ensure that patients whose procedure was reported to both organizations were not entered twice. Outcome data were compiled from the date of transplantation through the date of death or last contact. Median follow-up was 12 months (range, 3 to 40 months). Ninety percent of patients were followed for 8 months; 25% were followed for 23 months.
Patient characteristics according to transplant type
| Variable . | Blood stem cells transplant . | Bone marrow transplant . | P value . |
|---|---|---|---|
| Total no. of patients | [288] | [536] | .24 |
| Median age (range), y | 39 (20-61) [288] | 37 (20-63) [536] | .06 |
| Male sex | 159 (55) [288] | 288 (54) [536] | .68 |
| Karnofsky score < 90% | 50 (19) [264] | 112 (21) [534] | .50 |
| Disease, stage | [288] | [536] | .08 |
| AML, 1st CR | 95 (33) | 155 (29) | |
| AML, 2nd CR | 26 (9) | 29 (5) | |
| ALL, 1st CR | 25 (9) | 56 (10) | |
| ALL, 2nd CR | 13 (4) | 18 (4) | |
| CML, 1st CP | 101 (35) | 245 (46) | |
| CML, 2nd CP/AP | 28 (10) | 33 (6) | |
| Prior busulfan for CML | 12 (9) [129] | 27 (10) [277] | .42 |
| Prior interferon for CML | 65 (50) [129] | 131 (47) [277] | .16 |
| TBI for conditioning | 178 (62) [288] | 224 (42) [536] | < .0001 |
| Conditioning regimen | [288] | [536] | < .0001 |
| Cy + TBI ± other | 154 (53) | 162 (30) | |
| Busulfan + Cy ± other | 106 (37) | 289 (54) | |
| TBI ± other | 24 (8) | 56 (10) | |
| Cy ± other | — | 7 (2) | |
| Other | 4 (2) | 22 (4) | |
| G-CSF or GM-CSF in 1st 7 d after transplantation | 97 (37) [264] | 112 (21) [531] | < .001 |
| GVHD prophylaxis | [287] | [535] | < .001 |
| CsA ± other | 94 (33) | 56 (10) | |
| CsA + MTX ± corticosteroids | 174 (61) | 472 (88) | |
| FK506 ± other | 13 (4) | 1 (< 1) | |
| Other | 6 (2) | 6 (1) | |
| Region | [288] | [536] | < .001 |
| North America | 105 (37) | 165 (31) | |
| Europe | 152 (53) | 226 (42) | |
| Asia | 21 (7) | 59 (11) | |
| Australia/New Zealand | 7 (2) | 27 (5) | |
| Other | 2 (1) | 60 (11) | |
| Median follow-up, mo | 12 (3-39) [288] | 12 (3-40) [536] | .72 |
| Variable . | Blood stem cells transplant . | Bone marrow transplant . | P value . |
|---|---|---|---|
| Total no. of patients | [288] | [536] | .24 |
| Median age (range), y | 39 (20-61) [288] | 37 (20-63) [536] | .06 |
| Male sex | 159 (55) [288] | 288 (54) [536] | .68 |
| Karnofsky score < 90% | 50 (19) [264] | 112 (21) [534] | .50 |
| Disease, stage | [288] | [536] | .08 |
| AML, 1st CR | 95 (33) | 155 (29) | |
| AML, 2nd CR | 26 (9) | 29 (5) | |
| ALL, 1st CR | 25 (9) | 56 (10) | |
| ALL, 2nd CR | 13 (4) | 18 (4) | |
| CML, 1st CP | 101 (35) | 245 (46) | |
| CML, 2nd CP/AP | 28 (10) | 33 (6) | |
| Prior busulfan for CML | 12 (9) [129] | 27 (10) [277] | .42 |
| Prior interferon for CML | 65 (50) [129] | 131 (47) [277] | .16 |
| TBI for conditioning | 178 (62) [288] | 224 (42) [536] | < .0001 |
| Conditioning regimen | [288] | [536] | < .0001 |
| Cy + TBI ± other | 154 (53) | 162 (30) | |
| Busulfan + Cy ± other | 106 (37) | 289 (54) | |
| TBI ± other | 24 (8) | 56 (10) | |
| Cy ± other | — | 7 (2) | |
| Other | 4 (2) | 22 (4) | |
| G-CSF or GM-CSF in 1st 7 d after transplantation | 97 (37) [264] | 112 (21) [531] | < .001 |
| GVHD prophylaxis | [287] | [535] | < .001 |
| CsA ± other | 94 (33) | 56 (10) | |
| CsA + MTX ± corticosteroids | 174 (61) | 472 (88) | |
| FK506 ± other | 13 (4) | 1 (< 1) | |
| Other | 6 (2) | 6 (1) | |
| Region | [288] | [536] | < .001 |
| North America | 105 (37) | 165 (31) | |
| Europe | 152 (53) | 226 (42) | |
| Asia | 21 (7) | 59 (11) | |
| Australia/New Zealand | 7 (2) | 27 (5) | |
| Other | 2 (1) | 60 (11) | |
| Median follow-up, mo | 12 (3-39) [288] | 12 (3-40) [536] | .72 |
Values in square brackets are the numbers of patients evaluated in each major category, values in parentheses are percentages of patients unless otherwise indicated, and all other values are numbers of patients unless otherwise indicated.
AML indicates acute myelogenous leukemia; CR, complete remission; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; CP, chronic phase; AP, accelerated phase; TBI, total-body irradiation; Cy, cyclophosphamide; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; GVHD, graft versus host disease; CsA, cyclosporine; and MTX, methotrexate.
IBMTR
The IBMTR is a voluntary working group of more than 350 transplant teams worldwide that contribute detailed data on their allogeneic transplantations to the Statistical Center at the Medical College of Wisconsin. Participants are required to register all consecutive transplantations. The IBMTR database includes information on 40% to 45% of all patients who have undergone allogeneic transplantation since 1970. Patients are followed longitudinally. Computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality.
EBMT
The EBMT is a voluntary group of 436 transplant centers in Europe and associated members from non-European countries. Data quality of participating centers is checked by the Statistical Office of EBMT at the University College of London Hospitals and regular site visits to centers selected on a random basis. For this analysis, centers that had reported allogeneic peripheral blood stem cell transplantations in 1995 and 199623 24 were contacted and asked to provide data on all consecutive transplantations using unmanipulated allogeneic peripheral blood stem cells from HLA-identical sibling donors performed between January 1995 and June 1996. IBMTR Report Forms or EBMT Med-A/Med-B forms, with specific addenda to ensure that identical core data were reported, were used for this study.
Endpoints
The study focused on hematopoietic recovery, acute and chronic GVHD, treatment-related mortality (TRM), leukemia-free survival (LFS), and leukemia relapse after blood stem cell transplantation compared with bone marrow transplantation.25 The primary measure of hematopoietic recovery was the time after transplantation until a neutrophil count of at least 0.5 × 109/L was observed for 3 consecutive days. Also recorded were the times until a platelet count of at least 20 × 109/L and a platelet count of at least 50 × 109/L were achieved. The incidence and time to development of grades II to IV acute GVHD and grades III to IV acute GVHD were evaluated in patients surviving 21 days with evidence of engraftment.26 Time to occurrence of any chronic GVHD was evaluated in patients surviving 90 days or longer after transplantation with engraftment.27TRM was defined as death in continuous complete remission; patients were censored at relapse or, for patients in continuous complete remission, at last follow-up.28 LFS was defined as survival in continuous complete remission; relapse and death in remission were events, and patients surviving in continuous complete remission were censored at last contact. Treatment failure was the inverse of LFS. Relapse was defined as hematologic or clinical leukemia recurrence; patients never in remission after transplantation were considered to have had a recurrence on day 1.
Statistical methods
The association of graft type (blood stem cell compared with bone marrow) and other patient, disease, and transplant characteristics (Table 2) with each outcome was evaluated by using separate Cox proportional hazard regression models. Continuous variables were discretized using the cut point that minimized the −2-log likelihood of the 1-factor Cox proportional hazards regression model.29 Variables were tested by using a time-varying covariate method to determine whether the proportional hazards assumption was met. Adjustments for factors found to have nonproportional hazards used stratified proportional hazards models or time-dependent covariates. Interactions between each variable of interest and graft type were examined by fitting a proportional hazards model, stratified on transplant type, and examining the interaction term between the factor of interest and the type of transplant. Multivariate models were built by using a stepwise forward selection with a significance level of 0.05. Graft type was held in the model at each step. All multivariate models were examined for center effects by using a random effects or frailty model;30 there was no evidence of confounding of main effects by center effects. All analyses were done with PROC PHREG in SAS version 6.12 (SAS Institute Inc, Cary, NC).
Variables tested in Cox proportional hazards regression models
| Tested in both acute leukemia and CML models |
| Graft type: blood stem cells versus bone marrow |
| Age at transplantation: <40 y versus ≥40 y |
| Sex: male versus female |
| Karnofsky performance status: <90% versus 90%-100% |
| Disease stage at transplantation: second CR, second CP or AP versus first CR or CP |
| White blood cell count at diagnosis: high versus low* |
| Conditioning regimen: TBI versus non-TBI |
| GVHD prophylaxis: CsA ± other versus CsA + MTX ± other versus other |
| Growth factors given in first 7 d after transplantation: yes versus no |
| Tested in acute leukemia models only |
| Disease type: AML versus ALL |
| FAB classification: FAB M4 M5 versus other AML versus T-cell ALL versus other ALL |
| Tested in CML models only |
| Interval from diagnosis to transplantation: ≥1 y versus <1 y |
| Tested in both acute leukemia and CML models |
| Graft type: blood stem cells versus bone marrow |
| Age at transplantation: <40 y versus ≥40 y |
| Sex: male versus female |
| Karnofsky performance status: <90% versus 90%-100% |
| Disease stage at transplantation: second CR, second CP or AP versus first CR or CP |
| White blood cell count at diagnosis: high versus low* |
| Conditioning regimen: TBI versus non-TBI |
| GVHD prophylaxis: CsA ± other versus CsA + MTX ± other versus other |
| Growth factors given in first 7 d after transplantation: yes versus no |
| Tested in acute leukemia models only |
| Disease type: AML versus ALL |
| FAB classification: FAB M4 M5 versus other AML versus T-cell ALL versus other ALL |
| Tested in CML models only |
| Interval from diagnosis to transplantation: ≥1 y versus <1 y |
CML denotes chronic myelogenous leukemia; CR, complete remission; CP, chronic phase; AP, accelerated phase; TBI, total-body irradiation; GVHD, graft-versus-host disease; CsA, cyclosporine; MTX, methotrexate; AML, acute myelogenous leukemia; ALL, acute lymphocytic leukemia; and FAB, French-American-British.
High was above 75 × 109/L white blood cells for patients with acute leukemia and above 200 × 109/L white blood cells for patients with CML.
Univariate (unadjusted) probabilities of neutrophil and platelet recovery were calculated with the Kaplan-Meier estimator. Cumulative incidences were estimated for TRM and relapse. Ninety-five percent confidence intervals (CIs) were calculated using the SE of the survivor function by Greenwood formula. Adjusted probabilities for neutrophil and platelet recovery, acute and chronic GVHD, and LFS were calculated with the multivariate Cox models described previously, stratified on graft type, and weighted by the sample proportion value for each prognostic factor. The 95% CI for adjusted survival probabilities andP values of pair-wise comparisons were derived from point-wise estimates and calculated with standard techniques.31 These adjusted probabilities estimate likelihood of outcomes in populations with similar prognostic factors.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Two hundred eighty-eight patients received blood stem cell transplants and 536 received bone marrow transplants. Three hundred five patients (37%) had AML, 112 (14%) had ALL, and 407 (49%) had CML. Six hundred seventy-seven patients (82%) underwent transplantation while in first complete remission or first chronic phase, and the remaining 147 (18%) were in second complete remission or accelerated phase. Patient characteristics were relatively balanced between the blood stem cell and bone marrow transplant groups. There were significant differences in the following variables: the blood stem cell group had fewer CML patients in chronic phase; more patients with high leukocyte counts at diagnosis; a higher proportion of patients who received total-body irradiation for pretransplantation conditioning, hematopoietic growth factors after transplantation, or both; and a lower proportion of patients who received methotrexate for GVHD prophylaxis. A higher proportion of blood stem cell transplantations were performed in Europe. The distributions of leukemia subtypes and cytogenetic abnormalities were similar in the 2 groups.
Hematologic recovery
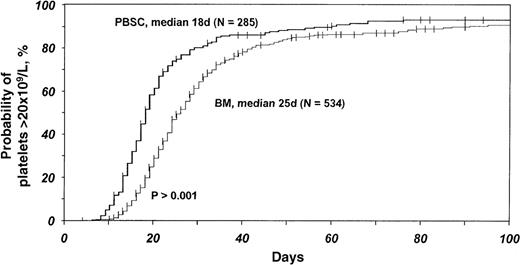
Patients who received blood stem cell transplants had significantly faster recovery of neutrophils and platelets (Table3). This difference was independent of growth factor and methotrexate use. Additionally, there was less variability in recovery times with blood stem cell transplantation (ie, fewer outliers with slow hematologic recovery) (Figures 1 and2). The median time to a neutrophil count of at least 0.5 × 109/L was 14 days (range, 10 to 40 days) with blood stem cells and 19 days (range, 11 to 35 days) with bone marrow (P < .001). The median time to a platelet count of at least 20 × 109/L was 18 days (range, 13 to 68 days) with blood stem cells and 25 days (range, 12 to 87 days) with bone marrow (P < .001). The median time to a platelet count of at least 50 × 109/L was 19 days (range, 11 to 70 days) with blood stem cells and 28 days (range, 20 to 79 days) with bone marrow (P < .001).
Results of multivariate analyses comparing hematopoietic recovery and graft versus host disease after HLA-identical sibling blood stem cell transplantation and bone marrow transplantation
| Outcome . | Relative risk (95% CI) . | P value . |
|---|---|---|
| Absolute neutrophil count ≥ 0.5 × 109/L3-150,3-164 | 1.90 (1.58-2.28) | .0001 |
| Platelets > 20 × 109/L3-151,3-164 | 1.82 (1.52-2.17) | .0001 |
| Platelets > 50 × 109/L3-152,3-164 | 1.73 (1.43-2.09) | .0001 |
| Grades II-IV acute GVHD3-153,3-164 | 1.19 (0.91-1.56) | .20 |
| Chronic GVHD (limited or extensive)3-154,3-161 | 1.30 (1.00-1.70) | .05 |
| Chronic GVHD (extensive only)3-160,3-162 | 1.44 (1.02-2.01) | .04 |
| Outcome . | Relative risk (95% CI) . | P value . |
|---|---|---|
| Absolute neutrophil count ≥ 0.5 × 109/L3-150,3-164 | 1.90 (1.58-2.28) | .0001 |
| Platelets > 20 × 109/L3-151,3-164 | 1.82 (1.52-2.17) | .0001 |
| Platelets > 50 × 109/L3-152,3-164 | 1.73 (1.43-2.09) | .0001 |
| Grades II-IV acute GVHD3-153,3-164 | 1.19 (0.91-1.56) | .20 |
| Chronic GVHD (limited or extensive)3-154,3-161 | 1.30 (1.00-1.70) | .05 |
| Chronic GVHD (extensive only)3-160,3-162 | 1.44 (1.02-2.01) | .04 |
GVHD indicates graft versus host disease.
Model stratified on Karnofsky performance score, GVHD prophylaxis, and use of growth factor granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor (G/GM-CSF); other significant variable was male sex (relative risk [RR], 0.80; P = .005).
Model stratified on GVHD prophylaxis; other significant variables were use of growth factor G/GM-CSF (RR, 0.79; P = .01) and Karnofsky performance score ≥ 90% (RR, 1.41; P = .002).
Model stratified on GVHD prophylaxis and use of growth factor G/GM-CSF; there were no other significant variables.
Model stratified on GVHD prophylaxis; there were no other significant variables.
Model stratified on sex and GVHD prophylaxis; other significant variable was age 40 years or older (RR, 1.36; P = .01).
Model stratified on sex and GVHD prophylaxis; there were no other significant variables.
No significant center effect was found.
Significant center effect was found; model adjusting for the influence of center showed higher risk of chronic GVHD for blood stem cell transplantation than for bone marrow transplantation (RR, 1.71; 95% confidence interval [CI], 1.08-2.71; P = .02).
Significant center effect was found; model adjusting for the influence of center showed higher risk of chronic GVHD for blood stem cell transplantation than for bone marrow transplantation (RR, 2.17; 95% CI, 1.13-4.18; P = .02).
Probabilities of neutrophil counts being at least 0.5 × 109/L after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation for acute leukemia and chronic myelogenous leukemia (CML).
Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table3).
Probabilities of neutrophil counts being at least 0.5 × 109/L after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation for acute leukemia and chronic myelogenous leukemia (CML).
Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table3).
Probabilities of platelet counts being at least 20 × 109/L after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation for acute leukemia and CML.
Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table3).
Probabilities of platelet counts being at least 20 × 109/L after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation for acute leukemia and CML.
Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table3).
In multivariate analyses stratified for GVHD prophylaxis, growth factor use, and Karnofsky performance score, the relative rate of neutrophil recovery to a count of at least 0.5 × 109/L with blood stem cells compared with bone marrow was 1.90 (95% CI, 1.58-2.28; P < .0001; Table 3). Similarly, relative rates of recovery to a platelet count of at least 20 × 109/L and at least 50 × 109/L were higher with blood stem cells than with bone marrow (Table 3). Probabilities of times to neutrophil and platelet recoveries after transplantation (adjusted for effects of other prognostic factors and derived from multivariate analyses) for each graft type are shown in Figures 1 and 2.
Acute GVHD
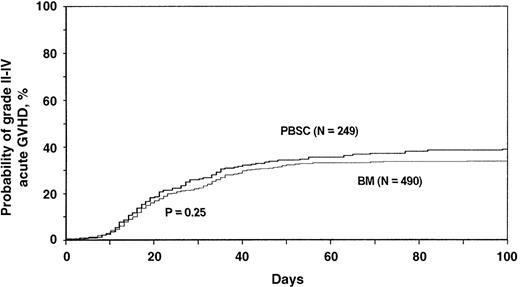
Risks of grades II to IV acute GVHD were similar with blood stem cell and bone marrow transplants (Table 3). Adjusted 100-day (after transplantation) probabilities of grades II to IV acute GVHD were 40% (95% CI, 33%-46%) with blood stem cells and 35% (95% CI, 31%-39%) with bone marrow (Figure 3). Results were similar for grades III to IV acute GVHD. Adjusted probabilities of grades III to IV acute GVHD after transplantation were 13% (95% CI, 8%-19%) with blood stem cells and 19% (95% CI, 15%-26%) with bone marrow.
Probabilities of grades II to IV acute graft versus host disease (GVHD) after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation for acute leukemia and CML.
Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table3).
Probabilities of grades II to IV acute graft versus host disease (GVHD) after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation for acute leukemia and CML.
Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table3).
Chronic GVHD
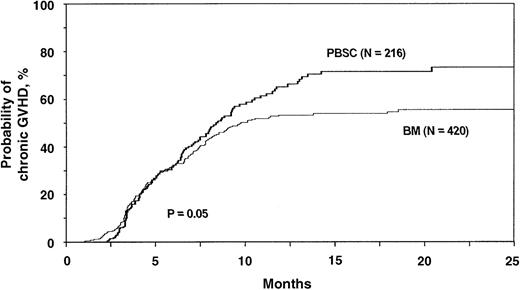
The risk of chronic GVHD in the first year after transplantation was higher in recipients of blood stem cell transplants than in recipients of bone marrow transplants. The probability of chronic GVHD at 1 year after transplantation was 65% (95% CI, 56%-72%) with blood stem cells and 53% (95% CI, 47%-59%) with bone marrow. Adjusted probabilities are shown in Figure4. The relative risk of having chronic GVHD after a blood stem cell transplantation compared with a bone marrow transplantation was 1.30 (95% CI, 1.00-1.70; Table 3). Similar results were obtained when only extensive chronic GVHD was considered. Data on the incidence, severity, and organ involvement of chronic GVHD are summarized in Table 4.
Probabilities of chronic GVHD (limited or extensive disease) after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation for acute leukemia and CML.
Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table3).
Probabilities of chronic GVHD (limited or extensive disease) after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation for acute leukemia and CML.
Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table3).
Comparison of severity and organ involvement among patients in whom chronic graft versus host disease developed after HLA-identical sibling blood stem cell transplantation or bone marrow transplantation
| Variable . | Bone marrow transplantation . | Blood stem cell transplantation . | P value4-150 . |
|---|---|---|---|
| Grade of graft versus host disease (GVHD) | [181] | [127] | .74 |
| Limited | 93 (51) | 62 (49) | |
| Extensive | 88 (49) | 65 (51) | |
| Severity of GVHD | [170] | [113] | .48 |
| Mild | 88 (52) | 56 (50) | |
| Moderate | 56 (33) | 44 (39) | |
| Severe | 26 (15) | 13 (11) | |
| Organ or system affected by GVHD | |||
| Skin | 120 (66) [181] | 81 (74) [109] | .15 |
| Eyes | 78 (44) [177] | 42 (38) [109] | .36 |
| Buccal mucosa | 111 (62) [179] | 68 (62) [109] | .95 |
| Lungs | 20 (11) [176] | 14 (13) [107] | .66 |
| Gastrointestinal | 63 (35) [178] | 27 (25) [108] | .07 |
| Genitourinary | 5 (3) [167] | 2 (2) [107] | .57 |
| Liver | 100 (55) [181] | 60 (55) [109] | .97 |
| Musculoskeletal | 18 (10) [177] | 8 (7) [107] | .45 |
| Variable . | Bone marrow transplantation . | Blood stem cell transplantation . | P value4-150 . |
|---|---|---|---|
| Grade of graft versus host disease (GVHD) | [181] | [127] | .74 |
| Limited | 93 (51) | 62 (49) | |
| Extensive | 88 (49) | 65 (51) | |
| Severity of GVHD | [170] | [113] | .48 |
| Mild | 88 (52) | 56 (50) | |
| Moderate | 56 (33) | 44 (39) | |
| Severe | 26 (15) | 13 (11) | |
| Organ or system affected by GVHD | |||
| Skin | 120 (66) [181] | 81 (74) [109] | .15 |
| Eyes | 78 (44) [177] | 42 (38) [109] | .36 |
| Buccal mucosa | 111 (62) [179] | 68 (62) [109] | .95 |
| Lungs | 20 (11) [176] | 14 (13) [107] | .66 |
| Gastrointestinal | 63 (35) [178] | 27 (25) [108] | .07 |
| Genitourinary | 5 (3) [167] | 2 (2) [107] | .57 |
| Liver | 100 (55) [181] | 60 (55) [109] | .97 |
| Musculoskeletal | 18 (10) [177] | 8 (7) [107] | .45 |
Values in square brackets are the numbers of patients evaluated in each category, values in parentheses are percentages of patients, and all other values are numbers of patients unless otherwise indicated.
By univariate analysis using χ2 statistic.
TRM
The relative risk of TRM with blood stem cell compared with bone marrow transplants differed according to the type and stage of leukemia. Among patients with acute leukemia in first remission, the risk of TRM in those who received blood stem cells was not different from the risk in those given bone marrow (Table5). The 1-year cumulative incidence of TRM was 18% (95% CI, 11%-26%) with blood stem cells and 28% (95% CI, 21%-35%) with bone marrow (Figure 5A). Among patients with acute leukemia in second remission, the risk of TRM was significantly lower after blood stem cell transplantation than after bone marrow transplantation (Table6). The 1-year cumulative incidence of TRM was 13% (95% CI, 5%-26%) with blood stem cells and 30% (95% CI, 16%-44%) with bone marrow (Figure 5A). Among patients with CML in first chronic phase, the risk of TRM was similar with blood stem cells and bone marrow (Table 5B). The 1-year cumulative incidences of TRM were 37% (95% CI, 25%-49%) and 27% (95% CI, 19%-32%), respectively, with blood stem cells and bone marrow (Figure 5B). Among patients who underwent transplantation while in accelerated or second chronic phase, TRM risk was significantly lower with blood stem cells (Table 6). The 1-year cumulative incidences of TRM were 26% (95% CI, 10%-46%) with blood stem cells and 67% (95% CI, 37%-85%) with bone marrow (Figure 6). Thus, use of blood stem cells rather than bone marrow decreased TRM in patients with advanced disease.
Relative risk of treatment-related mortality, disease-free survival, and relapse after blood stem cell transplantation compared with bone marrow transplantation for leukemia
| Outcome . | Patient group . | No. of patients . | RR5-150 [blood vs marrow] (95% CI) . | P value . | Adjusted 1-y probabilities, % (95% CI) . | ||
|---|---|---|---|---|---|---|---|
| Blood . | Bone marrow . | Blood . | Bone marrow . | ||||
| TRM5-151,5-152,5-160 | AL, CR1 | 112 | 204 | 0.59 (0.33-1.07) | .25 | 18 (11-26) | 28 (21-35) |
| AL, CR2 | 38 | 45 | 0.36 (0.13-0.99) | .04 | 13 (5-26) | 30 (16-44) | |
| CML, CP | 92 | 214 | 1.33 (0.82-2.15) | .27 | 37 (25-49) | 27 (19-32) | |
| CML, AP | 27 | 29 | 0.28 (0.12-0.67) | .004 | 26 (10-46) | 67 (37-85) | |
| TF/LFS5-151,5-153,5-160 | AL, CR1 | 82 | 181 | 0.76 (0.48-1.21) | .25 | 70 (56-80) | 61 (52-68) |
| AL, CR2 | 28 | 40 | 0.40 (0.18-0.92) | .03 | 77 (57-88) | 57 (40-71) | |
| CML, CP | 78 | 211 | 1.30 (0.82-2.08) | .27 | 63 (49-74) | 74 (66-80) | |
| CML, AP | 27 | 29 | 0.29 (0.14-0.59) | .0006 | 68 (45-84) | 23 (9-40) | |
| Relapse5-151,5-154,5-160 | AL, CR1 | 112 | 204 | 0.57 (0.04-7.66) | .67 | 14 (7-23) | 11 (7-17) |
| AL, CR2 | 38 | 45 | 0.24 (0.01-4.42) | .34 | 8 (2-20) | 13 (4-26) | |
| CML, CP | 92 | 214 | 0.48 (0.01-56.97) | .76 | 2 (1-8) | 1 (0.5-3) | |
| CML, AP | 27 | 29 | 1.02 (0.07-14.99) | .99 | 13 (3-30) | 21 (8-38) | |
| Outcome . | Patient group . | No. of patients . | RR5-150 [blood vs marrow] (95% CI) . | P value . | Adjusted 1-y probabilities, % (95% CI) . | ||
|---|---|---|---|---|---|---|---|
| Blood . | Bone marrow . | Blood . | Bone marrow . | ||||
| TRM5-151,5-152,5-160 | AL, CR1 | 112 | 204 | 0.59 (0.33-1.07) | .25 | 18 (11-26) | 28 (21-35) |
| AL, CR2 | 38 | 45 | 0.36 (0.13-0.99) | .04 | 13 (5-26) | 30 (16-44) | |
| CML, CP | 92 | 214 | 1.33 (0.82-2.15) | .27 | 37 (25-49) | 27 (19-32) | |
| CML, AP | 27 | 29 | 0.28 (0.12-0.67) | .004 | 26 (10-46) | 67 (37-85) | |
| TF/LFS5-151,5-153,5-160 | AL, CR1 | 82 | 181 | 0.76 (0.48-1.21) | .25 | 70 (56-80) | 61 (52-68) |
| AL, CR2 | 28 | 40 | 0.40 (0.18-0.92) | .03 | 77 (57-88) | 57 (40-71) | |
| CML, CP | 78 | 211 | 1.30 (0.82-2.08) | .27 | 63 (49-74) | 74 (66-80) | |
| CML, AP | 27 | 29 | 0.29 (0.14-0.59) | .0006 | 68 (45-84) | 23 (9-40) | |
| Relapse5-151,5-154,5-160 | AL, CR1 | 112 | 204 | 0.57 (0.04-7.66) | .67 | 14 (7-23) | 11 (7-17) |
| AL, CR2 | 38 | 45 | 0.24 (0.01-4.42) | .34 | 8 (2-20) | 13 (4-26) | |
| CML, CP | 92 | 214 | 0.48 (0.01-56.97) | .76 | 2 (1-8) | 1 (0.5-3) | |
| CML, AP | 27 | 29 | 1.02 (0.07-14.99) | .99 | 13 (3-30) | 21 (8-38) | |
CI indicates confidence interval; RR, relative risk; TRM, treatment-related mortality; AL, acute leukemia; CR1, 1st complete remission; CR2, 2nd complete remission; CML, chronic myelogenous leukemia; CP, chronic phase; AP, accelerated phase; and TF/LFS; treatment failure/leukemia-free survival.
Relative risks under 1.00 indicate a benefit for blood stem cell transplantation (lower risk of adverse outcome); relative risks greater than 1.00 indicate an adverse effect of blood stem cell transplantation (higher risk of adverse outcome).
Model stratified on the use of granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor.
Other significant variables were a Karnofsky performance score of 90 or higher (RR, 0.64; P = .01) and age 40 years or older (RR, 1.52; P = .006).
Other significant variables were a Karnofsky performance score of 90 or higher (RR, 0.64; P = .003) and age 40 years or older (RR, 1.33; P = .03).
No other significant variables.
No significant center effect.
Cumulative incidences of treatment-related mortality after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation, according to type of leukemia.
(A) Results in patients with acute leukemia. (B) Results in patients with CML (B).
Cumulative incidences of treatment-related mortality after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation, according to type of leukemia.
(A) Results in patients with acute leukemia. (B) Results in patients with CML (B).
Primary causes of death according to type of disease and transplant
| Disease/cause of death . | Blood stem cell transplant . | Bone marrow transplant . | P value . |
|---|---|---|---|
| Acute leukemia | [27] | [76] | |
| Primary disease | 16 | 33 | .24 |
| Infection | 22 | 15 | .37 |
| Interstitial pneumonitis | 12 | 11 | .93 |
| Graft-versus-host disease | 14 | 26 | .58 |
| Organ failure | 12 | 4 | .17 |
| Other | 24 | 11 | .06 |
| CML | [33] | [76] | |
| Primary disease | 8 | 3 | .16 |
| Infection | 30 | 25 | .73 |
| Interstitial pneumonitis | 17 | 18 | .81 |
| Graft-versus-host disease | 24 | 39 | .10 |
| Organ failure | 17 | 9 | .21 |
| New malignancy | — | 3 | .89 |
| Other | 4 | 3 | .60 |
| Disease/cause of death . | Blood stem cell transplant . | Bone marrow transplant . | P value . |
|---|---|---|---|
| Acute leukemia | [27] | [76] | |
| Primary disease | 16 | 33 | .24 |
| Infection | 22 | 15 | .37 |
| Interstitial pneumonitis | 12 | 11 | .93 |
| Graft-versus-host disease | 14 | 26 | .58 |
| Organ failure | 12 | 4 | .17 |
| Other | 24 | 11 | .06 |
| CML | [33] | [76] | |
| Primary disease | 8 | 3 | .16 |
| Infection | 30 | 25 | .73 |
| Interstitial pneumonitis | 17 | 18 | .81 |
| Graft-versus-host disease | 24 | 39 | .10 |
| Organ failure | 17 | 9 | .21 |
| New malignancy | — | 3 | .89 |
| Other | 4 | 3 | .60 |
Values in square brackets are numbers of patients evaluated; all other values are percentages of patients unless otherwise indicated.
Cumulative incidences of leukemia relapse after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation, according to type of leukemia.
(A) Results in patients with acute leukemia. (B) Results in patients with CML.
Cumulative incidences of leukemia relapse after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation, according to type of leukemia.
(A) Results in patients with acute leukemia. (B) Results in patients with CML.
Data on the duration of initial hospitalization were available for 224 blood stem cell transplant recipients and 491 bone marrow transplant recipients. Median time from transplantation to first hospital discharge was 23 days after blood stem cell transplantation and 28 days after bone marrow transplantation (P = .003).
Relapse
There was no apparent difference in the risk of relapse after blood stem cell and bone marrow transplantation, though the follow-up was relatively short to evaluate this outcome (Table 5). The 1-year cumulative incidences of relapse among patients with acute leukemia in first remission were 14% (95% CI, 7%-23%) with blood stems cells and 11% (95% CI, 7%-17%) with bone marrow (Figure 6A). Among patients who had transplantation while in second remission, the 1-year cumulative incidences of relapse were 8% (95% CI, 2%-20%) with blood stem cells and 13% (95% CI, 4%-26%) with bone marrow (Figure6A).
Patients with CML who underwent transplantation while in first chronic phase had a 1-year cumulative incidence of relapse of 2% (95% CI, 1%-8%) if they were given blood stem cells and 1% (95% CI, 0.5%-3%) if given bone marrow (Figure 6B). Among those who underwent transplantation while in accelerated phase, the 1-year cumulative incidences of relapse were 13% (95% CI, 3%-30%) with blood stem cells and 21% (95% CI, 8%-38%) with bone marrow (Figure 6B).
LFS
Similar to the results for TRM, the relation between graft type and LFS after transplantation varied according to the type and stage of leukemia. Among patients with acute leukemia in first remission, the risk of treatment failure (relapse or death; inverse of LFS) was similar with blood stem cell and bone marrow transplants (Table 5). Their adjusted 1-year probabilities of LFS were 70% (95% CI, 56%-80%) with blood stem cells and 61% (95% CI, 52%-68%) with bone marrow (Figure 7A). Among patients with acute leukemia in second remission, the risk of treatment failure was significantly lower after blood stem cell transplantation than after bone marrow transplantation (Table 5). Adjusted 1-year probabilities of LFS in these patients were 77% (95% CI, 57%-88%) with blood stem cells and 57% (95% CI, 40%-71%) with bone marrow (Figure 7A). Among patients with CML in first chronic phase, the risk of treatment failure was similar with blood stem cells and bone marrow (Table 5): their adjusted 1-year probabilities of LFS were 63% (95% CI, 49%-74%) and 74% (95% CI, 66%-80%), respectively, with blood stem cells and bone marrow (Figure 7B). Among patients who had transplantation while in accelerated or second chronic phase, treatment failure was significantly lower with blood stem cells (Table 5). Their adjusted 1-year probabilities of LFS were 68% (95% CI, 45%-84%) with blood stem cells and 23% (95% CI, 9%-40%) with bone marrow (Figure 7B). Causes of death after blood stem cell and bone marrow transplantation were similar (Table 6).
Probabilities of leukemia-free survival after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation, according to type of leukemia.
(A) Results in patients with acute leukemia. (B) Results in patients with CML. Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table 4).
Probabilities of leukemia-free survival after HLA-identical sibling blood stem cell transplantation compared with bone marrow transplantation, according to type of leukemia.
(A) Results in patients with acute leukemia. (B) Results in patients with CML. Probabilities were derived from multivariate Cox proportional hazards models and adjusted for effects of other significant covariates (Table 4).
Discussion
Previous studies of allogeneic blood stem cell transplantation indicated more rapid hematologic recovery than with allogeneic bone marrow transplantation and similar risks of acute GVHD. There are conflicting data on the risk of chronic GVHD. Most studies used historical bone marrow transplantation controls,6-15,22 although 1 small randomized trial has been reported.16 It is unclear from these data whether allogeneic blood stem cells have an advantage over bone marrow in TRM or overall survival.17 The current study compared early results of concurrent allogeneic blood stem cell and bone marrow transplantations by 105 teams that reported data to the IBMTR or EBMT. The source of allografts was chosen by the treatment center. All patients older than 20 years with acute leukemia in first or second remission or with CML in chronic or accelerated phase who underwent transplantation during the period studied were included. All patients had HLA-identical sibling donors and none received T-cell–depleted grafts. Although some differences existed, patient characteristics in the 2 groups were relatively well balanced. Multivariate analyses were used to adjust for potentially confounding effects of other variables. We found a more rapid hematologic recovery, with a shorter interval to recovery of neutrophils and platelets, with blood stem cell transplants than with bone marrow transplants. The risk of acute GVHD was comparable in the 2 groups, similar to results of previous studies.
An effect of graft type on TRM was observed in patients with advanced acute leukemia and accelerated CML. Patients with advanced leukemias who were given allogeneic bone marrow transplants were at a higher risk of both relapse and TRM than those given blood stem cells. The reduced TRM observed with blood stem cell transplants may have resulted from a more rapid and uniform hematopoietic recovery. Such recovery is probably more important in patients who are heavily pretreated or have active leukemia. Patients with acute leukemia in first remission or CML in chronic phase have intrinsically more stable conditions and a lower risk of death from toxicity and infection; thus, more rapid engraftment may not produce measurable survival benefits in them.
With a median follow-up time of 12 months and a maximum of 40 months, there was an increased risk of chronic GVHD with allogeneic blood stem cell transplants. This finding is consistent with several reports of a high incidence of chronic GVHD with blood stem cell grafts. Further follow-up is warranted to better assess this endpoint, to determine factors affecting its incidence, and to evaluate its effect on late mortality, leukemia relapse, and quality of life. Possible explanations are larger numbers of stem cells or T cells in the graft32or skewed cytokine production.33 Longer follow-up is also necessary to critically evaluate whether the risk of relapse is affected by the type of graft. It is possible that the higher rate of chronic GVHD with blood stem cell transplants may also be associated with greater graft versus leukemia effects, but no significant difference in relapse rates can currently be detected.
These data suggest that use of blood stem cells rather than bone marrow may improve the results of allogeneic transplantations in patients with advanced acute leukemia or CML, at least in the first year after transplantation. Overall outcome was similar for acute leukemia patients in first remission or CML patients in first chronic phase. Controlled clinical trials and longer follow-up, especially in good-risk patients, are necessary to better define the role of blood stem cells in allogeneic hematopoietic stem cell transplantation.
Supported by Public Health Service grants P01-CA-40053 and 1 U24 CA76518-01 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute, of the US Department of Health and Human Services; and grants from Amgen, Inc; Anonymous; Baxter Fenwal; Berlex Laboratories; BioWhitakker, Inc; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol-Myers Squibb Company; Cell Therapeutics, Inc; Center for Advanced Studies in Leukemia; Chimeric Therapies; Chiron Therapeutics; COBE BCT Inc; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Genentech, Inc; Human Genome Sciences; Immunex Corporation; Kettering Family Foundation; Kirin Brewery Company; Robert J. Kleberg Jr and Helen C. Kleberg Foundation; Herbert H. Kohl Charities, Inc; Nada and Herbert P. Mahler Charities; Milstein Family Foundation; Milwaukee Foundation/Elsa Schoeneich Research Fund; NeXstar Pharmaceuticals, Inc; Samuel Roberts Noble Foundation; Novartis Pharmaceuticals; Orphan Medical; Ortho Biotech, Inc; John Oster Family Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer, Inc; RGK Foundation; Roche Laboratories; SangStat Medical Corporation; Schering AG; Schering-Plough Oncology; Searle; SmithKline Beecham Pharmaceutical; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Foundation; Swiss National Research Foundation; SyStemix; United Resource Networks; and Wyeth-Ayerst Laboratories.
Reprints:Mary M. Horowitz, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Road, PO Box 26509, Milwaukee, WI 53226; e-mail: marymh@mcw.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal